Post-Processing Techniques for the Improvement of Liposome Stability
Abstract
1. Introduction
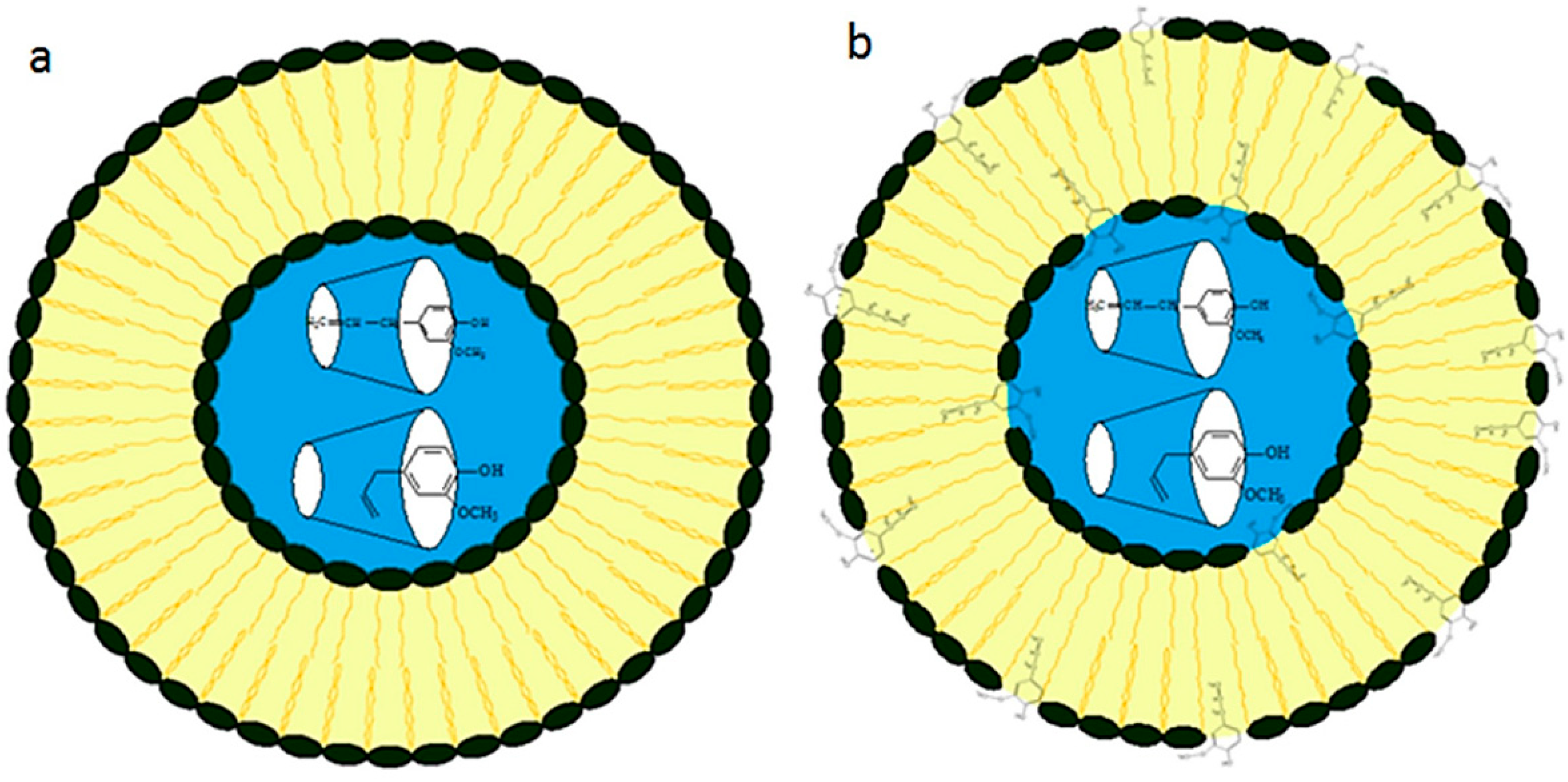
2. Liposome Formulation
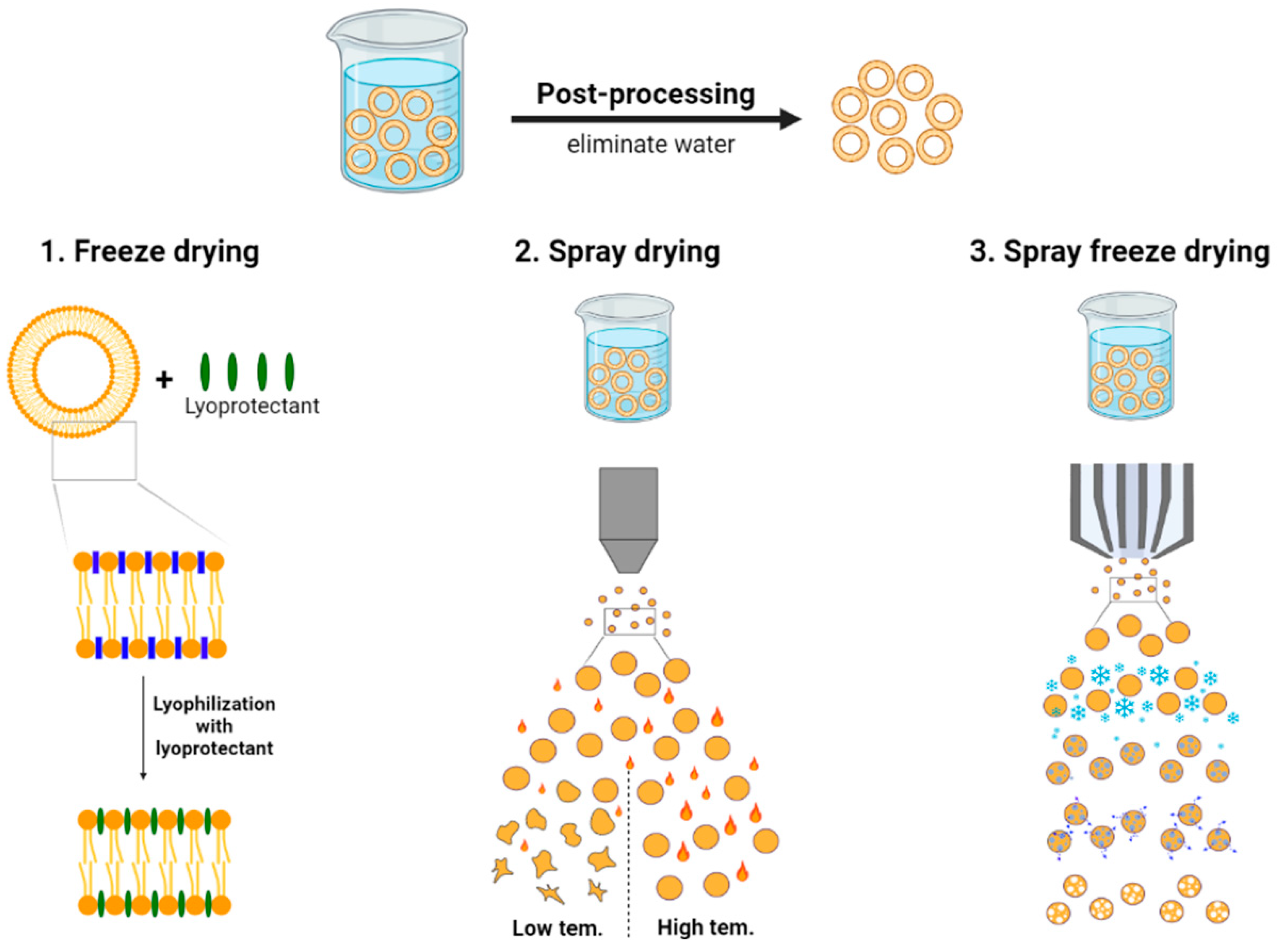
3. Post-Processing Techniques for Liposomes
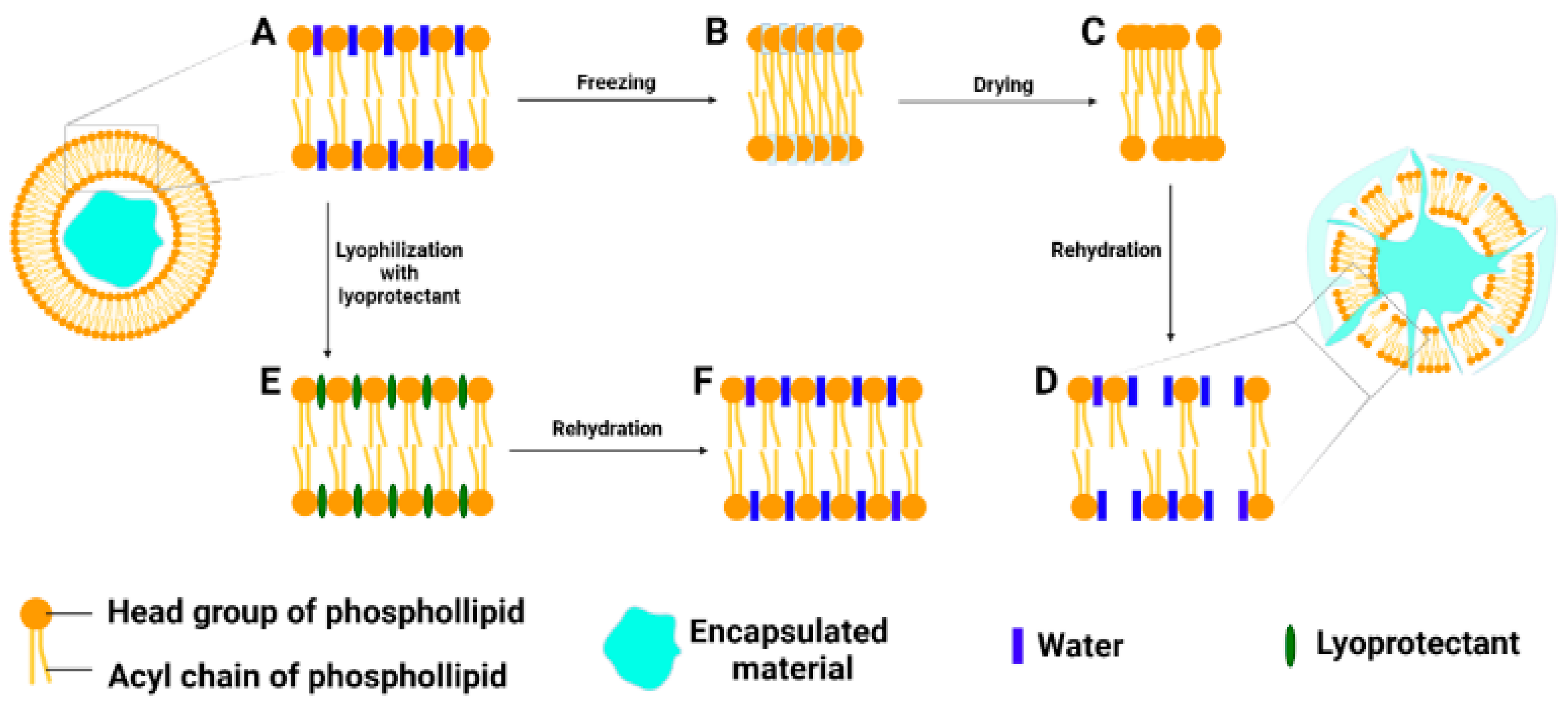
3.1. Freeze Drying Process
3.2. Spray Drying Process
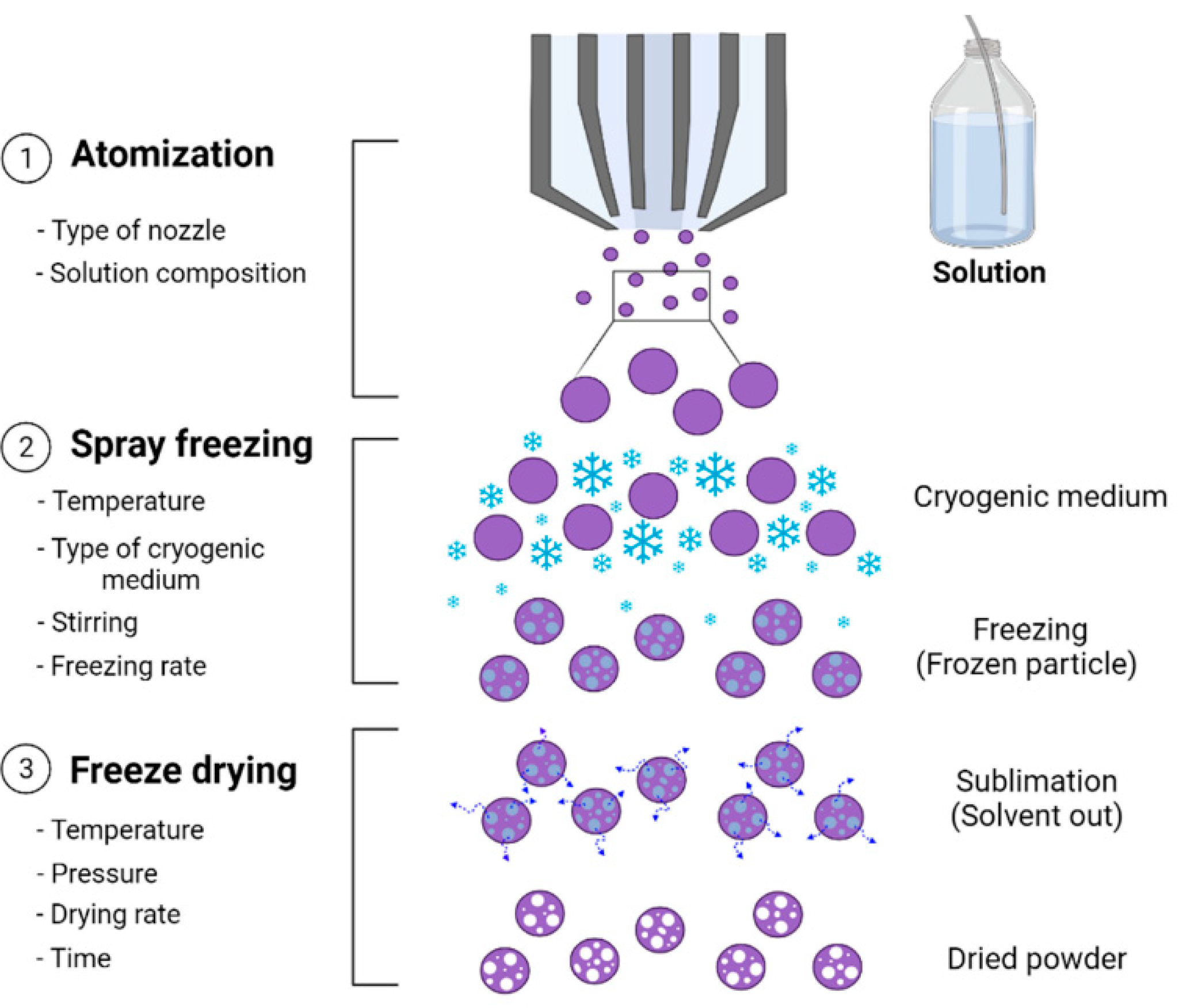
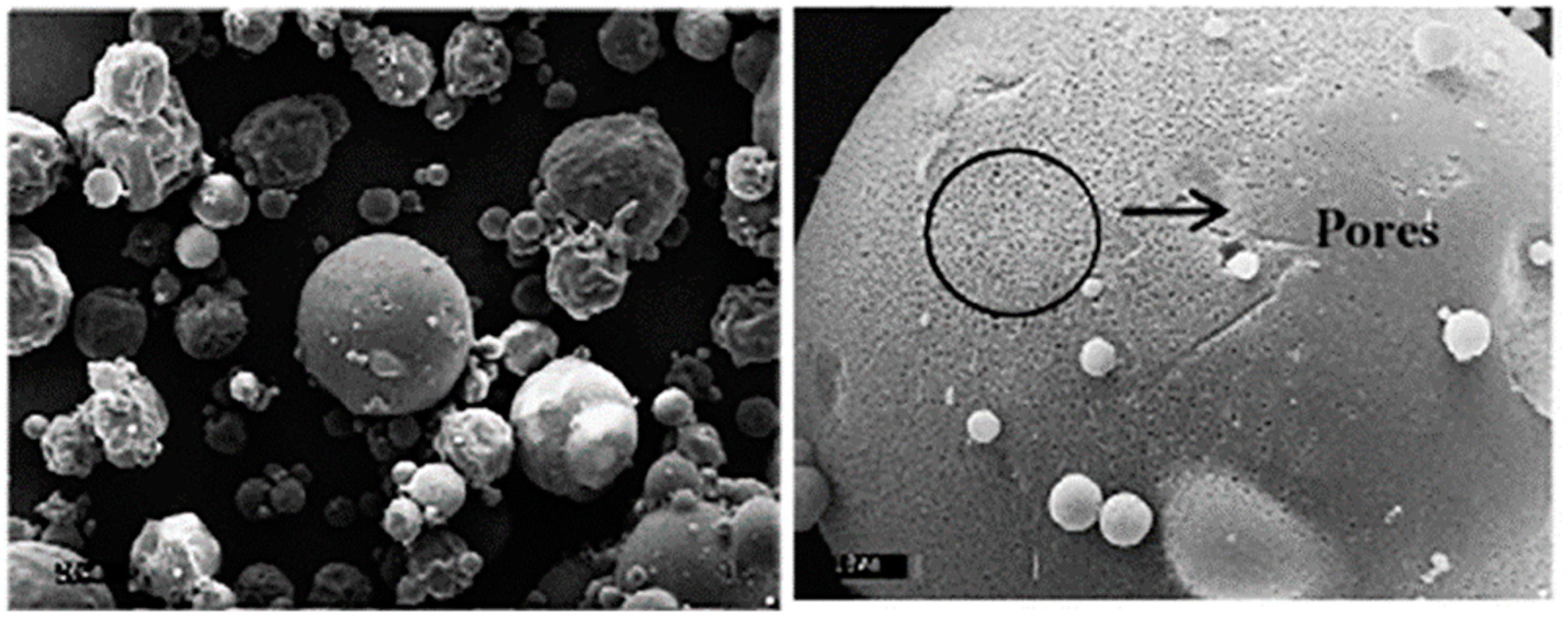
3.3. Spray Freeze Drying Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vishali, D.A.; Monisha, J.; Sivakamasundari, S.K.; Moses, J.A.; Anandharamakrishnan, C. Spray freeze drying: Emerging applications in drug delivery. J. Control. Release 2019, 300, 93–101. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef]
- Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of liposomal formulations: Still necessary, still challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef]
- Shin, G.H.; Kim, J.T.; Park, H.J. Recent developments in nanoformulations of lipophilic functional foods. Trends Food Sci. Technol. 2015, 46, 144–157. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Mochado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current applications of nanoemulsions in cancer therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef]
- Almeida, H.; Lobão, P.; Frigerio, C.; Fonseca, J.; Silva, R.; Lobo, J.M.S.; Amaral, M.H. Preparation, characterization and biocompatibility studies of thermoresponsive eyedrops based on the combination of nanostructured lipid carriers (NLC) and the polymer Pluronic F-127 for controlled delivery of ibuprofen. Pharm. Dev. Technol. 2015, 22, 336–349. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shin, G.H.; Chen, X.; Park, H.J. Modified curcumin with hyaluronic acid: Combination of pro-drug and nano-micelle strategy to address the curcumin challenge. Food Res. Int. 2015, 69, 202–208. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Vu, N.B.D.; Nguyen, T.H.N.; Le, H.S.; Le, H.T.; Tran, T.T.; Le, X.C.; Le, V.T.; Nguyen, T.T.; Bui, C.B.; et al. In vivo comparison of wound healing and scar treatment effect between curcumin–oligochitosan nanoparticle complex and oligochitosan-coated curcumin-loaded-liposome. J. Microencapsul. 2019, 36, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol. Adv. 2021, 48, 107727. [Google Scholar] [CrossRef]
- van den Hoven, J.M.; Metselaar, J.M.; Storm, G.; Beijnen, J.H.; Nuijen, B. Cyclodextrin as membrane protectant in spray-drying and freeze-drying of PEGylated liposomes. Int. J. Pharm. 2012, 438, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.K.; Shin, G.H.; Jung, M.K.; Hwang, I.C.; Park, H.J. Factors influencing the physicochemical characteristics of cationic polymer-coated liposomes prepared by high-pressure homogenization. Colloids Surf. A Physicochem. Eng. Asp. 2014, 454, 8–15. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Silva-Weiss, A.; Giménez, B.; Cantero-López, P.; Vega, R.; Osorio, F.A. Effect of lyophilization on the physicochemical and rheological properties of food grade liposomes that encapsulate rutin. Food Res. Int. 2020, 130, 108967. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K.; Amnuaikit, T. Liposomal encapsulated ethanolic coconut husk extract: Antioxidant and antibacterial properties. J. Food Sci. 2019, 84, 3664–3673. [Google Scholar] [CrossRef]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Li, X.; Yuan, F. The stabilization and release performances of curcumin-loaded liposomes coated by high and low molecular weight chitosan. Food Hydrocoll. 2020, 99, 105355. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Battino, M.; Benjakul, S. Effect of stabilizing agents on characteristics, antioxidant activities and stability of liposome loaded with hydrolyzed collagen from defatted Asian sea bass skin. Food Chem. 2020, 328, 127127. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Wang, X.; Feng, T.; Xia, S.; Zhang, X. Chitosan decoration improves the rapid and long-term antibacterial activities of cinnamaldehyde-loaded liposomes. Int. J. Biol. Macromol. 2021, 168, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Deng, Y.; Geng, Y.; Gao, Z.; Zou, J.; Wang, Z. Preparation of submicron unilamellar liposomes by freeze-drying double emulsions. Biochim. Biophys. Acta Biomembr. 2006, 1758, 222–231. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, W.; Han, C.; Sui, X.; Liu, C.; Ma, X.; Dong, Y. Preparation of glycyrrhetinic acid liposomes using lyophilization monophase solution method: Preformulation, optimization, and in vitro evaluation. Nanoscale Res. Lett. 2018, 13, 324. [Google Scholar] [CrossRef] [PubMed]
- Maniyar, M.G.; Kokare, C.R. Formulation and evaluation of spray dried liposomes of lopinavir for topical application. J. Pharm. Investig. 2019, 49, 259–270. [Google Scholar] [CrossRef]
- Jo, M.; Park, K.M.; Park, J.Y.; Yu, H.; Choi, S.J.; Chang, P.S. Microfluidic assembly of mono-dispersed liposome and its surface modification for enhancing the colloidal stability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124202. [Google Scholar] [CrossRef]
- Dag, D.; Guner, S.; Oztop, M.H. Physicochemical mechanisms of different biopolymers’ (lysozyme, gum arabic, whey protein, chitosan) adsorption on green tea extract loaded liposomes. Int. J. Biol. Macromol. 2019, 138, 473–482. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Silva-Weiss, A.; Zamorano, M.; Osorio, F.A. Humectability and physical properties of hydroxypropyl methylcellulose coatings with liposome-cellulose nanofibers: Food application. Carbohydr. Polym. 2020, 231, 115702. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Karadağ, A.; Duman, Ş.; ÖzKal, B.; Özçelik, B. Fortification of dark chocolate with spray dried black mulberry (Morus nigra) waste extract encapsulated in chitosan-coated liposomes and bioaccessability studies. Food Chem. 2016, 201, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Benjakul, S. Characteristics and storage stability of nanoliposomes loaded with shrimp oil as affected by ultrasonication and microfluidization. Food Chem. 2020, 310, 125916. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P.; Campardelli, R.; Aliakbarian, B.; Perego, P.; Reverchon, E. Supercritical assisted process for the encapsulation of olive pomace extract into liposomes. J. Supercrit. Fluids 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. A versatile supercritical assisted process for the one-shot production of liposomes. J. Supercrit. Fluids 2019, 146, 136–143. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M. Antioxidant activities of free and liposome-encapsulated green tea extracts on canola oil oxidation stability. J. Am. Oil Chem. Soc. 2020, 97, 1343–1354. [Google Scholar] [CrossRef]
- Savaghebi, D.; Barzegar, M.; Mozafari, M.R. Manufacturing of nanoliposomal extract from Sargassum boveanum algae and investigating its release behavior and antioxidant activity. Food Sci. Nutr. 2020, 8, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hens, A.; Fernández-Ramero, J.M. Analytical methods for the control of liposomal delivery systems. Trends Analyt. Chem. 2006, 25, 167–178. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Johnson, C.; Hatziantoniou, S.; Demetzos, C. Nanoliposomes and their applications in food nanotechnology. J. Liposome Res. 2008, 18, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, M.; Krolak, E.; Wagner, A.E.; Steffen-Heins, A. Physicochemical properties of WPI coated liposomes serving as stable transporters in a real food matrix. LWT-Food Sci. Technol. 2015, 63, 527–534. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M. Effect of chitosan coating on the properties of nanoliposomes loaded with flaxseed-peptide fractions: Stability during spray-drying. Food Chem. 2020, 310, 125951. [Google Scholar] [CrossRef]
- Hussain, M.T.; Forbes, N.; Perrie, Y.; Malik, K.P.; Duru, C.; Matejtschuk, P. Freeze-drying cycle optimization for the rapid preservation of protein-loaded liposomal formulations. Int. J. Pharm. 2020, 573, 118722. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Gauthier, M.; Yang, G.; Wang, Q. Recent advances in liposome surface modification for oral drug delivery. Nanomedicine 2016, 11, 1169–1185. [Google Scholar] [CrossRef]
- Tahara, K.; Nishio, M.; Takeuchi, H. Evaluation of liposomal behavior in the gastrointestinal tract after oral administration using real-time in vivo imaging. Drug Dev. Ind. Pharm. 2018, 44, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Ternullo, S.; Werning, L.V.S.; Holsæter, A.M.; Škalko-Basnet, N. Curcumin-in-deformable liposomes-in-chitosan-hydrogel as a novel wound dressing. Pharmaceutics 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Alihosseini, F.; Ghaffari, S.; Dabirsiaghi, A.R.; Haghighat, S. Freeze-drying of ampicillin solid lipid nanoparticles using mannitol as cryoprotectant. Braz. J. Pharm. Sci. 2015, 51, 797–802. [Google Scholar] [CrossRef]
- van Winden, E.C.A. Freeze-drying of liposomes: Theory and practice. Methods Enzymol. 2003, 367, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Susa, F.; Bucca, G.; Limongi, T.; Cauda, V.; Pisano, R. Enhancing the preservation of liposomes: The role of cryoprotectants, lipid formulations and freezing approaches. Cryobiology 2021, 98, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Glavas-Dodov, M.; Fredo-Kumbaradzi, E.; Goracinova, K.; Simonoska, M.; Calis, S.; Trajkovic-Jolevska, S.; Hincal, A.A. The effects of lyophilization on the stability of liposomes containing 5-FU. Int. J. Pharm. 2005, 291, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Sebaaly, C.; Charcosset, C.; Stainmesse, S.; Fessi, H.; Greige-Gerges, H. Clove essential oil-in-cyclodextrin-in-liposomes in the aqueous and lyophilized states: From laboratory to large scale using a membrane contactor. Carbohydr. Polym. 2016, 138, 75–85. [Google Scholar] [CrossRef]
- Sun, W.Q.; Leopold, A.C.; Crowe, L.M.; Crowe, J.H. Stability of dry liposomes in sugar glasses. Biophys. J. 1996, 70, 1769–1776. [Google Scholar] [CrossRef]
- Toniazzo, T.; Peres, M.S.; Ramos, A.P.; Pinho, S.C. Encapsulation of quercetin in liposomes by ethanol injection and physicochemical characterization of dispersions and lyophilized vesicles. Food Biosci. 2017, 19, 17–25. [Google Scholar] [CrossRef]
- Horn, J.; Friess, W. Detection of collapse and crystallization of saccharide, protein, and mannitol formulations by optical fibers in lyophilization. Front. Chem. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Ingvarsson, P.T.; Yang, M.; Nielsen, H.M.; Rantanen, J.; Foged, C. Stabilization of liposomes during drying. Expert Opin. Drug Deliv. 2011, 8, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Roh, S.H.; Park, H.J. Characterization of ferulic acid encapsulation complexes with maltodextrin and hydroxypropyl methylcellulose. Food Hydrocoll. 2021, 111, 106390. [Google Scholar] [CrossRef]
- Yu, J.Y.; Kim, J.A.; Joung, H.J.; Ko, J.A.; Park, H.J. Preparation and characterization of curcumin solid dispersion using HPMC. J. Food Sci. 2020, 85, 3866–3873. [Google Scholar] [CrossRef]
- Altin, G.; Gültekin-Özgüven, M.; Ozcelic, B. Chitosan coated liposome dispersions loaded with cacao hull waste extract: Effect of spray drying on physico-chemical stability and in vitro bioaccessibility. J. Food Eng. 2018, 223, 91–98. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Pérez-García, A.; Alemán, A.; Gómez-Guillén, M.C.; Montero, P. Drying soy phosphatidylcholine liposomal suspensions in alginate matrix: Effect of drying methods on physico-chemical properties and stability. Food Hydrocoll. 2021, 111, 106357. [Google Scholar] [CrossRef]
- Khatib, I.; Chow, M.Y.T.; Ruan, J.; Cipolla, D.; Chan, H.K. Modeling of a spray drying method to produce ciprofloxacin nanocrystals inside the liposomes utilizing a response surface methodology: Box-Behnken experimental design. Int. J. Pharm. 2021, 597, 120277. [Google Scholar] [CrossRef] [PubMed]
- Akgün, D.; Gültekin-Özgüven, M.; Yücetepe, A.; Altin, G.; Gibis, M.; Weiss, J.; Özçelik, B. Stirred-type yoghurt incorporated with sour cherry extract in chitosan-coated liposomes. Food Hydrocoll. 2020, 101, 105532. [Google Scholar] [CrossRef]
- Voronin, G.L.; Hettiarachchi, C.A.; Harte, F.M. High pressure jet spray drying of condensed skim milk results in powders with enhanced interfacial properties. J. Food Eng. 2021, 292, 110249. [Google Scholar] [CrossRef]
- Nijdam, J.J.; Langrish, T.A.G. The effect of surface composition on the functional properties of milk powders. J. Food Eng. 2006, 77, 919–925. [Google Scholar] [CrossRef]
- Cipolla, D.; Wu, H.; Salentinig, S.; Boyd, B.; Rades, T.; Vanhecke, D.; Petri-Fink, A.; Rothin-Rutishauser, B.; Eastman, S.; Redelmeier, T.; et al. Formation of drug nanocrystals under nanoconfinement afforded by liposomes. RSC Adv. 2016, 6, 6223–6233. [Google Scholar] [CrossRef][Green Version]
- Mertins, O.; Schneider, P.H.; Pohlmann, A.R.; Pesce de Silveira, N. Interaction between phospholipids bilayer and chitosan in liposomes investigated by 31P NMR spectroscopy. Colloids Surf. B Biointerfaces 2010, 75, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiva, B.; Zhao, Y.Y. Dry powder for pulmonary delivery: A comprehensive review. Pharmaceutics 2020, 13, 31. [Google Scholar] [CrossRef]
- Adali, M.B.; Barresi, A.A.; Boccardo, G.; Pisano, R. Spray freeze-drying as a solution to continuous manufacturing of pharmaceutical products in bulk. Processes 2020, 8, 709. [Google Scholar] [CrossRef]
- Engstrom, J.D.; Simpson, D.T.; Lai, E.S.; Williams III, R.O.; Johnston, K.P. Morphology of protein particles produced by spray freezing of concentrated solutions. Eur. J. Pharm. Biopharm. 2007, 65, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, S.P.; Anandharamakrishnan, C. Spray-freeze-drying approach for soluble coffee processing and its effect on quality characteristics. J. Food Eng. 2015, 149, 171–180. [Google Scholar] [CrossRef]
- Ali, M.E.; Lamprecht, A. Spray freeze drying as an alternative technique for lyophilization of polymeric and lipid-based nanoparticles. Int. J. Pharm. 2017, 516, 170–177. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, B. Principles of solidification. In Applied Solid State Physics; Springer: Boston, MA, USA, 1970; pp. 161–170. [Google Scholar] [CrossRef]
- Searles, J.A.; Carpenter, J.F.; Randolph, T.W. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J. Pharm. Sci. 2001, 90, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Yu, J.; Luo, Q.; Wang, S.; Chan, H.K. Inhalable clarithromycin liposomal dry powders using ultrasonic spray freeze drying. Powder Technol. 2017, 305, 63–70. [Google Scholar] [CrossRef]
- Fukushige, K.; Tagami, T.; Naito, M.; Goto, E.; Hirai, S.; Hatayama, N.; Yokota, H.; Yasui, T.; Baba, Y.; Ozeki, T. Developing spray-freeze-dried particles containing a hyaluronic acid-coated liposome-protamine-DNA complex for pulmonary inhalation. Int. J. Pharm. 2020, 583, 119338. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, M.F.; Olesen, M.T.; Gjelstrup, M.C.; Pakula, M.M.; Larsen, E.K.; Hansen, I.M.; Hansen, P.L.; Mollenhauer, J.; Malle, B.M.; Howard, K.A. Tunable CD44-specific cellular retargeting with hyaluronic acid nanoshells. Pharm. Res. 2015, 32, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
| Liposome Formulation | Liposome Preparation Technique | Core Materials | Shell Materials | Protectant | Main Result | Ref. |
|---|---|---|---|---|---|---|
| Liquid State | Thin Film Hydration (+ Extrusion/Sonication) | Curcumin | Soybean lecithin, cholesterol | Chitosan | Improved stability of curcumin-loaded liposomes | [16] |
| Fish hydrolyzed collagen | Soy phosphatidylcholine with cholesterol or glycerol | - | Enhanced bioactivities and stability of hydrolyzed collagen | [17] | ||
| Ethanol Injection | Ethanolic coconut husk extract | Phosphatidylcholine, cholesterol | - | Enhanced antibacterial properties, improved dark color | [15] | |
| Cinnamaldehyde | Egg yolk lecithin, Tween 80 | Chitosan | Increased encapsulation efficiency, antibacterial activity and storage stability | [18] | ||
| Microfluidization | Branched-chain amino acids | Phosphatidylcholine, cholesterol, palmitic acid, hexadecylamine | Chitosan, pectin | Improved colloidal and intestinal stabilities of encapsulated branched-chain amino acids | [22] | |
| Green tea extract | Soybean lecithin | Gum arabic, Whey protein, lysozyme, chitosan | Increased storage stability of liposomes | [23] | ||
| Heating (+ Sonication) | Rutin, glycerol, cellulose nanofibers | Soybean lecithin | HPMC | Improved appearance, increased apparent viscosity, decreased cohesive energy of the coating suspension | [24] | |
| High Shear Disperser | Black mulberry (Morus nigra) extract | Lecithin | Chitosan, maltodextrin | Protected anthocyanin content, enhanced in vitro bioaccessibility of anthocyanins | [25] | |
| Sonication | Shrimp oil | Phosphatidylcholine | - | Improved stability and nanoencapsulation efficiency, minimized fishy odor | [26] | |
| Superficial | Olive pomace extract | l-α-Phosphatidylcholine | - | Increased encapsulation efficiency of polyphenol compounds | [27] | |
| Limonene | l-α-Phosphatidylcholine | - | Increased encapsulation efficiency of limonene | [28] | ||
| Mozafari | Green tea extract | Lecithin, glycerol | - | Improved stability and antioxidant activity of green tea extract | [29] | |
| Algal extract | Soybean lecithin | - | Increased stability, maintained the antioxidant activity of algal extract | [30] | ||
| Solid State | Freeze Drying | Calcein, 5-fluorouracil, flurbiprofen | Soybean phosphatidylcholine, cholesterol | Sucrose, lactose, mannitol | Increased encapsulation efficiency | [19] |
| Glycyrrhetinic acid | Soybean phosphatidylcholine, cholesterol | Lactose, sucrose, trehalose, mannitol | Increased water solubility and encapsulation efficiency | [20] | ||
| Spray Drying | Lopinavir | Phospholipon 85G®, cholesterol | - | Increased stability and % entrapment efficiency | [21] |
| 1. Carbohydrate | 2. Protein [39] | 3. Polyol [39] | |
|---|---|---|---|
| 1.1. Mono and disaccharides [39,41] | 1.2. Oligo and polysaccharides [39] | Glycine | Mannitol |
| Glucose (dextrose) | Raffinose | Gelatin | Sorbitol |
| Fructose | Hydroxypropyl-β-cyclodextrin (HP-β-CD) | Proline | Glycerol |
| Mannose | Chitosan | Glutamine | Ethylene glycol |
| Maltose | Maltodextrin | Betaine | Propylene glycol |
| Sucrose | Inulin | Arginine | Polyvinyl alcohol |
| Trehalose | Dextran | Lysine | |
| Cellobiose | Hyaluronan | Histidine | |
| Lactose | |||
| Post-Processing Technique | Step | Advantages | Disadvantages |
|---|---|---|---|
| 1. Freeze Drying |
|
|
|
| 2. Spray Drying |
|
|
|
| 3. Spray Freeze Drying |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-Processing Techniques for the Improvement of Liposome Stability. Pharmaceutics 2021, 13, 1023. https://doi.org/10.3390/pharmaceutics13071023
Yu JY, Chuesiang P, Shin GH, Park HJ. Post-Processing Techniques for the Improvement of Liposome Stability. Pharmaceutics. 2021; 13(7):1023. https://doi.org/10.3390/pharmaceutics13071023
Chicago/Turabian StyleYu, Ji Young, Piyanan Chuesiang, Gye Hwa Shin, and Hyun Jin Park. 2021. "Post-Processing Techniques for the Improvement of Liposome Stability" Pharmaceutics 13, no. 7: 1023. https://doi.org/10.3390/pharmaceutics13071023
APA StyleYu, J. Y., Chuesiang, P., Shin, G. H., & Park, H. J. (2021). Post-Processing Techniques for the Improvement of Liposome Stability. Pharmaceutics, 13(7), 1023. https://doi.org/10.3390/pharmaceutics13071023





