Polymer-Based Scaffolds Loaded with Aloe vera Extract for the Treatment of Wounds
Abstract
:1. Introduction
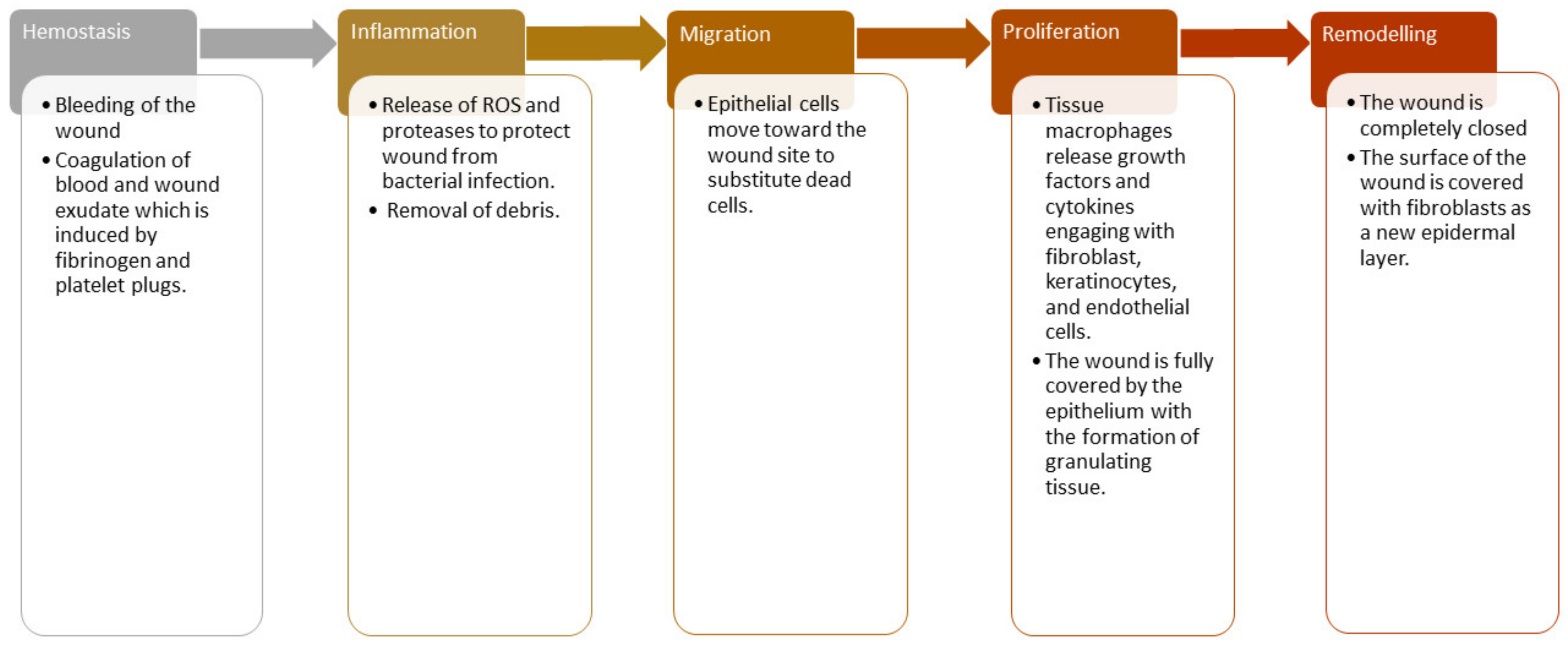
2. Phases of Wound Healing Process
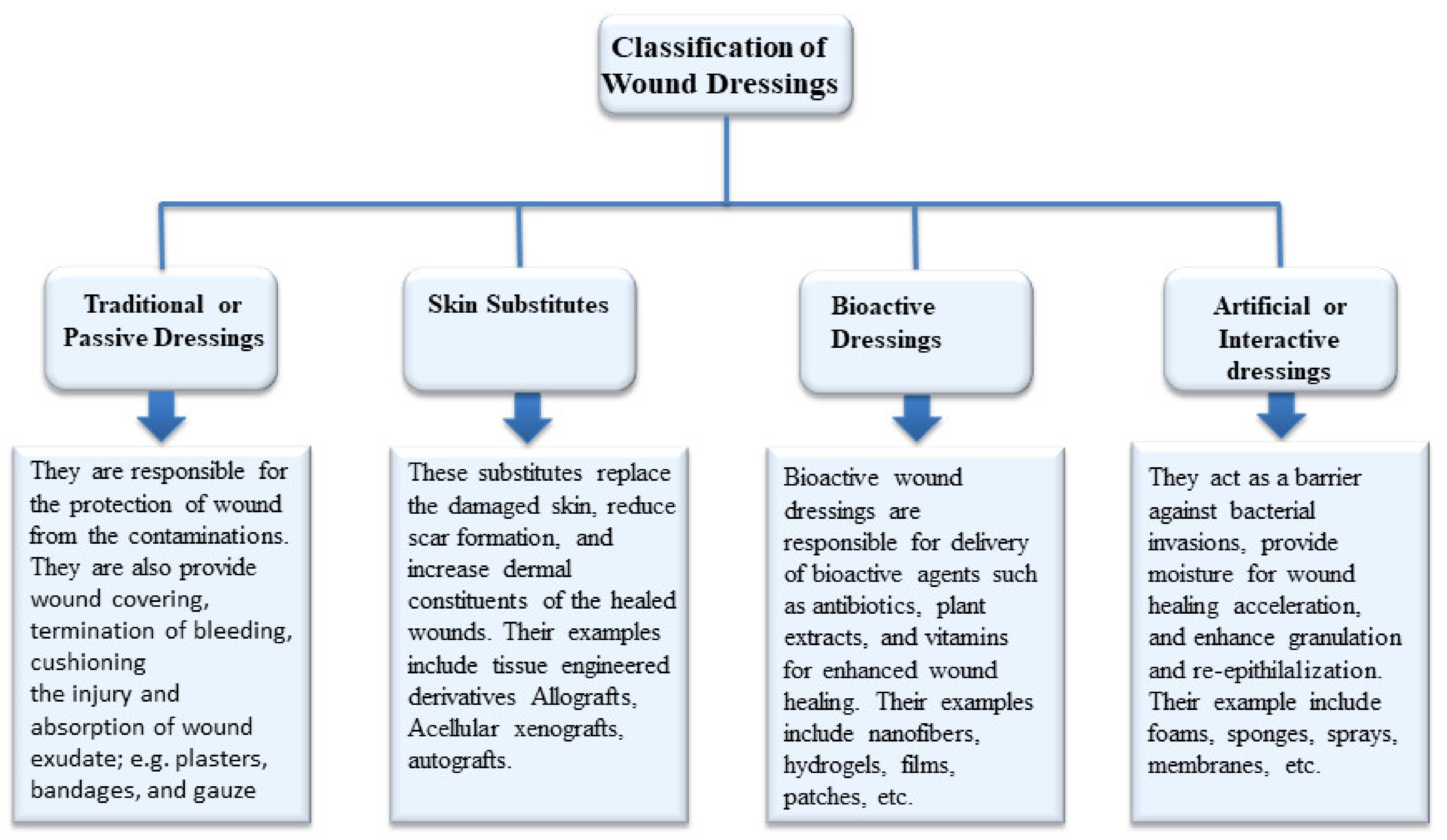
3. Classification of Wound Dressings
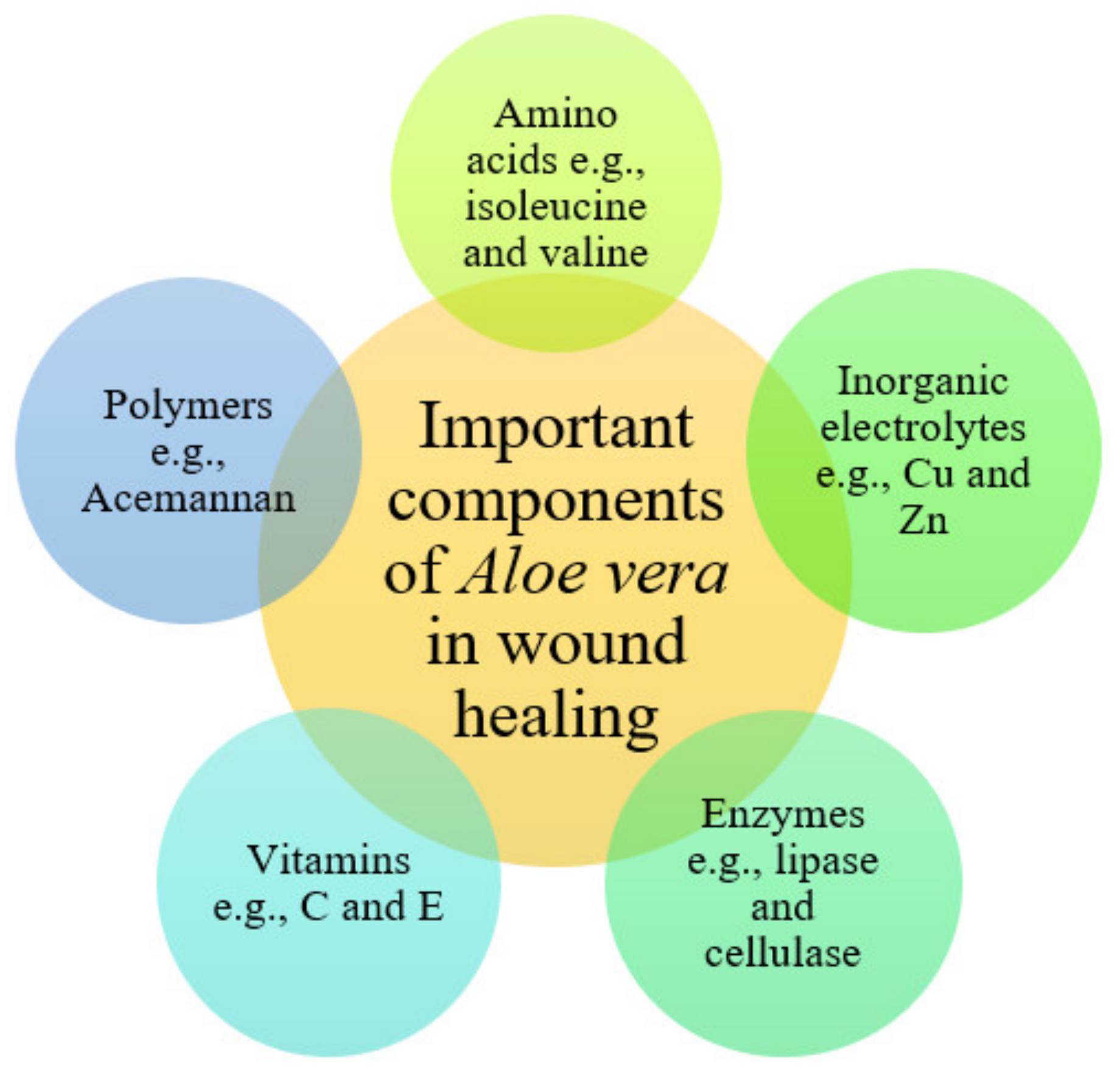
4. Biological Activities and Clinical Studies of Aloe vera in Wound Management
5. Polymer-Based Wound Dressings Scaffolds Enriched with Aloe vera
5.1. Nanofibers/Nanofibrous Materials
5.2. Films/Membranes
5.3. Hydrogels
5.4. Others
| Types of Wound Dressings Loaded with AV | Polymers Used | Effectiveness/Efficacy of Dressing | Harmfulness/Safety of Dressing | Ref |
|---|---|---|---|---|
| Nanofibers | Chitosan and PEO | Superior antibacterial efficacy against S. aureus and E. coli with fast full-thickness wound healing process. | The histological studies demonstrated high cell proliferation and increase blood vessels, indicating non-toxicity. | [67] |
| Silk fibroin and PVA | High antioxidant activity that can result in reduced toxic oxidation products in chronic wounds | These nanofibers were harmless when were incubated with fibroblasts, suggesting their safety in wound care. | [68] | |
| PVA, PVP, and PEG | No biological activities reported, but porosity was high and can promote acceleration of wound by stimulating high gaseous exchange and wound exudate absorption. | No cytotoxicity experiments reported. | [69] | |
| PVA and PAA | They were very effective against microbial strains (P. aeruginosa S. aureus, and E. coli)) | The cytotoxicity studies were not reported | [70] | |
| Gelatin and PCL | They were very effective against S. aureus and E. coli bacterial strains. | These scaffolds are safe because they showed high cell viability of fibroblasts. | [71] | |
| Chitosan and PEO | The initial burst drug release of AV can result in good biological efficacies. | The biocompatibility studies demonstrated non-toxicity on murine fibroblast cells. | [72] | |
| Chitosan and PVA | Excellent antibacterial efficacy against S. aureus and E. coli. | The nanofibers are safe to be used in wound healing due to their non-toxicity on murine fibroblasts. | [73] | |
| Gum tragacanth and PVA | These nanofibers can be effective in wound healing application due to their ability to absorb exudate | There was high cell proliferation of skin cells indicating good biocompatibility | [74] | |
| PVA | The fast release of AV can lead to good biological activities. | Not available | [75] | |
| PVA | Good antibacterial effectiveness against S. aureus and E. coli. | Not reported | [76] | |
| PCL | Excellent antibacterial efficacy against E. coli and S. aureus. | High cell proliferation and viability of human dermal fibroblasts indicating safety in the field of wound healing. | [77] | |
| Zein, PCL, and Collagen | High inhibition zones against S. aureus and E. coli, suggesting excellent antibacterial efficacy. | Cell adhesion and proliferation studies displayed no toxicity effect on fibroblasts, indicating that these nanofibers are harmless. | [78] | |
| Nanofiber membranes | Chitosan and PCL | Excellent bactericidal efficacy against E. coli. | Nanofibers were harmless on human umbilical vein endothelial cells, demonstrating their safety. | [79] |
| PLGA | Acceleration of full-thickness wound healing process. | The nanofibers were non-toxic although they showed a slightly low cell viability of 70%. | [80] | |
| PLGA | Fast wound recovery and reepithelization in full-thickness wound healing | Cell adhesion studies showed a high attachment of fibroblasts on nanofibers, showing non-toxicity. | [81] | |
| Nanofiber sponge | Chitosan and PVA | higher wound healing mechanism. | Cytocompatibility studies toward skin cells showed non-toxicity of nanofibers making them suitable for wound-healing applications. | [82] |
| Nanofiber pads | PVA | Drug release studies demonstrated that these pads could result in good biological activities. | Not available | [83] |
| Films | Chitosan | Good antibacterial synergistic activity against E. coli and S aureus with fast wound recovery. | High cell viability of about 112.49% of fibroblast cells, indicating that these films are very safe. | [85] |
| PVA | These films demonstrated favorable WVTR that can promote fast wound healing activity. | Good cell proliferation of fibroblasts showing non-toxicity. | [86] | |
| Alginate | High water uptake capacity that can reduce excess exudate to accelerate wound healing. | Not reported | [87] | |
| Chitosan | Appropriate WVTR that can lead to a fast wound-healing process | Not reported | [88] | |
| Alginate and PVA | Fast wound healing process | Not reported | [89] | |
| Alginate | Superior antibacterial efficacy against S. aureus than E. coli. Quick wound-healing process. | Excellent biocompatibility, indicating their safety. | [90] | |
| Chitosan and alginate | Excellent antibacterial activity against S. aureus and P. auregonosa | Good cytocompatibility, indicating non-toxicity. | [91] | |
| Alginate | Accelerated wound healing mechanism. | Not reported | [92] | |
| Alginate | faster wound healing mechanism. | Not reported | [93] | |
| Membranes | PVA, PEO, and carboxymethyl cellulose | High antibacterial activity against S. aureus and E. coli. | The drug release studies showed that these scaffolds are non-toxic. | [94] |
| PVA, PEO, and carboxymethyl cellulose | Moderate WVTR demonstrated that these dressings can promote fast wound healing. | The drug release profile displayed that these scaffolds are non-toxic. | [95] | |
| Chitosan | Quick MRSA-infected full-thickness wound healing process. | Histological studies demonstrated that these membranes are not harmful to skin cells. | [96] | |
| Dextran | Almost 100% bactericidal efficacy against both E. coli and S. aureus, with fast wound healing. | Good biocompatibility, showing safety to be used in wound treatment. | [97] | |
| Hydrogels | Polymethacrylic acid | High antimicrobial efficacy of 100% against S. aureus and more than 98% against E. coli, and good wound healing effects. | The histopathological experiment showed that these wound dressing are non-toxic to skin cells. | [102] |
| Alginate and gelatin | The quick biodegradation of these hydrogels can result in fast skin regeneration. | High cell viability and proliferation of fibroblast cells, indicating non-toxicity. | [103] | |
| poly (N-vinylpyrrolidone-Acrylamide) copolymer | Ability to induce wound healing. | Non-toxic. | [104] | |
| Alginate and PVA | Drug release profile demonstrated that these hydrogels could result in good biological activities. | Excellent biocompatibility and non-toxicity, indicating their safety | [105] | |
| Composite sponges | Chitosan | Higher inhibitory action against E. coli, S. aureus, K. pneumoniae, and B. subtilis. | Good cytocompatibility, confirming that they are harmless. | [106] |
| Cotton gauze | Cellulose | Good antibacterial activity against S. aureus and E. coli. | Non-toxicity effects when incubated with HepG2 cells. | [107] |
| Biocomposite dressing | Pectin and gelatin | Good radical scavenging and antibacterial efficacy with accelerated wound healing. | High cell viability when incubated with fibroblasts, indicating harmlessness. | [108] |
| Nanocapsules | Tragacanth gum | Rapid wound healing activity. | High cell viability of human fibroblasts, indicating non-toxicity. | [109] |
| Cotton fabric dressings | Tragacanth gum | Good antimicrobial efficacy against E. coli, S. aureus and C. albicans. | Good biocompatibility that can demonstrate safety in wound treatment. | [110] |
| Hollow fibers | Collagen | Excellent wound healing efficacy. | Cell migration rate, demonstrating non-toxicity. | [111] |
| Biocomposite wound dressing | Alginate and PEG | Good antibacterial activity against E. coli and S. aureus | High cell viability of human skin fibroblasts, suggesting safety. | [112] |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, 023267. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alven, S.; Nqoro, X.; Aderibigbe, B. Polymer-based materials loaded with curcumin for wound healing application. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A promising wound dressing material with excellent cytocompatibility and proangiogenesis action for wound healing: Strontium loaded Silk fibroin/Sodium alginate (SF/SA) blend films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Su, C.; Zhu, Z.; Wen, Y.; Shao, W. Development of gelatin/bacterial cellulose composite sponges as potential natural wound dressings. Int. J. Biol. Macromol. 2019, 133, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, X.; Xue, X.; Wu, D. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr. Polym. 2012, 88, 75–83. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Katas, H.; Hussain, F. Recent advances in polymer-based wound dressings for the treatment of diabetic foot ulcer: An overview of state-of-the-art. Curr. Drug Targets 2017, 18, 527–550. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Chand, N.; Ahuja, S.; Roy, M.K. Curcumin/cellulose micro crystals/chitosan films: Water absorption behavior and in vitro cytotoxicity. Int. J. Biol. Macromol. 2015, 75, 239–247. [Google Scholar] [CrossRef]
- Conzatti, G.; Chamary, S.; De Geyter, N.; Cavalie, S.; Morent, R.; Tourrette, A. Surface functionalization of plasticized chitosan fi lms through PNIPAM grafting via UV and plasma graft polymerization. Eur. Polym. J. 2018, 105, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Solaberrieta, I.; Jim, A.; Cacciotti, I.; Garrigos, M.G. Encapsulation of bioactive compounds from Aloe vera agrowastes in electrospun poly(ethylene oxide) nanofibers. Polymers 2020, 12, 1323. [Google Scholar] [CrossRef]
- Azevedo, J.; Juliao, E.; Dantas, J.; Reis, J. Is Aloe vera effective for wound healing? The state of the art abstract. J. Oral Diagn. 2019, 04, e20190005. [Google Scholar]
- Hashemi, S.A.; Madani, S.A.; Abediankenari, S. The review on properties of Aloe vera in healing of cutaneous wounds. Rev. Prop. Aloe Vera Heal. Cutan. Wounds 2015, 2015, 714216. [Google Scholar] [CrossRef] [Green Version]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Yaşayan, G.; Karaca, G.; Akgüner, Z.P.; Öztürk, A.B. Chitosan/collagen composite films as wound dressings encapsulating allantoin and lidocaine hydrochloride. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 623–635. [Google Scholar] [CrossRef]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Khan, G.; Pandey, V.K.; Bakade, B.V.; Mishra, B. Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: A potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound. Int. J. Pharm. 2019, 567, 118480. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hyper- trophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Horng, H.; Yeh, C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.; Yu, C.; Huang, Q.L.; Du, Y.Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotech. 2019, 17, 82. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of silk fibroin use in wound dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for wound dressings: An up-to-date overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Walker, R.M.; Gillespie, B.M.; Thalib, L.; Higgins, N.S.; Whitty, J.A. Foam dressings for treating pressure injuries in patients of any age in any care setting: An abridged Cochrane systematic review. Int. J. Nurs. Stud. 2018, 87, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2019, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound dressings: A comprehensive review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Dhivyaa, S.; Padmab, V.V.; Santhinia, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef]
- Mir, M.; Najabat, M.; Afifa, A.; Ayesha, B.; Munam, G.; Shizza, A. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf. B Biointerfaces 2017, 156, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, M.C.; Chifiriuc, M.; Marutescu, L.; Gheorghe, I.; Lazar, V.; Bolocan, A.; Bertesteanu, S. Bioactive wound dressings for the management of chronic wounds. Curr. Org. Chem. 2017, 21, 53–63. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Maria, Z. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Phytomedicine therapeutic potential of Aloe vera—A miracle gift of nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef]
- Gorsi, F.I.; Kausar, T.; Murtaza, M.A. Evaluation of antibacterial and antioxidant activity of Aloe vera (Aloe barbadensis Miller) gel powder using different solvents. Pure Appl. Biol. 2019, 8, 1265–1270. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Ansaria, M.A.; Khan, H.M.; Alzohairy, M.A.; Choi, I. Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci. Rep. 2016, 6, 20414. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, purification, structural characteristics, biological activities and pharmacological applications of acemannan, a polysaccharide from Aloe vera: A review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maenthaisong, R.; Chaiyakunapruk, N.; Niruntraporn, S.; Kongkaew, C. The efficacy of aloe vera used for burn wound healing: A systematic review. Burns 2007, 33, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, A.; Perrin, C.; Austin, S.; Vera, F.A. Quality and authenticity of commercial Aloe vera gel powders. Food Chem. 2007, 103, 22–30. [Google Scholar] [CrossRef]
- Tarameshloo, M.; Norouzian, M.; Zarein-dolab, S.; Dadpay, M. Aloe vera gel and thyroid hormone cream may improve wound healing in Wistar rats. Anat. Cell Biol. 2012, 45, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Eshun, K.; He, Q. Aloe vera: A valuable ingredient for the food, pharmaceutical and cosmetic industries—A review. Crit. Rev. Food Sci. Nutr. 2010, 44, 91–96. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tizard, I.R. Activation of a mouse macrophage cell line by acemannan: The major carbohydrate fraction from Aloe vera gel. Immunopharmacology 1996, 35, 119–128. [Google Scholar] [CrossRef]
- Moriyama, M.; Moriyama, H.; Uda, J.; Kubo, H.; Nakajima, Y.; Goto, A.; Akaki, J.; Yoshida, I.; Matsuoka, N.; Hayakawa, T. Beneficial effects of the genus aloe on wound healing, cell proliferation, and differentiation of epidermal keratinocytes. PLoS ONE 2016, 11, 0164799. [Google Scholar] [CrossRef]
- Liu, L.Y.; Chen, X.D.; Wu, B.; Jiang, Q. Influence of Aloe polysaccharide on proliferation and hyaluronic acid and hydroxyproline secretion of human fibroblasts in vitro. J. Chin. Integr. Med. 2010, 8, 256–262. [Google Scholar] [CrossRef]
- Moghbel, A.; Ghalambor, A.; Allipanah, S. Wound healing and toxicity evaluation of Aloe vera cream on outpatients with second degree burns. Iran. J. Pharm. Sci. Summer 2007, 3, 157–160. [Google Scholar]
- Somboonwong, J.; Thanamittramanee, S.; Jariyapongskul, A.; Patumraj, S. Therapeutic effects of Aloe vera on cutaneous microcirculation and wound healing in second degree burn model in rats. J. Med. Assoc. Thail. 2000, 83, 417–425. [Google Scholar]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The effect of Aloe vera clinical trials on prevention and healing of skin wound: A systematic review. Iran. J. Med. Sci. 2019, 44, 1–9. [Google Scholar]
- West, D.P.; Zhu, Y.F. Evaluation of Aloe vera gel gloves in the treatment of dry skin associated with occupational exposure. Am. J. Infect. Control 2003, 31, 40–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshghi, F.; Hosseinimehr, S.J.; Rahmani, N.; Khademloo, M.; Norozi, M.S.; Hojati, O. Effects of Aloe vera cream on posthemorrhoidectomy pain and wound healing: Results of a randomized, blind, placebo-control study. J. Altern. Complementary Med. 2010, 16, 647–650. [Google Scholar] [CrossRef] [Green Version]
- Burusapat, C.; Supawan, M.; Pruksapong, C.; Pitiseree, A.; Suwantemee, C. Topical Aloe vera gel for accelerated wound healing of split-thickness skin graft donor Sites: A double-blind, randomized, controlled trial and systematic review. Plast. Reconstr. Surg. 2018, 142, 217. [Google Scholar] [CrossRef]
- Molazem, Z.; Mohseni, F.; Masoumeh Younesi, M.; Keshavarzi, S. Aloe vera gel and cesarean wound healing; A randomized controlled clinical trial. Glob. J. Health Sci. 2015, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorasani, G.; Hosseinimehr, S.J.; Azadbakht, M.; Zamani, A.; Mahdavi, M.R. Aloe versus silver sulfadiazine creams for second-degree burns: A randomized controlled study. Surg. Today 2009, 39, 587–591. [Google Scholar] [CrossRef]
- Hosseini, A.M.; Ghaffarzadegan, R.; Alizadeh, S.A.; Ghaffarzadegan, R.; Agaei, R.H.; Ahmadlou, M. Effect of Aloe vera gel, compared to 1% silver sulfadiazine cream on second-degree burn wound healing. Complementary Med. J. Fac. Nurs. Midwif. 2013, 3, 418–428. [Google Scholar]
- Rahmani, N.; Khademloo, M.; Vosoughi, K.; Assadpour, S. Effects of Aloe vera cream on chronic anal fissure pain, wound healing and hemorrhaging upon defection: A prospective double blind clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1078–1084. [Google Scholar]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Electrospun starch nanofibers: Recent advances, challenges, and strategies for potential pharmaceutical application. J. Control. Release 2017, 252, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Arthanari, S.; Mani, G.; Jang, J.H.; Choi, J.O.; Cho, Y.H.; Lee, J.H.; Cha, S.E.; Oh, H.S.; Kwon, D.H.; Jang, H.T. Preparation and characterization of gatifloxacin- loaded alginate/poly (vinyl alcohol) electrospun nanofibers. Artif. Cells Nanomed. Biotechnol. Int. J. 2016, 44, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Nadem, S.; Ziyadi, H.; Hekmati, M.; Baghali, M. Cross-linked poly(vinyl alcohol) nanofibers as drug carrier of clindamycin. Polym. Bull. 2019, 77, 5615–5629. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Pathalamuthu, P.; Siddharthan, A.; Giridev, V.R.; Victoria, V.; Thangam, R.; Sivasubramanian, S.; Savariar, V.; Hemamalini, T. Enhanced performance of Aloe vera incorporated chitosan-polyethylene oxide electrospun wound scaffold produced using novel Spirograph based collector assembly. Int. J. Biol. Macromol. 2019, 140, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Kheradvar, S.A.; Nourmohammadi, J.; Tabesh, H.; Bagheri, B. Starch nanoparticle as a vitamin E-TPGS carrier loaded in silk fibroin-poly(vinyl alcohol)-Aloe vera nanofibrous dressing. Colloids Surf. B Biointerfaces 2018, 166, 9–16. [Google Scholar] [CrossRef]
- Uslu, İ.; Keskin, S.; Gül, A.; Karabulut, T.C.; Aksu, M.L. Preparation and properties of electrospun poly (vinyl alcohol) blended hybrid polymer with Aloe vera and HPMC as wound dressing. Hacet. J. Biol. Chem. 2010, 38, 19–25. [Google Scholar]
- Serinçay, H.; Özkan, S.; Yılmaz, N.; Koçyiğit, S.; Uslu, I.; Gürcan, S. PVA/PAA-based antibacterial wound dressing material with Aloe vera. Polym. Plast. Technol. Eng. 2013, 52, 1308–1315. [Google Scholar] [CrossRef]
- Baghersad, S.; Bahrami, S.H.; Ranjbar, M.; Reza, M.; Mojtahedi, M.; Brouki, P. Development of biodegradable electrospun gelatin/aloe-vera/poly (ε-caprolactone) hybrid nano fibrous scaffold for application as skin substitutes. Mater. Sci. Eng. C 2018, 93, 367–379. [Google Scholar] [CrossRef]
- Nikbakht, M.; Salehi, M.; Razayat, S.; Majidi, R. Various parameters in the preparation of chitosan/polyethylene oxide electrospun nanofibers containing Aloe vera extract for medical applications. Nanomedicine 2020, 7, 21–28. [Google Scholar]
- Rafieian, S.; Mahdavi, H.; Masoumi, M.E. Improved mechanical, physical and biological properties of chitosan films using Aloe vera and electrospun PVA nanofibers for wound dressing applications. J. Ind. Text. 2019. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M. Characteristics of aloe vera incorporated poly (e-caprolactone)/ gum tragacanth nanofibers as dressings for wound care. J. Ind. Eng. Chem. 2018, 47, 1464–1477. [Google Scholar] [CrossRef]
- Sirima, S.; Phiriyawirut, M.; Suttisintong, K. Comparison of the release of Aloe vera extracts from poly (vinyl alcohol) electrospun fibers and hydrogel films for wound healing applications. Key Eng. Mater. 2017, 751, 592–598. [Google Scholar] [CrossRef]
- Bootdee, K.; Nithitanakul, M. Poly(d,l-lactide-co-glycolide) nanospheres within composite poly ( vinyl alcohol )/ aloe vera electrospun nanofiber as a novel wound dressing for controlled release of drug. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 223–230. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Ramalingam, R.; Dhand, C.; Lakshminarayanan, R.; Sadiq, A.; Gandhimathi, C.; Ramakrishna, S.; Bay, B.H.; Venugopal, J.R.; Srinivasan, D.K. Biocompatible Aloe vera and tetracycline hydrochloride loaded hybrid nanofibrous scaffolds for skin tissue engineering. Int. J. Mol. Sci. 2019, 20, 5174. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera -loaded nanofibrous scaffold based on zein/polycaprolactone/collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef]
- Yin, J.; Xu, L. Batch preparation of electrospun polycaprolactone/ chitosan/aloe vera blended nanofiber membranes for novel wound dressing. Int. J. Biol. Macromol. 2020, 160, 352–363. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Garcia-Garcia, P.; Gutierrez, F.B.; Aguirre, J.J.; Hernandez, R.M.; Delgado, A.; Igartua, M. Composite nano fi brous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing applications. Int. J. Pharm. 2019, 556, 320–329. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Farzamfar, S.; Salehi, M.; Vaez, A.; Tajerian, R.; Azami, M. Erythropoietin/aloe polyvinyl alcohol/chitosan sponge-like wound dressing: In vitro and in vivo studies. J. Bioact. Compat. Polym. 2018, 33, 269–281. [Google Scholar] [CrossRef]
- Isfahani, F.R.; Tavanai, H.; Morshed, M. Release of Aloe vera from electrospun Aloe vera-PVA nanofibrous pad. Fibers Polym. 2017, 18, 264–271. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; You, L.; Tarafder, S.; Zou, L.; Fang, Z.; Chen, J.; Lee, C.H.; Zhang, Q. Curcumin-releasing chitosan/aloe membrane for skin regeneration. Chem. Eng. J. 2019, 359, 1111–1119. [Google Scholar] [CrossRef]
- Hajian, M.; Mahmoodi, M.; Imani, R. In vitro assessment of poly (Vinyl Alcohol) film incorporating Aloe vera for potential application as a wound dressing. J. Macromol. Sci. Part B Phys. 2017, 2348, 435–450. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bartolo, P. Preparation and characterization of Films based on alginate and Aloe vera. Int. J. Polym. Anal. Charact. 2011, 5341, 449–464. [Google Scholar] [CrossRef]
- Khoshgozaran-Abras, S.; Hossein, M.; Hamidy, Z.; Bagheripoor-Fellah, N. Mechanical, physicochemical and color properties of chitosan based-films as a function of Aloe vera gel incorporation. Carbohydr. Polym. 2012, 87, 2058–2062. [Google Scholar] [CrossRef]
- Pereira, G.G.; Guterres, S.S.; Balducci, A.G.; Colombo, P.; Sonvico, F. Polymeric films loaded with vitamin E and Aloe vera for topical application in the treatment of burn wounds. BioMed Res. Int. 2014, 2014, 641590. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.; Nath, M.S.; Mathew, N.; Reshmy, R.; Philip, E.; Latha, M.S. Alginate film modified with aloe vera gel and cellulose nanocrystals for wound dressing application: Preparation, characterization and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101894. [Google Scholar] [CrossRef]
- Gómez Chabala, L.F.; Cuartas, C.E.E.; López, M.E.L. Release behavior and antibacterial activity of chitosan/alginate blends with Aloe vera and silver nanoparticles. Mar. Drugs 2017, 15, 328. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.F.; Carvalho, A.; Gil, M.H.; Mendes, A.; Bártolo, P.J. Influence of Aloe vera on water absorption and enzymatic in vitro degradation of alginate hydrogel films. Carbohydr. Polym. 2013, 98, 311–320. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef]
- Gupta, B.; Agarwal, R.; Alam, M.S. Antimicrobial and release study of drug loaded PVA/PEO/CMC wound dressings. J. Mater. Sci. Mater. Med. 2014, 25, 1613–1622. [Google Scholar] [CrossRef]
- Gupta, B.; Agarwal, R.; Alam, M.S. Aloe vera loaded poly (vinyl alcohol)–poly (ethylene oxide)-carboxymethyl cellulose-polyester nonwoven membranes. J. Biomater. Tissue Eng. 2013, 3, 503–511. [Google Scholar] [CrossRef]
- Ranjbar, R.; Yousefi, A. Effects of Aloe vera and chitosan nanoparticle thin-film membranes on wound healing in full thickness infected wounds with methicillin resistant Staphylococcus aureus. Bull. Emerg. Trauma 2018, 6, 8–15. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.; Gupta, B. Scar free healing mediated by the release of aloe vera and manuka honey from dextran bionanocomposite wound dressings. Int. J. Biol. Macromol. 2018, 120, 1581–1590. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B. Combination therapy strategies for the treatment of malaria. Molecules 2019, 24, 3601. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.; Chu, C. Synthesis of photoresponsive hybrid alginate hydrogel with photo-controlled release behavior. Carbohydr. Polym. 2015, 119, 18–25. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modi fi ed chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 98, 217–233. [Google Scholar] [CrossRef]
- Anjum, S.; Gupta, A.; Sharma, D.; Gautam, D.; Bhan, S.; Sharma, A.; Kapil, A.; Gupta, B. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater. Sci. Eng. C 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Dadashzadeh, A.; Imani, R.; Moghassemi, S.; Omidfar, K.; Abolfathi, N. Study of hybrid alginate/gelatin hydrogel-incorporated niosomal Aloe vera capable of sustained release of Aloe vera. Polym. Bull. 2020, 77, 387–403. [Google Scholar] [CrossRef]
- Dey, A.; Bera, R.; Chakrabarty, D. Influence of Aloe vera on the properties of N-vinylpyrrolidone-Acrylamide copolymer hydrogel. Mater. Chem. Phys. 2015, 168, 168–179. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Malarz, K.; Mrozek-Wilczkiewicz, A. Advanced SA/PVA-based hydrogel matrices with prolonged release of Aloe vera as promising wound dressings. Mater. Sci. Eng. C 2020, 120, 111667. [Google Scholar] [CrossRef]
- Anbazhagan, S.; Thangavelu, K.P. Application of tetracycline hydrochloride loaded-fungal chitosan and Aloe vera extract based composite sponges for wound dressing. J. Adv. Res. 2018, 14, 63–71. [Google Scholar] [CrossRef]
- Salah, F.; El Ghoul, Y.; Alminderej, F.M.; El Golli-Bennour, E.; Ouanes, Z.; Maciejak, O.; Jarroux, N.; Majdoub, H.; Sakli, F. Development, characterization, and biological assessment of biocompatible cellulosic wound dressing grafted Aloe vera bioactive polysaccharide. Cellulose 2019, 26, 4957–4970. [Google Scholar] [CrossRef]
- Tummalapalli, M.; Berthet, M.; Verrier, B.; Deopura, B.L.; Alam, M.S.; Gupta, B. Composite wound dressings of pectin and gelatin with Aloe vera and curcumin as bioactive agents. Int. J. Biol. Macromol. 2016, 82, 104–113. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M.; Mahmoudi Rad, M. Encapsulation of Aloe Vera extract into natural Tragacanth Gum as a novel green wound healing product. Int. J. Biol. Macromol. 2016, 93, 344–349. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M.; Mahmoudi Rad, M. Simultaneous encapsulation and stabilization of Aloe vera extract on cotton fabric for wound dressing application. RSC Adv. 2016, 6, 111895. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Abdel-Rahman, R.M.; Kubena, I.; Kobera, L.; Spotz, Z.; Zboncak, M.; Prikryl, R.; Brus, J.; Jancar, J. Chitosan-glucan complex hollow fibers reinforced collagen wound dressing embedded with Aloe vera. Part I: Preparation and characterization. Carbohydr. Polym. 2020, 230, 115708. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Elizalde, I.; Bernáldez-Sarabia, J.; Moreno-Ulloa, A.; Vilanova, C.; Juárez, P.; Licea-Navarro, A.; Castro-Ceseña, A.B. Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications. Carbohydr. Polym. 2019, 206, 455–467. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alven, S.; Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Polymer-Based Scaffolds Loaded with Aloe vera Extract for the Treatment of Wounds. Pharmaceutics 2021, 13, 961. https://doi.org/10.3390/pharmaceutics13070961
Alven S, Khwaza V, Oyedeji OO, Aderibigbe BA. Polymer-Based Scaffolds Loaded with Aloe vera Extract for the Treatment of Wounds. Pharmaceutics. 2021; 13(7):961. https://doi.org/10.3390/pharmaceutics13070961
Chicago/Turabian StyleAlven, Sibusiso, Vuyolwethu Khwaza, Opeoluwa O. Oyedeji, and Blessing A. Aderibigbe. 2021. "Polymer-Based Scaffolds Loaded with Aloe vera Extract for the Treatment of Wounds" Pharmaceutics 13, no. 7: 961. https://doi.org/10.3390/pharmaceutics13070961
APA StyleAlven, S., Khwaza, V., Oyedeji, O. O., & Aderibigbe, B. A. (2021). Polymer-Based Scaffolds Loaded with Aloe vera Extract for the Treatment of Wounds. Pharmaceutics, 13(7), 961. https://doi.org/10.3390/pharmaceutics13070961









