Abstract
Coronavirus disease-2019 (COVID-19) is caused by coronavirus-2 (SARS-CoV-2) and has produced a global pandemic. As of 22 June 2021, 178 million people have been affected worldwide, and 3.87 million people have died from COVID-19. According to the Centers for Disease Control and Prevention (CDC) of the United States, COVID-19 virus is primarily transmitted between people through respiratory droplets and contact routes. Since the location of initial infection and disease progression is primarily through the lungs, the inhalation delivery of drugs directly to the lungs may be the most appropriate route of administration for treating COVID-19. This review article aims to present possible inhalation therapeutics and vaccines for the treatment of COVID-19 symptoms. This review covers the comparison between SARS-CoV-2 and other coronaviruses such as SARS-CoV/MERS, inhalation therapeutics for the treatment of COVID-19 symptoms, and vaccines for preventing infection, as well as the current clinical status of inhaled therapeutics and vaccines.
1. Introduction
Coronaviruses are single-stranded, positive-sense RNA viruses that can infect animals and humans [1]. Severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) were recognized earlier as strains of coronavirus that cause respiratory, gastrointestinal, hepatic, and neurologic diseases of erratic severity, and can be fatal to infants, older people, and immunocompromised individuals [2]. The most recent novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of the global pandemic of coronavirus disease 2019 (COVID-19) and was first reported in December 2019 in Wuhan, China [3]. As of 22 June 2021, there have been a total of 178,503,429 confirmed cases of COVID-19, including 3,872,457 deaths worldwide [4].
COVID-19 is transmitted to healthy individuals through small airborne droplets exhaled by an infected person, personal contact (shaking hands), and by touching contaminated surfaces [5,6]. The ingestion of droplets into the lungs leads to lower respiratory tract infections ranging from mild respiratory infections to severe acute respiratory syndrome [7]. The most common symptoms of COVID-19 infection include fever, headache, cough, sore throat, body aches, fatigue, dyspnea, and loss of taste or smell, while severe symptoms are accompanied by systemic infection and pneumonia [5,8].
Since the route of infection and disease progression is primarily through the lungs, the inhalation delivery of drugs directly to the lungs is the most appropriate route of administration for treating COVID-19. The International Society for Aerosols in Medicine (ISAM) has also called for the development of inhaled therapies for COVID-19 treatment because the symptoms of COVID-19 are mainly manifested in the respiratory system [9]. Currently, the inhalation delivery of drugs to the lungs is the most important route of administration for the treatment of severe lung diseases, such as asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), pneumonia, pulmonary hypertension, and respiratory distress syndrome [10]. The local delivery of drugs to the lungs may allow maximum pharmacological targeting with minimum systemic exposure [11,12,13,14,15,16,17].
This review article aims to present possible inhalation therapeutics for the treatment of COVID-19 symptoms and vaccines for preventing infection and is organized as follows. It covers a comparison between SARS-CoV-2 and other coronaviruses such as SARS-CoV/MERS, inhalation therapeutics for the treatment of COVID-19 symptoms and vaccines for preventing infection, and the current clinical status of inhaled therapeutics and vaccines.
3. Inhalational Drug Administration—An Overview
The inhalation delivery of drugs is one of the important routes of drug administration for the treatment of respiratory disorders from ancient times [11,27,28]. Today, the inhalation route is the most preferred route of administration for the treatment of many pulmonary conditions such as asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, pneumonia, etc. The human lungs’ surface area is large and has a highly permeable epithelium, making it easily accessed by an inhaled dose [8,29]. The pulmonary route (Figure 1) is considered a targeted lung delivery since it offers the administration of a drug directly to its site of action, resulting in a rapid onset of activity with smaller administered doses and higher concentrations delivered locally to the lungs’ disease site. Furthermore, minimizing systemic bioavailability reduces the potential incidence of adverse systemic toxicities and avoids the first-pass metabolism in the liver [8,30,31].
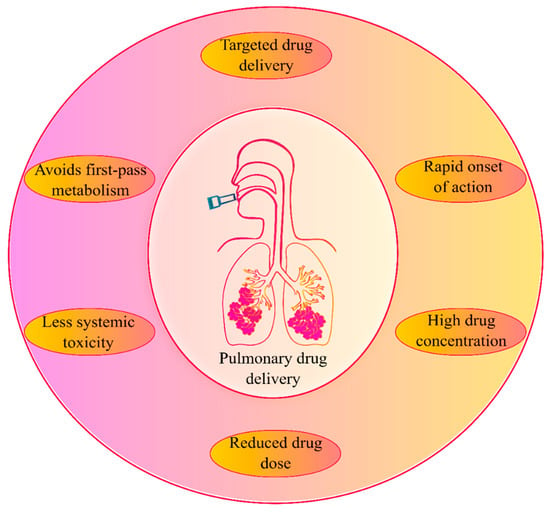
Figure 1.
An overview of pulmonary drug delivery and its advantages.
4. Inhaled COVID-19 Therapeutics
Inhaled drugs for pulmonary drug delivery are now extensive and highly recommended to treat lung disorders and diseases. Unsurprisingly, there are many FDA approved inhaled drugs for respiratory disorders in the market, such as inhaled zanamivir used for influenza and inhaled ribavirin for respiratory syncytial virus infection [8]. SARS-COV-2 patients face lung pneumonia and other acute respiratory tract disorders; thus, different drugs have been developed to manage lung pneumonia effectively, including steroidal, antibacterial, and antiviral drugs [8]. Below are examples (Table 2, Figure 2) mentioned in the literature for inhaled medications to treat different lungs’ complications associated with SARS-COV-2.

Table 2.
List of inhaled COVID-19 therapeutics and their physiological characteristics.
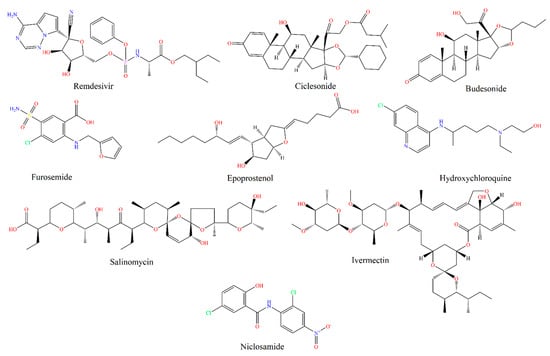
Figure 2.
Chemical structures (drawn using CambridgeSoft, Cambridge, MA, USA) of inhaled COVID-19 therapeutics.
4.1. Remdesivir
Remdesivir is a broad-spectrum antiviral agent and exhibits in vitro activity against SARS-CoV-2; thus, it was approved for emergency use. Shakijpijarna et al. [32], formulated remdesivir as a dry powder inhalation using thin-film freezing (TFF) technology to maximize delivery to the lung, the site of SARS-CoV-2 replication. TFF produces brittle matrix nanostructured aggregates that are sheared into respirable low-density microparticles upon aerosolization from a passive dry powder inhaler [8,32]. Vartak et al. [33] formulated a stable aerosolized nanoliposomal carrier for remdesivir (AL-Rem) using cholesterol, DSPE-PEG2000 (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [amino(polyethylene glycol)-2000]), and DOPC (1,2-dioleoyl-sn-glycero-3- phosphocholine) as lipids following a modified hydration technique. The formulated nanoliposomes (AL-Rem) have an optimal particle size of 71.46 ± 1.35 nm and showed an effective aerosol characteristic (fine particle fraction of 74.40 ± 2.96%) and high drug entrapment efficiency (99.79%). Further, this formulation showed minimal toxicity to lung epithelium and prolonged drug release characteristics that would benefit pulmonary administration and reduce frequent dosing.
4.2. Ciclesonide
Many systemic steroids were considered, such as methylprednisolone, dexamethasone, hydrocortisone, and ciclesonide. Ciclesonide is a safe drug that is considered superior to other systemic corticosteroids to decrease disease progression and rapidly control the symptoms with solid antiviral activity against SRAS-CoV-2, as ciclesonide primarily remains in the lung tissue and does not significantly enter the bloodstream [8,34]. Ciclesonide is used via inhalation to treat bronchial asthma and control other inflammation associated with the bronchial tract [35]. Thus, inhaled ciclesonide is sufficient to control inflammation associated with SARS-CoV-2 [8]. Iwabuchi et al. [35] reported the effect of the inhaled ciclesonide for three cases of COVID-19 pneumonia who were treated with ciclesonide inhalation and showed the presence of the steroid in the lungs for a relatively long time to control the local inflammation as well as inhibiting the virus proliferation via its antiviral activity [8,35].
4.3. Budesonide
Budesonide is a corticosteroid used in the long-term management of asthma and COPD [36]. In a recent in vitro study, pre-treatment of human respiratory epithelial cells (human nasal (HNE) and tracheal (HTE) epithelial cells) with a combination of budesonide, glycopyrronium, and formoterol has shown inhibitory actions on coronavirus HCoV-229E replication and cytokine production [37]. Currently, the inhaled budesonide alone and in combination with other drugs such as formoterol, a β2 agonist, and levamisole, an immunostimulatory are under investigation in various levels of clinical trials (NCT04355637, NCT04416399, NCT04193878, NCT04331470, and NCT04331054) to prevent an excessive local immune reaction in the lungs [38]. In a phase three clinical study by Oxford University, inhaled budesonide was found to shorten the recovery time in COVID-19 patients aged over 50 who were treated at home and in community settings [39]. Ramakrishnan et al. [40] conducted a randomized, open label trial of inhaled budesonide in adults within 7 days of the onset of mild COVID-19 symptoms. The results of this study indicate that early administration of inhaled budesonide reduced the likelihood of needing urgent medical care and reduced the time to recovery following early COVID-19 infection.
4.4. Furosemide
Furosemide is a diuretic that is a safe, globally available, inexpensive, and small molecule drug. It can be administered locally to the lungs by inhalation; pre-clinical data and in vitro experiments suggest that it may be a candidate for repurposing as an inhaled therapy against the immunopathology of COVID-19 [20,41]. As a part of its pre-clinical evaluation, Wang et al. [20] studied furosemide’s anti-inflammatory activity on multiple macrophage cell lines involved in innate immunity [20]. This study reported that inhaled furosemide can reduce the level of pro-inflammatory cytokines. They also proved that furosemide is a potent inhibitor of interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF alpha) release [8,20]. Other clinical studies by Grogono et al. [42] and Moosvai et al. [43] reported that inhaled furosemide has relieved air hunger in healthy individuals compared to inhaled saline [41,42,43]. Another study by Nishino et al. [44] demonstrated that furosemide alleviates dyspnea’s sensation in healthy subjects. The results of this study showed an increase in total breath-holding time and reduced respiratory discomfort during loaded breathing after the inhalation of furosemide compared to the inhalation of a placebo [44]. Other studies have reported the positive effects of inhaled furosemide via an anti-inflammatory mechanism in attenuating bronchoconstriction and asthma attacks [41].
It is essential to know that furosemide’s administration to COVID-19 patients has mainly the following two potential drawbacks: hypokalemia and electrolyte depletion because of SARS-CoV-2 induced pathology. However, the diuretic effect is anticipated to be negligible upon nebulized inhaled administration. Another potential issue of using a nebulized formulation is aerosol development that may promote viral spread if performed without physical distancing and appropriate protection. Despite this issue, inhaled furosemide decreases coughing and reduces disease spreading [41].
4.5. Nitric Oxide (NO) and Epoprostenol
Inhaled nitric oxide (iNO) and inhaled epoprostenol (iEPO) are two common pulmonary vasodilators that have been widely studied. Experience in patients with acute respiratory distress syndrome (ARDS) indicates that iNO can substantially reduce mean pulmonary arterial pressure and improve patients’ oxygenation. Furthermore, in vitro evidence of direct antiviral activity against SARS-CoV was studied, and the genetic similarity between SARS-CoV and SARS-CoV-2 suggests their potential effectiveness against SARS-CoV-2 [45]. Nitric oxide (NO) is an essential free radical in cardiovascular and immune systems whose role depends on its concentration and production site. Abnormal NO in vivo is mainly related to diseases, such as viral infection [19]. According to recent studies, NO levels reduced significantly in patients with COVID-19, which suggested a relation to vascular dysfunction and immune inflammation [19]. During the SARS outbreak in 2003, iNO was used to treat severe hypoxemia [46]. SARS-CoV-2 and SARS-CoV-1 share a similar infection process, as mentioned above; then, the inhibition of SARS-CoV-2 by NO may be identical to that of SARS-CoV-1 [19,46]. Furthermore, newborns with severe ARDS are treated with high-dose pulmonary surfactant, inhaled nitric oxide, high-frequency oscillatory ventilation, and extracorporeal membrane oxygenation. This approach might be effective for patients of COVID-19 as well [21].
Parikh et al. [46], utilized iNO therapy in spontaneously breathing COVID-19 patients. The starting dose of iNO was 30 parts per million for 2.1 days. The study results showed that more than half of the 39 spontaneously breathing patients with COVID-19 treated with iNO therapy did not require mechanical ventilation after treatment. These findings suggest that iNO therapy may help prevent the progression of hypoxic respiratory failure in COVID-19 patients. [46]. Additionally, clinical trials evaluating preventive and therapeutic options of iNO against SARS-CoV-2 are planned or underway (NCT04305457, NCT04306393, NCT03331445, NCT04312243) [21,45]. Furthermore, the FDA granted emergency expanded access, allowing its iNO delivery system (INOpulse®) to be immediately used to treat COVID-19 [21].
4.6. Hydroxychloroquine
Chloroquine (CQ) and hydroxychloroquine (HCQ) are antimalarial drugs that were among the earliest drugs to receive attention as possible repurposable treatment options for COVID-19 [47,48]. The two drugs impair in vitro the terminal glycosylation of ACE2 without significant change of the ACE2 cell surface; thus, they might be potent inhibitors of SARS-CoV-2 infections [49].
CQ and HCQ have been discussed as promising, cost-effective, and readily available agents in the treatment of COVID-19. In vitro cell cultures showed that HCQ seems to be a more potent inhibitor of infection with SARS-CoV-2 than CQ. However, both drugs, taken orally, may cause severe side effects such as ocular toxicity, psychiatric symptoms, and myocardial dysfunction. These side effects may be severe and, therefore, limit widespread application in vivo [49]. Thus, the authors proposed using a small dose of aerosolized HCQ (2–4 mg per inhalation) to reach sufficient therapeutic levels at the alveolar epithelial cells. The authors also explained that by using a non-systemic low-dose aerosol, oral administration’s adverse drug effects would significantly be reduced [8,49]. Kavanagh et al. [47] described an inhaled formulation of HCQ, which has passed safety studies in clinical trials for asthma treatment, and discussed how this approach might reduce side effects and improve efficacy [47]. The progression of a simple formulation to phase two studies would enable using the safety data to allow phase two trials in COVID-19 immediately [47]. As a conclusion to all of the above, one option to potentially improve HCQ efficacy at a lower dose is to deliver the drug directly to the lung as an inhaled formulation. HCQ was found to be effective as an antiviral in alveolar cells [8,47].
4.7. Plasminogen
Plasminogen is the zymogen of plasmin, the primary enzyme that degrades fibrin clots and interacts with cell surfaces. It is efficiently activated by the plasminogen activators, which is a protease system [50]. Plasminogen is a crucial regulator in many pathological processes, including fibrinolysis, wound healing, and infection [51]. The lungs of patients with COVID-19 have shown signs of acute respiratory distress syndrome, formation of hyaline membrane mainly composed of fibrin, and “ground-glass” opacity [51]. Thus, Wu Y et al. [51] have investigated the role of plasminogen in improving lung lesions and hypoxemia in COVID-19 patients. The inhalation of plasminogen has improved the lung lesions in five clinically moderate COVID-19 patients and oxygen saturation in six clinically severe COVID-19 patients. Finally, this study concludes that inhaled plasminogen might effectively treat the lung lesions and hypoxemia observed with COVID-19 infection [8,51].
4.8. Modified Angiotensin-Converting Enzyme 2 (ACE2)
ACE2 is a metallopeptidase, has been identified as a functional receptor for SARS-CoV-1 and a potent receptor for SARS-CoV-2. ACE2 is a renin-angiotensin system (RAS) component; it is a carboxypeptidase that potently degrades angiotensin II to angiotensin 1–7, a key player in RAS [52]. Earlier studies reported that recombinant ACE2 (rACE2) protects against severe acute lung injury and acute Ang II-induced hypertension. Recombinant ACE2 (rACE2) was also reported to attenuate Ang II-induced heart hypertrophy, cardiac dysfunction, and adverse myocardial remodeling in murine models, as well as renal oxidative stress, inflammation, and fibrosis [52]. Lei et al. [52] hypothesized that ACE2, especially the fusion protein, may have a neutralization potential for coronavirus SARS-CoV-2 based on the receptor function of ACE2 for coronavirus. The authors also investigated the therapeutic potential of ACE2 by constructing and generating a fusion protein (ACE2-Ig) composed of the extracellular domain of human ACE2 linked to the Fc domain of human IgG1 [52]. After they identified that ACE2 fusion proteins bind with high affinity to the RBD, they next tested the inhibitory activity of ACE2 fusion proteins against SARS-CoV-2 and compared it with that against SARS-CoV. Their data showed that ACE2-Ig and mACE2-Ig [52] potently neutralized both SARS-CoV and SARS-CoV-2 viruses. Wrapp et al. [23], provided biophysical and structural evidence that the SARS-CoV-2 S protein binds ACE2 with higher affinity than does SARS-CoV S [23].
Ameratunga et al. have investigated the potential of inhaled modified ACE2 as a decoy to mitigate SARS-CoV-2 infection [53]. They hypothesized that synthesizing modified recombinant soluble human ACE2 molecules (shACE2) by substituting two amino acids would increase the affinity for the RBD of inactivated SARS-CoV-2. The shACE2 was delivered via a lower shear stress inhaler, i.e., a Respimat® inhaler that lessens the denaturation of the protein [8,53]. Ultimately, the authors conclude that the inhalation delivery of modified shACE2 could alter the infection’s trajectory, delaying the destruction of pulmonary epithelium, and allowing appropriate protective immune responses against the virus [8].
4.9. Interferon-β
Interferon-β is a cytokine-induced by a viral infection, which primarily drives the innate immune responses in the human lung. SARS-CoV-2 suppresses the release of interferon-β [54,55,56]. SNG001 is an inhalable formulation of recombinant interferon-β delivered using a nebulizer and is in the developmental phases to treat virus-induced lower respiratory tract illnesses. The inhalation delivery will achieve a sufficient concentration of interferon-β in the lungs that results in an effective local antiviral response while minimizing systemic exposure. SNG001 has been shown to improve lung antiviral defenses, as evaluated in patients with and without respiratory viral infections, by sputum cell antiviral biomarkers. Phase two trials of SNG001 have demonstrated an improved lung function in asthma patients with respiratory viral infection compared to a placebo [57,58].
4.10. Anti-Microbial Colloidal Silver Formulations
Zachar et al. [59] studied the antiviral and antimicrobial effects of an inhaled silver nanoparticulate formulation for COVID-19 treatment and investigated the minimal inhibitory concentration (MIC) of these silver nanoparticles in various locations of the respiratory system [59]. The nanoparticles, 3–7 nm in size, are effective in virus attachment and the suppression of their infectious mechanism. This study concludes that delivering 25 µg/mL of nanoparticulate colloidal suspension is effective to achieve target tissue concentration. Depending on the silver dosage regimen’s safety information, the proposed formulations can be used as antiviral agent for the treatment of early-stage respiratory viral infections including COVID-19/SARS-CoV-2 [8,59].
4.11. Unfractionated Heparin (UFH)
A study performed by van Haren et al. [60] showed a trial to administer UFH via nebulizers. COVID-19-induced ARDS displays the features of diffuse alveolar damage with extensive pulmonary coagulation activation resulting in fibrin deposition in the microvasculature and formation of hyaline membranes in the air sacs. Furthermore, patients infected with SARS-CoV-2 have high inflammatory cytokines in plasma and bronchoalveolar lavage fluid and significant coagulopathy. The authors demonstrated that trials in patients with acute lung injury found that inhaled UFH reduced the pulmonary dead space, coagulation activation, and microvascular thrombosis. Moreover, UFH has anti-inflammatory, mucolytic, and anti-viral properties. Specifically, it has been shown to inactivate the SARS-CoV-2 virus and prevent its entry into mammalian cells due to that inhibiting the pulmonary infection by SARS-CoV-2 [8,60].
4.12. Salinomycin
Salinomycin, a carboxylic polyether ionophore isolated from Streptomyces albus, is a broad-spectrum antibiotic that had drawn attention in the selective targeting of cancer and viral infections [61]. Its antiviral activity was mediated via inhibition of endosomal acidification [62]. A recent study identified it as a potential antiviral agent for treating SARS-CoV-2 [63]. However, the oral administration of salinomycin for the treatment of COVID-19 is limited by its poor absorption, low oral bioavailability, and off-target effects. [61]. To overcome these limitations, Pindiprolu et al. [61] proposed the encapsulation of salinomycin in nanostructured carriers and delivering them directly to the lungs as an attractive strategy for the treatment of respiratory infections such as SARS-CoV-2 infections.
4.13. Ivermectin
Ivermectin is a potent anti-parasitic drug that has shown in vitro anti-viral activity against several DNA and RNA viruses, including SARS-CoV-2 [64,65]. It acts by inhibiting the interaction between the human immunodeficiency virus-1 (HIV-1), integrase protein (IN), and the importin (IMP) α/β. Caly et al. (2020) demonstrated that a single dose of ivermectin was able to reduce the replication of an Australian isolate of SARS-CoV-2 in Vero/hSLAM cells by 5000-fold [64]. However, a very high dose of drug is required as an oral dosage form to achieve a proper concentration at the site of infection, i.e., the respiratory system. Thus, an inhaled form of ivermectin is hypothesized to deliver the drug directly to the site of infection and as a best treatment option [66]. Currently, a phase two study is ongoing for inhaled ivermectin as nasal spray (1 mL in each nostril two times daily) at Tanta University, Egypt (NCT04510233) [67].
4.14. Niclosamide
Niclosamide, a narrow-spectrum anthelminthic drug, acts by inhibiting oxidative phosphorylation in the mitochondria [68,69]. Several research studies describe the potential use of niclosamide as an antiviral drug against several viruses, including coronaviruses [70,71,72,73,74]. It is hypothesized to act against SARS-CoV-2 by a similar mechanism of enhancing host cell autophagy against a MERS-CoV infection through inhibition of SKP2 (S-phase kinase associated protein 2) [73]. The poor water solubility and absorption of niclosamide limit its oral administration for effective delivery to the site of infection [75]. Thus, direct delivery of niclosamide to the lung could overcome the limitations of oral administration and achieve high drug concentration at the site of infection, i.e., the lungs. Brunaugh et al. [76] engineered composite particles containing niclosamide and an endogenous protein, human lysozyme, for inhalation delivery to the lungs. This study demonstrated that the co-formulation of niclosamide with lysozyme is four-fold more potent against coronaviruses compared to niclosamide alone. In a recent study, Jara et al. [77] developed a dry powder formulation of niclosamide in combination with hydrophilic excipients, mannitol and leucine, using a thin-film freezing method. These powders showed acceptable aerosol properties, with a fine particle fraction of 86%, suitable for deep lung delivery. Further, the pharmacokinetic study in a Syrian hamster model demonstrated that a single inhalation administration of the formulation maintains a drug concentration above IC90 levels for at least 24 h.
5. Clinical Trials on Inhaled COVID-19 Therapeutics
Along with the above-mentioned therapeutics, several other drugs are also in various phases of clinical trials. The current clinical trials on inhaled therapeutics for SARS-CoV-2 are collected from the website of ClinicalTrials.gov and listed in Table 3.

Table 3.
Summary of clinical trials on inhaled therapeutics for SARS-CoV-2 (collected from the website of ClinicalTrials.gov).
6. Inhaled COVID-19 Vaccines and Their Current Clinical Status
The intramuscularly administered COVID-19 vaccines that are being administered currently present a significant limitation, which is the lack of mucosal immunization: given that the primary route for transmission of SARS-CoV-2 is the respiratory mucosa in the respiratory tract via inhalation of small respiratory droplets from infected individuals [127]. To date, the COVID-19 vaccine that has advanced to phase three in clinical trials has no expectations to provide mucosal immunity in nasal cavities nor lung tissue, although they demonstrate T cell activation and the stimulation of serum neutralizing antibodies. Several COVID-19 inhaled vaccine candidates in development have shown good results in pre-clinical studies, as it has been mentioned in earlier sections; a selection of these vaccines, which have progressed to clinical trials, are presented in Table 4.

Table 4.
Summary of clinical trials on inhaled vaccines for SARS-CoV-2.
6.1. AdCOVID Vaccine
Altimmune is a clinical-stage biopharmaceutical company focused on developing intranasal vaccines, immune-modulating therapies, and treatments for liver disease. They have reported pre-clinical results of an intranasal adenovirus-vectored vaccine against COVID-19, which encodes the RBD as an alternative to the trimeric spike ectodomain for use as the target antigen.
The induction of a systemic and mucosal immune response following single-dose nasal inhalation in mice highlights multiple enormous advantages for this formulation. The first advantage is the non-invasive route of administration, and the second is the ability to activate an immune response in the upper and lower respiratory tract, thus leading to the acquisition of infection prevention at the site of virus entrance and also, the reduction in the probability of transmission between vaccinated individuals [134].
Phase one clinical trials have already begun for AdCOVID by Altimmune, Inc., early this year. The demographics for this study’s inclusion criteria are healthy men and women aged 18 to 55 years. The purpose of this first trial is to test the safety endpoint and tolerability after one to three intranasal doses and to evaluate its safety and immunogenicity. The main parameters for immunogenicity being tested in this study are serum IgG binding, neutralizing antibody titers, mucosal IgA antibody, and T cell responses.
6.2. MV-014-212 Meissa Vaccine
Meissa vaccines is using the same AttenuBlock® technology (Codon Deoptimization) employed for respiratory syncytial virus (RSV) (phase two in clinical trials) vaccine production [135] to develop a live attenuated chimeric virus-based intranasal vaccine for SARS-CoV-2, known as MV-014-212. Very promising results have shown that Meissa’s RSV vaccine was safe and well-tolerated among healthy adults. After a single dose, mucosal IgA RSV-specific binding was induced despite no detectable virus being found in cotton rats [136] and this is how the RSV vaccine differs from other live-attenuated vaccines. The SARS-CoV-2 spike (s) proteins replaced RSV surface proteins, which eliminates the expression of immune suppressors and increases antigen expression.
In accordance with previous clinical trial results of a similar RSV vaccine, the inhaled COVID-19 vaccine has advanced to phase one, which is stated to start at the end of March 2021, and will take approximately 18 months (ClinicalTrials.gov identifier NCT04798001) [129].
6.3. CoroFlu Vaccine
Bharat Biotech (India), in partnership with Biotech startup Precision Virologic and the University of Wisconsin-Madison (US), initiated clinical trials on the CoroFlu vaccine. The CoroFlu vaccine uses a chimpanzee adenovirus-vectored vaccine encoding a prefusion stabilized spike protein (ChAd-SARS-CoV-2-S) in challenge studies with SARS-CoV-2 and mice expressing the human ACE-2 receptors, [137]. Bricker et al. [138] compared the protective capacity of intranasal and intramuscular delivery of same vectored vaccine encoding a pre-fusion stabilized spike protein (ChAd-SARS-CoV-2-S) in Golden Syrian hamsters and Hassan et al. [139] did so in non-human primate rhesus macaques that were immunized with ChAd-Control or ChAd-SARS-CoV-2-S and challenged one month later by combined intranasal and intrabronchial routes with SARS-CoV-2. In all the preclinical tests, CoroFlu nasal spray induced neutralizing antibodies and T cell responses and limited or prevented infection in the upper and lower respiratory tract after the SARS-CoV-2 challenge. In a phase one clinical trial, they will evaluate the safety, reactogenicity, and immunogenicity of three groups of healthy volunteers who receive either a single intranasal dose (vaccine on day 0 and placebo on day 28) or two doses (vaccine on day 0 and 28) of the BBV154 vaccine or a placebo (on day 0 and day 28).
6.4. CanSinoBio Vaccine
A COVID-19 vaccine (adenovirus type 5 vector) from CanSino Biologics obtained the national drug regulator’s approval to start intranasal inhalation clinical trials of its latest recombinant intramuscular-applied COVID-19 vaccine. Stimulation of mucosal immunity will be achieved by the atomization of adenovirus into small particles in the respiratory tract after inhalation [140].
6.5. AstraZeneca COVID19 Vaccine
The current intramuscular-applied AZD12222 recombinant replication-defective chimpanzee adenovirus expressing the SARS-CoV-2 surface glycoprotein is facing phase three in clinical trials, showing statistically significant vaccine efficacy of 79% at preventing symptomatic COVID-19 and 100% efficacy at preventing severe disease and hospitalization. Oxford/AstraZeneca announced that they are going to phase one clinical trials for a new intranasal AZD12222 vaccine to investigate immune responses with 30 healthy volunteers [141]. Intranasal doses of ChAdOx1 nCov-19/AZD12222 administered to hamsters and rhesus macaques showed a decrease in the viral load in the lung tissue and bronchoalveolar lavage fluid (BALF) [142].
6.6. COVI-VAC
The US Codagenix company is advancing to a phase one clinical trial of its vaccine [143]. COVI-VAC is a live attenuated whole virus COVID-19 vaccine that is engineered to be structurally identical to wild-type SARS-CoV-2, but its replication rate is much slower, and it has the same amino acids sequence, it, therefore, has the potential to induce broad antibody, T-cell, and mucosal immunity with a single intranasal dose. In the clinical trial, they will evaluate the safety and immune response of COVI-VAC in healthy adults in two separate doses (28 days apart), the outcome measurements will be to record symptoms and oral temperature in a daily diary for 14 days after each dose. Safety laboratory tests, physical exams, ECGs, and a chest X-ray will also be performed, and peak expiratory flow and vital signs will be measured.
7. Inhaled Therapy in Long-Haul COVID
According to a report of 72,314 cases from the Chinese Center for Disease Control and Prevention, about 81% of patients (36160 cases) had mild to moderate disease (i.e., non-pneumonia and mild pneumonia), 14% had severe disease ((i.e., dyspnea, respiratory frequency ≥30/min, blood oxygen saturation ≤93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300, and/or lung infiltrates >50% within 24 to 48 h), and 5% were critically ill (i.e., respiratory failure, septic shock, and/or multiple organ dysfunction or failure) [144]. A few recovered COVID-19 patients develop one or more persistent symptoms or new symptoms lasting weeks or months, which is called “long-haul COVID”, “long COVID” or “post COVID syndrome” [145]. Long COVID is divided into acute COVID and chronic COVID, depending upon the duration of symptoms. In acute condition, COVID symptoms last beyond 3 weeks, but less than 12 weeks. In chronic COVID, symptoms extend beyond 12 weeks [146]. According to a report from Italy, fatigue, dyspnea, joint pain, and chest pain are the common persistent symptoms experienced by long haulers [147]. Other potential respiratory problems include chronic cough, fibrotic lung disease, bronchiectasis, and pulmonary vascular disease [148]. Inhalation delivery is the most appropriate route of administration for treating the above respiratory conditions in long haul COVID patients.
Recently, Ampio Pharmaceuticals has secured an approval to evaluate the use of inhaled Ampion (AP-018) in patients with prolonged respiratory symptoms due to long COVID [149,150]. Ampion is the filtrate of human serum albumin (low molecular weight), which has the potential to reduce inflammatory cytokines correlated with COVID-19 disease and respiratory complications, such as acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [150].
8. Conclusions and Future Opportunities
For decades, innovative inhaled drugs have been developed and continue to grow tremendously for lung diseases, as well as many other infectious and non-infectious diseases and syndromes. Over the last ten years, improvements in existing approaches have matured and new pathways have expanded the use of inhalation technology to effectively combat pulmonary diseases. Despite contributions from international medical and science communities’ and recent decreases in the number of hospitalizations, COVID-19 remains an unprecedented obstacle. The inhalation delivery of therapeutics and vaccines against SARS-CoV-2 is a promising, non-invasive route of administration with unique advantages. Generally, all vaccines and repurposed therapeutics for COVID-19 are currently administered via intramuscular and subcutaneous routes, but recently there has been an enormous interest in developing the non-invasive route of nasal and oral inhalation for immunization and treatment.
Currently, FluMist® (a live attenuated influenza vaccine) and Relenza® Diskhaler® dry powder inhaler are FDA-approved marketed pharmaceutical products for the prevention of influenza infection that were both approved years ago. There are several inhaled therapeutics currently in phase one and two clinical trials. It is advantageous to deliver a vaccine or therapeutic by inhalation directly to the respiratory tract since it is the primary route of initial viral infection and transmission. Furthermore, it is critical to discover alternative ways to mitigate the healthcare hazards associated with SARS-CoV-2, as well as to conduct further research into innovative inhaled drug and vaccine delivery for other respiratory pathogens.
Author Contributions
Invitation received, H.M.M.; conceptualization, B.B.E. and H.M.M.; writing and original draft preparation, B.B.E., W.A. and D.E.-B.; resources, J.G.L., R.P. and H.M.M.; writing, reviewing, and editing, B.B.E., R.P., J.G.L. and H.M.M.; project administration, H.M.M.; funding acquisition, J.G.L., R.P. and H.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH NIDA 5UG3DA047717 (HMM and RP), R01HL137282 (HMM), R21AG054766 (HMM), R21AI135935 (HMM and JGL), and P01HL103453 (HMM).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Omolo, C.A.; Soni, N.; Fasiku, V.O.; Mackraj, I.; Govender, T. Update on therapeutic approaches and emerging therapies for SARS-CoV-2 virus. Eur. J. Pharmacol. 2020, 883, 173348. [Google Scholar] [CrossRef]
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021, 170, 1–25. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Coronavirus Disease (COVID-19) Dashboard. World Health Organization website. Available online: https://covid19.who.int/ (accessed on 18 June 2021).
- Bhavana, V.; Thakor, P.; Singh, S.B.; Mehra, N.K. COVID-19: Pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life Sci. 2020, 261, 118336. [Google Scholar] [CrossRef]
- Edwards, D.; Hickey, A.; Batycky, R.; Griel, L.; Lipp, M.; Dehaan, W.; Clarke, R.; Hava, D.; Perry, J.; Laurenzi, B.; et al. A New natural defense against airborne pathogens. QRB Discov. 2020, 1, e5. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.H.; Tawfeek, H.M.; Abdelfattah, A.; El-Saber Batiha, G.; Hetta, H.F. Recent updates in COVID-19 with emphasis on inhalation therapeutics: Nanostructured and targeting systems. J. Drug Deliv. Sci. Technol. 2021, 63, 102435. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Berlinski, A.; Canisius, S.; Cipolla, D.; Dolovich, M.B.; Gonda, I.; Hochhaus, G.; Kadrichu, N.; Lyapustina, S.; Mansour, H.M. Urgent appeal from International Society for Aerosols in Medicine (ISAM) during COVID-19: Clinical decision makers and governmental agencies should consider the inhaled route of administration: A statement from the ISAM regulatory and standardization issues networking group. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.J. Back to the future: Inhaled drug products. J. Pharm. Sci. 2013, 102, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.L. The inhalation of drugs: Advantages and problems. Respir. Care 2005, 50, 367–382. [Google Scholar]
- Eedara, B.B.; Tucker, I.G.; Das, S.C. In vitro dissolution testing of respirable size anti-tubercular drug particles using a small volume dissolution apparatus. Int. J. Pharm. 2019, 559, 235–244. [Google Scholar] [CrossRef]
- Eedara, B.B.; Rangnekar, B.; Doyle, C.; Cavallaro, A.; Das, S.C. The influence of surface active l-leucine and 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) in the improvement of aerosolization of pyrazinamide and moxifloxacin co-spray dried powders. Int. J. Pharm. 2018, 542, 72–81. [Google Scholar] [CrossRef]
- Eedara, B.B.; Rangnekar, B.; Sinha, S.; Doyle, C.; Cavallaro, A.; Das, S.C. Development and characterization of high payload combination dry powders of anti-tubercular drugs for treating pulmonary tuberculosis. Eur. J. Pharm. Sci. 2018, 118, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Eedara, B.B.; Tucker, I.G.; Das, S.C. Phospholipid-based pyrazinamide spray-dried inhalable powders for treating tuberculosis. Int. J. Pharm. 2016, 506, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Eedara, B.B.; Tucker, I.G.; Zujovic, Z.D.; Rades, T.; Price, J.R.; Das, S.C. Crystalline adduct of moxifloxacin with trans-cinnamic acid to reduce the aqueous solubility and dissolution rate for improved residence time in the lungs. Eur. J. Pharm. Sci. 2019, 136, 104961. [Google Scholar] [CrossRef] [PubMed]
- Rangnekar, B.; Momin, M.A.M.; Eedara, B.B.; Sinha, S.; Das, S.C. Bedaquiline containing triple combination powder for inhalation to treat drug-resistant tuberculosis. Int. J. Pharm. 2019, 570, 118689. [Google Scholar] [CrossRef]
- Tu, Y.-F.; Chien, C.-S.; Yarmishyn, A.A.; Lin, Y.-Y.; Luo, Y.-H.; Lin, Y.-T.; Lai, W.-Y.; Yang, D.-M.; Chou, S.-J.; Yang, Y.-P.; et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020, 21, 2657. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Jiang, J.; Su, L.; Shu, T.; Liu, H.; Lai, S.; Ghiladi, R.A.; Wang, J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 2021, 163, 153–162. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Vilekar, P.; Yang, S.-P.; Gupta, M.; Oh, M.I.; Meek, A.; Doyle, L.; Villar, L.; Brennecke, A. Small molecule therapeutics for COVID-19: Repurposing of inhaled furosemide. PeerJ 2020, 8, e9533. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Hamblin, M.R.; Rezaei, N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin. Chim. Acta 2020, 508, 254–266. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020, 19, 141–154. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Amanat, F.; Krammer, F. SARS-CoV-2 vaccines: Status report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- Stein, S.W.; Thiel, C.G. The history of therapeutic aerosols: A chronological review. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.J. Emerging trends in inhaled drug delivery. Adv. Drug Deliv. Rev. 2020, 157, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Alabsi, W.; Al-Obeidi, F.A.; Polt, R.; Mansour, H.M. Organic solution advanced spray-dried microparticulate/nanoparticulate dry powders of lactomorphin for respiratory delivery: Physicochemical characterization, in vitro aerosol dispersion, and cellular studies. Pharmaceutics 2020, 13, 26. [Google Scholar] [CrossRef]
- Eedara, B.B.; Alabsi, W.; Encinas-Basurto, D.; Polt, R.; Mansour, H.M. Spray-dried inhalable powder formulations of therapeutic proteins and peptides. AAPS PharmSciTech 2021, 22, 185. [Google Scholar] [CrossRef] [PubMed]
- Sahakijpijarn, S.; Moon, C.; Koleng, J.J.; Christensen, D.J.; Williams III, R.O. Development of remdesivir as a dry powder for inhalation by thin film freezing. Pharmaceutics 2020, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Vartak, R.; Patil, S.M.; Saraswat, A.; Patki, M.; Kunda, N.K.; Patel, K. Aerosolized nanoliposomal carrier of remdesivir: An effective alternative for COVID-19 treatment in vitro. Nanomedicine 2021, 16, 1187–1202. [Google Scholar] [CrossRef]
- Matsuyama, S.; Kawase, M.; Nao, N.; Shirato, K.; Ujike, M.; Kamitani, W.; Shimojima, M.; Fukushi, S. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Iwabuchi, K.; Yoshie, K.; Kurakami, Y.; Takahashi, K.; Kato, Y.; Morishima, T. Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: Report of three cases. J. Infect. Chemother. 2020, 26, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.G.; Singh, D.; Hadfield, R.M. Inhaled corticosteroids and COVID-19: A systematic review and clinical perspective. Eur. Respir. J. 2020, 55, 2001009. [Google Scholar] [CrossRef]
- Yamaya, M.; Nishimura, H.; Deng, X.; Sugawara, M.; Watanabe, O.; Nomura, K.; Shimotai, Y.; Momma, H.; Ichinose, M.; Kawase, T. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir. Investig. 2020, 58, 155–168. [Google Scholar] [CrossRef]
- Nicolau, D.V.; Bafadhel, M. Inhaled corticosteroids in virus pandemics: A treatment for COVID-19? Lancet. Respir. Med. 2020, 8, 846–847. [Google Scholar] [CrossRef]
- Yu, L.-M.; Bafadhel, M.; Dorward, J.; Hayward, G.; Saville, B.R.; Gbinigie, O.; Van Hecke, O.; Ogburn, E.; Evans, P.H.; Thomas, N.P.; et al. Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: Interim analyses from the PRINCIPLE trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Nicolau, D.V.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; et al. Inhaled budesonide in the treatment of early COVID-19 illness: A randomised controlled trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Brennecke, A.; Villar, L.; Wang, Z.; Doyle, L.M.; Meek, A.; Reed, M.; Barden, C.; Weaver, D.F. Is inhaled furosemide a potential therapeutic for COVID-19? Am. J. Med. Sci. 2020, 360, 216–221. [Google Scholar] [CrossRef]
- Grogono, J.C.; Butler, C.; Izadi, H.; Moosavi, S.H. Inhaled furosemide for relief of air hunger versus sense of breathing effort: A randomized controlled trial. Respir. Res. 2018, 19, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosavi, S.H.; Binks, A.P.; Lansing, R.W.; Topulos, G.P.; Banzett, R.B.; Schwartzstein, R.M. Effect of inhaled furosemide on air hunger induced in healthy humans. Respir. Physiol. Neurobiol. 2007, 156, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Ide, T.; Sudo, T.; Sato, J. Inhaled furosemide greatly alleviates the sensation of experimentally induced dyspnea. Am. J. Respir. Crit. Care Med. 2000, 161, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, L.; Kuo, H.-C.D.; Shannar, A.; Peter, R.; Chou, P.J.; Li, S.; Hudlikar, R.; Liu, X.; Liu, Z. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. Rep. 2020, 6, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Wilson, C.; Weinberg, J.; Gavin, D.; Murphy, J.; Reardon, C.C. Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients. Ther. Adv. Respir. Dis. 2020, 14. [Google Scholar] [CrossRef]
- Kavanagh, O.; Healy, A.M.; Dayton, F.; Robinson, S.; O’Reilly, N.J.; Mahoney, B.; Arthur, A.; Walker, G.; Farragher, J.P. Inhaled hydroxychloroquine to improve efficacy and reduce harm in the treatment of COVID-19. Med. Hypotheses 2020, 143, 110110. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Klimke, A.; Hefner, G.; Will, B.; Voss, U. Hydroxychloroquine as an aerosol might markedly reduce and even prevent severe clinical symptoms after SARS-CoV-2 infection. Med. Hypotheses 2020, 142, 109783. [Google Scholar] [CrossRef]
- Miles, L.A.; Lighvani, S.; Baik, N.; Parmer, C.M.; Khaldoyanidi, S.; Mueller, B.M.; Parmer, R.J. New insights into the role of Plg-RKT in macrophage recruitment. Int. Rev. Cell Mol. Biol. 2014, 309, 259–302. [Google Scholar]
- Wu, Y.; Wang, T.; Guo, C.; Zhang, D.; Ge, X.; Huang, Z.; Zhou, X.; Li, Y.; Peng, Q.; Li, J. Plasminogen improves lung lesions and hypoxemia in patients with COVID-19. QJM: Int. J. Med. 2020, 113, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Fu, W.; Qian, K.; Li, T.; Zhang, S.; Ding, M.; Hu, S. Potent neutralization of 2019 novel coronavirus by recombinant ACE2-Ig. BioRxiv 2020. [Google Scholar] [CrossRef]
- Ameratunga, R.; Lehnert, K.; Leung, E.; Comoletti, D.; Snell, R.; Woon, S.-T.; Abbott, W.; Mears, E.; Steele, R.; McKee, J. Inhaled modified angiotensin converting enzyme 2 (ACE2) as a decoy to mitigate SARS-CoV-2 infection. New Zealand Med. J. (Online) 2020, 133, 112–118. [Google Scholar]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Monk, P.D.; Marsden, R.J.; Tear, V.J.; Brookes, J.; Batten, T.N.; Mankowski, M.; Gabbay, F.J.; Davies, D.E.; Holgate, S.T.; Ho, L.-P. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 196–206. [Google Scholar] [CrossRef]
- Yuen, C.K.; Lam, J.Y.; Wong, W.M.; Mak, L.F.; Wang, X.; Chu, H.; Cai, J.P.; Jin, D.Y.; To, K.K.; Chan, J.F.; et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 2020, 9, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Djukanović, R.; Harrison, T.; Johnston, S.L.; Gabbay, F.; Wark, P.; Thomson, N.C.; Niven, R.; Singh, D.; Reddel, H.K.; Davies, D.E.; et al. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am. J. Respir. Crit. Care Med. 2014, 190, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Olsson, M.; Aurell, M.; Lundin, C.; Paraskos, J.; Cavallin, A.; Kjerrulf, M.; Karlsson, K.; Marsden, R.; Malmgren, A.; Gustafson, P.; et al. On-demand inhaled interferon-beta 1a for the prevention of severa asthma exacerbations: Results of the INEXAS phase 2a study. In D12. IMMUNOTHERAPY IN LUNG DISEASE; American Thoracic Society: New York, NY, USA, 2018; p. A6165. [Google Scholar]
- Zachar, O. Formulations for COVID-19 early stage treatment via silver nanoparticles inhalation delivery at home and hospital. Sci. Prepr. 2020. [Google Scholar] [CrossRef]
- Van Haren, F.M.; Page, C.; Laffey, J.G.; Artigas, A.; Camprubi-Rimblas, M.; Nunes, Q.; Smith, R.; Shute, J.; Carroll, M.; Tree, J. Nebulised heparin as a treatment for COVID-19: Scientific rationale and a call for randomised evidence. Crit. Care 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Pindiprolu, S.K.S.; Kumar, C.S.P.; Golla, V.S.K.; Likitha, P.; Chandra, S.; Ramachandra, R. Pulmonary delivery of nanostructured lipid carriers for effective repurposing of salinomycin as an antiviral agent. Med. Hypotheses 2020, 143, 109858. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, J.S.; Yoon, Y.-S.; Go, Y.Y.; Lee, H.W.; Kwon, O.S.; Park, S.; Park, M.-S.; Kim, M. Salinomycin Inhibits Influenza Virus Infection by Disrupting Endosomal Acidification and Viral Matrix Protein 2 Function. J. Virol. 2018, 92, e01441–e01418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, M.; Chang, S.Y.; Byun, S.Y.; Choi, I.; d’Alexandry d’Orengiani, A.-L.P.H.; Shum, D.; Min, J.-Y.; Windisch, M.P. Screening of FDA-approved drugs using a MERS-CoV clinical isolate from South Korea identifies potential therapeutic options for COVID-19. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, Y.; Xiong, H.; Ci, X.; Li, H.; Yu, L.; Zhang, L.; Deng, X. Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2009, 9, 354–359. [Google Scholar] [CrossRef]
- Mittal, N.; Mittal, R. Inhaled route and anti-inflammatory action of ivermectin: Do they hold promise in fighting against COVID-19? Med. Hypotheses 2021, 146, 110364. [Google Scholar] [CrossRef]
- Okasha, K. Ivermectin Nasal Spray for COVID19 Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT04510233 (accessed on 19 June 2021).
- Weinbach, E.C.; Garbus, J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature 1969, 221, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Frayha, G.J.; Smyth, J.D.; Gobert, J.G.; Savel, J. The mechanisms of action of antiprotozoal and anthelmintic drugs in man. Gen. Pharmacol. Vasc. Syst. 1997, 28, 273–299. [Google Scholar] [CrossRef]
- Xu, J.; Shi, P.-Y.; Li, H.; Zhou, J. Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect. Dis. 2020, 6, 909–915. [Google Scholar] [CrossRef]
- New Delivery Method Could Make Niclosamide an Effective Antiviral to Treat COVID-19. Available online: https://news.utexas.edu/2020/04/06/new-delivery-method-could-make-niclosamide-an-effective-antiviral-to-treat-covid-19/ (accessed on 16 June 2021).
- Wu, C.-J.; Jan, J.-T.; Chen, C.-M.; Hsieh, H.-P.; Hwang, D.-R.; Liu, H.-W.; Liu, C.-Y.; Huang, H.-W.; Chen, S.-C.; Hong, C.-F.; et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 2004, 48, 2693–2696. [Google Scholar] [CrossRef] [Green Version]
- Gassen, N.C.; Niemeyer, D.; Muth, D.; Corman, V.M.; Martinelli, S.; Gassen, A.; Hafner, K.; Papies, J.; Mösbauer, K.; Zellner, A.; et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun. 2019, 10, 5770. [Google Scholar] [CrossRef]
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob. Agents Chemother. 2020, 64, e00819-00820. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS ONE 2018, 13, e0198389. [Google Scholar]
- Brunaugh, A.D.; Seo, H.; Warnken, Z.; Ding, L.; Seo, S.H.; Smyth, H.D.C. Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patient-adaptable treatment for coronavirus infections and sequalae. PLoS ONE 2021, 16, e0246803. [Google Scholar] [CrossRef]
- Jara, M.O.; Warnken, Z.N.; Sahakijpijarn, S.; Moon, C.; Maier, E.Y.; Christensen, D.J.; Koleng, J.J.; Peters, J.I.; Hackman Maier, S.D.; Williams Iii, R.O. Niclosamide inhalation powder made by thin-film freezing: Multi-dose tolerability and exposure in rats and pharmacokinetics in hamsters. Int. J. Pharm. 2021, 603, 120701. [Google Scholar] [CrossRef] [PubMed]
- Stuart-Harris, N. Nitric Oxide Inhalation Therapy for COVID-19 Infections in the ED (NO COV-ED). Available online: https://ClinicalTrials.gov/show/NCT04338828 (accessed on 10 June 2021).
- Andersson, D.P.; Blennow, O. Inhalation of Ciclesonide For patients with COVID-19: A Randomised Open Treatment Study (HALT COVID-19) (HALT). Available online: https://clinicaltrials.gov/ct2/show/NCT04381364 (accessed on 10 June 2021).
- Ezer, N.; Naderi, N. Inhaled Ciclesonide for Outpatients with COVID19 (CONTAIN). Available online: https://clinicaltrials.gov/ct2/show/NCT04435795 (accessed on 10 June 2021).
- A Study of the Safety and Efficacy of Ciclesonide in the Treatment of Non-Hospitalized COVID-19 Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT04377711 (accessed on 10 June 2021).
- Kaprin, A. Inhalation Low Dose Radionuclide Therapy in Treatment COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04724538 (accessed on 10 June 2021).
- Sjöbring, U. Study to Assess the Safety of Ascending Doses of UNI911 INHALATION in Healthy Volunteers in Preparation for Evaluation in Adults with COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04576312 (accessed on 10 June 2021).
- Elkazzaz, M.R.M. Efficacy of Aerosol Combination Therapy of 13 Cis Retinoic Acid and Captopril for Treating Covid-19 Patients via Indirect Inhibition of Transmembrane Protease, Serine 2 (TMPRSS2). Available online: https://clinicaltrials.gov/ct2/show/NCT04578236 (accessed on 10 June 2021).
- Elkazzaz, M.R.M. Combination of Chemopreventive Agents (all- Trans Retinoic Acid and Tamoxifen) as Potential Treatment for the Lung Complication of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04568096 (accessed on 10 June 2021).
- Elkazzaz, M.R.M. Combination Therapy with Isotretinoin and Tamoxifen Expected to Provide Complete Protection Against Severe Acute Respiratory Syndrome Coronavirus (Combination). Available online: https://clinicaltrials.gov/ct2/show/NCT04389580 (accessed on 10 June 2021).
- Elkazzaz, M.R.M. Clinical Role of Testosterone and Dihydrotestosterone and which of Them Should be Inhibited in COVID-19 Patients - A Double-Edged Sword? Available online: https://clinicaltrials.gov/ct2/show/NCT04623385 (accessed on 10 June 2021).
- Elkazzaz, M.R.M. Efficacy and Safety of Drug Combination Therapy of Isotretinoin and Some Antifungal Drugs as a Potential Aerosol Therapy for COVID-19: An innovative therapeutic approach COVID-19 (isotretinoin). Available online: https://clinicaltrials.gov/ct2/show/NCT04577378 (accessed on 10 June 2021).
- Xia, J. The Efficacy and Safety of Thalidomide Combined with Low-Dose Hormones in the Treatment of Severe COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04273581 (accessed on 10 June 2021).
- Elkazzaz, M.R.M. Aerosol Combination Therapy of All-Trans Retinoic Acid and Isotretinoin as a Novel Treatment for Inducing Neutralizing Antibodies in COVID -19 Infected Patients Better than Vaccine: An Innovative Treatment (Antibodies). Available online: https://clinicaltrials.gov/ct2/show/NCT04396067 (accessed on 10 June 2021).
- Ragab. New Treatment for COVID-19 Using Ethanol Vapor Inhalation. Available online: https://clinicaltrials.gov/ct2/show/NCT04554433 (accessed on 10 June 2021).
- A Clinical Study Evaluating Inhaled Aviptadil on COVID-19 (HOPE). Available online: https://clinicaltrials.gov/ct2/show/NCT04844580 (accessed on 10 June 2021).
- Leuppi, J.D.; Abig, K. Inhaled Aviptadil for the Treatment of COVID-19 in Patients at High Risk for ARDS. Available online: https://clinicaltrials.gov/ct2/show/NCT04536350 (accessed on 10 June 2021).
- Javitt, J.C. Inhaled ZYESAMI™ (Aviptadil Acetate) for the Treatment of Severe COVID-19 (AVICOVID-2). Available online: https://clinicaltrials.gov/ct2/show/NCT04360096 (accessed on 10 June 2021).
- El-Bendary, M. Inhaled Ivermectin and COVID-19 (CCOVID-19). Available online: https://clinicaltrials.gov/ct2/show/study/NCT04681053 (accessed on 10 June 2021).
- Kharma, N. INHALED Iloprost for Suspected COVID-19 Respiratory Failure (ILOCOVID). Available online: https://clinicaltrials.gov/ct2/show/NCT04445246 (accessed on 10 June 2021).
- First in Human SAD and MAD Study of Inhaled TD-0903, a Potential Treatment for ALI Associated with COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04350736 (accessed on 10 June 2021).
- Lanoix, J.-P. Treatment of COVID-19 by Nebulization of Inteferon Beta 1b Efficiency and Safety Study (COV-NI). Available online: https://clinicaltrials.gov/ct2/show/NCT04469491 (accessed on 10 June 2021).
- Varea, S. Inhaled Corticosteroid Treatment of COVID19 Patients with Pneumonia. Available online: https://clinicaltrials.gov/ct2/show/NCT04355637 (accessed on 10 June 2021).
- Bafadhel, M. Steroids in COVID-19 Study (STOIC). Available online: https://clinicaltrials.gov/ct2/show/NCT04416399 (accessed on 10 June 2021).
- Afazeli, S. Evaluation of Efficacy of Levamisole and Formoterol+Budesonide in Treatment of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04331470 (accessed on 10 June 2021).
- Taille, C. Protective role of Inhaled Steroids for Covid-19 Infection (INHASCO). Available online: https://clinicaltrials.gov/ct2/show/record/NCT04331054 (accessed on 10 June 2021).
- Levitt, J.; Festic, E.; Wilson, J. Arrest Respiratory Failure from Pneumonia (ARREST). Available online: https://clinicaltrials.gov/ct2/show/NCT04193878 (accessed on 10 June 2021).
- Zykov, K.A. Low-Doses Melphalan Inhalation in Patients with COVID-19 (Coronavirus Disease 2019) Pneumonia (MICOV). Available online: https://clinicaltrials.gov/ct2/show/NCT04380376 (accessed on 10 June 2021).
- Franco, V. VentaProst in Subjects with COVID-19 Requiring Mechanical Ventilation (VPCOVID). Available online: https://clinicaltrials.gov/ct2/show/NCT04452669 (accessed on 10 June 2021).
- Nebulized Heparin for the Treatment of COVID-19 (INHALE-HEP). Available online: https://clinicaltrials.gov/ct2/show/NCT04723563 (accessed on 10 June 2021).
- Haren, F.M.V. Inhaled Heparin for Hospitalised COVID-19 Patients (INHALE-HEP). Available online: https://clinicaltrials.gov/ct2/show/NCT04635241 (accessed on 10 June 2021).
- Qu, J.-M. A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia. Available online: https://clinicaltrials.gov/ct2/show/NCT04276987 (accessed on 10 June 2021).
- Wilkinson, T.; Francis, N. Trial of Inhaled Anti-Viral (SNG001) for SARS-CoV-2 (COVID-19) Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT04385095 (accessed on 10 June 2021).
- Holliday, Z.M.; Schrum, A. Dornase Alfa for ARDS in Patients with Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) (DORNASESARS2). Available online: https://clinicaltrials.gov/ct2/show/NCT04402970 (accessed on 10 June 2021).
- Porter, J. Nebulised Dornase Alfa for Treatment of COVID-19 (COVASE). Available online: https://clinicaltrials.gov/ct2/show/NCT04359654 (accessed on 10 June 2021).
- Astrelina, T. An Open Randomized Study of Dalargin Effectiveness in Patients with Severe and Critical manifestations of SARS-COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04346693 (accessed on 10 June 2021).
- Amen, R. Sodium Pyruvate Nasal Spray Treatment of COVID-19 and Influenza Infections. Available online: https://clinicaltrials.gov/ct2/show/NCT04824365 (accessed on 10 June 2021).
- Hawari, F. The Potential use of Inhaled hydroxychloroquine for the Treatment of COVID-19 in Cancer Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT04731051 (accessed on 10 June 2021).
- El-Sherbiny, I.M. Development and Validation of “Ready-to-Use” Inhalable forms of Hydroxychloroquine for Treatment of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04477083 (accessed on 10 June 2021).
- Bentur, O.S. A Study to Evaluate the Safety, Tolerability and Pharmacokinetics of Orally Inhaled Aerosolized Hydroxychloroquine Sulfate in Healthy Adult Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04461353 (accessed on 10 June 2021).
- Broom, C. The Use of PUL-042 Inhalation Solution to Reduce the Severity of COVID-19 in Adults Positive for SARS-CoV-2 Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT04312997 (accessed on 10 June 2021).
- Incalzi, R.A. Use of Inhaled High-Molecular Weight Hyaluronan in Patients with Severe COVID19: Feasibility and Outcomes (HA-COVID). Available online: https://clinicaltrials.gov/ct2/show/record/NCT04830020 (accessed on 10 June 2021).
- Study in Participants with Early Stage Coronavirus Disease 2019 (COVID-19) to Evaluate the Safety, Efficacy, and Pharmacokinetics of Remdesivir Administered by Inhalation. Available online: https://clinicaltrials.gov/ct2/show/NCT04539262 (accessed on 10 June 2021).
- Study of Ensifentrine or Placebo Delivered via pMDI in Hospitalized Patients with COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04527471 (accessed on 10 June 2021).
- Muscedere, J. Furosemide as Supportive Therapy for COVID-19 Respiratory Failure (FaST-1). Available online: https://clinicaltrials.gov/ct2/show/NCT04588792 (accessed on 10 June 2021).
- Phase 3 Inhaled Novaferon Study in Hospitalized Patients with Moderate to Severe COVID-19 (NOVATION-1). Available online: https://clinicaltrials.gov/ct2/show/NCT04669015 (accessed on 10 June 2021).
- Nebulised Rt-PA for ARDS due to COVID-19 (PACA). Available online: https://clinicaltrials.gov/ct2/show/NCT04356833 (accessed on 10 June 2021).
- Gong, Z. DAS181 for Severe COVID-19: Compassionate USE. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04324489 (accessed on 17 June 2021).
- Sargramostim Use in COVID-19 to Recover Patient Health (SCOPE). Available online: https://clinicaltrials.gov/ct2/show/NCT04707664?term=immunotherapy%2C+inhalation&cond=Covid19&draw=2&rank=3 (accessed on 17 June 2021).
- Inhalon Biopharma Receives $7 Million from USAMRDC to Study Inhaled “Muco-Trapping” Antibody for the Treatment of COVID-19. Available online: https://www.inhalon.com/26may2021 (accessed on 17 June 2021).
- Gomez, A.I.; Acosta, M.F.; Muralidharan, P.; Yuan, J.X.J.; Black, S.M.; Hayes, D.; Mansour, H.M. Advanced spray dried proliposomes of amphotericin B lung surfactant-mimic phospholipid microparticles/nanoparticles as dry powder inhalers for targeted pulmonary drug delivery. Pulm. Pharmacol. Ther. 2020, 64, 101975. [Google Scholar] [CrossRef]
- Safety and Immunogenicity of AdCOVID in Healthy Adults (COVID-19 Vaccine Study). Available online: https://clinicaltrials.gov/ct2/show/NCT04679909 (accessed on 10 June 2021).
- Medzihradsky, O. Safety and Immunogenicity of an Intranasal RSV Vaccine Expressing SARS-CoV-2 Spike Protein (COVID-19 Vaccine) in Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04798001 (accessed on 10 June 2021).
- Safety and immunogenicity of an intranasal SARS-CoV-2 vaccine (BBV154) for COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04751682 (accessed on 10 June 2021).
- Zhu, F. Phase I/II Clinical Trial of Recombinant novel Coronavirus (COVID-19) Vaccine (Adenovirus Type 5 Vector) for Inhalation. Available online: https://clinicaltrials.gov/ct2/show/NCT04840992 (accessed on 10 June 2021).
- Douglas, A. A Study of Intranasal ChAdOx1 nCOV-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04816019 (accessed on 10 June 2021).
- Bendel, D. Safety and Immunogenicity of COVI-VAC, a Live Attenuated Vaccine Against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04619628 (accessed on 10 June 2021).
- King, R.G.; Silva-Sanchez, A.; Peel, J.N.; Botta, D.; Meza-Perez, S.; Allie, S.R.; Schultz, M.D.; Liu, M.; Bradley, J.E.; Qiu, S.; et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 in mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Stobart, C.C.; Rostad, C.A.; Ke, Z.; Dillard, R.S.; Hampton, C.M.; Strauss, J.D.; Yi, H.; Hotard, A.L.; Meng, J.; Pickles, R.J.; et al. A live RSV vaccine with engineered thermostability is immunogenic in cotton rats despite high attenuation. Nat. Commun. 2016, 7, 13916. [Google Scholar] [CrossRef]
- Rostad, C.A.; Stobart, C.C.; Gilbert, B.E.; Pickles, R.J.; Hotard, A.L.; Meng, J.; Blanco, J.C.; Moin, S.M.; Graham, B.S.; Piedra, P.A. A recombinant respiratory syncytial virus vaccine candidate attenuated by a low-fusion F protein is immunogenic and protective against challenge in cotton rats. J. Virol. 2016, 90, 7508–7518. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 2020, 183, 169–184. [Google Scholar] [CrossRef]
- Bricker, T.; Darling, T.; Hassan, A.; Harastani, H.; Soung, A.; Jiang, X.; Dai, Y.-N.; Zhao, H.; Adams, L.; Holtzman, M. A single intranasal or intramuscular immunization with chimpanzee adenovirus vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. bioRxiv 2020. [Google Scholar] [CrossRef]
- Hassan, A.O.; Feldmann, F.; Zhao, H.; Curiel, D.T.; Okumura, A.; Tang-Huau, T.-L.; Case, J.B.; Meade-White, K.; Callison, J.; Chen, R.E.; et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep. Med. 2021, 2, 100230. [Google Scholar] [CrossRef]
- China Approves Inhaled CanSino Vaccine for Clinical Trials. Available online: https://medicalxpress.com/news/2021-03-china-inhaled-cansino-vaccine-clinical.html (accessed on 16 April 2021).
- Shabong, Y. Oxford to Test Inhaled Version of COVID-19 Vaccine with 30 Volunteers. Available online: https://www.reuters.com/article/us-health-coronavirus-astrazeneca-vaccin-idUSKBN2BH2PS (accessed on 10 June 2021).
- van Doremalen, N.; Purushotham, J.; Schulz, J.; Holbrook, M.; Bushmaker, T.; Carmody, A.; Port, J.; Yinda, K.C.; Okumura, A.; Saturday, G. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. bioRxiv 2021. [Google Scholar] [CrossRef]
- COVI-VAC for SARS-CoV-2 (COVID-19): CODAGENIX INC. Available online: https://codagenix.com/vaccine-programs/covid-19/ (accessed on 10 June 2021).
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: Summary of a report of 72,314 cases from the chinese center for disease control and prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute covid-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Fraser, E. Long term respiratory complications of covid-19. BMJ 2020, 370, m3001. [Google Scholar] [CrossRef]
- A Study to Evaluate Ampion in Patients with Prolonged Respiratory Symptoms Due to COVID-19 (long COVID). Available online: https://clinicaltrials.gov/ct2/show/NCT04880161 (accessed on 19 June 2021).
- A Study of Inhaled Ampion in Adults with Respiratory Distress Due to COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04868890 (accessed on 21 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).