Formulations Based on Drug Loaded Aptamer-Conjugated Liposomes as a Viable Strategy for the Topical Treatment of Basal Cell Carcinoma—In Vitro Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
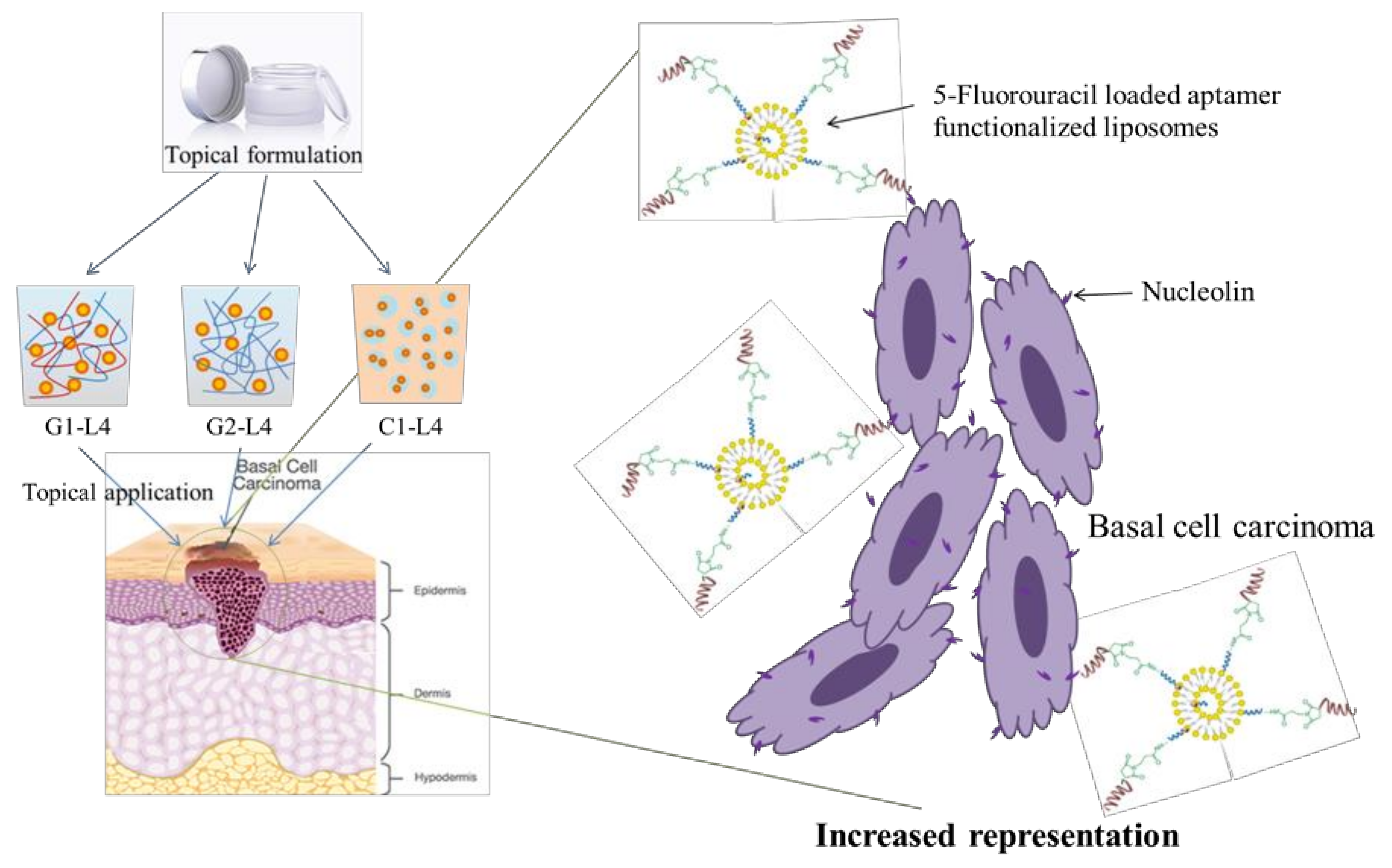
2.2. Preparation Methods
2.2.1. Preparation of Aptamer-Conjugated Liposomes Loaded with 5-Fluorouracil
2.2.2. Topical Formulations Preparation
2.3. Characterization
2.3.1. Rheological Studies of Topical Formulations
2.3.2. Transdermal Diffusion Assays across Strat-M Artificial Membrane
2.3.3. Mathematical Modelling
- -
- t = the time of drug release,
- -
- Mt = the amount of drug delivered at time t,
- -
- M∞ = the total amount of drug delivered after an infinite time interval,
- -
- kKP = a kinetic constant, a measure of release rate,
- -
- n = a diffusional exponent that provides an indication of the mechanism of drug release.
2.3.4. In Vitro Evaluation of Topical Formulaons Biocompatibility with Blood Components
2.3.5. The Skin Irritation Potential of the Topical Formulations
2.3.6. Cell Viability Assessment by MTT Method
2.3.7. Apoptosis Assay
2.3.8. Statistical Analysis
3. Results and Discussion
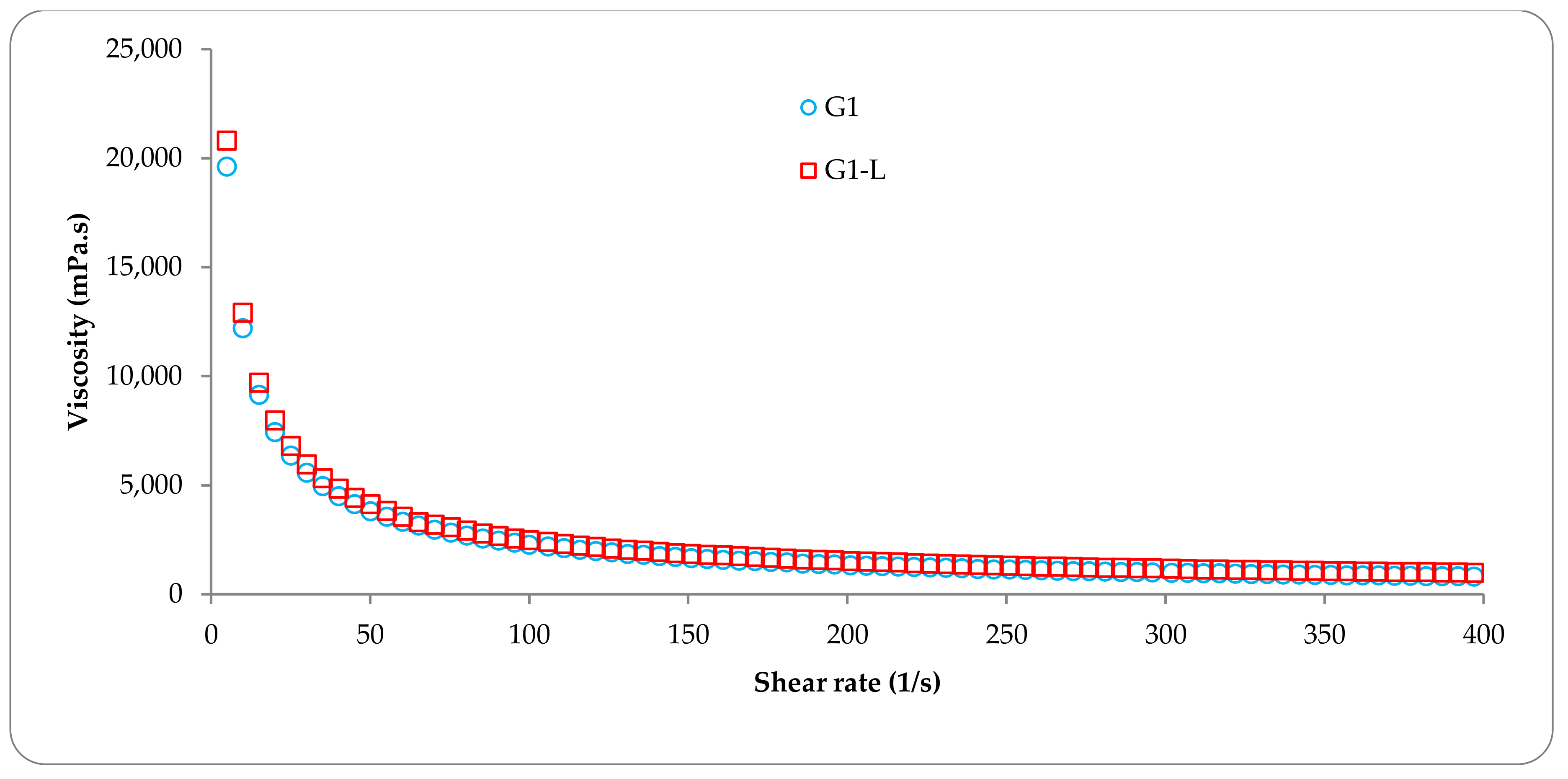
3.1. Rheological Studies of Topical Formulations
3.2. In vitro Transdermal Diffusion Assays
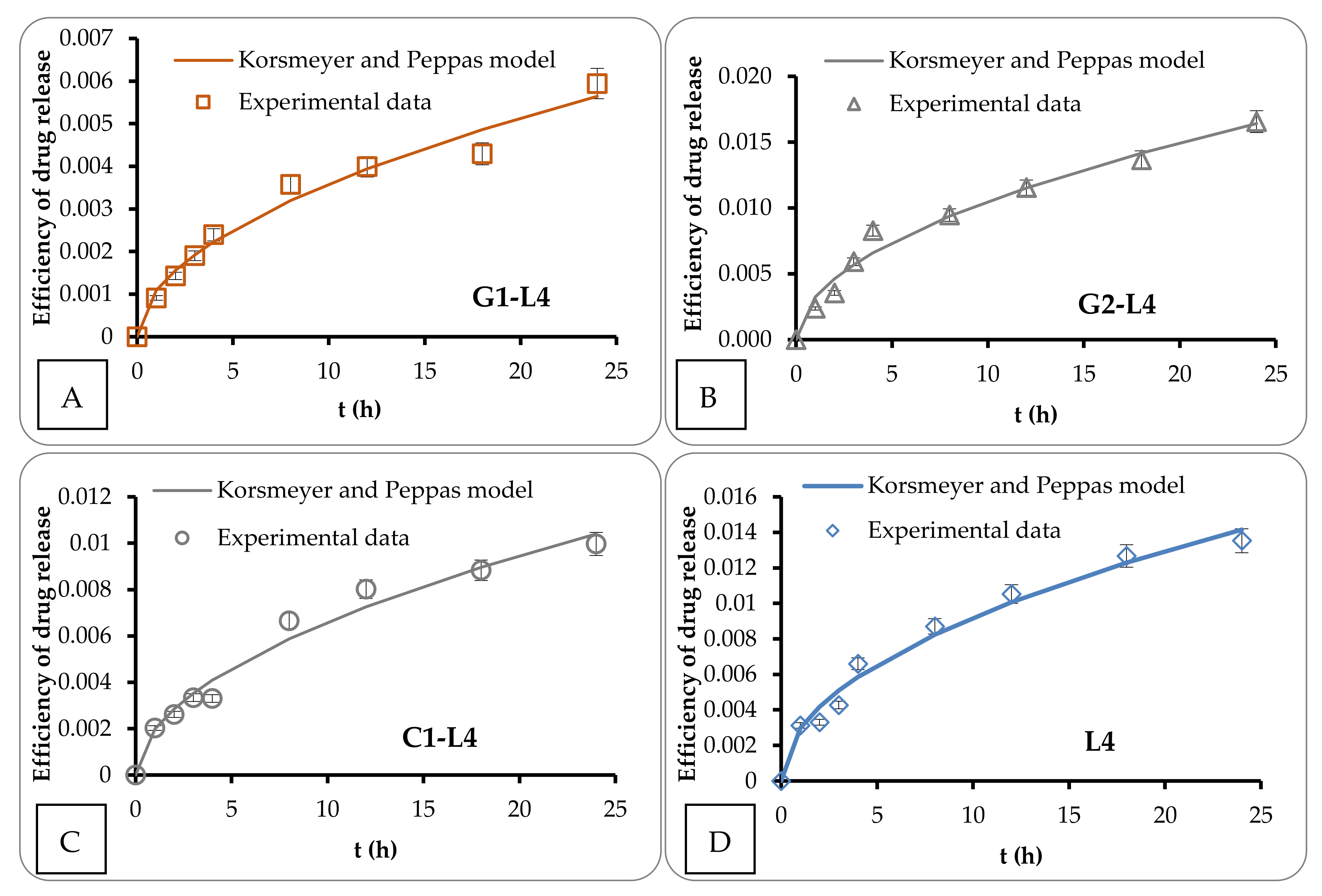
3.3. Mathematical Modeling
3.4. In Vitro Evaluation of Topical Formulations Biocompatibility with Blood Components
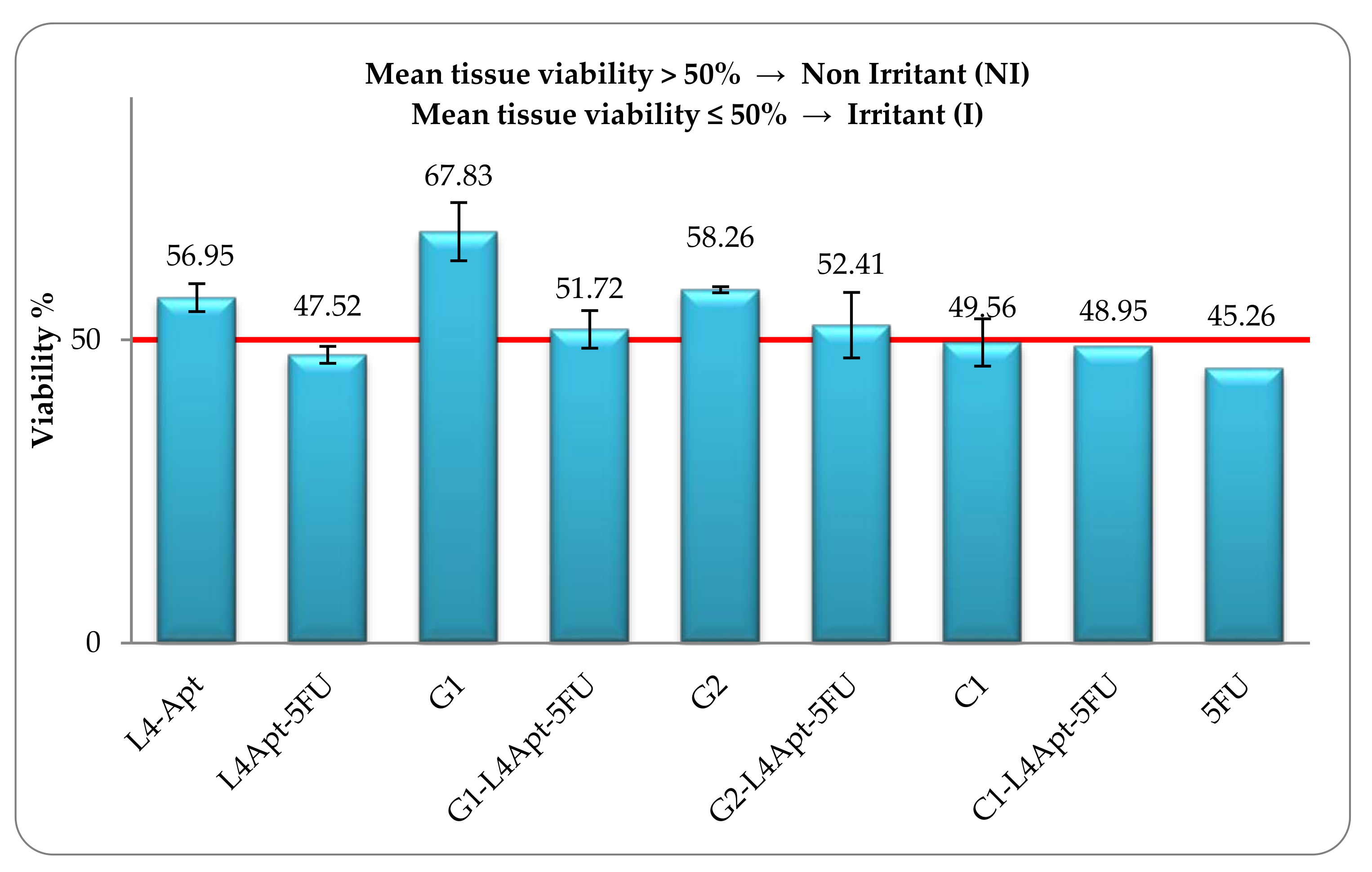
3.5. The Skin Irritation Potential of the Topical Formulations
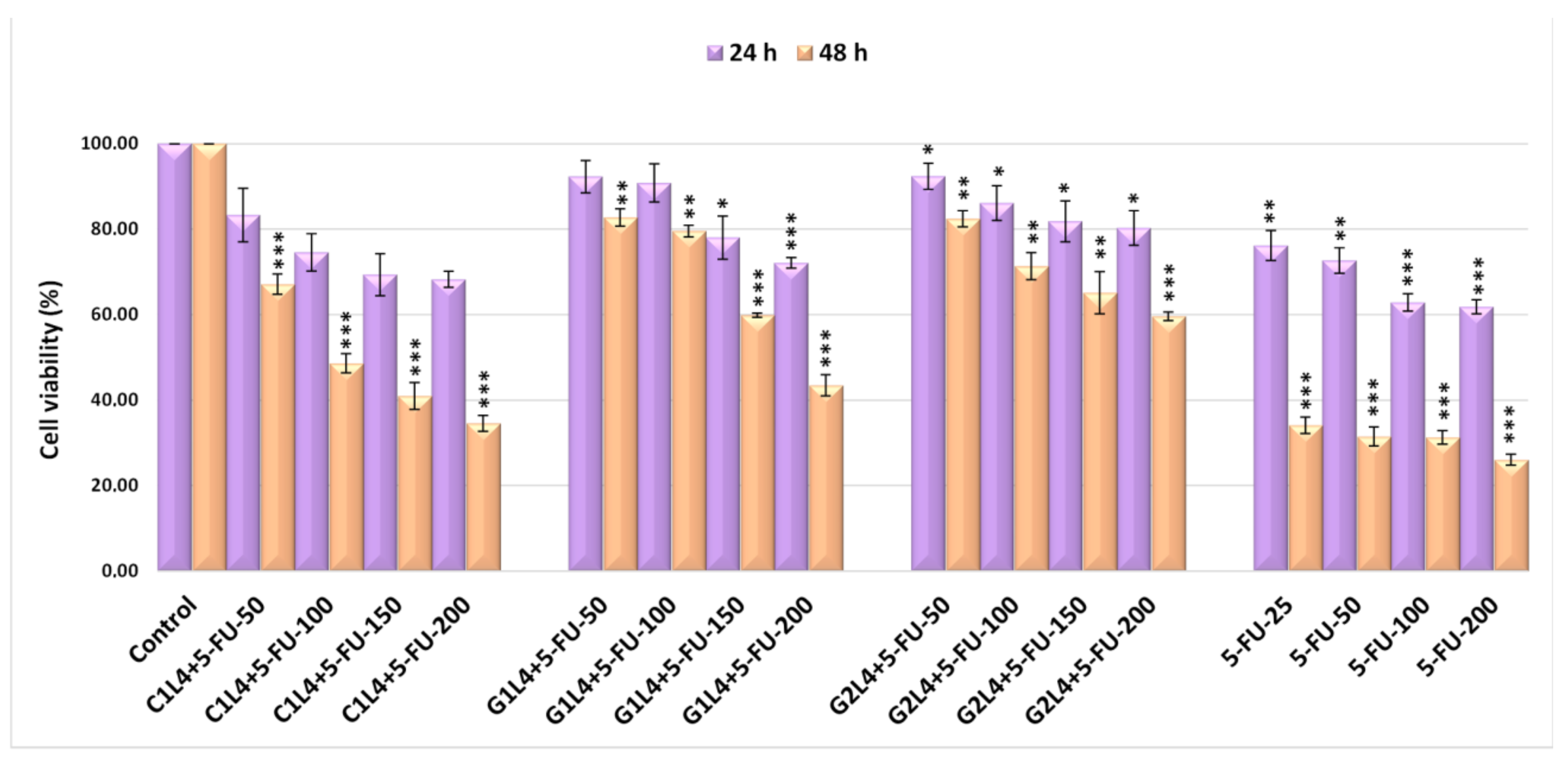
3.6. Cell Viability Assessment by the MTT Method
3.7. Apoptosis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability
Conflicts of Interest
References
- Skin cancer statistics. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/skin-cancer-statistics (accessed on 3 May 2021).
- Alam, M.; Goldber, L.H.; Silapunt, S.; Gardner, E.S.; Strom, S.S.; Rademaker, A.W.; Margolis, D.J. Delayed treatment and continued growth of nonmelanoma skin cancer. J. Am. Acad. Dermatol. 2011, 64, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci. 2020, 21, 5394. [Google Scholar] [CrossRef] [PubMed]
- McGillis, S.T.; Fein, H. Topical treatment strategies for non-melanoma skin cancer and precursor lesions. Semin. Cutan. Med. Surg. 2004, 23, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Firnhaber, J.M. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am. Fam. Physician. 2012, 86, 161–168. [Google Scholar] [PubMed]
- Sharquie, K.E.; Noaimi, A.A. Basal cell carcinoma: Topical therapy versus surgical treatment. J. Dermatol. Dermatol. Surg. 2012, 16, 41–51. [Google Scholar] [CrossRef][Green Version]
- Lopez, R.F.; Lange, N.; Guy, R.; Bentley, M.V. Photodynamic therapy of skin cancer: Controlled drug delivery of 5-ALA and its esters. Adv. Drug Deliv. Rev. 2004, 56, 77–94. [Google Scholar] [CrossRef]
- Taveira, S.F.; Lopez, R.F.V. Topical administration of anticancer drugs for skin cancer treatment. In Skin Cancer—Risk Factors, Prevention and Therapy; La Porta, C., Ed.; InTech: Rijeka, Croatia, 2011; pp. 247–272. [Google Scholar]
- Shende, P.; Vaidya, J.; Gaud, R.S. Pharmacotherapeutic approaches for transportation of anticancer agents via skin. Artif. Cells Nanomed. Biotechnol. 2018, 46, S423–S433. [Google Scholar] [CrossRef]
- Aldara. Available online: https://www.drugs.com/aldara.html (accessed on 3 May 2021).
- Carac. Available online: https://www.drugs.com/mtm/carac.html (accessed on 3 May 2021).
- Fluoroplex. Available online: https://www.drugs.com/mtm/fluoroplex.html (accessed on 3 May 2021).
- Efudex. Available online: https://www.drugs.com/mtm/efudex.html (accessed on 3 May 2021).
- Dianzani, C.; Zara, G.P.; Maina, G.; Pettazzoni, P.; Pizzimenti, S.; Rossi, F.; Gigliotti, C.L.; Ciamporcero, E.S.; Daga, M.; Barrera, G. Drug Delivery Nanoparticles in Skin Cancers. BioMed Res. Int. 2014, 2014, 895986. [Google Scholar] [CrossRef]
- Uchechi, O.; Ogbonna JDNAttama, A.A. Nanoparticles for dermal and transdermal drug delivery. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; InTech: Rijeka, Croatia, 2014; pp. 193–235. [Google Scholar]
- Gupta, S.; Bansal, R.; Gupta, S.; Jindal, N.; Jindal, A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013, 4, 267–272. [Google Scholar] [CrossRef]
- Gupta, V.; Trivedi, P. Dermal Drug Delivery for Cutaneous Malignancies: Literature at a Glance. J. Pharm. Innov. 2016, 11, 1–33. [Google Scholar] [CrossRef]
- Sapkota, R.; Dash, A.K. Liposomes and transferosomes: A breakthrough in topical and transdermal delivery. Ther Deliv. 2021, 12, 2. [Google Scholar] [CrossRef]
- Sabitha, M.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Development and evaluation of 5-fluorouracil loaded chitin nanogels for treatment of skin cancer. Carbohydr. Polym. 2013, 91, 48–57. [Google Scholar] [CrossRef]
- Tiwari, R.; Tiwari, G.; Wal, A.; Gupta, C. Liposomal delivery of 5 Fluorouracil and Tretinoin: An Aspect of Topical treatment of skin warts. ARS Pharm. 2019, 60, 139–146. [Google Scholar]
- Maghfour, J.; Kuraitis, D.; Murina, A. Intralesional 5-Fluorouracil for Treatment of Non-Melanoma Skin Cancer: A Systematic Review. J. Drugs Dermatol. 2021, 20, 192–198. [Google Scholar] [CrossRef]
- Manalo, I.F.; Lowe, M.C.; Nelson, K.C.; Chen, S.C. Triple therapy with intralesional 5-fluorouracil, chemowraps, and acitretin: A well-tolerated option for treatment of widespread cutaneous squamous cell carcinomas on the legs. JAAD Case Rep. 2019, 5, 1051–1054. [Google Scholar] [CrossRef][Green Version]
- Voiculescu, V.M.; Lisievici, C.V.; Lupu, M.; Vajaitu, C.; Draghici, C.C.; Popa, A.V.; Solomon, I.; Sebe, T.I.; Constantin, M.M.; Caruntu, C. Mediators of Inflammation in Topical Therapy of Skin Cancers. Mediat. Inflamm. 2019, 2019, 8369690. [Google Scholar] [CrossRef]
- Morse, L.G.; Kendrick, C.; Hooper, D.; Ward, H.; Parry, E. Treatment of Squamous Cell Carcinoma with Intralesional 5-Fluorouracil. Dermatol. Surg. 2003, 29, 1150–1153. [Google Scholar]
- Mercuri, S.R.; Brianti, P.; Dattola, A.; Bennardo, L.; Silvestri, M.; Schipani, G.; Nisticò, S.P. CO2 laser and photodynamic therapy: Study of efficacy in periocular BCC. Dermatol. Ther. 2018, 31, e12616. [Google Scholar] [CrossRef]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers 2019, 11, 1515. [Google Scholar] [CrossRef]
- Guideline on the Pharmacokinetic and Clinical Evaluation of Modified Release Dosage forms (EMA/CPMP/EWP/280/96 Corr1). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmacokinetic-clinical-evaluation-modified-release-dosage-forms_en.pdf (accessed on 3 May 2021).
- ICH Guideline Q8 (R2) on Pharmaceutical Development (EMA/CHMP/ICH/167068/2004). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-11.pdf (accessed on 3 May 2021).
- Guideline on Quality of Transdermal Patches (EMA/CHMP/QWP/608924/2014). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-transdermal-patches_en.pdf (accessed on 3 May 2021).
- Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization. Polymers 2020, 12, 1018. [Google Scholar] [CrossRef]
- Bacaita, E.S.; Ciobanu, B.C.; Popa, M.; Agop, M.; Desbrieres, J. Phases in the temporal multiscale evolution of the drug release mechanism in IPN-type chitosan based hydrogels. Phys. Chem. Chem. Phys. 2014, 16, 25896–25905. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanism of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ozyazici, M.; Gökçe, E.H.; Ertan, G. Release and diffusional modeling of metronidazole lipid matrices. Eur. J. Pharm. Biopharm. 2006, 63, 331–339. [Google Scholar] [CrossRef]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.M.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef]
- Alupei, L.; Lisa, G.; Butnariu, A.; Desbrieres, J.; Cadinoiu, A.N.; Peptu, C.A.; Calin, G.; Popa, M. New folic acid-chitosan derivatives based nanoparticles—Potential applications in cancer therapy. Cell Chem. Technol. 2017, 51, 631–648. [Google Scholar]
- Severino, P.; Fangueiro, J.F.; Ferreira, S.V.; Basso, R.; Chaud, M.V.; Santana, M.H.A.; Rosmaninho, A.; Souto, E.B. Nanoemulsions and nanoparticles for non-melanoma skin cancer: Effects of lipid materials. Clin. Transl. Oncol. 2013, 15, 417–424. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil—An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C 2021, 119, 111591. [Google Scholar] [CrossRef]
- The Episkin Validated Protocol for EpiSkinTM Small Model. Available online: https://www.episkin.com/skin-irritation (accessed on 3 May 2021).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Laville, N.; Aït-Aïssa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blazquez-Castro, A.; Canete, M.; Horobin, R.W.; Villanueva, A. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
- Pozarowski, P.; Grabarek, J.; Darzynkiewicz, Z. Flow Cytometry of Apoptosis. Curr. Protoc. Cell Biol. 2003, 21, 18.8.1–18.8.33. [Google Scholar]
- Cann, A.J. Maths from Scratch for Biologists; Jon Willey & Sons Ltd.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Bacaita, E.S.; Agop, M. A multiscale mechanism of drug release from polymeric matrices: Confirmation through a nonlinear theoretical model. Phys. Chem. Chem. Phys. 2016, 18, 21809–21816. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Z.; Yang, K.; Zhou, Y.; Chen, X.; Zhang, Y.; Wang, F.; Liu, Y.; Ren, L. Self-Assembled Polymeric Micellar Nanoparticles as Nanocarriers for Poorly Soluble Anticancer Drug Ethaselen. Nanoscale Res. Lett. 2009, 4, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- OECD Guidelines for the Testing of Chemicals. Available online: http://www.oecd.org/env/ehs/testing/tg439-revised-in-vitro-skin-irritation-reconstructed-human-epidermis-test-method.pdf (accessed on 3 May 2021).
- United Nations (UN). Globally Harmonised System of Classification and Labelling of Chemicals (GHS); New York and Geneva. Seventh Revised Edition. Available online: https://www.unece.org/trans/danger/publi/ghs/ghs_rev07/07files_e0.html (accessed on 3 May 2021).
- Leiter, J.; Abbott, D.J.; Schepartz, S.A. Screening data from the Cancer Chemotherapy National Service Center Screening Laboratories. XXVIII. Cancer Res. Supp. 1965, 25, 1626–1769. [Google Scholar]
- Dold, U. Criteria for the evaluation of cytostatic chemotherapy. Int. J. Clin. Pharmacol. Biopharm. 1978, 16, 68–71. [Google Scholar] [PubMed]
- De Vita, V.T., Jr.; Hellman, S.; Rosenberg, S.A. Cancer: Principles and Practice of Oncology, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Udofot, O.; Affram, K.; Israel, B.; Agyare, E. Cytotoxicity of 5-fluorouracil-loaded pH-sensitive liposomal nanoparticles in colorectal cancer cell lines. Integr. Cancer Sci. Therap. 2015, 2, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lollo, G.; Matha, K.; Bocchiardo, M.; Bejaud, J.; Marigo, I.; Virgone-Carlotta, A.; Dehoux, T.; Rivière, C.; Rieu, J.P.; Briançon, S.; et al. Drug delivery to tumours using a novel 5-FU derivative encapsulated into lipid nanocapsules. J. Drug Target. 2019, 27, 634–645. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Di Marzio, L.; Carafa, M.; Ventura, C.A.; Fresta, M. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int. J. Pharm. 2015, 489, 1–10. [Google Scholar] [CrossRef]
- Calienni, M.N.; Temprana, C.F.; Prieto, M.J.; Paolino, D.; Fresta, M.; Tekinay, A.Y.; Del Valle Alonso, S.; Montanari, J. Nano-formulation for topical treatment of precancerous lesions: Skinpenetration, in vitro, and in vivo toxicological evaluation. Drug Deliv. Transl. Res. 2018, 8, 496–514. [Google Scholar] [CrossRef]


| Parameter | Viscosity × 10−3 (mPa.s) | ||||||
|---|---|---|---|---|---|---|---|
| G1 | G1-L4 | G2 | G2-L4 | C1 | C1-L4 | ||
| Shear rate a (1/s) | 50 | 4.12 | 4.11 | 0.97 | 0.91 | 3.63 | 3.62 |
| 100 | 2.27 | 2.25 | 0.97 | 0.92 | 1.82 | 1.82 | |
| 200 | 1.52 | 1.51 | 0.98 | 0.92 | 0.77 | 0.78 | |
| 300 | 1.18 | 1.17 | 0.97 | 0.91 | 0.50 | 0.49 | |
| 400 | 0.99 | 0.98 | 0.97 | 0.92 | 0.38 | 0.37 | |
| Temperature b (°C) | 20 | 6.43 | 5.91 | 0.22 | 0.24 | 1.55 | 1.50 |
| 30 | 6.71 | 6.01 | 0.12 | 0.14 | 1.41 | 1.28 | |
| 40 | 6.33 | 5.57 | 0.09 | 0.09 | 1.45 | 1.36 | |
| Sample | kKP | n |
|---|---|---|
| G1-L4 | 1.088 × 10−3 | 0.518 |
| G2-L4 | 3.245 × 10−3 | 0.510 |
| C1-L4 | 1.986 × 10−3 | 0.521 |
| L4 | 2.963 × 10−3 | 0.492 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Mihai, C.T.; Bacaita, S.E.; Popa, M. Formulations Based on Drug Loaded Aptamer-Conjugated Liposomes as a Viable Strategy for the Topical Treatment of Basal Cell Carcinoma—In Vitro Tests. Pharmaceutics 2021, 13, 866. https://doi.org/10.3390/pharmaceutics13060866
Cadinoiu AN, Rata DM, Atanase LI, Mihai CT, Bacaita SE, Popa M. Formulations Based on Drug Loaded Aptamer-Conjugated Liposomes as a Viable Strategy for the Topical Treatment of Basal Cell Carcinoma—In Vitro Tests. Pharmaceutics. 2021; 13(6):866. https://doi.org/10.3390/pharmaceutics13060866
Chicago/Turabian StyleCadinoiu, Anca N., Delia M. Rata, Leonard I. Atanase, Cosmin T. Mihai, Simona E. Bacaita, and Marcel Popa. 2021. "Formulations Based on Drug Loaded Aptamer-Conjugated Liposomes as a Viable Strategy for the Topical Treatment of Basal Cell Carcinoma—In Vitro Tests" Pharmaceutics 13, no. 6: 866. https://doi.org/10.3390/pharmaceutics13060866
APA StyleCadinoiu, A. N., Rata, D. M., Atanase, L. I., Mihai, C. T., Bacaita, S. E., & Popa, M. (2021). Formulations Based on Drug Loaded Aptamer-Conjugated Liposomes as a Viable Strategy for the Topical Treatment of Basal Cell Carcinoma—In Vitro Tests. Pharmaceutics, 13(6), 866. https://doi.org/10.3390/pharmaceutics13060866









