Transdermal Delivery of Chemotherapeutics: Strategies, Requirements, and Opportunities
Abstract
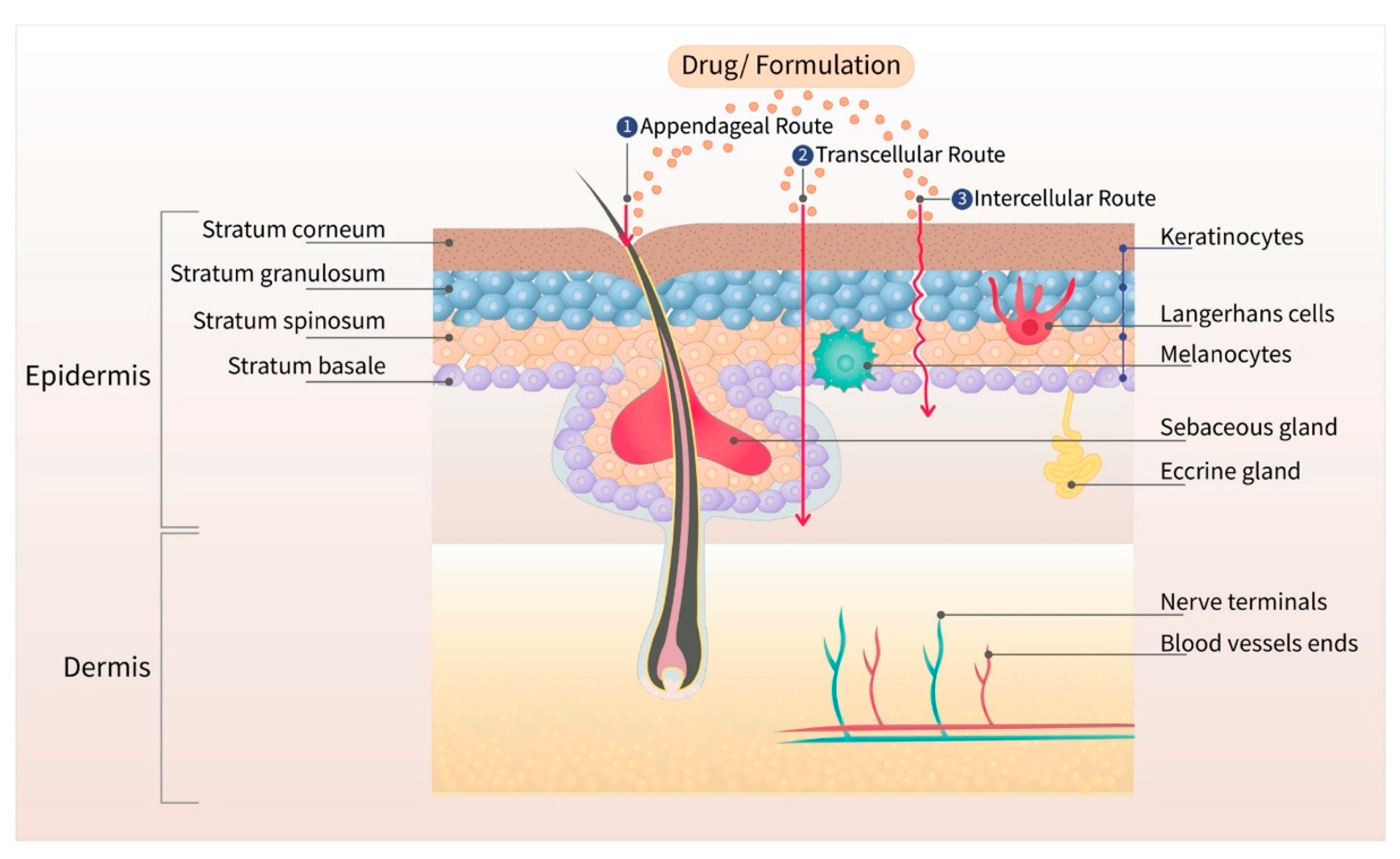
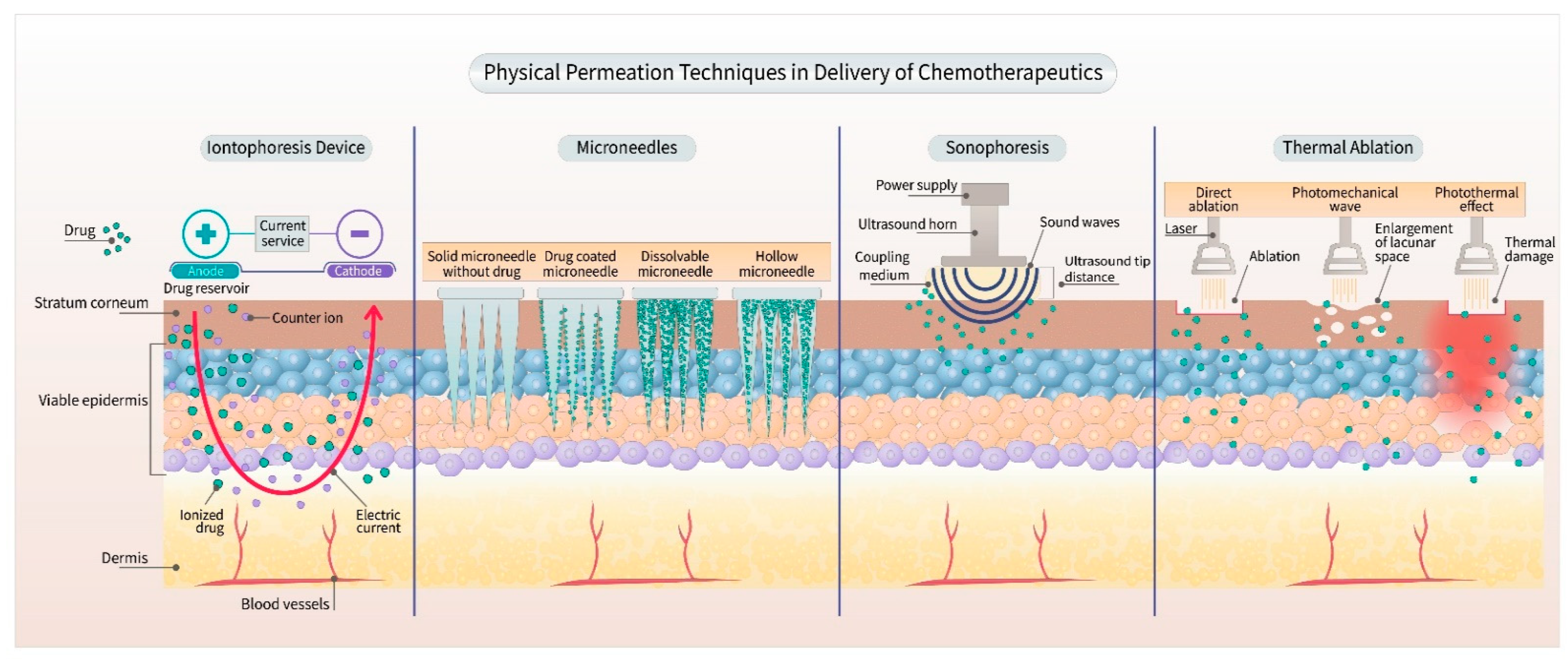
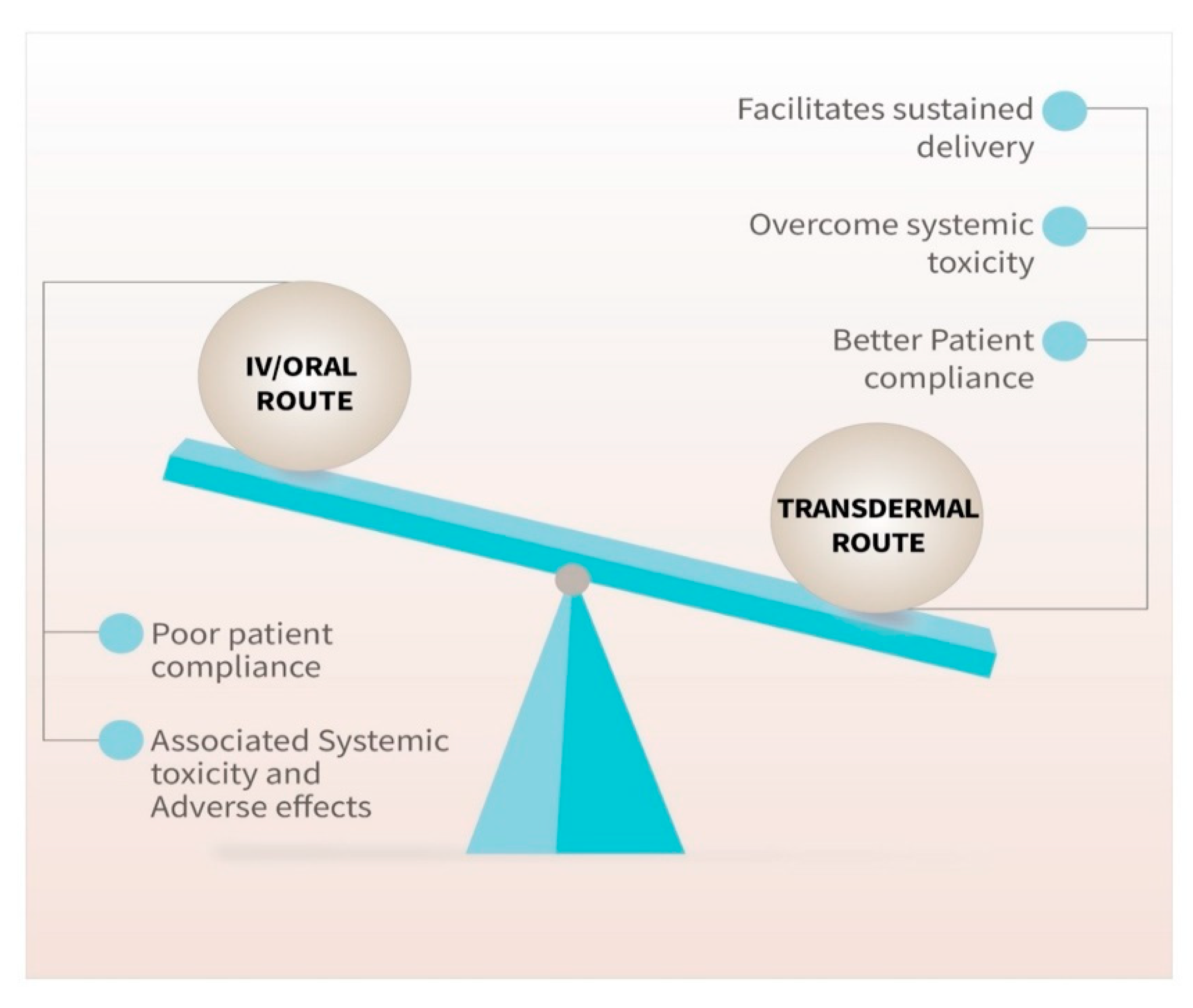
1. Introduction
2. Transdermal Chemotherapeutics for Breast Cancer
2.1. Tamoxifen Citrate
2.2. Letrozole
2.3. Anastrozole
3. Transdermal Chemotherapeutics for Melanoma
3.1. Imatinib Mesylate (IM)
3.2. Vemurafenib
3.3. Five-Aminolevulinic Acid (5-ALA) Hydrochloride
4. Plant Product–Based Transdermal Chemotherapeutics
4.1. Curcumin
4.2. Resveratrol
5. Transdermal-Vaccine-Based Cancer Management
6. Transdermal Permeation Study Using Selected Chemotherapeutic Agents
6.1. Five-Fluorouracil (5-FU)
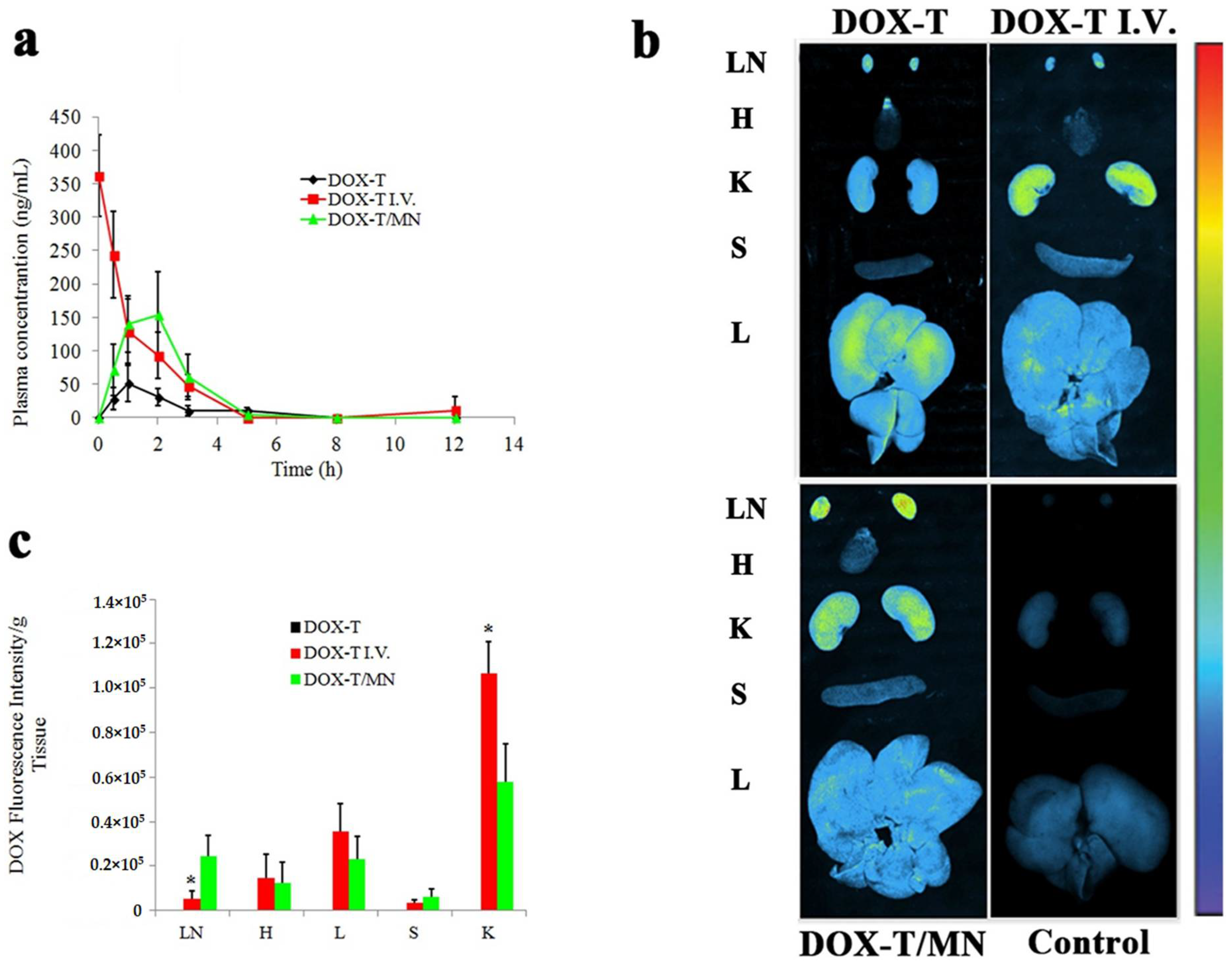
6.2. Doxorubicin Hydrochloride (DOX)
6.3. Methotrexate (MTX)
6.4. Paclitaxel (PTX)
7. Formulating for Efficacy™ (FFE)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Langer, R. Drug delivery and targeting. Nature 1998, 392 (Suppl. 6679), 5–10. [Google Scholar]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Experimental Dermatology: Viewpoint 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Wiedersberg, S.; Guy, R.H. Transdermal drug delivery: 30+ years of war and still fighting! J. Control. Release 2014, 190, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Neubert, R. Overcoming the stratum corneum: The modulation of skin penetration. Ski. Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Elias, P.M.; Friend, D.S. The permeability barrier in mammalian epidermis. J. Cell Biol. 1975, 65, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; Swartzendruber, D.C.; Squier, C.A. Regional variation in the structure and permeability of oral mucosa and skin. Adv. Drug Deliv. Rev. 1993, 12, 1–12. [Google Scholar] [CrossRef]
- Iyer, R.; Mok, S.; Savkovic, S.; Turner, L.; Fraser, G.; Desai, R.; Jayadev, V.; Conway, A.; Handelsman, D. Pharmacokinetics of testosterone cream applied to scrotal skin. Andrology 2017, 5, 725–731. [Google Scholar] [CrossRef]
- Basaria, S.; Dobs, A.S. New modalities of transdermal testosterone replacement. Treat. Endocrinol. 2003, 2, 1–9. [Google Scholar] [CrossRef]
- Ibrahim, S.A. Spray-on transdermal drug delivery systems. Expert Opin. Drug Deliv. 2015, 12, 195–205. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Skin permeation: The years of enlightenment. Int. J. Pharm. 2005, 305, 2–12. [Google Scholar] [CrossRef]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef]
- Castro, G.A.; Oréfice, R.L.; Vilela, J.M.; Andrade, M.S.; Ferreira, L.A. Development of a new solid lipid nanoparticle formulation containing retinoic acid for topical treatment of acne. J. Microencapsul. 2007, 24, 395–407. [Google Scholar] [CrossRef]
- Chourasia, R.; Jain, S.K. Drug targeting through pilosebaceous route. Curr. Drug Targets 2009, 10, 950–967. [Google Scholar] [CrossRef]
- Toll, R.; Jacobi, U.; Richter, H.; Lademann, J.; Schaefer, H.; Blume-Peytavi, U. Penetration profile of microspheres in follicular targeting of terminal hair follicles. J. Investig. Dermatol. 2004, 123, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S. Solute-vehicle-skin interactions in percutaneous absorption: The principles and the people. Ski. Pharmacol. Physiol. 2013, 26, 356–370. [Google Scholar] [CrossRef]
- Chu, K.A.; Yalkowsky, S.H. An interesting relationship between drug absorption and melting point. Int. J. Pharm. 2009, 373, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting drugs across biological barriers. Adv. Mater. 2017, 29, 1606596. [Google Scholar] [CrossRef]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- WHO. Radiation: Ultraviolet (UV) Radiation and Skin Cancer. Available online: https://www.who.int/news-room/q-a-detail/ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 6 July 2020).
- AIM at Melanoma Foundation. Melanoma Stat, Facts and Fig. Available online: https://www.aimatmelanoma.org/about-melanoma/melanoma-stats-facts-and-figures/ (accessed on 6 July 2020).
- American Cancer Society. “Cancer Facts and Figures 2021”. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 8 May 2021).
- Breast Cancer Research Foundation. Breast Cancer Statistics and Resources. 2020. Available online: https://www.bcrf.org/breast-cancer-statistics-and-resources (accessed on 23 June 2021).
- World Health Organization. Press Release. Latest World Cancer Statistics. Available online: https://www.iarc.who.int/wp-content/uploads/2018/07/pr223_E.pdf (accessed on 8 May 2021).
- Jiang, T.; Xu, G.; Chen, G.; Zheng, Y.; He, B.; Gu, Z. Progress in transdermal drug delivery systems for cancer therapy. Nano Res. 2020, 13, 1810–1824. [Google Scholar] [CrossRef]
- Kurakula, M.; Chen, L.; Tiwari, A.K.; Srinivas, N.R.; Dash, R.P.; Panizzi, P.R.; Arnold, R.D.; Babu, R.J. Recent Advances in Lipid-Based Nanovesicular Delivery Systems for Melanoma Therapy. Crit. Rev. Ther. Drug Carr. Syst. 2021, 38, 1–38. [Google Scholar] [CrossRef]
- Pei, P.; Yang, F.; Liu, J.; Hu, H.; Du, X.; Hanagata, N.; Zhao, S.; Zhu, Y. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater. Sci. 2018, 6, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef]
- Daftardar, S.; Neupane, R.; Boddu, S.H.; Renukuntla, J.; Tiwari, A.K. Advances in ultrasound mediated transdermal drug delivery. Curr. Pharm. Des. 2019, 25, 413–423. [Google Scholar] [CrossRef]
- Lim, D.-J.; Vines, J.B.; Park, H.; Lee, S.-H. Microneedles: A versatile strategy for transdermal delivery of biological molecules. Int. J. Biol. Macromol. 2018, 110, 30–38. [Google Scholar] [CrossRef]
- Franz, T.J. Percutaneous absorption. On the relevance of in vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef]
- Clemons, M.; Danson, S.; Howell, A. Tamoxifen (‘Nolvadex’): A review: Antitumour treatment. Cancer Treat. Rev. 2002, 28, 165–180. [Google Scholar] [CrossRef]
- Jordan, V.C. Tamoxifen (ICI46, 474) as a targeted therapy to treat and prevent breast cancer. Br. J. Pharmacol. 2006, 147, S269–S276. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Cawthorn, S.; Hamed, H.; Holli, K.; Howell, A.; Forbes, J.F.; Investigators, I.-I. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015, 16, 67–75. [Google Scholar] [CrossRef]
- Waters, E.A.; Cronin, K.A.; Graubard, B.I.; Han, P.K.; Freedman, A.N. Prevalence of tamoxifen use for breast cancer chemoprevention among US women. Cancer Epidemiol. Prev. Biomark. 2010, 19, 443–446. [Google Scholar] [CrossRef]
- Brauch, H.; Schroth, W.; Goetz, M.P.; Mürdter, T.E.; Winter, S.; Ingle, J.N.; Schwab, M.; Eichelbaum, M. Tamoxifen use in postmenopausal breast cancer: CYP2D6 matters. J. Clin. Oncol. 2013, 31, 176. [Google Scholar] [CrossRef] [PubMed]
- Teft, W.A.; Gong, I.Y.; Dingle, B.; Potvin, K.; Younus, J.; Vandenberg, T.A.; Brackstone, M.; Perera, F.E.; Choi, Y.-H.; Zou, G. CYP3A4 and seasonal variation in vitamin D status in addition to CYP2D6 contribute to therapeutic endoxifen level during tamoxifen therapy. Breast Cancer Res. Treat. 2013, 139, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.; Page, K.; Ivancic, D.; Helenowski, I.; Parini, V.; Sullivan, M.E.; Margenthaler, J.A.; Chatterton, R.T.; Jovanovic, B.; Dunn, B.K. A randomized phase II presurgical trial of transdermal 4-hydroxytamoxifen gel versus oral tamoxifen in women with ductal carcinoma in situ of the breast. Clin. Cancer Res. 2014, 20, 3672–3682. [Google Scholar] [CrossRef]
- Helland, T.; Hagen, K.B.; Haugstøyl, M.E.; Kvaløy, J.T.; Lunde, S.; Lode, K.; Lind, R.A.; Gripsrud, B.H.; Jonsdottir, K.; Gjerde, J. Drug monitoring of tamoxifen metabolites predicts vaginal dryness and verifies a low discontinuation rate from the Norwegian Prescription Database. Breast Cancer Res. Treat. 2019, 177, 185–195. [Google Scholar] [CrossRef]
- Fontein, D.; Seynaeve, C.; Hadji, P.; Hille, E.; van de Water, W.; Putter, H.; Kranenbarg, E.; Hasenburg, A.; Paridaens, R.J.; Vannetzel, J.-M. Specific adverse events predict survival benefit in patients treated with tamoxifen or aromatase inhibitors: An international tamoxifen exemestane adjuvant multinational trial analysis. J. Clin. Oncol. 2013, 31, 2257–2264. [Google Scholar] [CrossRef]
- Pathan, I.B.; Setty, C.M. Enhancement of transdermal delivery of tamoxifen citrate using nanoemulsion vehicle. Int. J. Pharm. Tech. Res. 2011, 3, 287–297. [Google Scholar]
- Lin, S.L.; Chan, W.P.; Choy, C.-S.; Leung, T.-K. Enhancement of transdermal delivery of indomethacin and tamoxifen by far-infrared ray-emitting ceramic material (Bioceramic): A pilot study. Transl. Med. 2013. [Google Scholar] [CrossRef]
- Lee, O.; Ivancic, D.; Chatterton, R.T., Jr.; Rademaker, A.W.; Khan, S.A. In vitro human skin permeation of endoxifen: Potential for local transdermal therapy for primary prevention and carcinoma in situ of the breast. Breast Cancer: Targets Ther. 2011, 3, 61. [Google Scholar] [CrossRef]
- Yang, Y.; Pearson, R.M.; Lee, O.; Lee, C.W.; Chatterton, R.T., Jr.; Khan, S.A.; Hong, S. Dendron-Based Micelles for Topical Delivery of Endoxifen: A Potential Chemo-Preventive Medicine for Breast Cancer. Adv. Funct. Mater. 2014, 24, 2442–2449. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Choudhary, M.; Kandasubramanian, B. Recent advances in dendrimer-based nanoplatform for cancer treatment: A review. Eur. Polym. J. 2020, 126, 109546. [Google Scholar] [CrossRef]
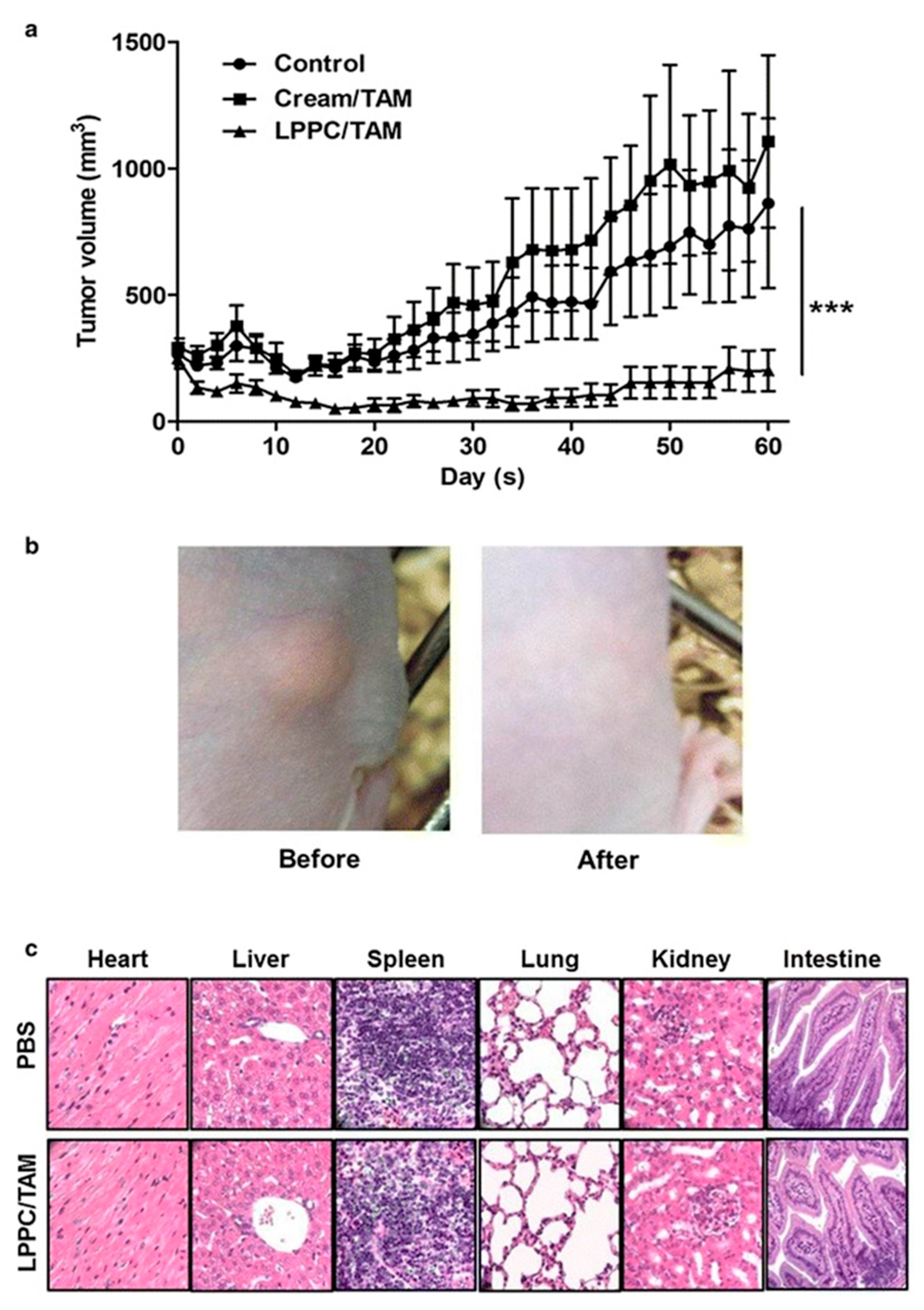
- Lin, Y.-L.; Chen, C.-H.; Wu, H.-Y.; Tsai, N.-M.; Jian, T.-Y.; Chang, Y.-C.; Lin, C.-H.; Wu, C.-H.; Hsu, F.-T.; Leung, T.K. Inhibition of breast cancer with transdermal tamoxifen-encapsulated lipoplex. J. Nanobiotechnol. 2016, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.S. The discovery and mechanism of action of letrozole. Breast Cancer Res. Treat. 2007, 105, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Lønning, P.; Dowsett, M.; Powles, T. Postmenopausal estrogen synthesis and metabolism: Alterations caused by aromatase inhibitors used for the treatment of breast cancer. J. Steroid Biochem. 1990, 35, 355–366. [Google Scholar] [CrossRef]
- Perez, E.A. The balance between risks and benefits: Long-term use of aromatase inhibitors. Eur. J. Cancer Suppl. 2006, 4, 16–25. [Google Scholar] [CrossRef]
- Chetrite, G.; Cortes-Prieto, J.; Philippe, J.; Wright, F.; Pasqualini, J. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J. Steroid Biochem. Mol. Biol. 2000, 72, 23–27. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Fang, L.; Liu, Y.; Sun, Y.; Wang, M.; Zhao, N.; He, Z. The transdermal patches for site-specific delivery of letrozole: A new option for breast cancer therapy. AAPS PharmSciTech 2010, 11, 1054–1057. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Fang, L.; Xu, X.; Liu, Y.; Sun, Y.; He, Z. Formulation and biopharmaceutical evaluation of a transdermal patch containing letrozole. Biopharm. Drug Dispos. 2010, 31, 138–149. [Google Scholar] [CrossRef]
- Maniyar, M.; Chakraborty, A.; Kokare, C. Formulation and evaluation of letrozole-loaded spray dried liposomes with PEs for topical application. J. Liposome Res. 2020, 30, 274–284. [Google Scholar] [CrossRef]
- Geisler, J. Differences between the non-steroidal aromatase inhibitors anastrozole and letrozole–of clinical importance? Br. J. Cancer 2011, 104, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Yang, Y.; Zhao, D.; Fang, L.; Sun, L.; Mu, L.; Liu, J.; Zhao, N.; Zhao, Y.; Zheng, N. Transdermal patches for site-specific delivery of anastrozole: In vitro and local tissue disposition evaluation. Int. J. Pharm. 2010, 391, 73–78. [Google Scholar] [CrossRef]
- Regenthal, R.; Voskanian, M.; Baumann, F.; Teichert, J.; Brätter, C.; Aigner, A.; Abraham, G. Pharmacokinetic evaluation of a transdermal anastrozole-in-adhesive formulation. Drug Des. Dev. Ther. 2018, 12, 3653. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.D.; Hamamoto, D.; Ilha, J.; dos Santos Pereira, A.; De Nucci, G. Anastrozole quantification in human plasma by high-performance liquid chromatography coupled to photospray tandem mass spectrometry applied to pharmacokinetic studies. J. Chromatogr. B 2007, 850, 553–559. [Google Scholar] [CrossRef]
- An, X.; Tiwari, A.K.; Sun, Y.; Ding, P.-R.; Ashby, C.R., Jr.; Chen, Z.-S. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: A review. Leuk. Res. 2010, 34, 1255–1268. [Google Scholar] [CrossRef]
- Waller, C.F. Imatinib mesylate. In Small Molecules in Hematology; Springer: Cham, Switzerland, 2018; pp. 1–27. [Google Scholar]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on gold nanoparticles and their applications. Toxicol. Environ. Health Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Larese Filon, F.; Crosera, M.; Adami, G.; Bovenzi, M.; Rossi, F.; Maina, G. Human skin penetration of gold nanoparticles through intact and damaged skin. Nanotoxicology 2011, 5, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Filon, F.L.; Crosera, M.; Timeus, E.; Adami, G.; Bovenzi, M.; Ponti, J.; Maina, G. Human skin penetration of cobalt nanoparticles through intact and damaged skin. Toxicol. In Vitro 2013, 27, 121–127. [Google Scholar] [CrossRef]
- Labala, S.; Mandapalli, P.K.; Kurumaddali, A.; Venuganti, V.V.K. Layer-by-layer polymer coated gold nanoparticles for topical delivery of imatinib mesylate to treat melanoma. Mol. Pharm. 2015, 12, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A two-stage poly (ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef]
- Labala, S.; Jose, A.; Chawla, S.R.; Khan, M.S.; Bhatnagar, S.; Kulkarni, O.P.; Venuganti, V.V.K. Effective melanoma cancer suppression by iontophoretic co-delivery of STAT3 siRNA and imatinib using gold nanoparticles. Int. J. Pharm. 2017, 525, 407–417. [Google Scholar] [CrossRef]
- Bollag, G.; Tsai, J.; Zhang, J.; Zhang, C.; Ibrahim, P.; Nolop, K.; Hirth, P. Vemurafenib: The first drug approved for BRAF-mutant cancer. Nat. Rev. Drug Discov. 2012, 11, 873–886. [Google Scholar] [CrossRef]
- Wellbrock, C.; Hurlstone, A. BRAF as therapeutic target in melanoma. Biochem. Pharmacol. 2010, 80, 561–567. [Google Scholar] [CrossRef]
- da Rocha Dias, S.; Salmonson, T.; van Zwieten-Boot, B.; Jonsson, B.; Marchetti, S.; Schellens, J.H.; Giuliani, R.; Pignatti, F. The European Medicines Agency review of vemurafenib (Zelboraf®) for the treatment of adult patients with BRAF V600 mutation-positive unresectable or metastatic melanoma: Summary of the scientific assessment of the Committee for Medicinal Products for Human Use. Eur. J. Cancer 2013, 49, 1654–1661. [Google Scholar] [PubMed]
- Spengler, E.K.; Kleiner, D.E.; Fontana, R.J. Vemurafenib-induced granulomatous hepatitis. Hepatology 2017, 65, 745–748. [Google Scholar] [CrossRef]
- Launay-Vacher, V.; Zimner-Rapuch, S.; Poulalhon, N.; Fraisse, T.; Garrigue, V.; Gosselin, M.; Amet, S.; Janus, N.; Deray, G. Acute renal failure associated with the new BRAF inhibitor vemurafenib: A case series of 8 patients. Cancer 2014, 120, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ding, W.; Zhang, Y.; Cheng, S.; Li, F.; Ruan, R.; Wei, P.; Qiu, B. Peptide-modified vemurafenib-loaded liposomes for targeted inhibition of melanoma via the skin. Biomaterials 2018, 182, 1–12. [Google Scholar] [CrossRef]
- Ruan, R.; Jin, P.; Zhang, L.; Wang, C.; Chen, C.; Ding, W.; Wen, L. Peptide-chaperone-directed transdermal protein delivery requires energy. Mol. Pharm. 2014, 11, 4015–4022. [Google Scholar] [CrossRef]
- Leman, J.; Dick, D.; Morton, C. Topical 5-ALA photodynamic therapy for the treatment of cutaneous T-cell lymphoma. Clin. Exp. Dermatol. 2002, 27, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.; Szeimies, R.M.; Sidoroff, A.; Braathen, L. European guidelines for topical photodynamic therapy part 1: Treatment delivery and current indications–actinic keratoses, Bowen’s disease, basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 536–544. [Google Scholar] [CrossRef]
- Lopez, R.F.V.; Lange, N.; Guy, R.; Bentley, M.V.L.B. Photodynamic therapy of skin cancer: Controlled drug delivery of 5-ALA and its esters. Adv. Drug Deliv. Rev. 2004, 56, 77–94. [Google Scholar] [CrossRef]
- Chen, H.M.; Liu, C.M.; Yang, H.; Chou, H.Y.; Chiang, C.P.; Kuo, M.Y.P. 5-aminolevulinic acid induce apoptosis via NF-κB/JNK pathway in human oral cancer Ca9–22 cells. J. Oral Pathol. Med. 2011, 40, 483–489. [Google Scholar] [CrossRef]
- Pierre, M.B.R.; Tedesco, A.C.; Marchetti, J.M.; Bentley, M.V.L. Stratum corneum lipids liposomes for the topical delivery of 5-aminolevulinic acid in photodynamic therapy of skin cancer: Preparation and in vitro permeation study. BMC Dermatol. 2001, 1, 1–6. [Google Scholar] [CrossRef]
- Lin, M.-W.; Huang, Y.-B.; Chen, C.-L.; Wu, P.-C.; Chou, C.-Y.; Wu, P.-C.; Hung, S.-Y. A formulation study of 5-aminolevulinic encapsulated in DPPC liposomes in melanoma treatment. Int. J. Med. Sci. 2016, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Russo, S.; Gerace, D.; Alonci, A.; Musolino, C. Anticancer activity of curcumin and its analogues: Preclinical and clinical studies. Cancer Investig. 2017, 35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Young, P.M.; Traini, D.; Mason, R.S.; Rohanizadeh, R. Recent advances in curcumin nanoformulation for cancer therapy. Expert Opin. Drug Deliv. 2014, 11, 1183–1201. [Google Scholar] [CrossRef]
- Sun, Y.; Du, L.; Liu, Y.; Li, X.; Li, M.; Jin, Y.; Qian, X. Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-β-cyclodextrin for melanoma treatment. Int. J. Pharm. 2014, 469, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Labala, S.; Venuganti, V.V.K. Co-delivery of curcumin and STAT3 siRNA using deformable cationic liposomes to treat skin cancer. J. Drug Target. 2017, 25, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Kortylewski, M.; Jove, R.; Yu, H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005, 24, 315–327. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, M.; Dai, E.; Luo, Y. Molecular targets of curcumin in breast cancer. Mol. Med. Rep. 2019, 19, 23–29. [Google Scholar] [CrossRef]
- Atlan, M.; Neman, J. Targeted transdermal delivery of curcumin for breast cancer prevention. Int. J. Environ. Res. Public Health 2019, 16, 4949. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Curcumin-loaded ultradeformable nanovesicles as a potential delivery system for breast cancer therapy. Colloids Surf. B Biointerfaces 2018, 167, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cyclodextrin nanosponge based hydrogel for the transdermal co-delivery of curcumin and resveratrol: Development, optimization, in vitro and ex vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 55–64. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.J.S. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef]
- Fontecave, M.; Lepoivre, M.; Elleingand, E.; Gerez, C.; Guittet, O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 1998, 421, 277–279. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Lu, I.-J.; Fu, Y.-S.; Fang, Y.-P.; Huang, Y.-B.; Wu, P.-C. Nanocarriers enhance the transdermal bioavailability of resveratrol: In-vitro and in-vivo study. Colloids Surf. B Biointerfaces 2016, 148, 650–656. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Q.; Ma, C.; Xia, Q. Non-aqueous self-double-emulsifying drug delivery system: A new approach to enhance resveratrol solubility for effective transdermal delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 360–369. [Google Scholar] [CrossRef]
- Qi, X.; Wang, L.; Zhu, J.; Hu, Z.; Zhang, J.J.I.j.o.p. Self-double-emulsifying drug delivery system (SDEDDS): A new way for oral delivery of drugs with high solubility and low permeability. Int. J. Pharm. 2011, 409, 245–251. [Google Scholar] [CrossRef]
- Park, S.N.; Jo, N.R.; Jeon, S.H. Chitosan-coated liposomes for enhanced skin permeation of resveratrol. J. Ind. Eng. Chem. 2014, 20, 1481–1485. [Google Scholar] [CrossRef]
- Pentek, T.; Newenhouse, E.; O’Brien, B.; Chauhan, A.S. Development of a topical resveratrol formulation for commercial applications using dendrimer nanotechnology. Molecules 2017, 22, 137. [Google Scholar] [CrossRef]
- Carletto, B.; Berton, J.; Ferreira, T.N.; Dalmolin, L.F.; Paludo, K.S.; Mainardes, R.M.; Farago, P.V.; Favero, G.M. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surf. B Biointerfaces 2016, 144, 65–72. [Google Scholar] [CrossRef]
- Palliyage, G.H.; Hussein, N.; Mimlitz, M.; Weeder, C.; Alnasser, M.H.A.; Singh, S.; Ekpenyong, A.; Tiwari, A.K.; Chauhan, H. Novel Curcumin-Resveratrol Solid Nanoparticles Synergistically Inhibit Proliferation of Melanoma Cells. Pharm. Res. 2021, 38, 851–871. [Google Scholar] [CrossRef]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Munger, K.; Baldwin, A.; Edwards, K.M.; Hayakawa, H.; Nguyen, C.L.; Owens, M.; Grace, M.; Huh, K. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004, 78, 11451–11460. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Jia, R.; Zhou, L.; Guo, J.; Fan, M. Fusion of CTLA-4 with HPV16 E7 and E6 enhanced the potency of therapeutic HPV DNA vaccine. PLoS ONE 2014, 9, e108892. [Google Scholar] [CrossRef] [PubMed]
- Kashem, S.W.; Haniffa, M.; Kaplan, D.H. Antigen-presenting cells in the skin. Annu. Rev. Immunol. 2017, 35, 469–499. [Google Scholar] [CrossRef]
- Cheung, Y.-K.; Cheng, S.C.-S.; Sin, F.W.-Y.; Xie, Y. Plasmid encoding papillomavirus Type 16 (HPV16) DNA constructed with codon optimization improved the immunogenicity against HPV infection. Vaccine 2004, 23, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Kines, R.C.; Zarnitsyn, V.; Johnson, T.R.; Pang, Y.-Y.S.; Corbett, K.S.; Nicewonger, J.D.; Gangopadhyay, A.; Chen, M.; Liu, J.; Prausnitz, M.R. Vaccination with human papillomavirus pseudovirus-encapsidated plasmids targeted to skin using microneedles. PLoS ONE 2015, 10, e0120797. [Google Scholar] [CrossRef]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.; Ali, A.A.; McCrudden, C.M.; McBride, J.W.; McCaffrey, J.; Robson, T.; Kett, V.L.; Dunne, N.J.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer: Strategic optimisation of RALA mediated gene delivery from a biodegradable microneedle system. Eur. J. Pharm. Biopharm. 2018, 127, 288–297. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef]
- McGrath, M.G.; Vucen, S.; Vrdoljak, A.; Kelly, A.; O’Mahony, C.; Crean, A.M.; Moore, A. Production of dissolvable microneedles using an atomised spray process: Effect of microneedle composition on skin penetration. Eur. J. Pharm. Biopharm. 2014, 86, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.; McCaffrey, J.; Ali, A.A.; McBride, J.W.; McCrudden, C.M.; Vincente-Perez, E.M.; Donnelly, R.F.; McCarthy, H.O. Dissolving microneedles for DNA vaccination: Improving functionality via polymer characterization and RALA complexation. Hum. Vaccines Immunother. 2017, 13, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.B.; Cheng, Y.C. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol. Ther. 1990, 48, 381–395. [Google Scholar] [CrossRef]
- Diasio, R.B.; Harris, B.E. Clinical pharmacology of 5-fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Grem, J.L. 5-Fluorouracil: Forty-plus and still ticking. A review of its preclinical and clinical development. Investig. New Drugs 2000, 18, 299–313. [Google Scholar] [CrossRef]
- Gross, K.; Kircik, L.; Kricorian, G. 5% 5-Fluorouracil cream for the treatment of small superficial Basal cell carcinoma: Efficacy, tolerability, cosmetic outcome, and patient satisfaction. Dermatol. Surg. 2007, 33, 433–440. [Google Scholar] [CrossRef]
- Lee, W.R.; Shen, S.C.; Wang, K.H.; Hu, C.H.; Fang, J.Y. The effect of laser treatment on skin to enhance and control transdermal delivery of 5-fluorouracil. J. Pharm. Sci. 2002, 91, 1613–1626. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Huang, C.-T.; Tsou, H.-Y.; Fu, L.-T.; Fu, Y.-S.; Tsai, Y.-H.; Wu, P.-C. The transport effect of submicron emulsions on 5-flurouracil topical application. J. Microencapsul. 2013, 30, 425–431. [Google Scholar] [CrossRef]
- Raviraj, V.; Pham, B.T.; Kim, B.J.; Pham, N.T.; Kok, L.F.; Painter, N.; Delic, N.C.; Jones, S.K.; Hawkett, B.S.; Lyons, J.G. Non-invasive transdermal delivery of chemotherapeutic molecules in vivo using superparamagnetic iron oxide nanoparticles. Cancer Nanotechnol. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Guo, P.; Pi, C.; Zhao, S.; Fu, S.; Yang, H.; Zheng, X.; Zhang, X.; Zhao, L.; Wei, Y. Oral co-delivery nanoemulsion of 5-fluorouracil and curcumin for synergistic effects against liver cancer. Expert Opin. Drug Deliv. 2020, 17, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Neervannan, S. Preclinical formulations for discovery and toxicology: Physicochemical challenges. Expert Opin. Drug Metab. Toxicol. 2006, 2, 715–731. [Google Scholar] [CrossRef]
- Anirudhan, T.; Nair, A.S.; Bino, S.J. Nanoparticle assisted solvent selective transdermal combination therapy of curcumin and 5-flurouracil for efficient cancer treatment. Carbohydr. Polym. 2017, 173, 131–142. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Abilash, V.; PB, T.P.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy—An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Bankar, N.G.; Kulkarni, M.V.; Venuganti, V.V.K. Dissolvable microneedle patch containing doxorubicin and docetaxel is effective in 4T1 xenografted breast cancer mouse model. Int. J. Pharm. 2019, 556, 263–275. [Google Scholar] [CrossRef]
- Yang, H.; Wu, X.; Zhou, Z.; Chen, X.; Kong, M. Enhanced transdermal lymphatic delivery of doxorubicin via hyaluronic acid based transfersomes/microneedle complex for tumor metastasis therapy. Int. J. Biol. Macromol. 2019, 125, 9–16. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Gadiraju, P.; Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Microsecond thermal ablation of skin for transdermal drug delivery. J. Control. Release 2011, 154, 58–68. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Mansur, A.A.; Capanema, N.S.; Carvalho, I.C.; Chagas, P.; de Oliveira, L.C.A.; Mansur, H.S. Synthesis and in vitro assessment of anticancer hydrogels composed by carboxymethylcellulose-doxorubicin as potential transdermal delivery systems for treatment of skin cancer. J. Mol. Liq. 2018, 266, 425–440. [Google Scholar] [CrossRef]
- Asgharzadeh, M.R.; Barar, J.; Pourseif, M.M.; Eskandani, M.; Niya, M.J.; Mashayekhi, M.R.; Omidi, Y.J.B.B. Molecular machineries of pH dysregulation in tumor microenvironment: Potential targets for cancer therapy. Bioimpacts 2017, 7, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.J.C.r. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.C.; Francis, R.E.; Guest, S.K.; Costa, J.R.; Gomes, A.R.; Myatt, S.S.; Brosens, J.J.; Lam, E.W. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol. Cancer Ther. 2008, 7, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Wong, Z.W.; Sandanaraj, E.; Xiang, X.; Ang, P.C.S.; Lee, E.J.; Chowbay, B. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008, 99, 816–823. [Google Scholar] [CrossRef]
- Amawi, H.; Sim, H.-M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC transporter-mediated multidrug-resistant cancer. Drug Transp. Drug Dispos. Eff. Toxic. 2019, 1141, 549–580. [Google Scholar]
- Sims, J.T.; Ganguly, S.S.; Bennett, H.; Friend, J.W.; Tepe, J.; Plattner, R. Imatinib reverses doxorubicin resistance by affecting activation of STAT3-dependent NF-κB and HSP27/p38/AKT pathways and by inhibiting ABCB1. PLoS ONE 2013, 8, e55509. [Google Scholar] [CrossRef]
- Zhou, Z.-y.; Wan, L.-l.; Yang, Q.-j.; Han, Y.-l.; Li, D.; Lu, J.; Guo, C. Nilotinib reverses ABCB1/P-glycoprotein-mediated multidrug resistance but increases cardiotoxicity of doxorubicin in a MDR xenograft model. Toxicol. Lett. 2016, 259, 124–132. [Google Scholar] [CrossRef]
- Qiu, J.-G.; Zhang, Y.-J.; Li, Y.; Zhao, J.-M.; Zhang, W.-J.; Jiang, Q.-W.; Mei, X.-L.; Xue, Y.-Q.; Qin, W.-M.; Yang, Y. Trametinib modulates cancer multidrug resistance by targeting ABCB1 transporter. Oncotarget 2015, 6, 15494. [Google Scholar] [CrossRef]
- Kim, K.B.; Kefford, R.; Pavlick, A.C.; Infante, J.R.; Ribas, A.; Sosman, J.A.; Fecher, L.A.; Millward, M.; McArthur, G.A.; Hwu, P. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J. Clin. Oncol. 2013, 31, 482. [Google Scholar] [CrossRef]
- Lugowska, I.; Koseła-Paterczyk, H.; Kozak, K.; Rutkowski, P. Trametinib: A MEK inhibitor for management of metastatic melanoma. OncoTargets Ther. 2015, 8, 2251. [Google Scholar]
- Huang, S.; Liu, H.; Huang, S.; Fu, T.; Xue, W.; Guo, R. Dextran methacrylate hydrogel microneedles loaded with doxorubicin and trametinib for continuous transdermal administration of melanoma. Carbohydr. Polym. 2020, 246, 116650. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Shan, X.; Mao, J.; Qiu, L.; Chen, J. Derma roller® microneedles-mediated transdermal delivery of doxorubicin and celecoxib co-loaded liposomes for enhancing the anticancer effect. Mater. Sci. Eng. C 2019, 99, 1448–1458. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Banga, A.K. Fabrication, characterization and application of sugar microneedles for transdermal drug delivery. Ther. Deliv. 2017, 8, 249–264. [Google Scholar] [CrossRef]
- Degim, I.T.; Burgess, D.J.; Papadimitrakopoulos, F. Carbon nanotubes for transdermal drug delivery. J. Microencapsul. 2010, 27, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of carbon nanotubes in drug delivery: A comprehensive review. Charact. Biol. Nanomater. Drug Deliv. 2019, 113–135. [Google Scholar]
- Monteiro-Riviere, N.A.; Inman, A.O. Challenges for assessing carbon nanomaterial toxicity to the skin. Carbon 2006, 44, 1070–1078. [Google Scholar] [CrossRef]
- Blagus, T.; Markelc, B.; Cemazar, M.; Kosjek, T.; Preat, V.; Miklavcic, D.; Sersa, G. In vivo real-time monitoring system of electroporation mediated control of transdermal and topical drug delivery. J. Control. Release 2013, 172, 862–871. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Ya, S.; Hu, Y.; Zhi, D.; Wang, W.; Xu, M.; Qiu, B.; Ding, W. An Iron Oxide Nanoparticle-Based Transdermal Nanoplatform for Dual-Modal Imaging-Guided Chemo-Photothermal Therapy of Superficial Tumors. Acta Biomater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Abolmaali, S.S.; Tamaddon, A.M.; Dinarvand, R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother. Pharmacol. 2013, 71, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Goločorbin-Kon, S.; Pavlović, N.; Stanimirov, B.; Vukmirović, S.; Milijašević, B.; Al-Salami, H.; Mikov, M. Methotrexate-an old drug with new pharmaceutical formulations and new indications. Your Hosts Maced. Pharm. Assoc. Fac. Pharm. Ss Cyril Methodius Univ. Skopje 2016, 62, 575–576. [Google Scholar]
- Yang, C.; Daoping, Z.; Xiaoping, X.; Jing, L.; Chenglong, Z. Magnesium oil enriched transdermal nanogel of methotrexate for improved arthritic joint mobility, repair, and reduced inflammation. J. Microencapsul. 2020, 37, 77–90. [Google Scholar] [CrossRef]
- Zeb, A.; Qureshi, O.S.; Kim, H.-S.; Cha, J.-H.; Kim, H.-S.; Kim, J.-K. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int. J. Nanomed. 2016, 11, 3813. [Google Scholar]
- Chauhan, N.; Kumar, K.; Pant, N.C. An updated review on transfersomes: A novel vesicular system for transdermal drug delivery. Univers. J. Pharm. Res. 2017, 2, 49–52. [Google Scholar] [CrossRef]
- Vemulapalli, V.; Yang, Y.; Friden, P.M.; Banga, A.K. Synergistic effect of iontophoresis and soluble microneedles for transdermal delivery of methotrexate. J. Pharm. Pharmacol. 2008, 60, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Banga, A.K. Electrically and ultrasonically enhanced transdermal delivery of methotrexate. Pharmaceutics 2018, 10, 117. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Banga, A.K. Delivery of methotrexate and characterization of skin treated by fabricated PLGA microneedles and fractional ablative laser. Pharm. Res. 2018, 35, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Neupane, R.; Boddu, S.H.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Anand, S.; Koul, V. Biophysical assessment of DC iontophoresis and current density on transdermal permeation of methotrexate. Int. J. Pharm. Investig. 2011, 1, 234. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Hamishehkar, H. Enhancing percutaneous delivery of methotrexate using different types of surfactants. Colloids Surf. B Biointerfaces 2011, 82, 422–426. [Google Scholar] [CrossRef]
- Panchagnula, R.; Desu, H.; Jain, A.; Khandavilli, S. Effect of lipid bilayer alteration on transdermal delivery of a high-molecular-weight and lipophilic drug: Studies with paclitaxel. J. Pharm. Sci. 2004, 93, 2177–2183. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Sapra, B.; Jain, S.; Tiwary, A. Percutaneous permeation enhancement by terpenes: Mechanistic view. AAPS J. 2008, 10, 120–132. [Google Scholar] [CrossRef]
- Brambilla, L.; Romanelli, A.; Bellinvia, M.; Ferrucci, S.; Vinci, M.; Boneschi, V.; Miedico, A.; Tedeschi, L. Weekly paclitaxel for advanced aggressive classic Kaposi sarcoma: Experience in 17 cases. Br. J. Dermatol. 2008, 158, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Tulpule, A.; Groopman, J.; Saville, M.W.; Harrington, W., Jr.; Friedman-Kien, A.; Espina, B.M.; Garces, C.; Mantelle, L.; Mettinger, K.; Scadden, D.T. Multicenter trial of low-dose paclitaxel in patients with advanced AIDS-related Kaposi sarcoma. Cancer 2002, 95, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Willson, M.L.; Burke, L.; Ferguson, T.; Ghersi, D.; Nowak, A.K.; Wilcken, N. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Antman, K.; Chang, Y. Kaposi’s sarcoma. New Engl. J. Med. 2000, 342, 1027–1038. [Google Scholar] [CrossRef]
- Hosmer, J.M.; Steiner, A.A.; Lopes, L.B. Lamellar liquid crystalline phases for cutaneous delivery of Paclitaxel: Impact of the monoglyceride. Pharm. Res. 2013, 30, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, J.M.; Shin, S.H.; Nornoo, A.; Zheng, H.; Lopes, L.B. Influence of internal structure and composition of liquid crystalline phases on topical delivery of paclitaxel. J. Pharm. Sci. 2011, 100, 1444–1455. [Google Scholar] [CrossRef]
- Utreja, P.; Jain, S.; Tiwary, A. Localized delivery of paclitaxel using elastic liposomes: Formulation development and evaluation. Drug Deliv. 2011, 18, 367–376. [Google Scholar] [CrossRef]
- Szebeni, J.; Alving, C.R.; Muggia, F.M. Complement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: An in vitro study. JNCI J. Natl. Cancer Inst. 1998, 90, 300–306. [Google Scholar] [CrossRef] [PubMed]
- CHEMISTS CORNER. The Best Formulation Software Options for Cosmetic Chemist. Available online: https://chemistscorner.com/the-best-formulation-software-options-for-cosmetic-chemists/ (accessed on 14 June 2021).
- Maharao, N.; Antontsev, V.; Hou, H.; Walsh, J.; Varshney, J. Scalable in silico Simulation of Transdermal Drug Permeability: Application of BIOiSIM Platform. Drug Des. Dev. Ther. 2020, 14, 2307. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, J.W.; Kelly, C.L.; Blease, T.G.; Dederen, J.C. Formulating for efficacy 1. Int. J. Cosmet. Sci. 2004, 26, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Chandler, M.; Michniak-Kohn, B. Solubility-physicochemical-thermodynamic theory of penetration enhancer mechanism of action. Int. J. Pharm. 2020, 575, 118920. [Google Scholar] [CrossRef]
- JW Solutions Software. A Detailed Description of What “Formulating for Efficacy™, the Software” Can Do. 2020. Available online: https://www.jwsolutionssoftware.com/ (accessed on 28 August 2020).
- Jameel, B.M.; Huynh, A.; Chadha, A.; Pandey, S.; Duncan, J.; Chandler, M.; Baki, G. Computer-based formulation design and optimization using Hansen solubility parameters to enhance the delivery of ibuprofen through the skin. Int. J. Pharm. 2019, 569, 118549. [Google Scholar] [CrossRef]
- Burger, C.; Gerber, M.; Du Preez, J.L.; Du Plessis, J. Optimised transdermal delivery of pravastatin. Int. J. Pharm. 2015, 496, 518–525. [Google Scholar] [CrossRef]
| Critical Properties | Ideal Limits |
|---|---|
| Aqueous solubility | >1 mg/mL |
| Lipophilicity (log octanol/water P) | >1 and <4 |
| Molecular weight | <500 Da |
| Melting point | <200 °C |
| pH of the saturated aqueous solution | 5–9 |
| Daily dose | <20 mg |
| Skin irritation or sensitization | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neupane, R.; Boddu, S.H.S.; Abou-Dahech, M.S.; Bachu, R.D.; Terrero, D.; Babu, R.J.; Tiwari, A.K. Transdermal Delivery of Chemotherapeutics: Strategies, Requirements, and Opportunities. Pharmaceutics 2021, 13, 960. https://doi.org/10.3390/pharmaceutics13070960
Neupane R, Boddu SHS, Abou-Dahech MS, Bachu RD, Terrero D, Babu RJ, Tiwari AK. Transdermal Delivery of Chemotherapeutics: Strategies, Requirements, and Opportunities. Pharmaceutics. 2021; 13(7):960. https://doi.org/10.3390/pharmaceutics13070960
Chicago/Turabian StyleNeupane, Rabin, Sai H. S. Boddu, Mariam Sami Abou-Dahech, Rinda Devi Bachu, David Terrero, R. Jayachandra Babu, and Amit K. Tiwari. 2021. "Transdermal Delivery of Chemotherapeutics: Strategies, Requirements, and Opportunities" Pharmaceutics 13, no. 7: 960. https://doi.org/10.3390/pharmaceutics13070960
APA StyleNeupane, R., Boddu, S. H. S., Abou-Dahech, M. S., Bachu, R. D., Terrero, D., Babu, R. J., & Tiwari, A. K. (2021). Transdermal Delivery of Chemotherapeutics: Strategies, Requirements, and Opportunities. Pharmaceutics, 13(7), 960. https://doi.org/10.3390/pharmaceutics13070960







