Quantification of Ceftaroline in Human Plasma Using High-Performance Liquid Chromatography with Ultraviolet Detection: Application to Pharmacokinetic Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Samples
2.2. Chromatographic Equipment and Conditions
2.3. Working Solutions, Calibration Curves and Quality Control Samples
2.4. Sample Preparation
2.5. Method Validation
2.6. Stability
2.7. Application of the Method to Pharmacokinetic Studies
3. Results
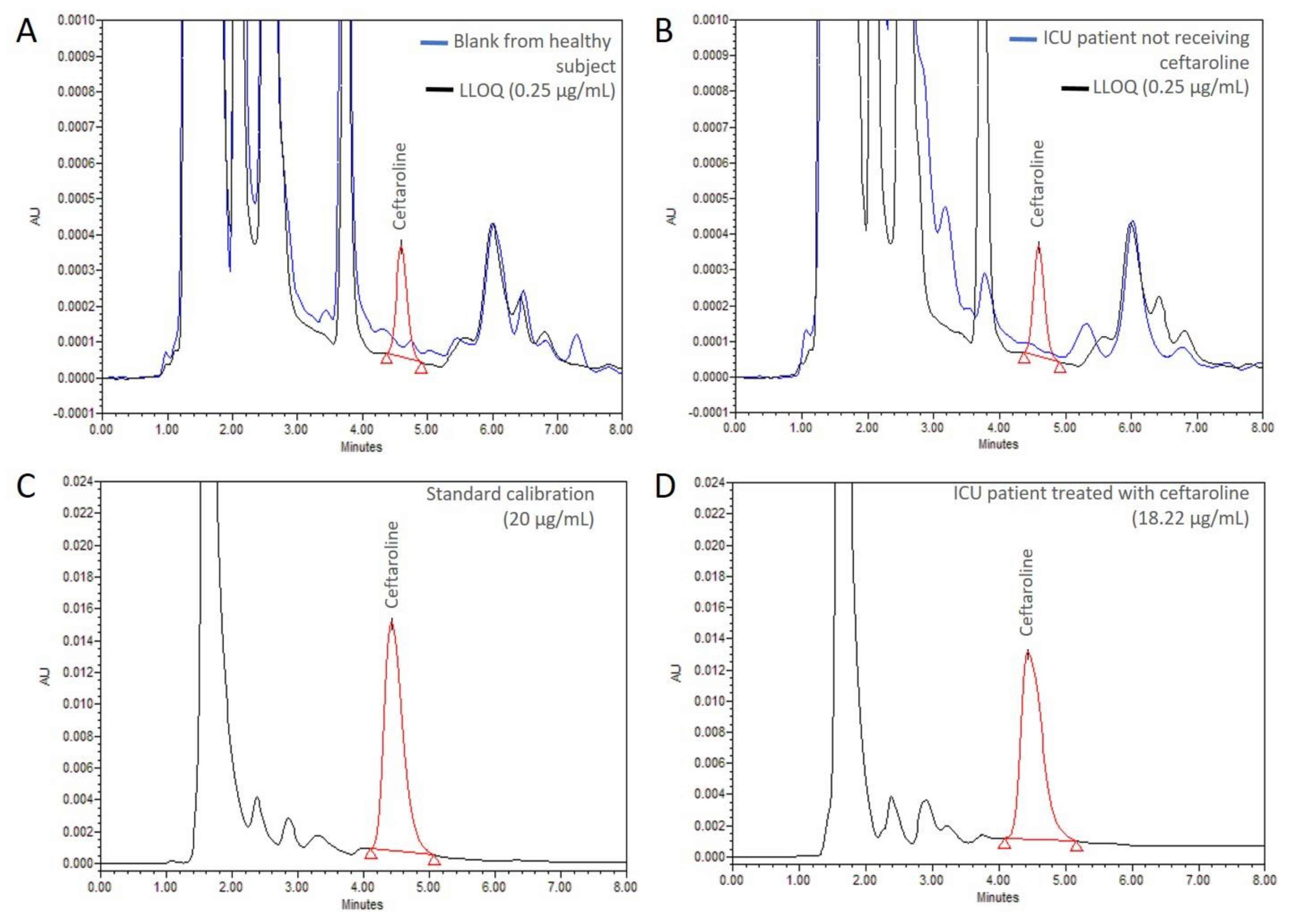
3.1. Chromatography and Detection
3.2. Validation
3.3. Stability
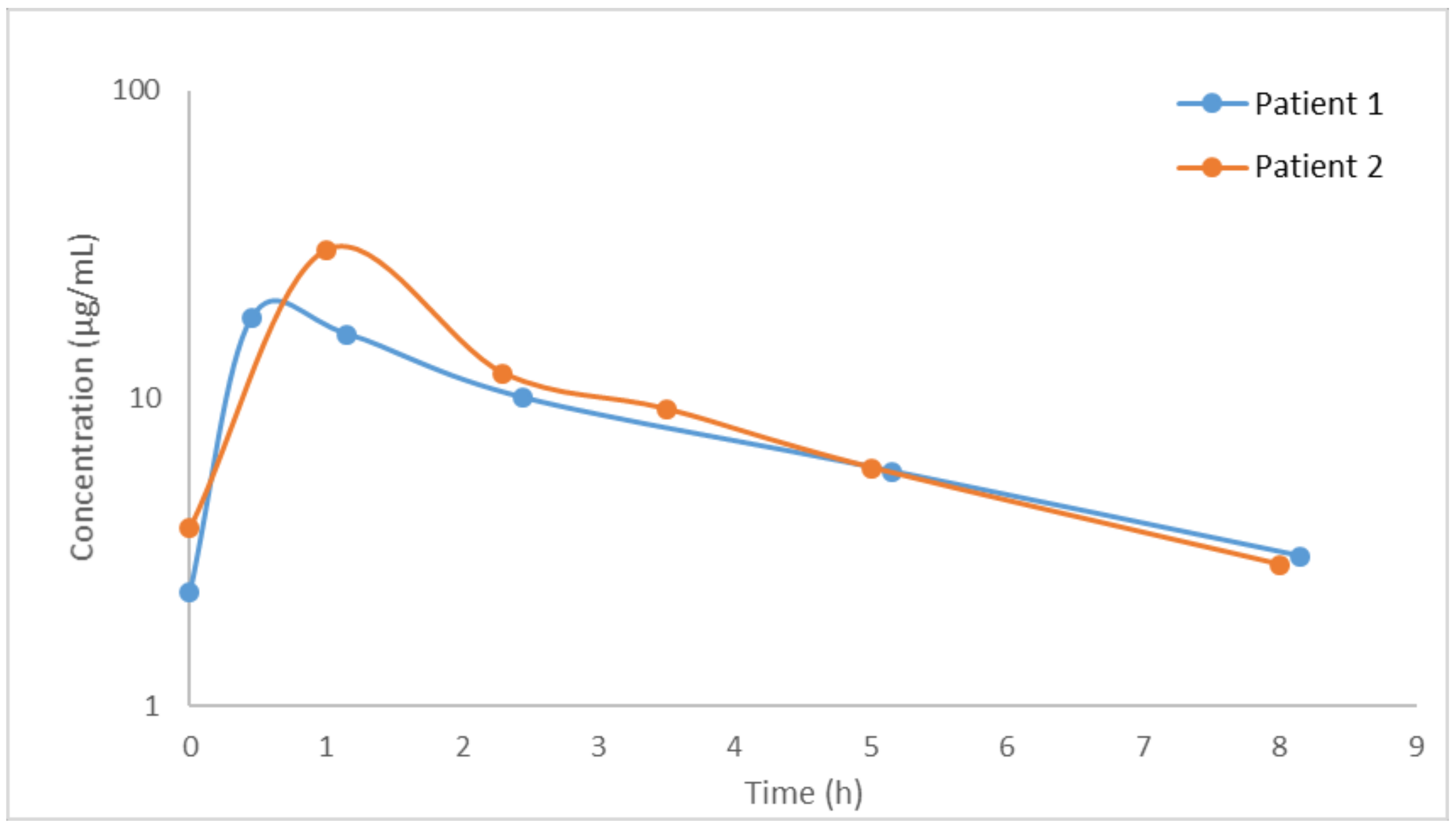
3.4. Analysis of Patient Samples
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ge, Y.; Biek, D.; Talbot, G.H.; Sahm, D.F. In Vitro Profiling of Ceftaroline against a Collection of Recent Bacterial Clinical Isolates from across the United States. Antimicrob. Agents Chemother. 2008, 52, 3398–3407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, R.N.; Farrell, D.J.; Mendes, R.E.; Sader, H.S. Comparative ceftaroline activity tested against pathogens associated with community-acquired pneumonia: Results from an international surveillance study. J. Antimicrob. Chemother. 2011, 66 (Suppl. 3), iii69–iii80. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Fritsche, T.R.; Jones, R.N. Antimicrobial Activities of Ceftaroline and ME1036 Tested against Clinical Strains of Community-Acquired Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 1153–1155. [Google Scholar] [CrossRef] [PubMed]
- Biek, D.; Critchley, I.A.; Riccobene, T.A.; Thye, D.A. Ceftaroline fosamil: A novel broad-spectrum cephalosporin with expanded anti-Gram-positive activity. J. Antimicrob. Chemother. 2010, 65, iv9–iv16. [Google Scholar] [CrossRef]
- Villegas-Estrada, A.; Lee, M.; Hesek, D.; Vakulenko, S.B.; Mobashery, S. Co-opting the cell wall in fighting methicillin-resistant Staphylococcus aureus: Potent inhibition of PBP 2a by two anti-MRSA β-Lactam Antibiotics. J. Am. Chem. Soc. 2008, 130, 9212–9213. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Sniezek, G.; Schweizer, F.; Zelenitsky, S.; Lagacé-Wiens, P.R.S.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; Karlowsky, J.A. Ceftaroline: A novel broad-spectrum cephalosporin with activity against meticillin-resistant Staphylococcus aureus. Drugs 2009, 69, 809–831. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Food and Drug Administration. Teflaro. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/200327s016s017lbl.pdf (accessed on 31 May 2021).
- European Medicines Agency. Science Medicines Health. Zinforo. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zinforo (accessed on 31 May 2021).
- Sader, H.S.; Carvalhaes, C.G.; Mendes, R.E. Ceftaroline activity against Staphylococcus aureus isolated from patients with infective endocarditis, worldwide (2010–2019). Int. J. Infect. Dis. 2021, 102, 524–528. [Google Scholar] [CrossRef]
- Chan, P.L.S.; McFadyen, L.; Quaye, A.; Leister-Tebbe, H.; Hendrick, V.M.; Hammond, J.; Raber, S. The Use of Extrapolation Based on Modeling and Simulation to Support High-Dose Regimens of Ceftaroline Fosamil in Pediatric Patients with Complicated Skin and Soft-Tissue Infections. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 551–563. [Google Scholar] [CrossRef]
- Merker, A.; Danziger, L.H.; Rodvold, K.A.; Glowacki, R.C. Pharmacokinetic and pharmacodynamic evaluation of ceftaroline fosamil. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1741–1750. [Google Scholar] [CrossRef]
- Riccobene, T.; Jakate, A.; Rank, D. A series of pharmacokinetic studies of ceftaroline fosamil in select populations: Normal subjects, healthy elderly subjects, and subjects with renal impairment or end-stage renal disease requiring hemodialysis. J. Clin. Pharmacol. 2014, 54, 742–752. [Google Scholar] [CrossRef]
- Kiang, T.K.L.; Wilby, K.J.; Ensom, M.H.H. A Critical Review on the Clinical Pharmacokinetics, Pharmacodynamics, and Clinical Trials of Ceftaroline. Clin. Pharmacokinet. 2015, 54, 915–931. [Google Scholar] [CrossRef]
- Riccobene, T.A.; Khariton, T.; Knebel, W.; Das, S.; Li, J.; Jandourek, A.; Carrothers, T.J.; Bradley, J.S. Population PK Modeling and Target Attainment Simulations to Support Dosing of Ceftaroline Fosamil in Pediatric Patients With Acute Bacterial Skin and Skin Structure Infections and Community-Acquired Bacterial Pneumonia. J. Clin. Pharmacol. 2017, 57, 345–355. [Google Scholar] [CrossRef]
- Canut, A.; Isla, A.; Rodríguez-Gascón, A. Pharmacokinetic/pharmacodynamic analysis to evaluate ceftaroline fosamil dosing regimens for the treatment of community-acquired bacterial pneumonia and complicated skin and skin-structure infections in patients with normal and impaired renal function. Int. J. Antimicrob. Agents 2015, 45, 399–405. [Google Scholar] [CrossRef]
- Riccobene, T.A.; Carrothers, T.J.; Knebel, W.; Raber, S.; Chan, P.L.S. Pharmacokinetic and Pharmacodynamic Target Attainment in Adult and Pediatric Patients Following Administration of Ceftaroline Fosamil as a 5-Minute Infusion. Clin. Pharmacol. Drug Dev. 2021, 10, 420–427. [Google Scholar] [CrossRef]
- Grégoire, M.; Leroy, A.G.; Bouquié, R.; Malandain, D.; Dailly, E.; Boutoille, D.; Renaud, C.; Jolliet, P.; Caillon, J.; Deslandes, G. Simultaneous determination of ceftaroline, daptomycin, linezolid and rifampicin concentrations in human plasma by on-line solid phase extraction coupled to high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2016, 118, 17–26. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9852981, Ceftaroline Fosamil. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ceftaroline-fosamil (accessed on 18 June 2021).
- High-Quality Physico-Chemical Calculations and Predictions for Drug Discovery. ChemAxon. Available online: https://chemaxon.com/products/calculators-and-predictors#pka (accessed on 18 June 2021).
- Virtual Computational Chemistry Laboratory. Available online: http://www.vcclab.org/lab/alogps/ (accessed on 28 May 2021).
- U.S. Department of Health and Human Services. Food and Drug Administration. 2018. Bioanalytical Method Validation. Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 31 May 2021).
- European Medicines Agency. Guideline on Bioanalytical Method Validation. 2012. EMEA/CHMP/EWP/192217/2009. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation (accessed on 31 May 2021).
- Ates, H.C.; Roberts, J.A.; Lipman, J.; Cass, A.E.G.; Urban, G.A.; Dincer, C. On-Site Therapeutic Drug Monitoring. Trends Biotechnol. 2020, 38, 1262–1277. [Google Scholar] [CrossRef]
- Kalaria, S.; Williford, S.; Guo, D.; Shu, Y.; Medlin, C.; Li, M.; Yeung, S.Y.A.; Ali, F.; Jean, W.; Gopalakrishnan, M.; et al. Optimizing ceftaroline dosing in critically ill patients undergoing continuous renal replacement therapy. Pharmacotherapy 2021, 41, 205–211. [Google Scholar] [CrossRef]
- EUCAST Clinical Breakpoint v. 11.0. Available online: http://www.eucast.org (accessed on 21 May 2021).
- Barrasa, H.; Soraluce, A.; Usón, E.; Sainz, J.; Martín, A.; Sánchez-Izquierdo, J.Á.; Maynar, J.; Rodríguez-Gascón, A.; Isla, A. Impact of augmented renal clearance on the pharmacokinetics of linezolid: Advantages of continuous infusion from a pharmacokinetic/pharmacodynamic perspective. Int. J. Infect. Dis. 2020, 93, 329–338. [Google Scholar] [CrossRef]
- Soraluce, A.; Barrasa, H.; Asín-prieto, E.; Sánchez-izquierdo, J.Á.; Maynar, J.; Isla, A.; Rodríguez-Gascón, A. Novel population pharmacokinetic model for linezolid in critically ill patients and evaluation of the adequacy of the current dosing recommendation. Pharmaceutics 2020, 12, 54. [Google Scholar] [CrossRef]
- Soraluce, A.; Asín-Prieto, E.; Rodríguez-Gascón, A.; Barrasa, H.; Maynar, J.; Carcelero, E.; Soy, D.; Isla, A. Population pharmacokinetics of daptomycin in critically ill patients. Int. J. Antimicrob. Agents 2018, 52, 158–165. [Google Scholar] [CrossRef]
- Phe, K.; Heil, E.L.; Tam, V.H. Optimizing pharmacokinetics-pharmacodynamics of antimicrobial management in patients with sepsis: A review. J. Infect. Dis. 2021, 222, S132–S141. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Sime, F.B.; Taccone, F.S.; Roberts, J.A. How to optimize antibiotic pharmacokinetic/pharmacodynamics for Gram-negative infections in critically ill patients. Curr. Opin. Infect. Dis. 2018, 31, 555–565. [Google Scholar] [CrossRef]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; Van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: A two-center prospective study (EXPAT). Crit. Care 2020, 24, 558. [Google Scholar] [CrossRef]
- Bilbao-Meseguer, I.; Rodríguez-Gascón, A.; Barrasa, H.; Isla, A.; Solinís, M.Á. Augmented Renal Clearance in Critically Ill Patients: A Systematic Review. Clin. Pharmacokinet. 2018, 57, 1107–1121. [Google Scholar] [CrossRef]
- Abdulla, A.; Ewoldt, T.M.J.; Purmer, I.M.; Muller, A.E.; Gommers, D.; Endeman, H.; Koch, B.C.P. A narrative review of predictors for β-lactam antibiotic exposure during empirical treatment in critically ill patients. Expert Opin. Drug Metab. Toxicol. 2021, 17, 359–368. [Google Scholar] [CrossRef]
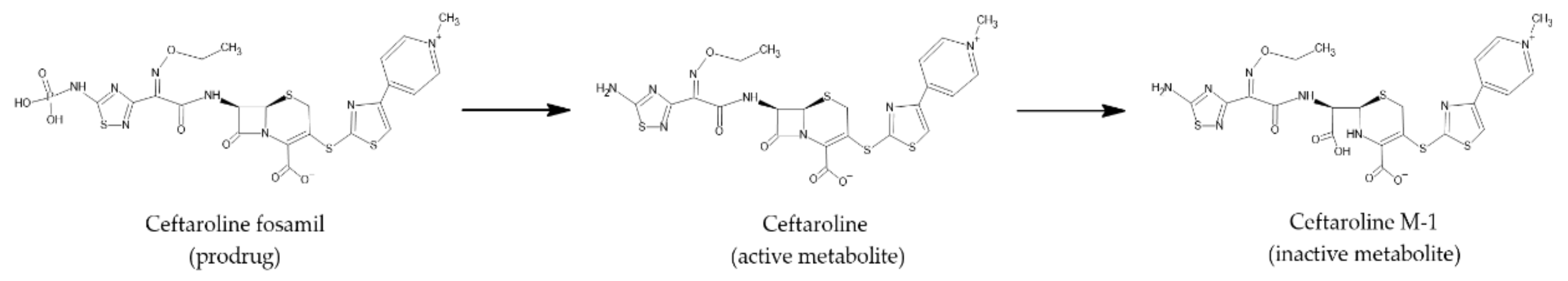
| Molecular Weight (g/mol) [18] | Solubility (mg/mL) [18] | pKa (Strongest Acidic) [19] | pKa (Strongest Basic) [19] | logP [19] | logP [20] |
|---|---|---|---|---|---|
| 684.7 | >100 | 1.31 | 0.31 | −2.7 | −0.84 |
| Patient | 1 | 2 |
|---|---|---|
| Age (year) | 72 | 74 |
| Weight (kg) | 90 | 75 |
| Sex | M | M |
| Body surface area (m2) | 2.06 | 1.86 |
| Dosage | 600 mg every 8 h | 600 mg every 8 h |
| Previous doses | 4 | 3 |
| APACHE II score | 14 | 30 |
| Creatinine clearance (mL/min/1.73 m2) | 129 | 87 |
| Glucose (mg/dL) | 110 | 94 |
| Albumin (g/dL) | 2 | 2.7 |
| Total protein (g/dL) | 5.9 | 4.8 |
| Haemoglobin (g/dL) | 10.8 | 9.5 |
| Haematocrit (%) | 34.7 | 29.8 |
| Ceftaroline MIC (MRSA) (mg/L) | 0.5 | 0.25 |
| Y = bx + a | R1 | R2 | R3 |
|---|---|---|---|
| a | −681 | 87.9 | 1310 |
| b | 15,200 | 15,800 | 15,700 |
| R | 0.999 | 0.998 | 1.000 |
| Response factor (CV, %) | 6.72 | 5.71 | 10.78 |
| Nominal Concentration (µg/mL) | Concentration Measured (µg/mL) mean ± SD | Precision CV (%) | RE (%) | |
|---|---|---|---|---|
| Intra-day (n = 5) | 0.25 (LLOQ) | 0.26 ± 0.01 | 2.62 | 2.96 |
| 0.75 | 0.76 ± 0.02 | 2.05 | 1.39 | |
| 15 | 15.20 ± 0.54 | 3.56 | 1.35 | |
| 30 | 28.94 ± 0.34 | 1.17 | 3.52 | |
| Inter-day (n = 15) | 0.25 (LLOQ) | 0.26 ± 0.01 | 3.77 | 4.08 |
| 0.75 | 0.77 ± 0.04 | 5.11 | 3.03 | |
| 15 | 14.64 ± 0.78 | 5.30 | 2.37 | |
| 30 | 28.06 ± 0.91 | 3.23 | 6.47 |
| Ceftaroline Concentration | Low QC 0.75 µg/mL (n = 3) | Medium QC 15 µg/mL (n = 3) | High QC 30 µg/mL (n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | CV (%) | RE (%) | Mean ± SD | CV (%) | RE (%) | Mean ± SD | CV (%) | RE (%) | |
| −20 °C (2 weeks) | 0.68 ± 0.02 | 2.95 | 9.33 | 13.01 ± 0.17 | 1.29 | 13.28 | 25.85 ± 0.12 | 0.47 | 13.85 |
| −80 °C (3 months) | 0.65 ± 0.01 | 1.13 | 13.38 | 13.90 ± 0.16 | 1.12 | 7.35 | 27.36 ± 0.35 | 1.28 | 8.79 |
| Three freeze/thaw cycles | 0.68 ± 0.03 | 4.68 | 9.51 | 13.50 ± 0.15 | 1.08 | 10.03 | 26.68 ± 1.16 | 4.34 | 11.07 |
| Sample processing (2 h) | 0.66 ± 0.02 | 2.48 | 12.47 | 15.65 ± 0.02 | 0.15 | 4.30 | 32.30 ± 0.48 | 1.49 | 7.65 |
| Autosampler 4 °C | 8 h | ||||||||
| Patient | Dose (mg Every 8 h) | Cmax (µg/mL) | Cmin (µg/mL) | CLT (L/h) | t1/2 (h) | Vss (L) | AUCτ (mg h/L) |
|---|---|---|---|---|---|---|---|
| 1 | 600 | 18.22 | 2.34 | 8.84 | 3.33 | 36.40 | 67.85 |
| 2 | 600 | 30.28 | 2.87 | 7.33 | 2.73 | 23.61 | 81.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarcia-Lacalle, A.; Barrasa, H.; Maynar, J.; Canut-Blasco, A.; Gómez-González, C.; Solinís, M.Á.; Isla, A.; Rodríguez-Gascón, A. Quantification of Ceftaroline in Human Plasma Using High-Performance Liquid Chromatography with Ultraviolet Detection: Application to Pharmacokinetic Studies. Pharmaceutics 2021, 13, 959. https://doi.org/10.3390/pharmaceutics13070959
Alarcia-Lacalle A, Barrasa H, Maynar J, Canut-Blasco A, Gómez-González C, Solinís MÁ, Isla A, Rodríguez-Gascón A. Quantification of Ceftaroline in Human Plasma Using High-Performance Liquid Chromatography with Ultraviolet Detection: Application to Pharmacokinetic Studies. Pharmaceutics. 2021; 13(7):959. https://doi.org/10.3390/pharmaceutics13070959
Chicago/Turabian StyleAlarcia-Lacalle, Ana, Helena Barrasa, Javier Maynar, Andrés Canut-Blasco, Carmen Gómez-González, María Ángeles Solinís, Arantxazu Isla, and Alicia Rodríguez-Gascón. 2021. "Quantification of Ceftaroline in Human Plasma Using High-Performance Liquid Chromatography with Ultraviolet Detection: Application to Pharmacokinetic Studies" Pharmaceutics 13, no. 7: 959. https://doi.org/10.3390/pharmaceutics13070959
APA StyleAlarcia-Lacalle, A., Barrasa, H., Maynar, J., Canut-Blasco, A., Gómez-González, C., Solinís, M. Á., Isla, A., & Rodríguez-Gascón, A. (2021). Quantification of Ceftaroline in Human Plasma Using High-Performance Liquid Chromatography with Ultraviolet Detection: Application to Pharmacokinetic Studies. Pharmaceutics, 13(7), 959. https://doi.org/10.3390/pharmaceutics13070959








