An Updated Risk Assessment as Part of the QbD-Based Liposome Design and Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Elements of the QbD Design
Development of the Knowledge Space and Determination of the QTPP
Determination of the CQAs, the CMAs and the CPPs
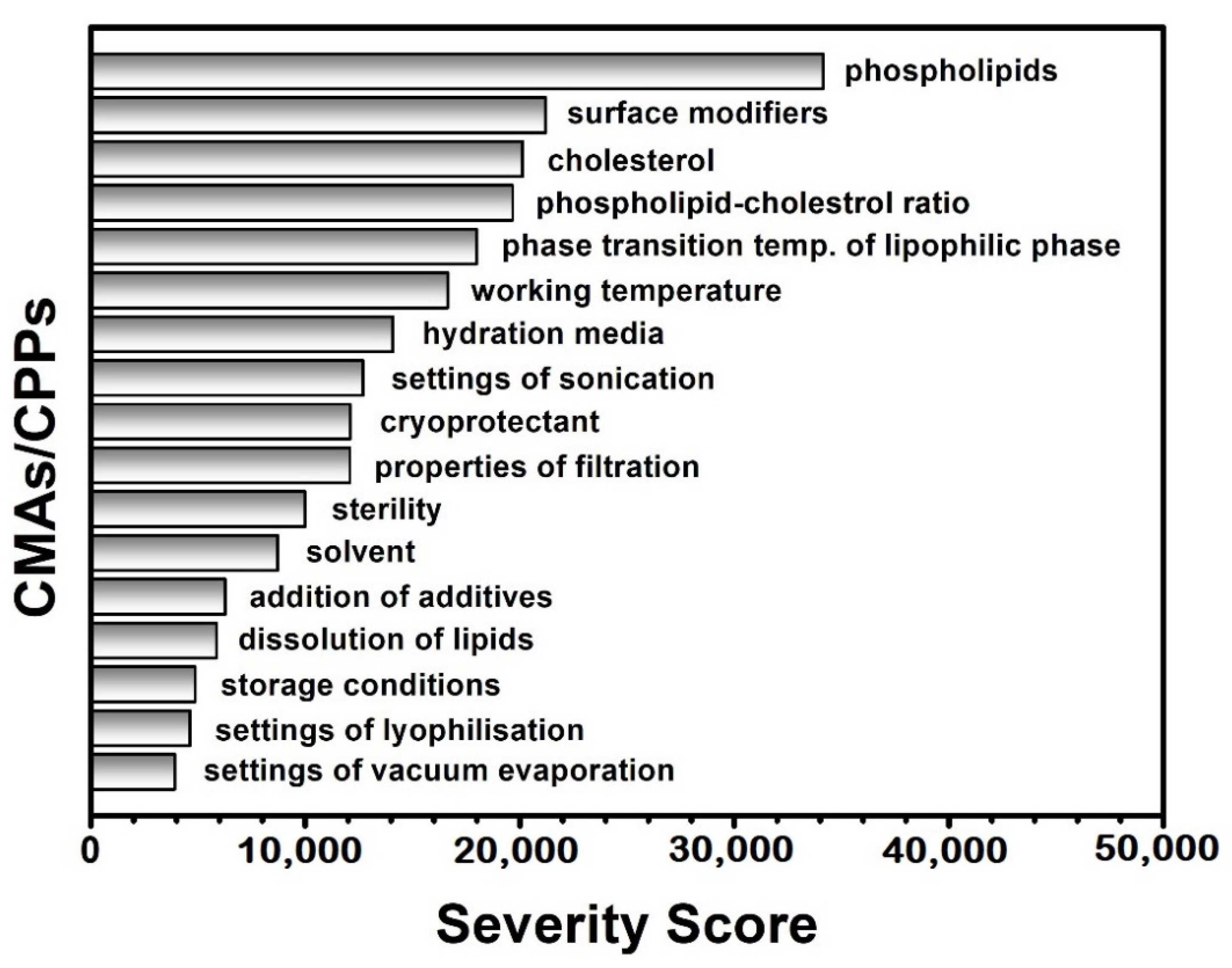
Risk Assessment
Design of the Experiments
2.2.2. Preparation of Liposomes and Process of Lyophilisation
2.2.3. Characterisation of the Liposomes
Vesicle Size and Zeta Potential Analysis
Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) Investigations
Fourier-Transform Infrared (FT-IR) Spectroscopy Measurements
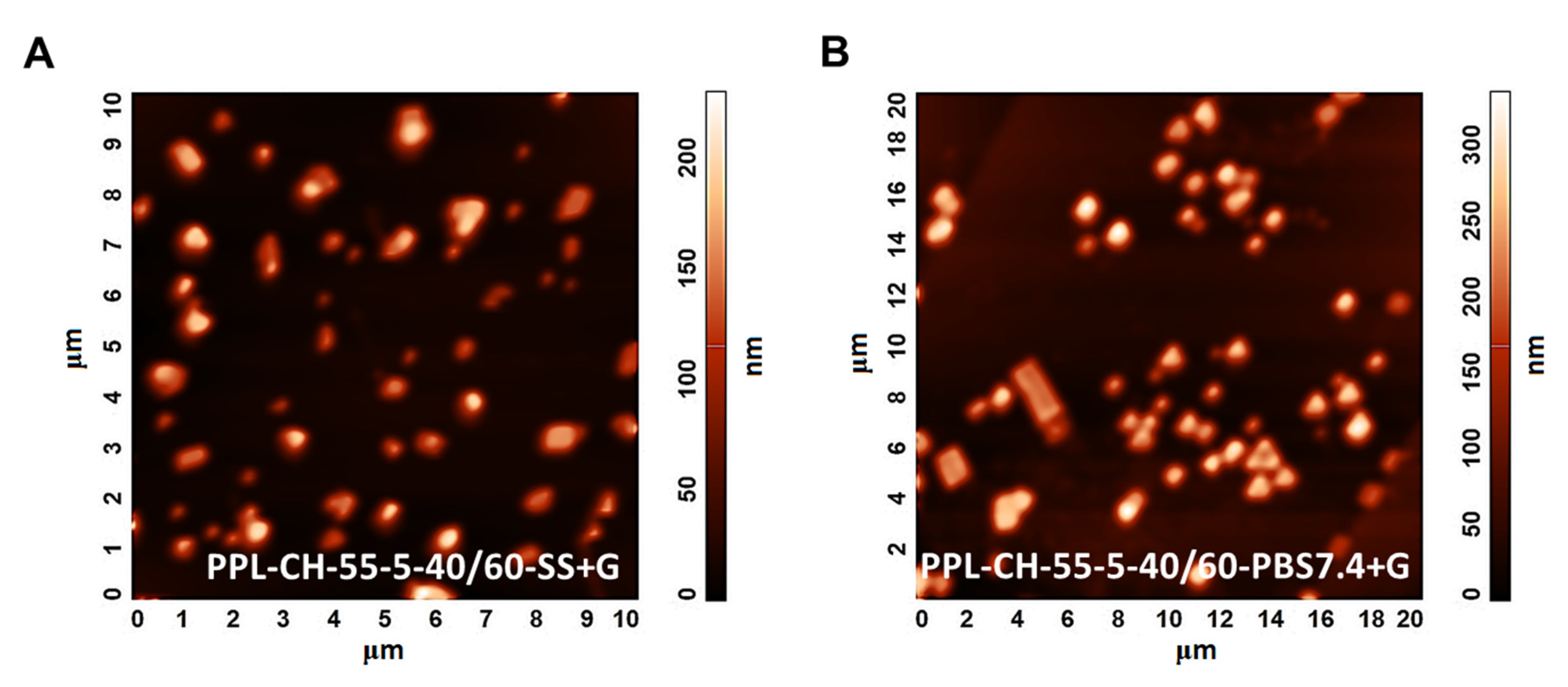
Atomic Force Microscopy (AFM) Measurements
Residual Ethanol Measurements via Gas Chromatography-Mass Spectrometry (GC-MS)
2.2.4. Statistical Analysis
3. Results
3.1. Development of the Knowledge Space, the Definition of the QTPP and the Identification of the CQAs, CMAs and CPPs
3.2. Risk Assessment
3.3. Characterisation Results of the Liposomal Products
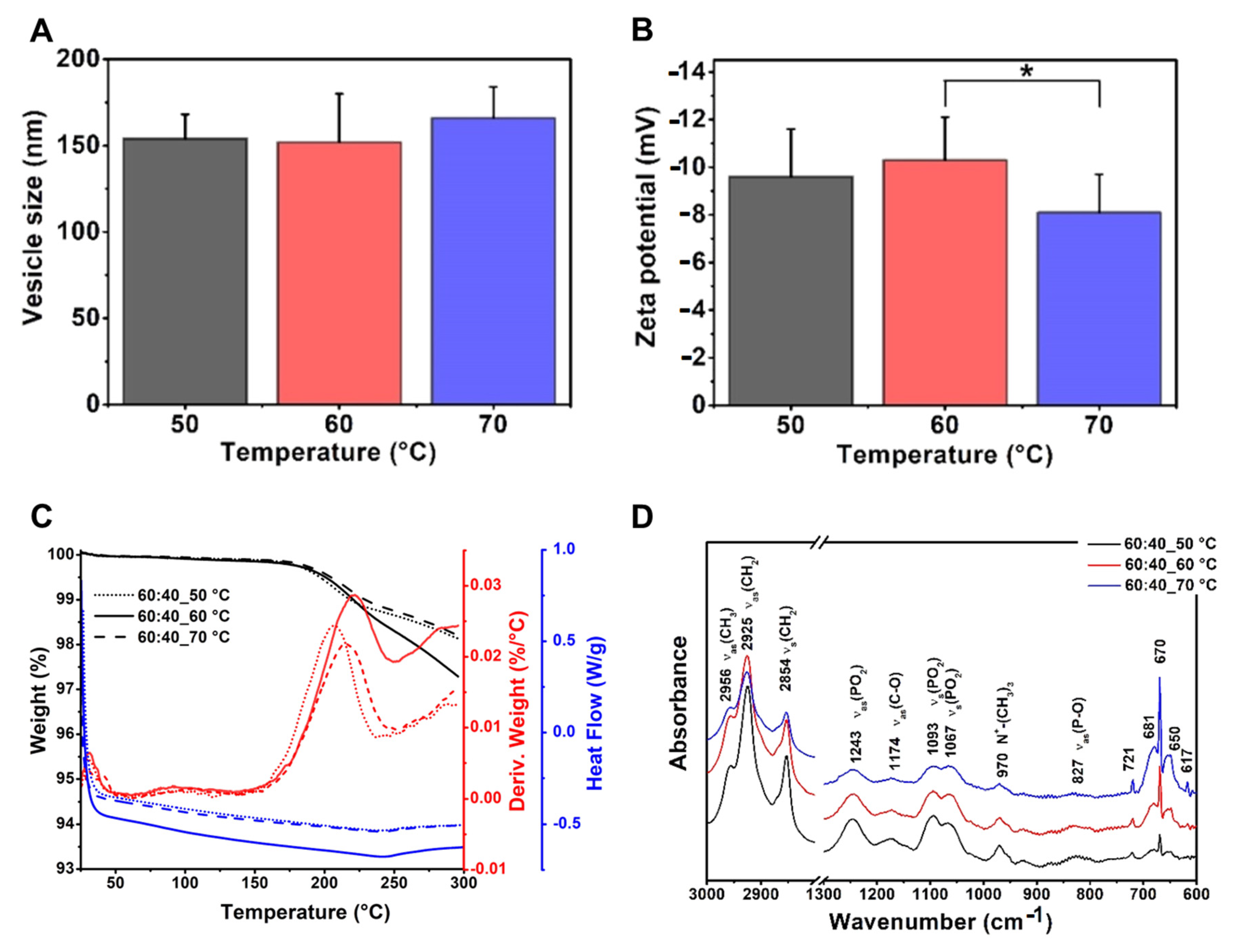
3.3.1. Effects of Using Different Temperature Values
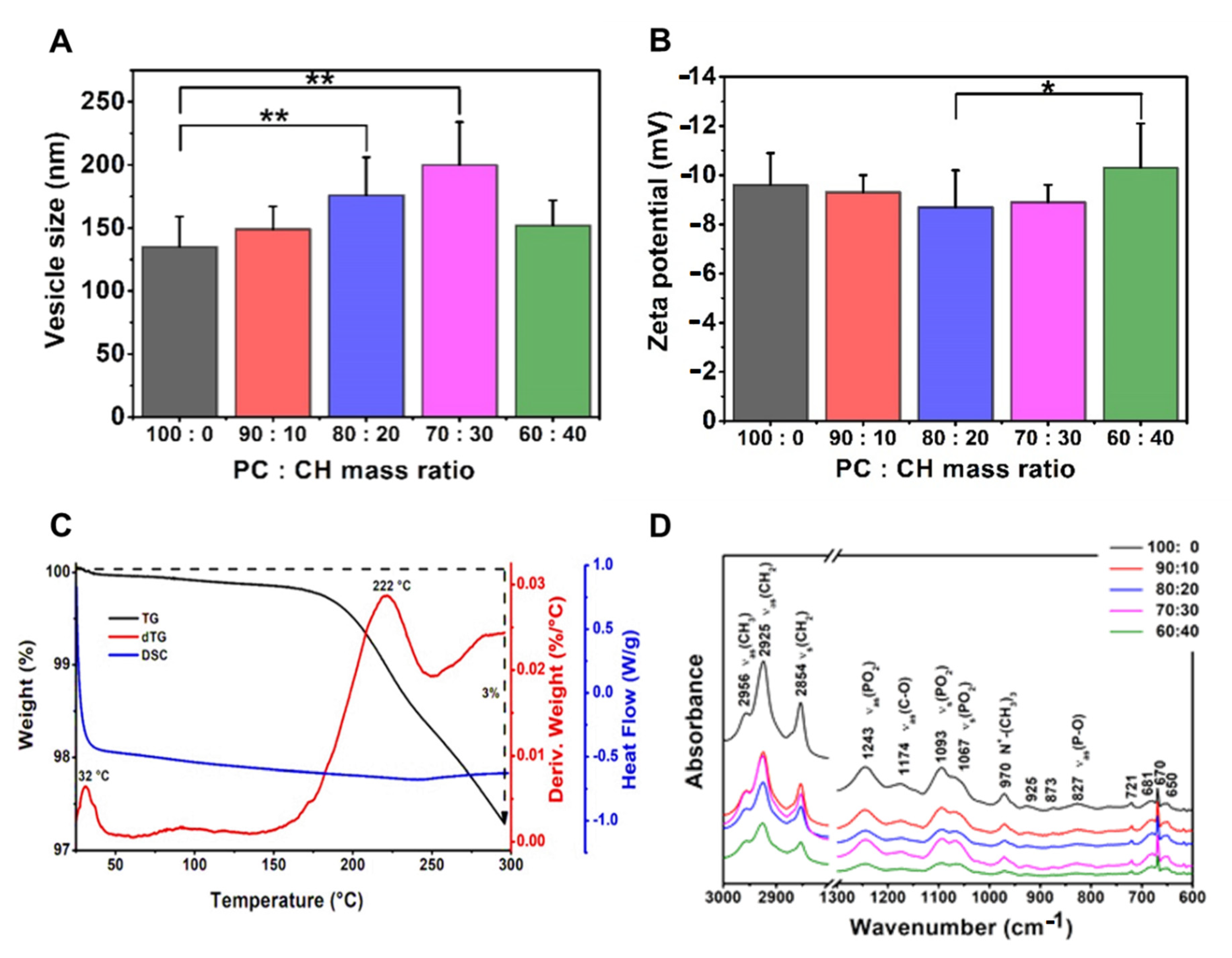
3.3.2. Effects of Using Different Ratios of Wall-Forming Agents
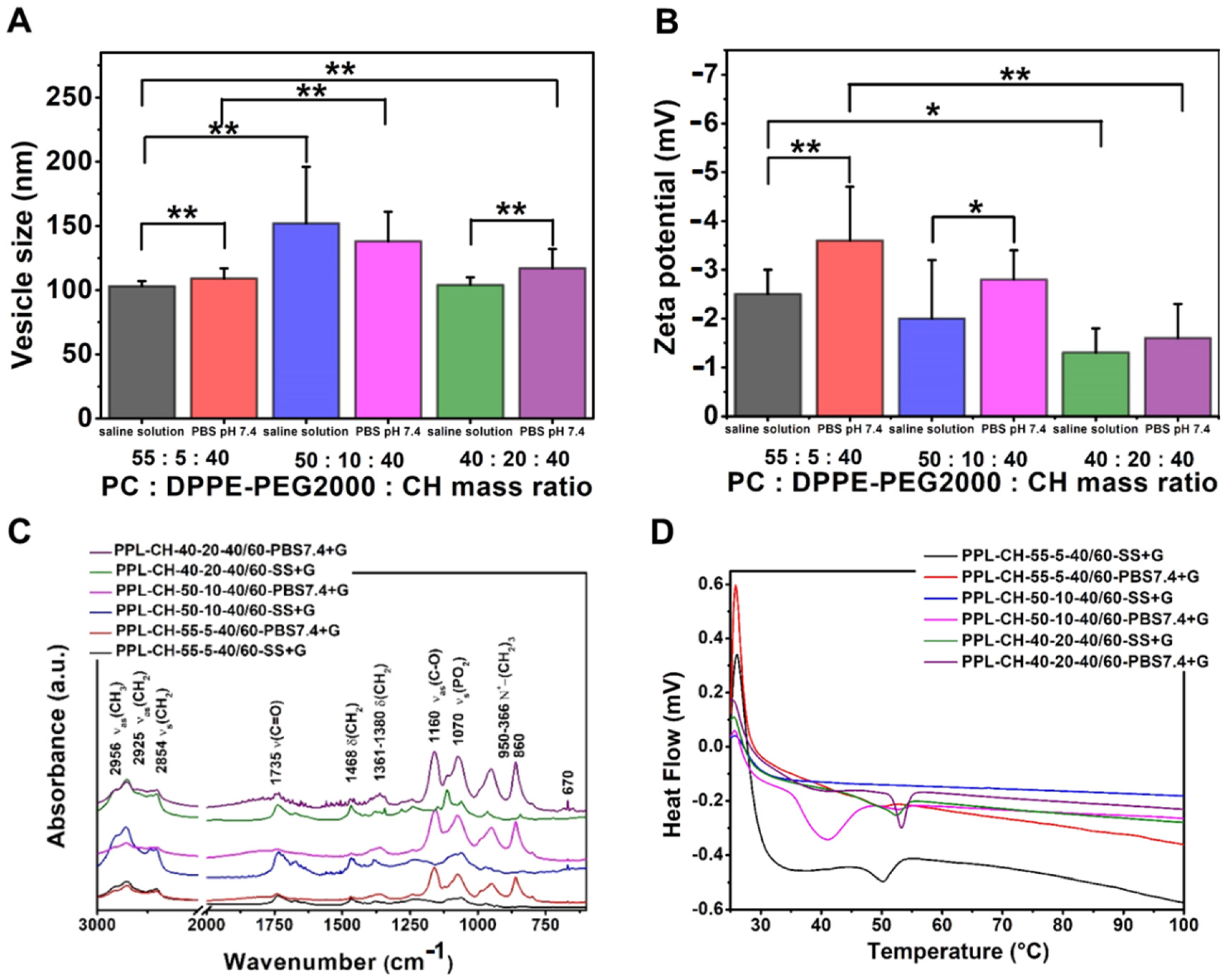
3.3.3. Effect of Using Different Concentrations of PEGylated Phospholipid
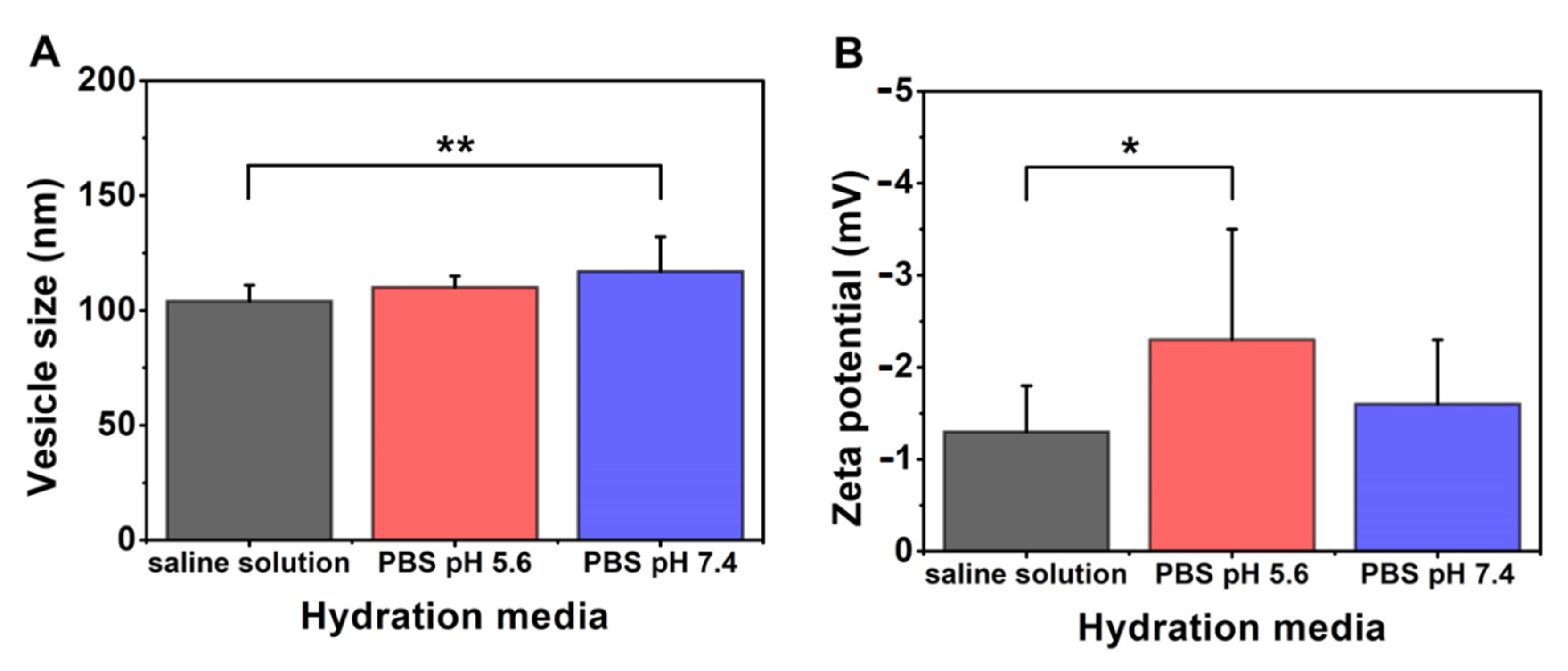
3.3.4. Effect of Using Different Types of Hydration Media
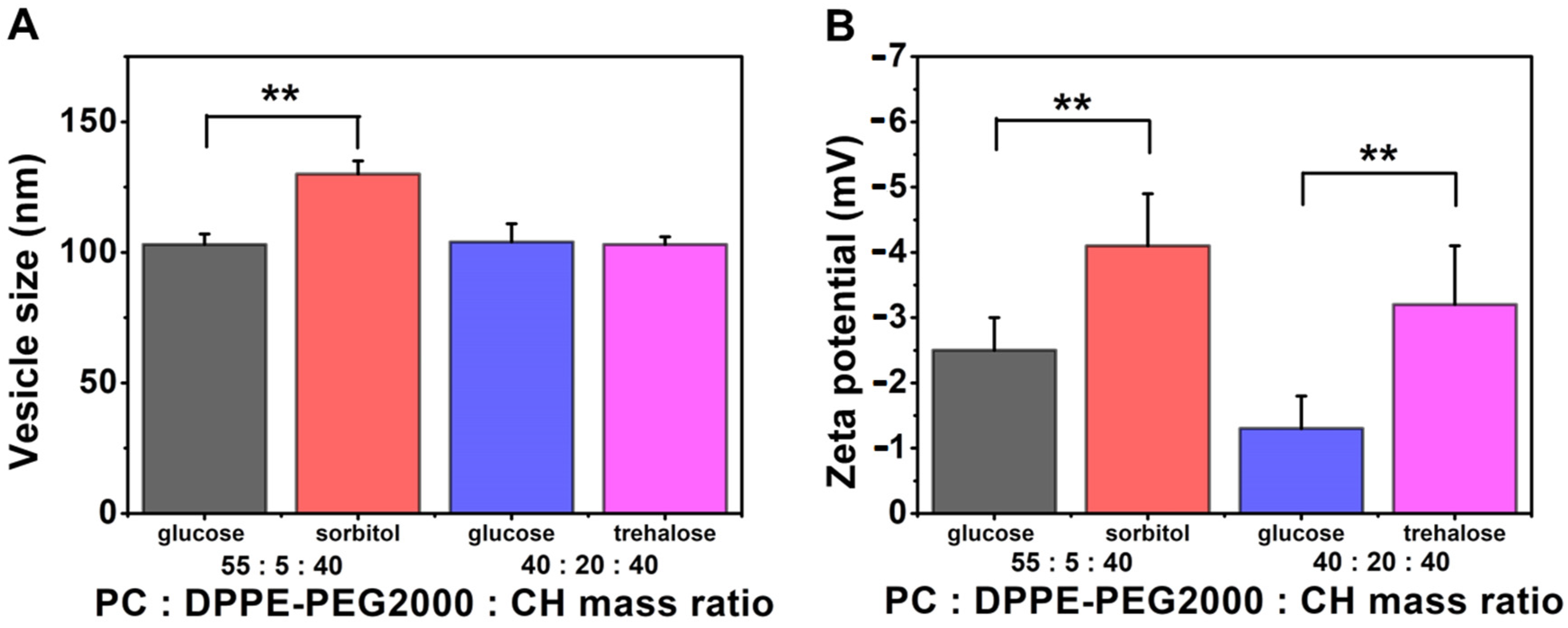
3.3.5. Effect of Using Different Types of Cryoprotectants
3.4. Residual Ethanol Measurements via Gas Chromatography-Mass Spectrometry (GC-MS)
4. Discussion
4.1. Development of the Knowledge Space, the Definition of the QTPP and the Identification of the CQAs, CMAs and CPPs
4.2. Risk Assessment and Design of Experiment
4.3. Characterisation Results of the Liposomal Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| API | active pharmaceutical ingredient |
| CH | cholesterol |
| CMAs | Critical Material Attributes |
| CPPs | Critical Process Parameters |
| CQAs | Critical Quality Attributes |
| DLS | dynamic light scattering |
| DoE | Design of Experiments |
| DPPE-PEG2000 | 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt) |
| DS | Design Space |
| DSC | differential scanning calorimetry |
| DSPE–PEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (ammonium salt) |
| dTG | derivative thermogravimetry |
| EMA | European Medicine Agency |
| FT-IR | Fourier-transform infrared spectroscopy |
| ICH | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| LUV | large unilamellar vesicles |
| PBS | phosphate-buffered saline |
| PC | L-α-phosphatidylcholine |
| PdI | Polydispersity Index |
| PE | phosphatidylethanolamine |
| PEG | polyethylene glycol |
| QbD | Quality by Design |
| QTPP | Quality Target Product Profile |
| R&D | Research and Development |
| RA | Risk Assessment |
| Tc | gel to liquid-crystalline phase transition temperature |
| Tg | glass transitions temperature |
| TGA | thermogravimetric analysis |
| Tm | phase transition temperature |
References
- European Medicine Agency. Reflection paper on the data requirements for intravenous liposomal products developed with reference to an innovator liposomal product. EMA/Committee Hum. Med. Prod. 2013, 44, 1–13. [Google Scholar]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W. From “banghasomes” to liposomes: A memoir of Alec Bangham, 1921–2010. FASEB J. 2010, 24, 1308–1310. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Németh, Z.; Pallagi, E.; Dobó, D.G.; Csóka, I. A proposed methodology for a risk assessment-based liposome development process. Pharmaceutics 2020, 12, 1164. [Google Scholar] [CrossRef]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Csóka, I.; Pallagi, E.; Paál, T.L. Extension of quality-by-design concept to the early development phase of pharmaceutical R&D processes. Drug Discov. Today 2018, 23, 1340–1343. [Google Scholar] [CrossRef]
- Sipos, B.; Katona, G.; Csóka, I. A Systematic, Knowledge Space-Based Proposal on Quality by Design-Driven Polymeric Micelle Development. Pharmaceutics 2021, 13, 702. [Google Scholar] [CrossRef]
- ICH. Pharmaceutical Development Q8. ICH Harmon. Tripart. Guidel. 2009, 8, 1–28. [Google Scholar]
- European Medicines Agency (EMA). ICH Guideline Q9 on quality risk management. ICH Harmon. Tripart. Guidel. 2014, 44, 1–20. [Google Scholar]
- EMA. ICH guideline Q10 on pharmaceutical quality system. Eur. Med. Agency 2015, 44, 1–20. [Google Scholar]
- Jain, S. Quality by Design (QbD): A Comprehensive Understanding of Implementation and Challenges in Pharmaceutical Development. Int. J. Pharm. Pharm. Sci. 2014, 6, 29–35. [Google Scholar]
- Pallagi, E.; Ambrus, R.; Szabó-Révész, P.; Csóka, I. Adaptation of the quality by design concept in early pharmaceutical development of an intranasal nanosized formulation. Int. J. Pharm. 2015, 491. [Google Scholar] [CrossRef]
- Pallagi, E.; Karimi, K.; Ambrus, R.; Szabó-Révész, P.; Csóka, I. New aspects of developing a dry powder inhalation formulation applying the quality-by-design approach. Int. J. Pharm. 2016, 511. [Google Scholar] [CrossRef]
- Karimi, K.; Pallagi, E.; Szabó-Révész, P.; Csóka, I.; Ambrus, R. Development of a microparticle-based dry powder inhalation formulation of ciprofloxacin hydrochloride applying the quality by design approach. Drug Des. Devel. Ther. 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Gieszinger, P.; Csóka, I.; Pallagi, E.; Katona, G.; Jójárt-Laczkovich, O.; Szabó-Révész, P.; Ambrus, R. Preliminary study of nanonized lamotrigine containing products for nasal powder formulation. Drug Des. Devel. Ther. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Arora, P.; Sharma, S.; Garg, S. Permeability issues in nasal drug delivery. Drug Discov. Today 2002, 7, 967–975. [Google Scholar] [CrossRef]
- Pallagi, E.; Ismail, R.; Paál, T.L.; Csóka, I. Initial Risk Assessment as part of the Quality by Design in peptide drug-containing formulation development. Eur. J. Pharm. Sci. 2018, 122, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Sipos, B.; Szabó-Révész, P.; Csóka, I.; Pallagi, E.; Dobó, D.G.; Bélteky, P.; Kónya, Z.; Deák, Á.; Janovák, L.; Katona, G. Quality by design based formulation study of meloxicam-loaded polymeric micelles for intranasal administration. Pharmaceutics 2020, 12, 697. [Google Scholar] [CrossRef]
- Sabir, F.; Katona, G.; Pallagi, E.; Dobó, D.G.; Akel, H.; Berkesi, D.; Kónya, Z.; Csóka, I. Quality-by-Design-Based Development of n-Propyl-Gallate-Loaded Hyaluronic-Acid-Coated Liposomes for Intranasal Administration. Molecules 2021, 26, 1429. [Google Scholar] [CrossRef] [PubMed]
- Akel, H.; Ismail, R.; Katona, G.; Sabir, F.; Ambrus, R.; Csóka, I. A comparison study of Lipid and Polymeric Nanoparticles in the Nasal Delivery of Meloxicam: Formulation, Characterization, and In-Vitro Evaluation. Int. J. Pharm. 2021. [Google Scholar] [CrossRef]
- Porfire, A.; Achim, M.; Barbalata, C.; Rus, I.; Tomuta, I.; Cristea, C. Pharmaceutical Development of Liposomes Using the QbD Approach. Liposomes Adv. Perspect. 2019, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Costa, A.P.; Khan, M.A.; Burgess, D.J. Application of quality by design to formulation and processing of protein liposomes. Int. J. Pharm. 2012, 434, 349–359. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Kurakula, M.; Banjar, Z.M.; Afouna, M.I.; Zidan, A.S. Quality by design coupled with near infrared in formulation of transdermal glimepiride liposomal films. J. Pharm. Sci. 2015, 104, 2062–2075. [Google Scholar] [CrossRef] [PubMed]
- Pallagi, E.; Jójárt-Laczkovich, O.; Németh, Z.; Szabó-Révész, P.; Csóka, I. Application of the QbD-based approach in the early development of liposomes for nasal administration. Int. J. Pharm. 2019, 562, 11–22. [Google Scholar] [CrossRef]
- de Barros, C.; Aranha, N.; Severino, P.; Souto, E.B.; Zielińska, A.; Lopes, A.; Rios, A.; Batain, F.; Crescencio, K.; Chaud, M.; et al. Quality by design approach for the development of liposome carrying ghrelin for intranasal administration. Pharmaceutics 2021, 13, 686. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.P.; Karande, K.P.; Sonawane, R.O.; Deshmukh, P.K. Applying quality by design (QbD) concept for fabrication of chitosan-coated nanoliposomes. J. Liposome Res. 2014, 24, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Merlo-Mas, J.; Tomsen-Melero, J.; Corchero, J.L.; González-Mira, E.; Font, A.; Pedersen, J.N.; García-Aranda, N.; Cristóbal-Lecina, E.; Alcaina-Hernando, M.; Mendoza, R.; et al. Application of Quality by Design to the robust preparation of a liposomal GLA formulation by DELOS-susp method. J. Supercrit. Fluids 2021, 173, 105204. [Google Scholar] [CrossRef] [PubMed]
- Reddi, B.A.J. Why is saline so acidic (and does it really matter?). Int. J. Med. Sci. 2013, 10, 747–750. [Google Scholar] [CrossRef] [Green Version]
- Kuntsche, J.; Freisleben, I.; Steiniger, F.; Fahr, A. Temoporfin-loaded liposomes: Physicochemical characterization. Eur. J. Pharm. Sci. 2010, 40, 305–315. [Google Scholar] [CrossRef]
- Braem, A.; Turner, G. Applications of quality risk assessment in quality by design (QbD) drug substance process development. Chem. Eng. Pharm. Ind. 2019, 1073–1089. [Google Scholar] [CrossRef]
- Powell, T.; Sammut-Bonnic, T. Pareto Analysis. In Wiley Encyclopedia of Management; Cooper, C.L., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. In Liposomes. Methods in Molecular Biology; D’Souza, G., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1522, pp. 17–22. [Google Scholar]
- Mozafari, M.R. Chapter 2-Nanoliposomes: Preparation and Analysis. Liposomes-Methods Mol. Biol. 2010, 605, 41–62. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.G.; Morris, R.M. Thermal analysis of phase transition behaviour in liposomes. Thermochim. Acta 1995, 248, 289–301. [Google Scholar] [CrossRef]
- Elgharbawy, H.; Morsy, R. Preparation and Physicochemical Evaluation of Magnetoliposomes as Drug Carriers for 5-Fluorouracile Preparation and Physicochemical Evaluation of Magnetoliposomes as Drug Carriers for 5-Fluorouracile. J. Biophys. Biomed. Sci. 2016, 9, 901–906. [Google Scholar]
- Biltonen, R.L.; Lichtenberg, D. The use of differential scanning calorimetry as a tool to characterize liposome preparations. Chem. Phys. Lipids 1993, 64, 129–142. [Google Scholar] [CrossRef]
- Mobley, W.C.; Schreier, H. Phase transition temperature reduction and glass formation in dehydroprotected lyophilized liposomes. J. Control. Release 1994, 31, 73–87. [Google Scholar] [CrossRef]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des. Devel. Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef] [Green Version]
- López-Pinto, J.M.; González-Rodríguez, M.L.; Rabasco, A.M. Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int. J. Pharm. 2005, 298, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Magarkar, A.; Dhawan, V.; Kallinteri, P.; Viitala, T.; Elmowafy, M.; Róg, T.; Bunker, A. Cholesterol level affects surface charge of lipid membranes in saline solution. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, E.; Chapman, D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972, 23, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Erdei, L.; Csorba, I.; Thuyen, H.X. Simple, rapid method for detecting phase transitions of lipids. Lipids 1975, 10, 115–117. [Google Scholar] [CrossRef]
- Derenne, A.; Claessens, T.; Conus, C.; Goormaghtigh, E. Infrared Spectroscopy of Membrane Lipids; Roberts, G.C.K., Ed.; Spinger: Berlin/Heidelberg, Germany, 2013; ISBN 9783642167126. [Google Scholar]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Garbuzenko, O.; Barenholz, Y.; Priev, A. Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer. Chem. Phys. Lipids 2005, 135, 117–129. [Google Scholar] [CrossRef]
- Viitala, L.; Pajari, S.; Gentile, L.; Määttä, J.; Gubitosi, M.; Deska, J.; Sammalkorpi, M.; Olsson, U.; Murtomäki, L. Shape and Phase Transitions in a PEGylated Phospholipid System. Langmuir 2019, 35, 3999–4010. [Google Scholar] [CrossRef]
- Akizuki, H.; Kaneko, T. Characteristics of Liposomes Made by Phosphatidylethanolamine. Biophys. J. 2016, 110, 71a. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Tsermentseli, S.K.; Kontogiannopoulos, K.N.; Papageorgiou, V.P.; Assimopoulou, A.N. Comparative study of pEgylated and conventional liposomes as carriers for shikonin. Fluids 2018, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Lei, G.; MacDonald, R.C. Effects on interactions of oppositely charged phospholipid vesicles of covalent attachment of polyethylene glycol oligomers to their surfaces: Adhesion, hemifusion, full fusion and “endocytosis”. J. Membr. Biol. 2008, 221, 97–106. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH guideline Q3C (R6) on impurities: Guideline for Residual Solvents. Int. Conf. Harmon. Tech. Requir. Regist. Pharm. Hum. Use 2019, 31, 24. [Google Scholar]
- Dhoble, S.; Patravale, V. Development of anti-angiogenic erlotinib liposomal formulation for pulmonary hypertension: A QbD approach. Drug Deliv. Transl. Res. 2019, 9, 980–996. [Google Scholar] [CrossRef]
- Kan, P.; Wang, A.-J.; Chen, W.-K.; Tsao, C.-W. Liposome for incorporating large amounts of hydrophobic substances. U.S. Patent US 7485,320 B2, 3 February 2009. [Google Scholar]
- Sahu, A.K.; Jain, V. Screening of process variables using Plackett–Burman design in the fabrication of gedunin-loaded liposomes. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1011–1022. [Google Scholar] [CrossRef]
- Teo, Y.Y.; Misran, M.; Low, K.H. Effect of pH on Physicochemical Properties and Encapsulation Efficiency of PEGylated Linoleic Acid Vesicles. J. Chem. 2012, 9, 729–738. [Google Scholar] [CrossRef]
- Hau, Z.Z.; Li, B.G.; Liu, Z.J.; Sun, D.W. Freeze-drying of liposomes with cryoprotectants and its effect on retention rate of encapsulated ftorafur and vitamin A. Dry. Technol. 2003, 21, 1491–1505. [Google Scholar] [CrossRef]
- Sylvester, B.; Porfire, A.; Achim, M.; Rus, L.; Tomuţă, I. A step forward towards the development of stable freeze-dried liposomes: A quality by design approach (QbD). Drug Dev. Ind. Pharm. 2018, 44, 385–397. [Google Scholar] [CrossRef]




| Compositions | Phosphatidylcholine–Cholesterol Liposomes | ||||
|---|---|---|---|---|---|
| Phospholipid:Cholesterol Mass Ratio | |||||
| 100:0 | 90:10 | 80:20 | 70:30 | 60:40 | |
| PC (w/w%) | 100 | 90 | 80 | 70 | 60 |
| cholesterol (w/w%) | - | 10 | 20 | 30 | 40 |
| solvent of the stock solution | |||||
| EtOH 96% | + | ||||
| hydration media | |||||
| saline solution (mL) | 100 | ||||
| Compositions | PEGylated Liposomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PC:DPPE-PEG2000:Cholesterol Mass Ratio | |||||||||
| 55:5:40 | 50:10 | 40:20:40 | |||||||
| PC (w/w%) | 55 | 50 | 40 | ||||||
| DPPE-PEG2000 (w/w%) | 5 | 10 | 10 | ||||||
| cholesterol (w/w%) | 40 | 40 | 40 | ||||||
| solvent of the stock solution | |||||||||
| EtOH 96% | + | ||||||||
| hydration media | |||||||||
| saline solution (mL) | 100 | - | 100 | 100 | - | 100 | - | 100 | - |
| PBS pH 5.6 (mL) | - | 100 | - | - | - | - | - | - | 100 |
| PBS pH 7.4 (mL) | - | - | - | - | 100 | - | 100 | - | - |
| cryoprotectant | |||||||||
| glucose (%) | 5 | 5 | - | 5 | 5 | 5 | 5 | - | 5 |
| sorbitol (%) | - | - | 5 | - | - | - | - | - | - |
| trehalose (%) | - | - | - | - | - | - | - | 5 | - |
| (A) Sample Name | Composition (m/m%) | Hydration Media | Cryoprotectant (5% of Total PPL. Mass) | |||||
| PC | CH | |||||||
| PPL-CH-60-40/50-SS | 60 | 40 | saline solution | - | ||||
| PPL-CH-60-40/60-SS | 60 | 40 | saline solution | - | ||||
| PPL-CH-70-30/60-SS | 70 | 30 | saline solution | - | ||||
| PPL-CH-80-20/60-SS | 80 | 20 | saline solution | - | ||||
| PPL-CH-90-10/60-SS | 90 | 10 | saline solution | - | ||||
| PPL-CH-100-0/60-SS | 100 | 0 | saline solution | - | ||||
| PPL-CH-60-40/70-SS | 60 | 40 | saline solution | - | ||||
| (B) Sample Name | Composition (m/m%) | Hydration Media | Cryoprotectant (5% of Total PPL. Mass) | |||||
| PC | DPPE- PEG2000 | CH | ||||||
| PPL-CH-55-5-40/60-SS+G | 55 | 5 | 40 | saline solution | glucose | |||
| PPL-CH-55-5-40/60-PBS7.4+G | 55 | 5 | 40 | pH 7.4 PBS | glucose | |||
| PPL-CH-50-10-40/60-SS+G | 50 | 10 | 40 | saline solution | glucose | |||
| PPL-CH-50-10-40/60-PBS7.4+G | 50 | 10 | 40 | pH 7.4 PBS | glucose | |||
| PPL-CH-40-20-40/60-SS+G | 40 | 20 | 40 | saline solution | glucose | |||
| PPL-CH-40-20-40/60-PBS7.4+G | 40 | 20 | 40 | pH 7.4 PBS | glucose | |||
| PPL-CH-40-20-40/60-SS+T | 40 | 20 | 40 | saline solution | trehalose | |||
| PPL-CH-40-20-40/60-PBS5.6+G | 40 | 20 | 40 | pH 5.6 PBS | glucose | |||
| PPL-CH-55-5-40/60-SS+S | 55 | 5 | 40 | saline solution | sorbitol | |||
| Critical Factors | Investigated Levels or Parameters | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C P P | working temperature | 50 °C | 60 °C | 70 °C | ||||||||||||
| C M A | Phosphatidylcholine:cholesterol mass ratio | 100:0 | 90:10 | 80:20 | 70:30 | 60:40 | ||||||||||
| PEGylated phospholipid content PC:DPPE-PEG2000:cholesterol mass ratio | 5% 55:5:40 | 10% 50:10:40 | 20% 40:20:40 | |||||||||||||
| quality of hydration media pH ionic strength | saline solution pH 5.5 0.15 M | PBS pH 5.6 pH 5.6 0.40 M | PBS pH 7.4 pH 7.4 0.16 M | |||||||||||||
| quality of cryoprotectants | glucose | sorbitol | trehalose | |||||||||||||
| QTPP Factors | Details | Comments/Justifications |
|---|---|---|
| dosage form/ appearance | lyophilised powder |
|
| physical attributes | morphology, large unilamellar structured, liposomes (LUV), optimal particle size, proper zeta potential |
|
| stable structure | in aqueous solution |
|
| in freeze-dried powder form | ||
| homogeneoussystem | homogenous formulation |
|
| CQAs | Details | Comments/Justification |
| type of liposomes | conventional, cationic, immune, bioresponsive, magnetic | determine the quality of the lipids |
| targeted delivery compatibility | knowledge about the possible administration route | formulation needs to be suitable for the requirements of the later API |
| size of the vesicles | mean particle size: 100–200 nm | large vesicles (LUV) |
| number of lamellas | 1 lamella | unilamellar vesicles (LUV) |
| morphology | shape and structure | spherical unilamellar vesicles |
| polydispersity index (PdI) | acceptable: below: 0.3 | monodisperse system |
| zeta potential | the higher in absolute value, the more stable the formulation | indicates stability |
| surface modifications | attachment of polyethylene glycol (PEG) chains, monoclonal antibodies, antibody fragments peptides, nucleic acids, carbohydrates or small molecules | maintain targeted delivery |
| specific surface area | surface area-to-volume ratio | determines the properties of the later drug release |
| phase transition temperature (Tm) | working temperature is recommended to be higher than Tm | different value for each composition |
| sterility | meets the microbiological requirements | depends on the chosen administration route |
| stability | stable under given circumstances | in aqueous solution/in freeze-dried powder form |
| Compositions | Phosphatidylcholine–Cholesterol Liposomes (Mass Ratio: 60:40) | |||||
|---|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | ||||
| mean | SD | mean | SD | mean | SD | |
| vesicle size (nm) | 154 | 14 | 152 | 28 | 166 | 18 |
| PdI | 0.24 | 0.02 | 0.18 | 0.08 | 0.21 | 0.04 |
| zeta potential (mV) | −9.6 | 2.0 | −10.3 | 1.8 | −8.1 | 1.6 |
| TG% | 2 | 3 | 2 | |||
| sample name | PPL-CH-60-40/50-SS | PPL-CH-60-40/60-SS | PPL-CH-60-40/70-SS | |||
| Compositions | Phosphatidylcholine–Cholesterol Liposomes (60 °C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylcholine:Cholesterol Mass Ratio | ||||||||||
| 100:0 | 90:10 | 80:20 | 70:30 | 60:40 | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| vesicle size (nm) | 135 | 24 | 149 | 18 | 176 | 30 | 200 | 34 | 152 | 20 |
| PdI | 0.24 | 0.02 | 0.25 | 0.03 | 0.26 | 0.03 | 0.30 | 0.08 | 0.18 | 0.08 |
| zeta potential (mV) | −9.6 | 1.3 | −9.3 | 0.7 | −8.7 | 1.5 | −8.9 | 0.7 | −10.3 | 1.8 |
| TG% | 4 | 3 | 4 | 2 | 3 | |||||
| sample name | PPL-CH-100-0/60-SS | PPL-CH-90-10/60-SS | PPL-CH-80-20/60-SS | PPL-CH-70-30/60-SS | PPL-CH-60-40/60-SS | |||||
| Compositions | Cryoprotectant: Glucose; 60 °C | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC:DPPE-PEG2000:Cholesterol Mass Ratio | ||||||||||||
| 55:5:40 | 50:10:40 | 40:20:40 | ||||||||||
| Hydration Media | ||||||||||||
| Saline Solution | PBS pH 7.4 | Saline Solution | PBS pH 7.4 | Saline Solution | PBS pH 7.4 | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| vesicle size (nm) | 103 | 4 | 109 | 8 | 152 | 44 | 138 | 23 | 104 | 6 | 117 | 15 |
| PdI | 0.25 | 0.05 | 0.20 | 0.02 | 0.27 | 0.01 | 0.27 | 0.07 | 0.29 | 0.07 | 0.26 | 0.06 |
| zeta potential (mV) | −2.5 | 0.5 | −3.6 | 1.1 | −2.0 | 1.2 | −2.8 | 0.6 | −1.3 | 0.5 | −1.6 | 0.7 |
| TG% | 4 | 4 | 5 | 6 | 5 | 7 | ||||||
| sample name | PPL-CH-55-5-40/60-SS+G | PPL-CH-55-5-40/60-PBS7.4+G | PPL-CH-50-10-40/60-SS+G | PPL-CH-50-10-40/60-PBS7.4+G | PPL-CH-40-20-40/60-SS+G | PPL-CH-40-20-40/60-PBS7.4+G | ||||||
| Compositions | Phospholipid:Cholesterol Mass Ratio 60:40 Liposomes; Saline Solution; 60° | |||
|---|---|---|---|---|
| PC: Cholesterol 60:40 | PC:DPPE-PEG2000: Cholesterol 55:5:40 | |||
| Mean | SD | Mean | SD | |
| vesicle size (nm) | 152 | 28 | 103 | 5 |
| PdI | 0.18 | 0.08 | 0.25 | 0.05 |
| zeta potential (mV) | −10.3 | 2.0 | −2.5 | 0.5 |
| TG% | 3 | 4 | ||
| sample name | PPL-CH-60-40/60-SS | PPL-CH-55-5-40/60-SS+G | ||
| Compositions | PC:DPPE-PEG2000:Cholesterol Mass Ratio 40:20:40; Cryoprotectant: Glucose; 60 °C | |||||
|---|---|---|---|---|---|---|
| Hydration Media | ||||||
| Saline Solution 0.154 M | PBS pH 5.6 0.40 M | PBS pH 7.4 0.16 M | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| vesicle size (nm) | 104 | 7 | 110 | 5 | 117 | 15 |
| PdI | 0.29 | 0.07 | 0.33 | 0.05 | 0.26 | 0.06 |
| zeta potential (mV) | −1.3 | 0.5 | −2.3 | 1.2 | −1.6 | 0.7 |
| TG% | 5 | 15 | 7 | |||
| sample name | PPL-CH-40-20-40/60-SS+G | PPL-CH-40-20-40/60-PBS7.4+G | PPL-CH-40-20-40/60-PBS5.6+G | |||
| Compositions | Hydration Media: Saline Solution; 60 °C | |||||||
|---|---|---|---|---|---|---|---|---|
| PC:DPPE-PEG2000:Cholesterol Mass Ratio | ||||||||
| 55:5:40 | 40:20:40 | |||||||
| Glucose | Sorbitol | Glucose | Trehalose | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| vesicle size (nm) | 103 | 4 | 130 | 5 | 104 | 7 | 103 | 3 |
| PdI | 0.25 | 0.05 | 0.30 | 0.05 | 0.29 | 0.07 | 0.29 | 0.03 |
| zeta potential (mV) | −2.5 | 0.5 | −4.1 | 0.8 | −1.3 | 0.5 | −3.2 | 0.9 |
| TG% | 4 | 25 | 5 | 6 | ||||
| sample name | PPL-CH-55-5-40/60-SS+G | PPL-CH-55-5-40/60-SS+S | PPL-CH-40-20-40/60-SS+G | PPL-CH-40-20-40/60-SS+T | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, Z.; Pallagi, E.; Dobó, D.G.; Kozma, G.; Kónya, Z.; Csóka, I. An Updated Risk Assessment as Part of the QbD-Based Liposome Design and Development. Pharmaceutics 2021, 13, 1071. https://doi.org/10.3390/pharmaceutics13071071
Németh Z, Pallagi E, Dobó DG, Kozma G, Kónya Z, Csóka I. An Updated Risk Assessment as Part of the QbD-Based Liposome Design and Development. Pharmaceutics. 2021; 13(7):1071. https://doi.org/10.3390/pharmaceutics13071071
Chicago/Turabian StyleNémeth, Zsófia, Edina Pallagi, Dorina Gabriella Dobó, Gábor Kozma, Zoltán Kónya, and Ildikó Csóka. 2021. "An Updated Risk Assessment as Part of the QbD-Based Liposome Design and Development" Pharmaceutics 13, no. 7: 1071. https://doi.org/10.3390/pharmaceutics13071071
APA StyleNémeth, Z., Pallagi, E., Dobó, D. G., Kozma, G., Kónya, Z., & Csóka, I. (2021). An Updated Risk Assessment as Part of the QbD-Based Liposome Design and Development. Pharmaceutics, 13(7), 1071. https://doi.org/10.3390/pharmaceutics13071071










