Effectiveness of Antimicrobial Photodynamic Therapy in the Treatment of Periodontitis: A Systematic Review and Meta-Analysis of In Vivo Human Randomized Controlled Clinical Trials
Abstract
Highlights
- Limitations of scaling and root planing (SRP) have directed the research to assess alternative comprehensive treatment strategies.
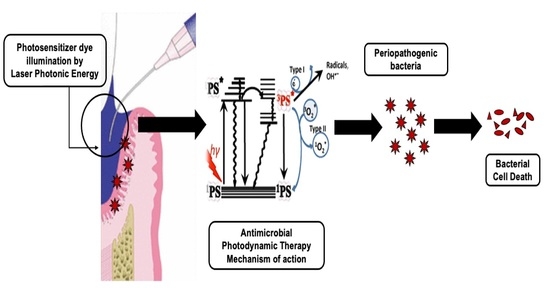
- Antimicrobial Photodynamic therapy (aPDT) involves photo-excitation of photosensitizer dye upon illumination by a light of a matched wavelength.
- This systematic review and meta-analysis evaluated the effectiveness of aPDT in the treatment of periodontitis.
- In spite of the inconsistencies in their findings and methodological bias, the majority of the studies have demonstrated aPDT effectiveness.
- The efficacy of aPDT in improving treatment outcomes when it is utilized in the non-surgical management of periodontitis remains debatable.
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Population (P), Intervention (I), Comparison (C) and Outcomes (O)—PICO
- Population: Patients diagnosed with Periodontitis (CP or AgP) [26]
- Intervention: Utilisation of aPDT as a monotherapy or as an adjunct to SRP
- Comparison: Utilisation of SRP alone or SRP with adjunctive AB therapy
- Outcome: Evaluation of clinical and/or microbiological and/or immunological profiles
2.3. Focused Research Question
2.4. Search Strategy
2.5. Search Algorithms
2.6. Eligibility Criteria
2.6.1. Inclusion Criteria
- Subjects diagnosed with CP or AgP according to 1999 AAP Classification of Periodontal diseases and conditions [26].
- Studies included: In vivo human RCT’s comparing the efficacy of aPDT in CP or AgP as monotherapy or adjunctive to SRP compared to SRP alone or in combination with AB.
- Parallel group (PG) and split-mouth (SM) studies.
- Age group >18 years, fit and healthy subjects.
- No language restrictions for search strategy.
- Studies that have utilized any PS dye (regardless dose and incubation period) and laser wavelength combination.
- Studies reporting at least one of the following parameters as an outcome variable: probing pocket depth (PPD), loss of clinical attachment level (CAL), bleeding on probing (BOP), plaque index (PI), gingival index (GI), microbiological profile, or immunological profile.
- Studies with a minimum follow-up period of at least one month after treatment.
2.6.2. Exclusion Criteria
- Subjects with systemic diseases or on medications that can influence the outcome variables.
- Subjects who have undergone any periodontal therapy and/or antibiotic therapy in the last six months prior to RCT enrolment.
- Studies utilizing low level laser therapy or laser therapy alone, as one of the intervention groups as compared to aPDT.
- Studies involving utilization of aPDT for residual pockets or in supportive periodontal therapy (SPT).
- Studies that have utilized light emitting diodes (LEDs) as a light source.
- No outcome variable of interest.
- Pregnancy.
- Smoking.
- Narrative and systematic reviews, in vitro studies, in vivo animal studies, commentaries, interviews, updates, case series and case reports.
2.7. Systematic Review Outcomes
2.7.1. Primary Outcome Measures
2.7.2. Secondary Outcome Measures
2.8. Data Extraction
2.9. Qualitative Analysis
2.10. Statistical Analysis of Data
3. Results
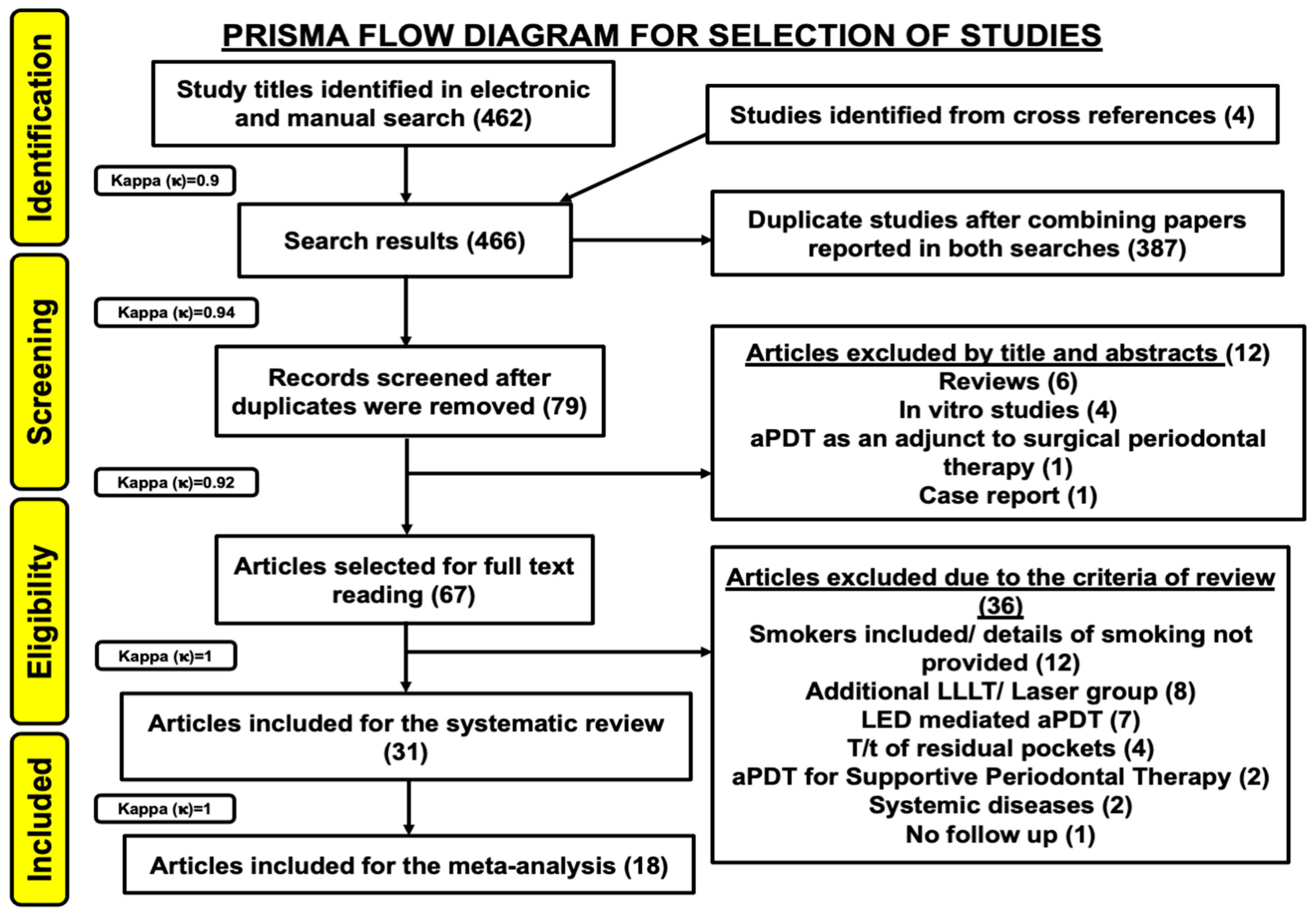
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Country of Origin
3.2.2. Study Design
3.2.3. Selection Criteria
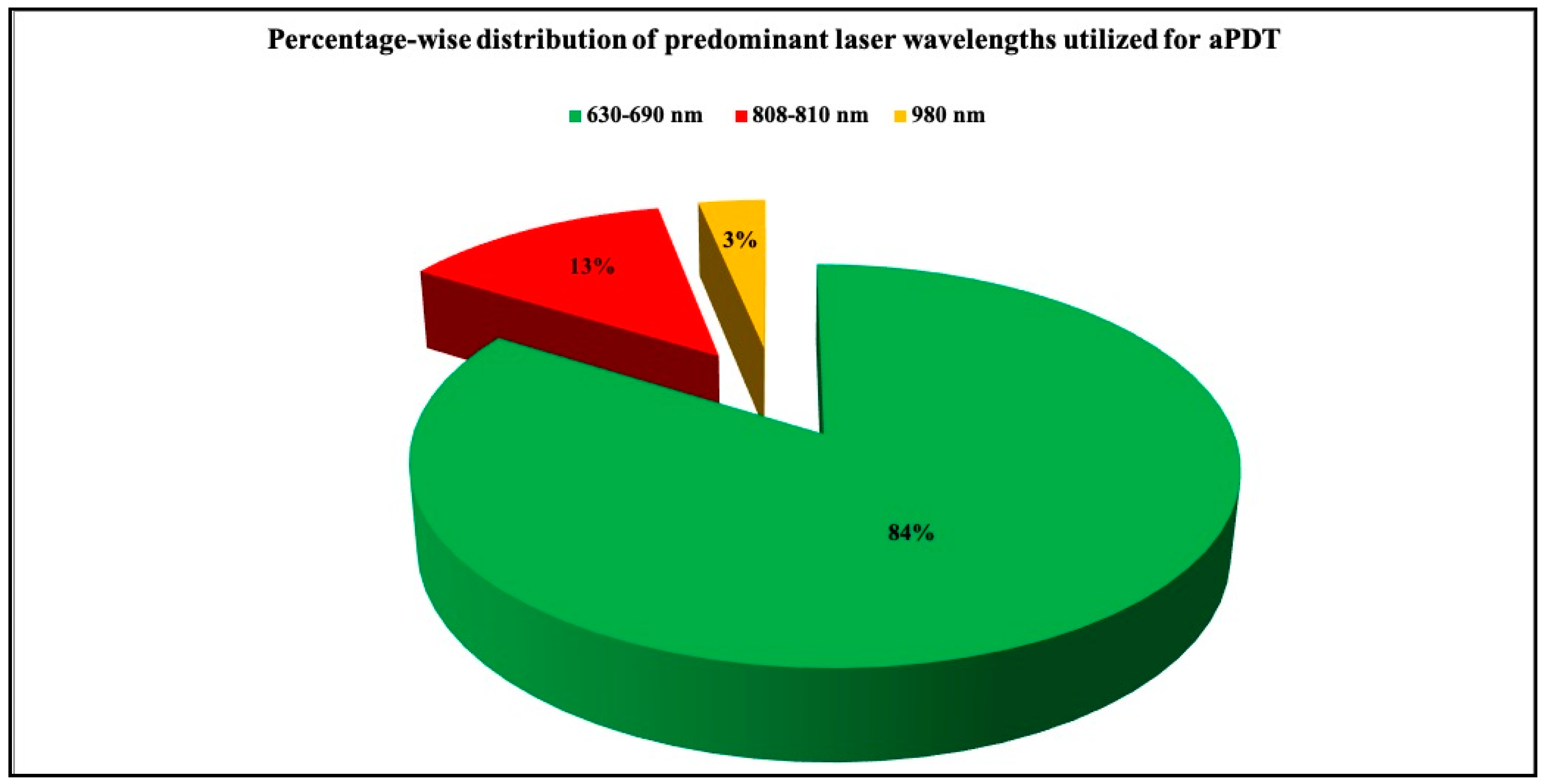
3.2.4. Documentation of Laser Parameters
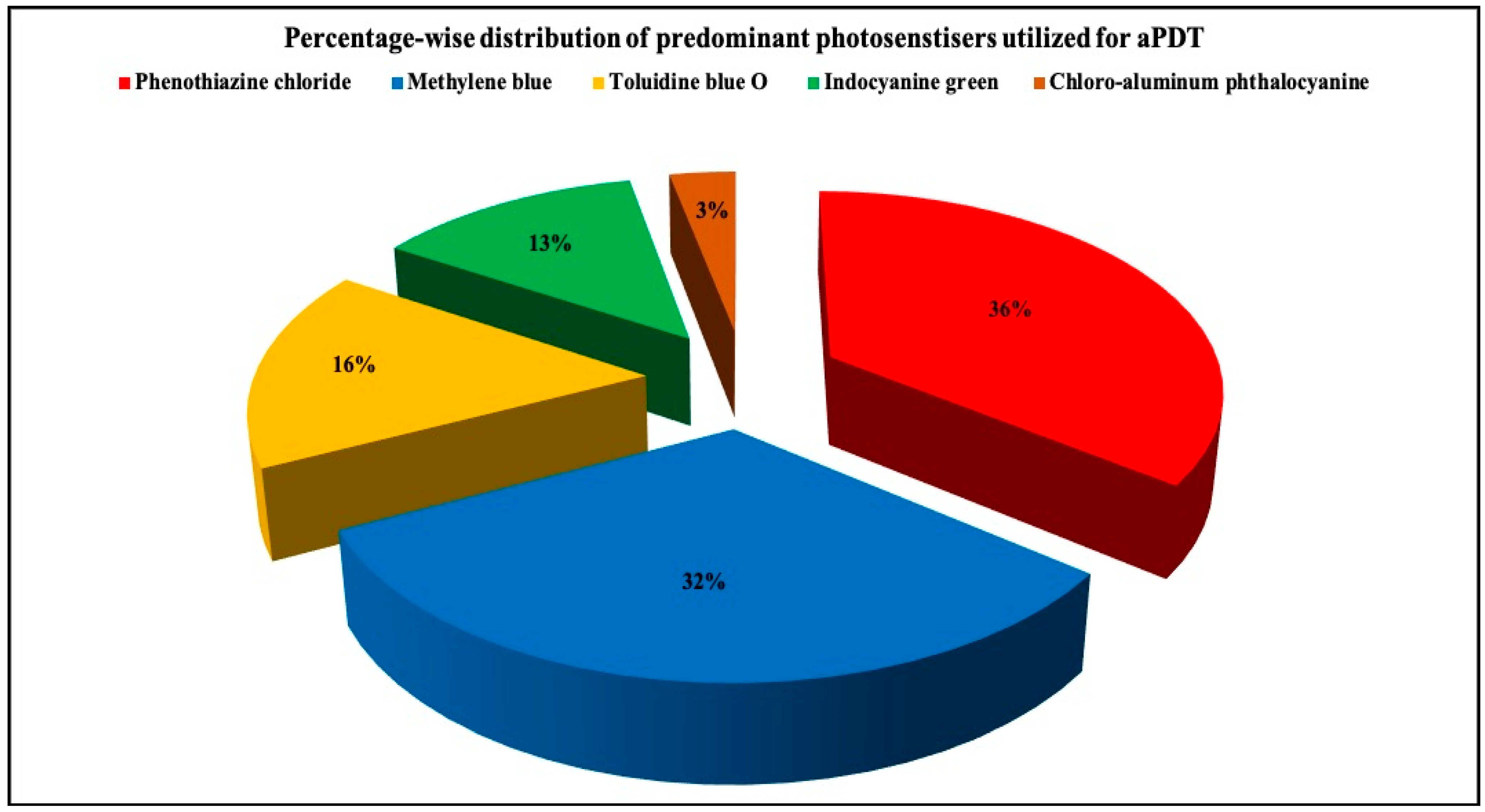
3.2.5. PS Utilized
3.2.6. Utilization of aPDT as a Mono-Therapeutic or an Adjunctive Therapeutic Agent
3.2.7. Comparison in between SRP+ aPDT versus SRP+AB
3.2.8. Number of aPDT Sessions
3.2.9. Follow-Up Assessment
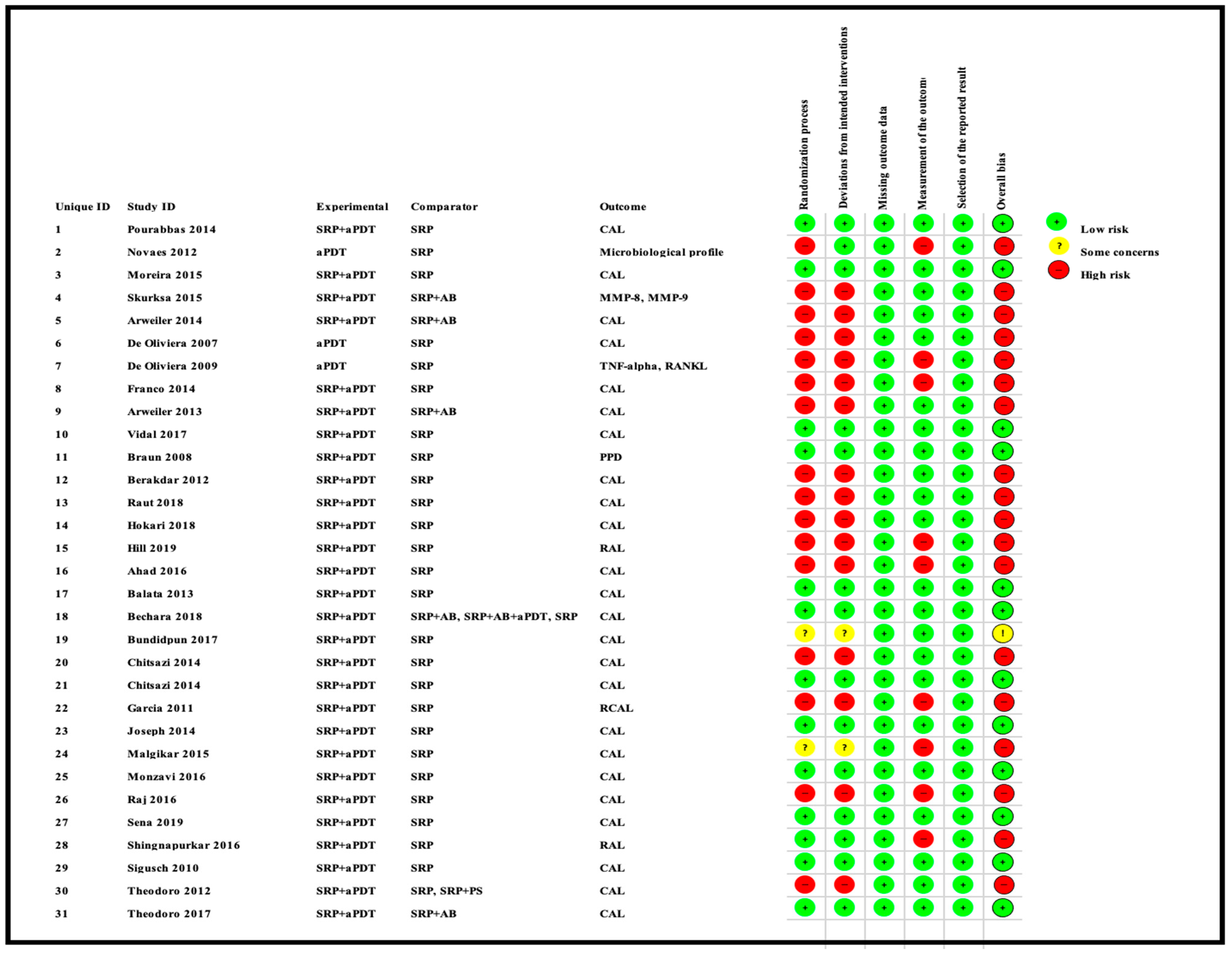
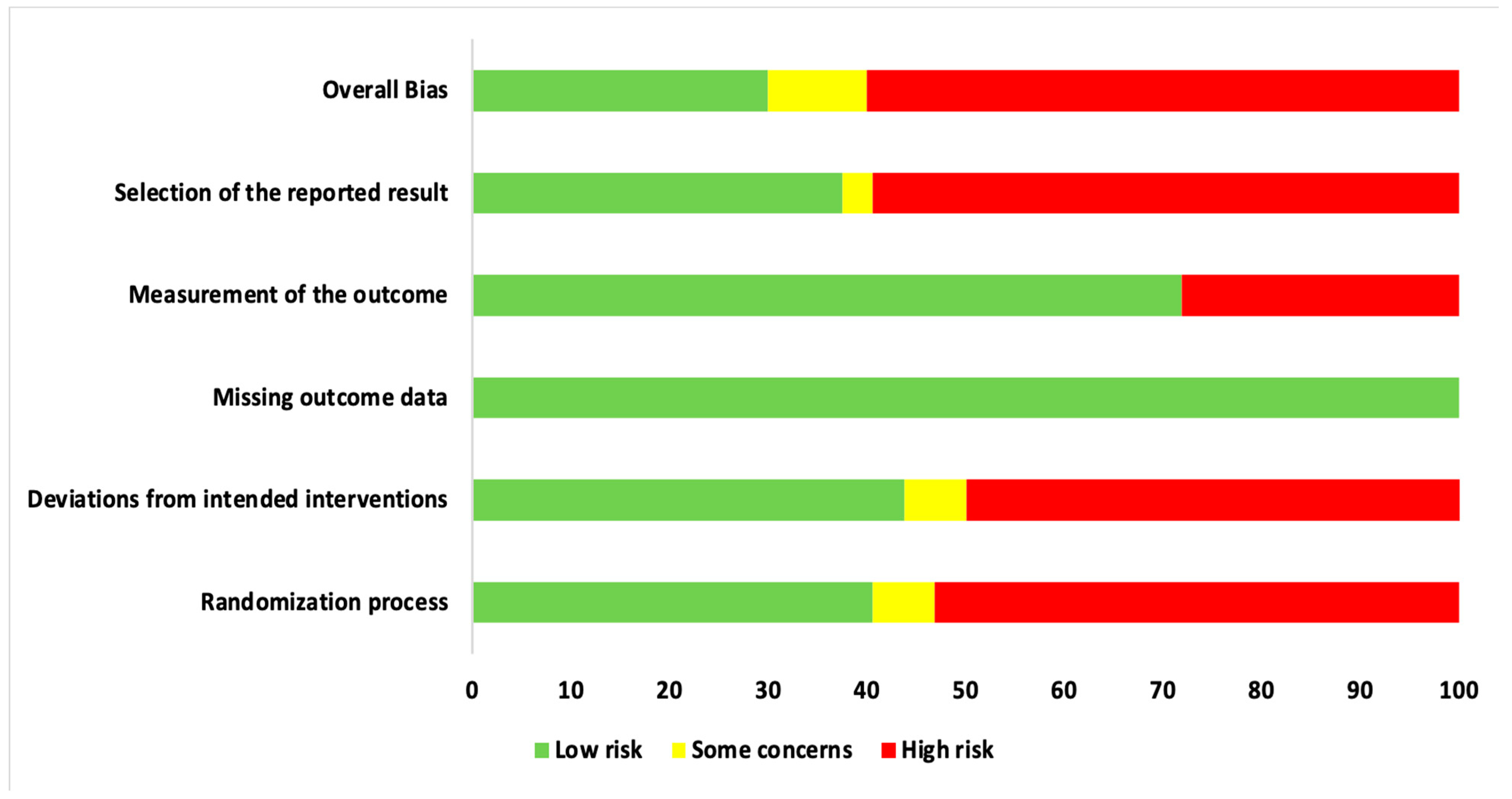
3.3. Qualitative Assessment
3.4. Quantitative Assessment
3.4.1. Outcome Variables
3.4.2. Sensitivity Analysis
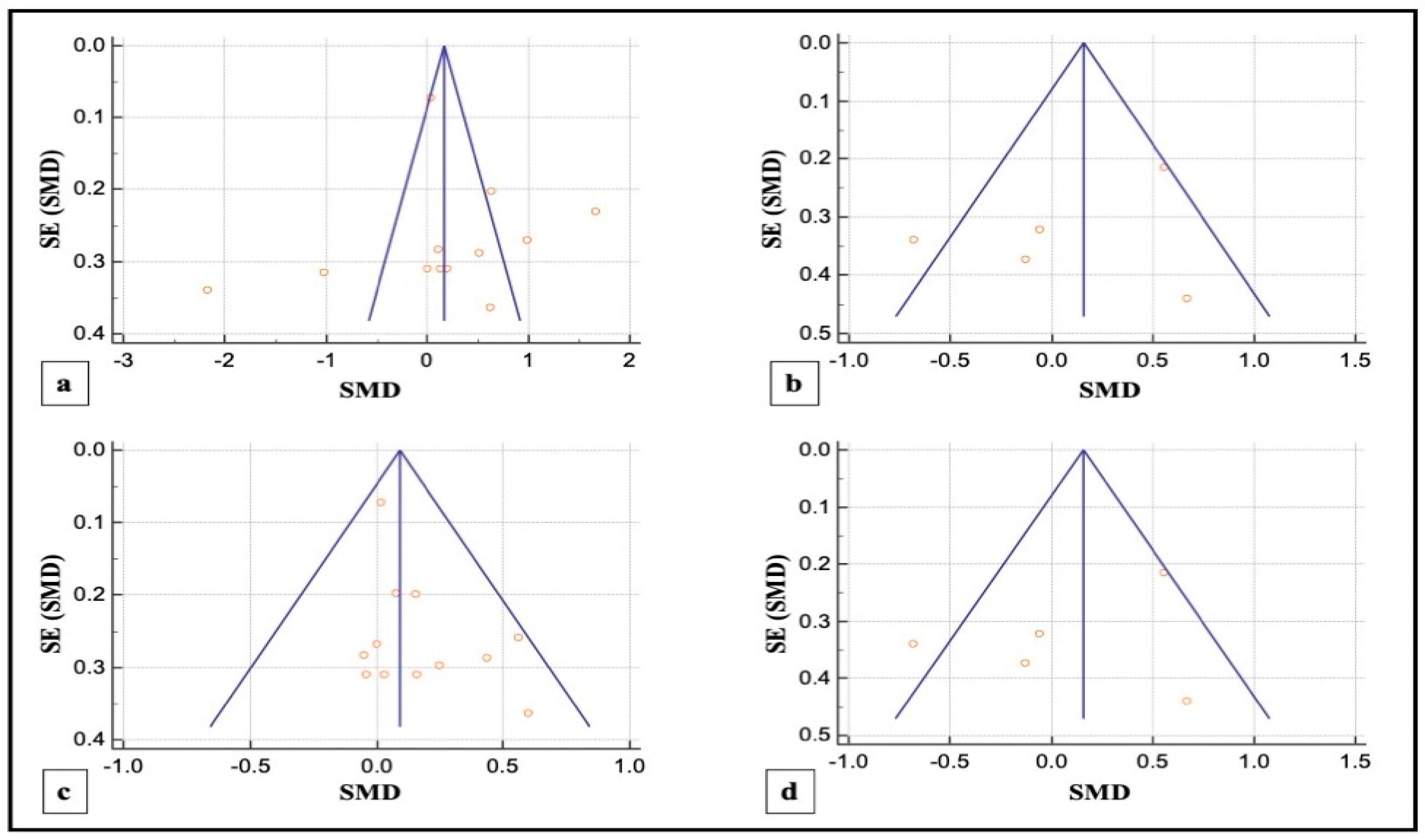
3.4.3. Publication Bias
4. Discussion
4.1. Role of Baseline Characteristics
4.2. Assessment Methods for Various Parameters and Their Inferences to Determine aPDT Efficacy
4.3. Representation of the Treatment Outcomes
4.4. Role of Laser Parameters
4.5. Role of RoB Assessment
4.6. Limitations of the Present Systematic Review
4.7. Future Scope
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rajesh, S.; Koshi, E.; Philip, K.; Mohan, A. Antimicrobial photodynamic therapy: An overview. J. Indian Soc. Periodontol. 2011, 15, 323–327. [Google Scholar] [CrossRef]
- De Oliveira, R.R.; Schwartz-Filho, H.O.; Novaes, A.B., Jr.; de Souza, R.F.; Taba, M., Jr.; Scombatti de Souza, S.L.; Ribeiro, F.J. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: Cytokine profile in gingival crevicular fluid, preliminary results. J. Periodontol. 2009, 80, 98–105. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.R.; Schwartz-Filho, H.O.; Novaes, A.B., Jr.; Taba, M., Jr. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: A preliminary randomized controlled clinical study. J. Periodontol. 2007, 78, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, U. What exactly distinguishes aggressive from chronic periodontitis: Is it mainly a difference in the degree of bacterial invasiveness? Periodontology 2000 2017, 75, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Novaes, A.B., Jr.; Schwartz-Filho, H.O.; de Oliveira, R.R.; Feres, M.; Sato, S.; Figueiredo, L.C. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: Microbiological profile. Lasers Med. Sci. 2012, 27, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A. Photodynamic therapy as an adjunctive treatment for chronic periodontitis: A meta-analysis. Lasers Med. Sci. 2010, 4, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Graziani, F.; Gatto, R.; Monaco, A. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: A systemic review and meta-analysis. J. Clin. Periodontol. 2013, 40, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Azaripour, A.; Dittrich, S.; Van Noorden, C.J.F.; Willershausen, C. Efficacy a photodynamic therapy as adjunct treatment of chronic periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2018, 33, 407–423. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Gatto, R.; Mazzo, G.; Monaco, A. Photodynamic therapy in the treatment of chronic periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2013, 28, 669–682. [Google Scholar] [CrossRef]
- Souza, E.; Medeiros, A.C.; Gurgel, B.C.; Sarmento, C. Antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2016, 31, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Azarpazhooh, A.; Shah, P.S.; Tenenbaum, H.C.; Goldberg, M.B. The effect of photodynamic therapy for periodontitis: A systematic review and meta-analysis. J. Periodontol. 2010, 81, 4–14. [Google Scholar] [CrossRef]
- Akram, Z.; Hyder, T.; Al-Hammoudi, N.; Binshabaib, M.S.; Alharthi, S.S.; Hanif, A. Efficacy of photodynamic therapy versus antibiotics as an adjunct to scaling and root planing in the treatment of periodontitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 19, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Akram, Z.; Safii, S.H.; Vaithilingam, R.D.; Ghanem, A.; Sergis, K.; Javed, F. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: A systematic review. Photodiagnosis Photodyn. Ther. 2016, 13, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulos, G.S.; Doufexi, A.E. Photodynamic therapy in the treatment of aggressive periodontitis: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e192–e200. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Reddy, C.; Krishna Kumar, R.V.S.; Sudhir, K.M.; Srinivasulu, G. Effect of photodynamic therapy on aggressive periodontitis—A systematic review. Int. J. Sci. Res. Manag. 2017, 5, 7177–7185. [Google Scholar]
- Pal, A.; Paul, S.; Perry, R.; Puryer, J. Is the use of antimicrobial photodynamic therapy or systemic antibiotics more effective in improving periodontal health when used in conjunction with localised non-surgical periodontal therapy? A systematic review. Dent. J. 2019, 7, 108. [Google Scholar] [CrossRef]
- Franco, E.J.; Pogue, R.E.; Sakamoto, L.H.; Cavalcante, L.L.; Carvalho, D.R.; de Andrade, R.V. Increased expression of genes after periodontal treatment with photodynamic therapy. Photodiagnosis Photodyn. Ther. 2014, 11, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, N.; Van Essche, M.; Pauwels, M.; Teughels, W.; Quirynen, M. Do periodontopathogens disappear after full-mouth tooth extraction? J. Clin. Periodontol. 2009, 36, 1043–1047. [Google Scholar] [CrossRef]
- Guerrero, A.; Griffiths, G.S.; Nibali, L.; Suvan, J.; Moles, D.R.; Laurell, L.; Tonetti, M.S. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2005, 32, 1096–1107. [Google Scholar] [CrossRef]
- Sigusch, B.; Beier, M.; Klinger, G.; Pfister, W.; Glockmann, E. A 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis. J. Periodontol. 2001, 72, 275–283. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Jepsen, S.; Needleman, I.; Roldán, S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J. Clin. Periodontol. 2002, 29, 136–162. [Google Scholar] [CrossRef]
- Pallasch, T.J. Antibiotic resistance. Dent. Clin. N. Am. 2003, 47, 623–639. [Google Scholar] [CrossRef]
- Andersen, R.C.; Loebel, N.G. Photodynamic disinfection in the treatment of chronic adult periodontitis: A multicenter clinical trial. J. Dent. Health Oral Disord. Ther. 2017, 8, 00289. [Google Scholar] [CrossRef][Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 The Cochrane Collaboration. 2011. Available online: http://www.cochrane-handbook.org (accessed on 12 February 2021).
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Inter-rate reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Schulz, K.F.; Moher, D.; Egger, M.; Davidoff, F.; Elbourne, D.; Gøtzsche, P.C.; Lang, T.; CONSORT GROUP (Consolidated Standards of Reporting Trials). The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann. Intern Med. 2001, 134, 663–694. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Eldridge, S.; Li, T. Chapter 23: Including variants on randomized trials. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; (updated July 2019); Cochrane: Oxford, UK, 2019; Available online: www.training.cochrane.org/handbook (accessed on 12 February 2021).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Lau, J.; Ioannidis, J.P.; Schmid, C.H. Quantitative synthesis in systematic reviews. Ann. Intern Med. 1997, 127, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Assem, N.Z.; Longo, M.; Alves, M.L.F.; Duque, C.; Stipp, R.N.; Vizoto, N.L.; Garcia, V.G. Treatment of periodontitis in smokers with multiple sessions of antimicrobial photodynamic therapy or systemic antibiotics: A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2018, 22, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, M.S.; Austah, O.N. Photodynamic therapy as an adjunctive to scaling and root planing in treatment of chronic periodontitis in smokers. Saudi Med. J. 2011, 32, 1183–1188. [Google Scholar] [PubMed]
- Tabenski, L.; Moder, D.; Cieplik, F.; Schenke, F.; Hiller, K.A.; Buchalla, W.; Schmalz, G.; Christgau, M. Antimicrobial photodynamic therapy vs. local minocycline in addition to non-surgical therapy of deep periodontal pockets: A controlled randomized clinical trial. Clin. Oral Investig. 2017, 21, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Shu, R.; Li, Y.; Li, C.; Luo, L.; Song, Z.; Xie, Y.; Liu, D. Adjunctive effect of photodynamic therapy to scaling and root planing in the treatment of chronic periodontitis. Photomed. Laser Surg. 2011, 29, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Loebel, N.G.; Galler, C.C.; Andersen, R.C. Antimicrobial photodynamic therapy in the treatment of chronic adult periodontitis. Oral Health Dental Sci. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Harmouche, L.; Courval, A.; Mathieu, A.; Peti, C.; Huck, O.; Severac, F.; Davideau, J.L. Impact of tooth-related factors on photodynamic therapy effectiveness during active periodontal therapy: A 6-months split-mouth randomized clinical trial. Photodiagnosis Photodyn. Ther. 2019, 27, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, N.; Nikolidakis, D.; Chondros, P.; Becker, J.; Schwarz, F.; Rossler, R.; Sculean, A. Photodynamic therapy as an adjunct to non-surgical periodontal treatment: A randomized, controlled clinical trial. J. Periodontol. 2008, 79, 1638–1644. [Google Scholar] [CrossRef]
- Polansky, R.; Haas, M.; Heschl, A.; Wimmer, G. Clinical effectiveness of photodynamic therapy in the treatment of periodontitis. J. Clin. Periodontol. 2009, 36, 575–580. [Google Scholar] [CrossRef]
- Badea, M.E.; Serbanescu, A.; Hedesiu, M.; Badea, A.F. Photodynamic Laser therapy in patients with periodontitis. TMJ 2010, 60, 18–22. [Google Scholar]
- Andersen, R.; Loebel, N.; Hammond, D.; Wilson, M. Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J. Clin. Dent. 2007, 18, 34–38. [Google Scholar]
- Alwaeli, H.A.; Al-Khateeb, S.N.; Al-Sadi, A. Long-term clinical effect of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. Lasers Med. Sci. 2015, 30, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Annaji, S.; Sarkar, I.; Rajan, P.; Pai, J.; Malagi, S.; Bharmappa, R.; Kamath, V. Efficacy of photodynamic therapy and lasers as an adjunct to scaling and root planing in the treatment of aggressive periodontitis—A clinical and microbiologic short term study. J. Clin. Diagn. Res. 2016, 10, ZC08–ZC12. [Google Scholar] [CrossRef] [PubMed]
- Dilsiz, A.; Canakci, V.; Aydin, T. Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2013, 84, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.; Corbet, E.F.; Jin, L. Combined photodynamic and low-level laser therapies as an adjunct to nonsurgical treatment of chronic periodontitis. J. Periodontal Res. 2011, 46, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Pereira, R.; Kini, V.; Padhye, A. Effect of combined therapy using diode laser and photodynamic therapy on levels of IL-17 in gingival crevicular fluid in patients with chronic periodontitis. J. Lasers Med. Sci. 2016, 7, 250–255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Srikanth, K.; Chandra, R.V.; Reddy, A.A.; Reddy, B.H.; Reddy, C.; Naveen, A. Effect of a single session of antimicrobial photodynamic therapy using indocyanine green in the treatment of chronic periodontitis: A randomized controlled pilot trial. Quintessence Int. 2015, 46, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Grzech-Leśniak, K.; Matys, J.; Dominiak, M. Comparison of the clinical and microbiological effects of antibiotic therapy in periodontal pockets following laser treatment: An in vivo study. Adv. Clin. Exp. Med. 2018, 27, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Kamma, J.J.; Vasdekis, V.G.; Romanos, G.E. The effect of diode laser (980 nm) treatment on aggressive periodontitis: Evaluation of microbial and clinical parameters. Photomed. Laser Surg. 2009, 27, 11–19. [Google Scholar] [CrossRef]
- Matarese, G.; Ramaglia, L.; Cicciù, M.; Cordasco, G.; Isola, G. The effects of diode laser therapy as an adjunct to scaling and root planing in the treatment of aggressive periodontitis: A 1-year randomized controlled clinical trial. Photomed. Laser Surg. 2017, 35, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Annaji, S.; Sarkar, I.; Rajan, P.; Pai, J.; Malagi, S.; Kamath, V.; Barmappa, R. Comparative evaluation of the efficacy of curcumin gel with and without photo activation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A split mouth clinical and microbiological study. J. Nat. Sci. Biol. Med. 2015, 6, S102–S109. [Google Scholar] [CrossRef]
- Borekci, T.; Meseli, S.E.; Noyan, U.; Kuru, B.E.; Kuru, L. Efficacy of adjunctive photodynamic therapy in the treatment of generalized aggressive periodontitis: A randomized controlled clinical trial. Lasers Surg. Med. 2019, 51, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Pulikkotil, S.J.; Toh, C.G.; Mohandas, K.; Leong, K. Effect of photodynamic therapy adjunct to scaling and root planing in periodontitis patients: A randomized clinical trial. Aust. Dent. J. 2016, 61, 440–445. [Google Scholar] [CrossRef]
- Saitawee, D.; Teerakapong, A.; Morales, N.P.; Jitprasertwong, P.; Hormdee, D. Photodynamic therapy of Curcuma longa extract stimulated with blue light against Aggregatibacter actinomycetemcomitans. Photodiagnosis Photodyn. Ther. 2018, 22, 101–105. [Google Scholar] [CrossRef]
- Bassir, S.H.; Moslemi, N.; Jamali, R.; Mashmouly, S.; Fekrazad, R.; Chiniforush, N.; Shamshiri, A.R.; Nowzari, H. Photoactivated disinfection using light-emitting diode as an adjunct in the management of chronic periodontitis: A pilot double-blind split-mouth randomized clinical trial. J. Clin. Periodontol. 2013, 40, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Husejnagic, S.; Lettner, S.; Laky, M.; Georgopoulos, A.; Moritz, A.; Rausch-Fan, X. Photoactivated disinfection in periodontal treatment: A randomized controlled clinical split-mouth trial. J. Periodontol. 2019, 90, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Maria, A.M.; Ursarescu, I.G.; Solomon, S.; Foia, L. Evaluation the effects of led photo-activated disinfection on periodontal clinical parameters in patients with chronic periodontitis. Balk. J. Dent. Med. 2016, 20, 29–32. [Google Scholar] [CrossRef][Green Version]
- Campos, G.N.; Pimentel, S.P.; Ribeiro, F.V.; Casarin, R.C.; Cirano, F.R.; Saraceni, C.H.; Casati, M.Z. The adjunctive effect of photodynamic therapy for residual pockets in single- rooted teeth: A randomized controlled clinical trial. Lasers Med. Sci. 2013, 28, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, M.G.; Oliveira, D.H.; Saraceni, C.H.; Ribeiro, F.V.; Pimentel, S.P.; Cirano, F.R.; Casarin, R.C. Short-term microbiological effects of photodynamic therapy in non-surgical periodontal treatment of residual pockets: A split-mouth RCT. Lasers Surg. Med. 2016, 48, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Petelin, M.; Perkič, K.; Seme, K.; Gašpirc, B. Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis. Lasers Med. Sci. 2015, 30, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Cappuyns, I.; Cionca, N.; Wick, P.; Giannopoulou, C.; Mombelli, A. Treatment of residual pockets with photodynamic therapy, diode laser, or deep scaling. A randomized, split-mouth controlled clinical trial. Lasers Med. Sci. 2012, 27, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Lulic, M.; Leiggener, G.I.; Salvi, G.E.; Ramseier, C.A.; Mattheos, N.; Lang, N.P. One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: A proof-of-principle randomized-controlled clinical trial. J. Clin. Periodontol. 2009, 36, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Rühling, A.; Fanghänel, J.; Houshmand, M.; Kuhr, A.; Meisel, P.; Schwahn, C.; Kocher, T. Photodynamic therapy of persistent pockets in maintenance patients-A clinical study. Clin. Oral Investig. 2010, 14, 637–644. [Google Scholar] [CrossRef]
- Barbosa, F.I.; Araújo, P.V.; Machado, L.J.C.; Magalhães, C.S.; Guimarães, M.M.; Moreira, A.N. Effect of photodynamic therapy as an adjuvant to non-surgical periodontal therapy: Periodontal and metabolic evaluation in patients with type 2 diabetes mellitus. Photodiagnosis Photodyn. Ther. 2018, 22, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ramos, U.D.; Ayub, L.G.; Reino, D.M.; Grisi, M.F.; Taba, M., Jr.; Souza, S.L.; Palioto, D.B.; Novaes, A.B., Jr. Antimicrobial photodynamic therapy as an alternative to systemic antibiotics: Results from a double-blind, randomized, placebo-controlled, clinical study on type 2 diabetics. J. Clin. Periodontol. 2016, 43, 147–155. [Google Scholar] [CrossRef]
- Alvarenga, L.H.; Gomes, A.C.; Carribeiro, P.; Godoy-Miranda, B.; Noschese, G.; Simões Ribeiro, M.; Kato, I.T.; Bussadori, S.K.; Pavani, C.; Geraldo, Y.G.; et al. Parameters for antimicrobial photodynamic therapy on periodontal pocket- Randomized clinical trial. Photodiagnosis Photodyn. Ther. 2019, 27, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Pourabbas, R.; Kashefimehr, A.; Rahmanpour, N.; Babaloo, Z.; Kishen, A.; Tenenbaum, H.C.; Azarpazhooh, A. Effects of photodynamic therapy on clinical and gingival crevicular fluid inflammatory biomarkers in chronic periodontitis: A split-mouth randomized clinical trial. J. Periodontol. 2014, 85, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.; Novaes, A.B., Jr.; Grisi, M.F.; Taba, M., Jr.; Souza, S.L.; Palioto, D.B.; de Oliveira, P.G.; Casati, M.Z.; Casarin, R.C.; Messora, M.R. Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: A split-mouth randomized controlled trial. J. Periodontol. 2015, 86, 376–386. [Google Scholar] [CrossRef]
- Skurska, A.; Dolinska, E.; Pietruska, M.; Pietruski, J.K.; Dymicka, V.; Kemona, H.; Arweiler, N.B.; Milewsk, R.; Sculean, A. Effect of nonsurgical periodontal treatment in conjunction with either systemic administration of amoxicillin and metronidazole or additional photodynamic therapy on the concentration of matrix metalloproteinases 8 and 9 in gingival crevicular fluid in patients with aggressive periodontitis. BMC Oral Health 2015, 15, 63. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Pietruska, M.; Pietruski, J.; Skurska, A.; Dolińsk, E.; Heumann, C.; Auschill, T.M.; Sculean, A. Six-month results following treatment of aggressive periodontitis with antimicrobial photodynamic therapy or amoxicillin and metronidazole. Clin. Oral Investig. 2014, 18, 2129–2135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arweiler, N.B.; Pietruska, M.; Skurska, A.; Dolińska, E.; Pietruski, J.K.; Bläs, M.; Auschill, T.M.; Sculean, A. Nonsurgical treatment of aggressive periodontitis with photodynamic therapy or systemic antibiotics. Three-month results of a randomized, prospective, controlled clinical study. Schweiz. Monatsschr. Zahnmed. 2013, 123, 532–544. [Google Scholar] [PubMed]
- Segarra-Vidal, M.; Guerra-Ojeda, S.; Vallés, L.S.; López-Roldán, A.; Mauricio, M.D.; Aldasoro, M.; Alpiste-Illueca, F.; Vila, J.M. Effects of photodynamic therapy in periodontal treatment: A randomized, controlled clinical trial. J. Clin. Periodontol. 2017, 44, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Dehn, C.; Krause, F.; Jepsen, S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. J. Clin. Periodontol. 2008, 35, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Berakdar, M.; Callaway, A.; Eddin, M.F.; Ross, A.; Willershausen, B. Comparison between scaling-root-planing (SRP) and SRP/Photodynamic therapy: Six-month study. Head Face Med. 2012, 8, 12. [Google Scholar] [CrossRef]
- Raut, C.P.; Sethi, K.S.; Kohale, B.R.; Mamajiwala, A.; Warang, A. Indocyanine green- mediated photothermal therapy in treatment of chronic periodontitis: A clinico- microbiological study. J. Indian Soc. Periodontol. 2018, 22, 221–227. [Google Scholar] [CrossRef]
- Hokari, T.; Morozumi, T.; Komatsu, Y.; Shimizu, T.; Yoshino, T.; Tanaka, M.; Tanaka, Y.; Nohno, K.; Kubota, T.; Yoshie, H. Effects of antimicrobial photodynamic therapy and local administration of minocycline on clinical, microbiological, and inflammatory markers of periodontal pockets: A Pilot Study. Int. J. Dent. 2018, 1748584. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.; Dehn, C.; Hinze, A.V.; Frentzen, M.; Meister, J. Indocyanine green-based adjunctive antimicrobial photodynamic therapy for treating chronic periodontitis: A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2019, 26, 29–35. [Google Scholar] [CrossRef]
- Ahad, A.; Lamba, A.K.; Faraz, F.; Tandon, S.; Chawla, K.; Yadav, N. Effect of antimicrobial photodynamic therapy as an adjunct to nonsurgical treatment of deep periodontal pockets: A clinical study. J. Lasers Med. Sci. 2016, 7, 220–226. [Google Scholar] [CrossRef]
- Balata, M.L.; Andrade, L.P.; Santos, D.B.; Cavalcanti, A.N.; Tunes Uda, R.; Ribeiro Édel, P.; Bittencourt, S. Photodynamic therapy associated with full-mouth ultrasonic debridement in the treatment of severe chronic periodontitis: A randomized-controlled clinical trial. J. Appl. Oral Sci. 2013, 21, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Bechara Andere, N.M.R.; Dos Santos, N.C.C.; Araujo, C.F.; Mathias, I.F.; Rossato, A.; de Marco, A.C.; Santamaria, M., Jr.; Jardini, M.A.N.; Santamaria, M.P. Evaluation of the local effect of nonsurgical periodontal treatment with and without systemic antibiotic and photodynamic therapy in generalized aggressive periodontitis. A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2018, 24, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bundidpun, P.; Srisuwantha, R.; Laosrisin, N. Clinical effects of photodynamic therapy as an adjunct to full-mouth ultrasonic scaling and root planing in treatment of chronic periodontitis. Laser Ther. 2018, 27, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Chitsazi, M.T.; Shirmohammadi, A.; Pourabbas, R.; Abolfazli, N.; Farhoudi, I.; Azar, B.D.; Farhadi, F. Clinical and microbiological effects of photodynamic therapy associated with non- surgical treatment in aggressive periodontitis. J. Dent. Res. Dent. Clin. Dent. Prospects 2014, 8, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chitsazi, M.T.; Kashefimehr, A.; Pourabbas, R.; Shirmohammadi, A.; Ghasemi Barghi, V.; Daghigh Azar, B. Efficacy of subgingival application of xanthan-based chlorhexidine gel adjunctive to full-mouth root planing assessed by real-time PCR: A Microbiologic and Clinical Study. J. Dent. Res. Dent. Clin. Dent. Prospects 2013, 7, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.B.; Dias, A.T.; Tinoco, B.E.M.; Fischeer, R.G. Evaluation of the efficacy of photodynamic therapy as adjunct to periodontal treatment in patients with aggressive periodontitis. Rev. Periodontia 2011, 21, 12–19. [Google Scholar]
- Betsy, J.; Prasanth, C.S.; Baiju, K.V.; Prasanthila, J.; Subhash, N. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: A randomized controlled clinical trial. J. Clin. Periodontol. 2014, 4, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Malgikar, S.; Reddy, S.H.; Babu, P.R.; Sagar, S.V.; Kumar, P.S.; Reddy, G.J. A randomized controlled clinical trial on efficacy of photodynamic therapy as an adjunct to nonsurgical treatment of chronic periodontitis. J. Dent. Lasers 2015, 9, 75–79. [Google Scholar] [CrossRef]
- Monzavi, A.; Chinipardaz, Z.; Mousavi, M.; Fekrazad, R.; Moslemi, N.; Azaripour, A.; Bagherpasand, O.; Chiniforush, N. Antimicrobial photodynamic therapy using diode laser activated indocyanine green as an adjunct in the treatment of chronic periodontitis: A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2016, 14, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.R.; Musalaiah, S.; Nagasri, M.; Kumar, P.A.; Reddy, P.I.; Greeshma, M. Evaluation of efficacy of photodynamic therapy as an adjunct to nonsurgical periodontal therapy in treatment of chronic periodontitis patients: A clinico-microbiological study. Indian J. Dent. Res. 2016, 27, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Sena, I.A.A.; Silva, D.N.A.; Azevedo, M.L.D.S.; da Silva, N.T.; Longo, J.P.F.; de Moraes, M.; de Aquino Martins, A.R.L. Antimicrobial photodynamic therapy using a chloro-aluminum phthalocyanine adjuvant to nonsurgical periodontal treatment does not improve clinical parameters in patients with chronic periodontitis. Photobiomodul. Photomed. Laser Surg. 2019, 37, 729–735. [Google Scholar] [CrossRef]
- Shingnapurkar, S.H.; Mitra, D.K.; Kadav, M.S.; Shah, R.A.; Rodrigues, S.V.; Prithyani, S.S. The effect of indocyanine green-mediated photodynamic therapy as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A comparative split-mouth randomized clinical trial. Indian J. Dent. Res. 2016, 27, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Sigusch, B.W.; Engelbrecht, M.; Völpel, A.; Holletschke, A.; Pfister, W.; Schütze, J. Full-mouth antimicrobial photodynamic therapy in Fusobacterium nucleatum-infected periodontitis patients. J. Periodontol. 2010, 81, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Theodoro, L.H.; Silva, S.P.; Pires, J.R.; Soares, G.H.G.; Pontes, A.E.F.; Zuza, E.P.; Spolidório, D.M.; de Toledo, B.E.; Garcia, V.G. Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment: A 6-month follow-up. Lasers Med. Sci. 2012, 27, 687–693. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Lopes, A.B.; Nuernberg, M.A.A.; Cláudio, M.M.; Miessi, D.M.J.; Alves, M.L.F.; Duque, C.; Mombelli, A.; Garcia, V.G. Comparison of repeated applications of aPDT with amoxicillin and metronidazole in the treatment of chronic periodontitis: A short-term study. J. Photochem. Photobiol. B 2017, 174, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Festic, E.; Rawal, B.; Gajic, O. How to improve assessment of balance in baseline characteristics of clinical trial participants-example from PROSEVA trial data? Ann. Transl. Med. 2016, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Tripepi, G.; Jager, K.J.; Dekker, F.W.; Zoccali, C. Selection bias and information bias in clinical research. Nephron. Clin. Pract. 2010, 115, c94–c99. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.K.; Hill, M. Cigarette smoking and the periodontal patient. J. Periodontol. 2004, 75, 196–209. [Google Scholar] [CrossRef]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef]
- Arigbede, A.O.; Babatope, B.O.; Bamidele, M.K. Periodontitis and systemic diseases: A literature review. J. Indian Soc. Periodontol. 2012, 16, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Komine-Aizawa, S.; Aizawa, S.; Hayakawa, S. Periodontal diseases and adverse pregnancy outcomes. J. Obstet. 2019, 45, 5–12. [Google Scholar] [CrossRef]
- Blasini, M.; Movsas, S.; Colloca, L. Placebo hypoalgesic effects in pain: Potential applications in dental and orofacial pain management. Semin. Orthod. 2018, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Smaïl-Faugeron, V.; Fron-Chabouis, H.; Courson, F.; Durieux, P. Erratum to: Comparison of intervention effects in split-mouth and parallel-arm randomized controlled trials: A meta-epidemiological study. BMC Med. Res. Methodol. 2015, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Joss, A.; Orsanic, T.; Gusberti, F.A.; Siegrist, B.E. Bleeding on probing. A predictor for the progression of periodontal disease? J. Clin. Periodontol. 1986, 13, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, G. Contemporary interpretation of probing depth assessments: Diagnostic and therapeutic implications. A literature review. J. Periodontol. 1997, 68, 1194–1205. [Google Scholar] [CrossRef]
- Ramfjord, S.P.; Caffesse, R.G.; Morrison, E.C.; Hill, R.W.; Kerry, G.J.; Appleberry, E.A.; Nissle, R.R.; Stults, D.L. 4 modalities of periodontal treatment compared over 5 years. J. Clin. Periodontol. 1987, 14, 445–452. [Google Scholar] [CrossRef]
- Kaldahl, W.B.; Kalkwarf, K.L.; Patil, K.D.; Molvar, M.P.; Dyer, J.K. Long-term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities. J. Periodontol. 1996, 67, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mailoa, J.; Lin, G.H.; Khoshkam, V.; MacEachern, M.; Chan, H.L.; Wang, H.L. Long-term effect of four surgical periodontal therapies and one non-surgical therapy: A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 1150–1158. [Google Scholar] [CrossRef]
- Singh, S.; Uppoor, A.; Nayak, D. A comparative evaluation of the efficacy of manual, magnetostrictive and piezoelectric ultrasonic instruments- An in vitro profilometric and SEM study. J. Appl. Oral Sci. 2012, 20, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Needleman, I. Endpoints of active periodontal therapy. J. Clin. Periodontol. 2020, 47, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Manresa, C.; Sanz-Miralles, E.C.; Twigg, J.; Bravo, M. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst. Rev. 2018, 1, CD009376. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, H.J.; Zhang, Y.; Li, X.E.; Jia, Y.R.; Wang, C.M. Prediction of loss to follow-up in long-term supportive periodontal therapy in patients with chronic periodontitis. PLoS ONE 2018, 13, e0192221. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Westfelt, E.; Nyman, S.; Socransky, S.S.; Haffajee, A.D. Long-term effect of surgical/non-surgical treatment of periodontal disease. J. Clin. Periodontol. 1984, 11, 448–458. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [PubMed]
- Meisel, P.; Kocher, T. Photodynamic therapy for periodontal diseases: State of the art. J. Photochem. Photobiol. B 2005, 79, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, L.; Ramaglia, L.; Gulletta, E.; Iacono, V. Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J. Periodontol. 1990, 61, 579–584. [Google Scholar] [CrossRef]
- Carrera, E.T.; Dias, H.B.; Corti, S.C.T.; Marcantonio, R.A.C.; Bernardi, A.C.A.; Bagnato, V.S. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: A critical review. Laser Phys. 2016, 26, 1–23. [Google Scholar] [CrossRef]
- Fumes, A.C.; Romualdo, P.C.; Monteiro, R.M.; Watanabe, E.; Corona, S.A.M.; Borsatto, M.C. Influence of pre-irradiation time employed in antimicrobial photodynamic therapy with diode laser. Lasers Med. Sci. 2018, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
| Study, Year, Origin and Citation | Journal Name/ Impact Factor (IF) | Study Design | Type of Periodontitis | Sample Size (n) | Gender M/F | Age (Years) (Mean ± SD) | Intervention Groups | Evaluation Period | Parameters Assessed | Conclusions | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Oliveira et al., 2009 (Brazil) [2] | Journal of Periodontology IF 2020: 3.742 IF 2009: 2.580 | SM-RCT | AgP (A minimum of 20 teeth (mean, 26 teeth) with at least one tooth in each posterior sextant and at least one posterior sextant with a minimum of three natural teeth; ≥5 mm of attachment loss around at least seven teeth involved, excluding first molars and central incisors) | 10 | 2/8 | 18–35 Mean: 31.01 ± 4.43 | SRP (Hand instruments) (10 teeth) | aPDT (10 teeth) | −7 (baseline), 0 (immediately after interventions), +1, +7, +30 and +90 days. | TNF-α and RANKL assessment | NSPT with PDT or SRP led to statistically significant reductions in TNF-a level 30 days following treatment (p < 0.05) with no statistically significant intergroup differences (p > 0.5). | ||
| De Oliveira et al., 2007 (Brazil) [3] | Journal of Periodontology IF 2020: 3.742 IF 2007: 2.426 | SM-RCT | AgP (A minimum of 20 teeth (mean, 26 teeth) with at least one tooth in each posterior sextant and at least one posterior sextant with a minimum of three natural teeth; ≥5 mm of attachment loss around at least seven teeth involved, excluding first molars and central incisors) | 10 | 2/8 | 18–35 Mean: 31.01 ± 4.43 | SRP (Hand instruments) (10 teeth) | aPDT (10 teeth) | Baseline, 3 months | PD, RCAL, GR, PI, GI, BOP | PDT and SRP showed statistically significant clinical results (p < 0.05) in the non-surgical treatment of aggressive periodontitis with no statistically significant differences (p > 0.5) in intergroup comparison. | ||
| Novaes et al., 2012 (Brazil) [5] | Lasers in Medical Science IF 2019: 2.574 IF 2012: 2.645 | SM-RCT | AgP (A minimum of 20 teeth (mean, 26 teeth) with at least one tooth in each posterior sextant, and at least one posterior sextant with a minimum of three natural teeth; ≥5 mm of attachment loss around at least seven teeth involved, excluding first molars and central incisors) | 10 | 2/8 | 18–35 Mean: 31 | SRP (Hand instruments) | aPDT | −7, 0 (Baseline), and 3 months | Plaque sample analysis for estimation of 40 subgingival species using DNA-DNA hybridization. | aPDT was more effective in reducing the counts of A.a (p = 0.00) whereas, SRP reduced red complex bacteria. Combination of both treatment methods would be beneficial for the non-surgical treatment of AgP | ||
| Franco et al., 2014 (Brazil) [17] | Photodiagnosis and Photodynamic Therapy IF 2020: 2.894 IF 2014: 2.359 | SM-RCT | CP (At least 20 teeth with at least one posterior tooth in each quadrant, and periodontal pockets ≥ 5 mm on at least seven teeth) | 15 | NI | 39.5 | SRP (Hand instruments) | SRP+aPDT | Baseline and 90 days | BOP, PI, PD, CAL, qPCR gene expression analysis. | Significant improvement in BOP was noted with aPDT group (p = 0.03). PDT increased the expression of RANK and OPG, which could indicate a reduction in osteoclastogenesis. Furthermore, the use of PDT in conjunction with conventional treatment significantly increased the expression of FGF2, which has an important role in the periodontal repair process. | ||
| Pourabbas et al., 2014 (Iran) [70] | Journal of Periodontology IF 2020: 3.742 IF 2014: 2.900 | SM-RCT | CP (≥12 natural teeth with a minimum of three in each quadrant; ≥3 mm attachment loss in about a minimum of 30% of the existing teeth; ≥1 site per quadrant with PPD of ≥4 mm and BOP) | 24 | 10/14 | 46 ± 8 | SRP (Sonic and hand instruments) | SRP+aPDT | Baseline and 3 months | PD, BOP, CAL, GR, IL-1β, TNF-α, MMP-8 and MMP-9 analysis | Intragroup comparison showed significant improvements (p < 0.001) for all variables in 3-month follow-up compared with baseline. TNF-α was significantly improved in the SRP+aPDT versus SRP group (p < 0.001). Total levels of PMNs were reduced for all patients compared with baseline levels (p < 0.001). | ||
| Moreira et al., 2015 (Brazil) [71] | Journal of Periodontology IF 2020: 3.742 IF 2015: 3.159 | SM-RCT | AgP (A minimum of 20 teeth and two pairs of single rooted contralateral teeth with proximal sites presenting PD and CAL ≥ 5 mm) | 20 | 2/18 | 18–35 30.6 ± 4.25 | SRP + sham procedure (Hand and ultrasonic instruments) 40 teeth/128 sites | SRP+aPDT 40 teeth/135 sites | Baseline,3 months | PD, CAL, GR, PI, BOP Microbiological analysis for counts of 40 bacterial species using DNA- DNA Hybridization Immunological evaluation for GCF levels of IL-1β, IL- 10 and TNF-α. | In deep periodontal pockets analysis (PD ≥ 7 mm at baseline), Test Group presented a decrease in PD and a clinical attachment gain significantly higher than Control Group at 90 days (p < 0.05). Test Group also demonstrated significantly less periodontal pathogens of red and orange complexes and a lower ratio IL-1β/IL-10 than Control Group (p < 0.05). Four adjunctive sessions of aPDT after SRP have clinical, microbiological and immunological benefits over SRP alone in management of AgP. | ||
| Skurska et al., 2015 (Poland) [72] | BMC Oral Health IF 2019: 1.911 IF 2015: 1.605 | PG-RCT | AgP (At least 3 sites with PD ≥ 6 mm) | 35 SRP+AB: 17 SRP+aPDT:18 | 12/24 SRP+aPDT: 7/10 SRP+AB: 5/13 | 23–55 SRP+aPDT: 37.3 ± 8.0 SRP+AB: 34.7 ± 9.0 | SRP+ AB 141 sites AB: 375 mg of amoxicillin + 250 mg of metronidazole TDS for 7 days, starting on the day of SRP (Hand and ultrasonic instruments) | SRP+aPDT 137 sites | Baseline, 3 and 6 months | MMP-8 and MMP-9 assessment | In the AB group, patients showed a statistically significant (p = 0.01) decrease of MMP-8 GCF level at both 3- and 6-months post treatment. In the PDT group, the change of MMP-8 GCF level was not statistically significant. Both groups showed at 3 and 6 months a decrease in MMP-9 levels. However, this change did not reach statistical significance. SRP+AB is more effective in reducing GCF MMP-8 levels compared to SRP+aPDT. | ||
| Arweiler et al., 2014 (Poland) [73] | Clinical Oral Investigations IF 2019: 2.903 IF 2014: 2.704 | PG-RCT | AgP (At least 3 sites with PD ≥ 6 mm) | 35 SRP+aPDT: 17 SRP+AB: 18 | 12/24 SRP+aPDT: 7/10 SRP+AB: 5/13 | 23–55 SRP+aPDT: 37.3 ± 8.0 SRP+AB: 34.7 ± 9.0 | SRP+AB 141 sites AB: 375 mg Amoxicillin + 250 mg Metronidazole TDS for 7 days (starting from day of SRP) (Hand and ultrasonic instruments) | SRP+aPDT 137 sites | Baseline, 6 months | PD, CAL, GR, PI, BOP, FMPI, FMBOP | Intragroup comparison revealed statistically significant PD reduction from baseline (p < 0.001). SRP+AB showed significant differences in PD reduction and lower number of deep pockets ≥ 7 mm (p < 0.001) as compared to SRP+aPDT (p = 0.03). | ||
| Arweiler et al., 2013 (Poland) [74] | Schweiz Monatsschr Zahnmed IF 2020: NA IF 2013: NA | PG-RCT | AgP (At least 3 sites with PD ≥ 6 mm) | 35 SRP+aPDT: 17 SRP+AB: 18 | 12/24 SRP+aPDT: 7/10 SRP+AB: 5/13 | 23–55 SRP+aPDT: 37.3 ± 8.0 SRP+AB: 34.7 ± 9.0 | SRP+AB 141 sites AB: 375mg Amoxicillin+250 mg MTZ TDS for 7 days (starting from day of SRP) (Hand and ultrasonic instruments) | SRP+aPDT 137 sites | Baseline, 3 months | PD, CAL, GR, PI, BOP, FMPI, FMBOP | SRP+AB showed significant differences in PD reduction, CAL gain and lower number of deep pockets ≥ 7 mm as compared to SRP+aPDT (p < 0.001). | ||
| Vidal et al., 2017 (Spain) [75] | Journal of Clinical Periodontology IF 2020: 5.241 IF 2017: 4.165 | PG-RCT | CP (Four or more periodontal pockets with a PPD ≥ 5 mm and BOP) | 37 | 11/26 | 55 ± 2 | SRP (Hand and ultrasonic instruments) | SRP+aPDT | Baseline, 5, 13 and 25 weeks | PI, PD, GR, CAL, BOP, GCF volume, microbiological and biochemical parameters | RANKL and abundance of A.a was significantly decreased in the SRP+aPDT group compared with the SRP group (p < 0.05). Except of a reduction in A.a, SRP+ aPDT resulted in no additional improvement compared with SRP alone. | ||
| Braun et al., 2008 (Germany) [76] | Journal of Clinical Periodontology IF 2020: 5.241 IF 2008: 3.525 | SM-RCT | CP (At least one premolar and one molar in every quadrant with a minimum of four teeth each; at least one tooth with an attachment loss of >3 mm in every quadrant) | 20 | 9/11 | 46.6 ± 6.1 | SRP (Hand and piezo- electric ultrasonic instruments) | SRP+aPDT | Baseline, 1 week, 3 months | SFFR, BOP, RAL PD, GR | Values for RAL, PD, SFFR and BOP decreased significantly 3 months after treatment in the control group with a higher impact on the sites treated with adjunctive aPDT (p < 0.05). GR increased 3 months after treatment with and without adjunctive aPDT, with no difference between the groups (p > 0.05). In patients with CP, clinical outcomes can be improved by adjunctive aPDT. | ||
| Berakdar et al., 2012 (Germany) [77] | Head and Face Medicine IF 2020: 1.492 IF 2012: 1.519 | SM-RCT | CP (At least four teeth with a PPD of ≥5 mm) | 22 | 12/10 | 59.3 ± 11.7 | SRP (Hand instruments) | SRP+aPDT | Baseline, 1, 3 and 6 months | BOP, PI, PD, CAL | At 1, 3 and 6 months after both types of treatment, an improvement in BOP and CAL was observed. The greater reduction of the PD, achieved by a combination of SRP/PDT, was statistically significant after 6 months (p = 0.007). | ||
| Raut et al., 2018 (India) [78] | Journal of Indian Society of Periodontology IF 2020: 0.460 IF 2018: 0.44 | PG-RCT | CP (PPD > 5 mm and CAL > 4 mm) | 50 | SRP group: 12/13 SRP+aPDT group: 16/9 | SRP group: 46.90 ± 4.32 SRP+aPDT group: 51 ± 2.83 | SRP+ sham procedure (Hand and ultrasonic instruments) | SRP+aPDT | Baseline and 6 months | PI, BOP, CAL, PD, microbiological analysis | Significant reduction was seen in PD, CAL and BOP in the test group as compared to control group after 6 months (p < 0.05). However, intergroup comparison of PI showed nonsignificant results (p > 0.05). Anaerobic culture of plaque samples of test group also revealed a significant reduction of microorganisms in comparison with control group. | ||
| Hokari et al., 2018 (Japan) [79] | International Journal of Dentistry IF 2019: 0.58 IF 2018: 0.58 | PG-RCT | CP (Moderate: 3–4 mm clinical attachment loss, severe: ≥5 mm loss, generalized: >30% of sites affected) | 30 | aPDT group: 7/8 MO group: 6/9 | aPDT group: 61.4 ± 10.2 MO group: 66.7 ± 9.5 | SRP+ Minocycline ointment (MO) (Ultrasonic instruments) | SRP+aPDT | Baseline, 1 and 4 weeks | BOP, PD, CAL, PI, GI, microbiological and inflammatory marker analysis | Local MO administration exhibited a significant decrease in scores for clinical parameters (p < 0.01) and a significant reduction in bacterial counts (p < 0.01) and IL-1β and IF-γ levels at 1 and 4 weeks after treatment (p < 0.01). No significant changes were observed in the aPDT group, except in clinical parameters. | ||
| Hill et al., 2019 (Germany) [80] | Photodiagnosis and Photodynamic Therapy IF 2020: 2.894 IF 2019: 2.821 | SM-RCT | CP (At least one single and one multi-rooted tooth with at least 4 mm PPD in each quadrant) | 20 | 3/17 | 61.1 | SRP (Hand and piezo- electric ultrasonic instruments) | SRP+aPDT | Baseline, 2 week, 3 and 6 months | BOP, SFFR, PD, GR, RAL, Microbiological analysis | Median values for BOP, RAL, PD, decreased significantly in both groups (p < 0.05) after three months of treatment without significant difference between the groups (p > 0.05). Two weeks after treatment, the SFFR showed significantly lower mean values in the test group (aPDT). With the applied parameters, this study does not conclusively support ICG-based aPDT, though it is promising because no adverse effects occurred. | ||
| Ahad et al., 2016 (India) [81] | Journal of Lasers in Medical Sciences IF 2020: 1.570 IF 2016: 0.68 | SM-RCT | CP (At least 2 teeth in different quadrants with PD ≥ 6 mm, and BOP) | 30 | 21/9 | 38.67 ± 10.52 | SRP (Hand and ultrasonic instruments) | SRP+aPDT | Baseline, 1 and 3 months | PI, mSBI, PD, CAL | At 1 month follow-up, intergroup difference in mean change was statistically significant in terms of mSBI and PD for the adjunctive aPDT group (p < 0.05), at 3 months interval, no statistically significant difference was observed between test and control groups except in terms of mSBI (p > 0.05), thus proving that aPDT improved the gingival status in the nonsurgical management of CP. | ||
| Balata et al., 2013 (Brazil) [82] | Journal of Applied Oral Science IF 2019: 2.005 IF 2013: 1.153 | SM-RCT | CP (Periodontal pockets with CAL ≥ 5 mm, BOP and radiographic bone loss; minimum of 2 teeth with PD ≥ 7 mm and 2 other teeth with a PD ≥ 5 mm, all with BOP and located on opposite sides of the mouth; and ≥16 teeth in both jaws) | 22 | 8/14 | 43.18 | SRP (Ultrasonic instruments) | SRP+aPDT | Baseline, 1, 3 and 6 months | PI, GI, BOP, GR, CAL | Both groups revealed statistically significant improvement in the clinical parameters (p < 0.05) with no statistically significant differences upon intergroup comparison (p > 0.05). aPDT did not provide any additional benefit to those obtained with full-mouth ultrasonic debridement used alone. | ||
| Bechara et al., 2018 (Brazil) [83] | Photodiagnosis and Photodynamic Therapy IF 2020: 2.894 IF 2018: 2.624 | PG-RCT | AgP (Single-rooted teeth in multiple quadrants, with both PPD and CAL ≥ 5 mm, and with BOP) | 36 patients (72 sites) | CLM group: 1/17 Placebo group: 1/17 | <35 years CLM group: 33.11 ± 4.26 Placebo group: 31.26 ± 4.73 | CLM group (n = 18) Clarithromycin 500 mg BD for 3 days | Placebo group (n = 18) | Baseline, 3 months and 6 months | PD, CAL, BOP, GR | At 3 months, UPD+aPDT, UPD+CLM and UPD + CLM + aPDT groups all exhibited reduced PD relative to the UPD group (p < 0.05). However, at 6 months, the mean PD reduction was greater in the antibiotic groups (UPD+CLM and UPD+CLM+aPDT) than in the UPD and UPD+aPDT groups (p < 0.05). Regarding clinical attachment level, only the UPD+CLM+aPDT group presented a significant gain relative to the UPD and UPD+aPDT groups (p < 0.05). | ||
| UPD + CLM (18 sites) | UPD+ CLM+ aPDT (18 sites) | UPD (18 sites) | UPD+ aPDT (18 sites) | ||||||||||
| Bundidpun et al., 2017 (Thailand) [84] | Laser Therapy IF 2020: 0.43 IF 2017: 0.53 | SM-RCT | CP (Generalized moderate to severe chronic periodontitis, presence of at least 20 teeth, at least one molar tooth in each quadrant with a minimum of four teeth, at least two teeth and one molar tooth presented with PD > 6 mm in each quadrant) | 20 | 7/13 | 47.25 ± 8.91 | SRP (Piezo-electric ultrasonic instruments) | SRP+aPDT | Baseline, 1, 3 and 6 months | PD, CAL, PI, GBI, GI | All parameters in test group were better than that control group, with statistically significant differences of GBI and GI (p < 0.05) at 3 and 6 months after treatment but no statistically significant differences of PD, CAL and PI. | ||
| Chitsazi et al., 2014 (Iran) [85] | Journal of Dental Research, Dental Clinics, Dental Prospects IF 2020: 0.69 IF 2014: 1.30 | SM-RCT | AgP (Minimum of 12 teeth with at least 3 teeth in each quadrant with ≥4 mm of probing depth) | 24 | 9/15 | 29 | SRP (Piezo-electric ultrasonic instruments) | SRP+aPDT | Baseline, 3 months | PD, CAL, GR, PI, GI, BOP, Microbiological analysis for A.a | Intragroup comparison showed an improvement in all the clinical parameters and a significant reduction in the counts of A.a at 90 days compared to baseline (p < 0.05). None of the periodontal parameters exhibited significant differences between the two groups (p > 0.05). | ||
| Chitsazi et al., 2014 (Iran) [86] | Journal of Advanced Periodontology and Implant Dentistry IF 2020: NA IF 2014: NA | SM-RCT | CP (At least one site per quadrant exhibiting pocket depth of ≥4 mm with bleeding on probing) | 22 | 10/12 | 46.1 | SRP (Sonic instruments) | SRP+aPDT | Baseline, 1 and 3 months | PD, CAL, BOP, GR, microbiological analysis | PD values decreased significantly in both groups after 1 month (p = 0.001) and 3 months (p = 0.001) in the SRP and (p = 0.001) in the PDT groups the inter-group differences were not significant after 1 (p = 0.25) and 3 months (p = 0.51). Clinical measurements showed significant decreases after 1 and 3 months at both sites, without inter-group differences, except for BOP after 1 (p = 0.004) and 3 months (p = 0.0001). | ||
| Garcia et al., 2011 (Brazil) [87] | Revista Periodontia IF 2020: NA IF 2011: NA | SM-RCT | AgP (Bone loss first molars and incisors, and other teeth adjacent, with PPD ≥ 5 mm and loss of CAL ≥ 2 mm) | 10 | 4/6 | 39.3 ± 5.84 | SRP (Hand and ultrasonic instruments) | SRP+aPDT | Baseline, 3 months | PD, RCAL, furcation involvement, tooth mobility | Both groups showed improved clinical results in the nonsurgical treatment of AgP with no statistically significant intergroup differences (p > 0.05). | ||
| Joseph et al., 2014 (India) [88] | Journal of Clinical Periodontology IF 2020: 5.241 IF 2014: 4.641 | PG-RCT | CP (A minimum of 20 teeth; PPD 4–6 mm at least in two different quadrants of the mouth) | 90 | 39/51 | 39.6 ± 8.7 | SRP (Hand and ultrasonic instruments) | SRP+aPDT | Baseline, 2 weeks, 1, 3 and 6 months | PPD, CAL, GI, GBI, PI, halitosis. | PD and CAL showed statistically significant reduction in the test group on evaluation at 3 months and 6 months as compared to the control group (p < 0.05). A statistically significant improvement in GI and GBI was seen for the test group after 2 weeks and 1 month of aPDT (p < 0.01), whereas the improvement in GI and GBI at 3 months and in plaque index at 2 weeks after aPDT was less (p < 0.05). In addition, a significant difference was detected for the test group at 1 month in terms of halitosis, which did not persist for long (p < 0.05). | ||
| Malgikar et al., 2015 (India) [89] | Journal of Dental Lasers IF 2020: 0.696 IF 2015: NA | SM-RCT | CP (At least one site in each quadrant of the mouth having deep PPD ≥ 5 mm and radiographic signs of alveolar bone loss) | 24 | 15/9 | M: 36.73 ± 8.46 F: 34.33 ± 6.80 | SRP (Hand and piezo- electric ultrasonic instruments) | SRP+aPDT | Baseline, 1, 3 and 6 months. | PI, GI, mSBI, PD, CAL. | A statistically significant decrease in PD, CAL, PI, GI, mSBI scores was seen in SRP+aPDT at the end of 6 months (p < 0.001). | ||
| Monzavi et al., 2016 (Iran) [90] | Photodiagnosis and Photodynamic Therapy IF 2020: 2.894 IF 2016: 2.503 | SM-RCT | CP (At least three teeth exhibiting residual pocket depth of ≥ 5 mm with bleeding on probing) | 50 | 25/25 | 49.6 ± 8.5 | SRP (Hand and ultrasonic instruments) | SRP+aPDT | Baseline, 1 and 3 months | BOP, PI, CAL, PPD, FMPS, FMBS | There were no significant differences between two groups at baseline. BOP, PPD and FMBS showed significant improvements in the test group (p ≤ 0.001). In terms of PI, FMPS and CAL, no significant differences were observed between both groups (p ≥ 0.05). | ||
| Raj et al., 2016 (India) [91] | Indian Journal of Dental Research IF 2020: 0.37 IF 2016: 0.08 | PG-RCT | CP (More than 16 natural teeth; PPD ≥ 5 mm) | 20 | 8/12 | NI | SRP (Type of instruments utilized-NI) | SRP+aPDT | Baseline and 3 months | PI, GI, PD, CAL and microbiological analysis | There was a significant reduction in PI, GI, PD, CAL and microbiologic parameters in test group, following SRP and PDT, when compared with SRP alone in control group (p < 0.001). SRP+aPDT has shown additional improvement in periodontal parameters when compared to SRP alone and has a beneficial effect in chronic periodontitis patients. | ||
| Sena et al., 2019 (Brazil) [92] | Photobiomodulation, Photomedicine and Laser Surgery IF 2019: 1.913 | SM-RCT | CP (At least six sites with PD 5–9 mm; and BOP) | 9 (6 sites/ patient: total-54 sites) | NI | NI | SRP+ placebo procedure (Hand and ultrasonic instruments) | SRP+aPDT | Baseline and 3 months | BOP, PD, CAL, VPI | There was a statistically significant decrease in BOP for test group (p = 0.003) and control group (p = 0.001). Intragroup comparison for PD and CAL showed statistically significant differences from baseline (p < 0.05) with no intergroup differences (p > 0.05). Hence, SRP+aPDT did not show any additional benefits over SRP alone. | ||
| Shingnapurkar et al., 2016 (India) [93] | Indian Journal of Dental Research IF 2020: 0.37 IF 2016: 0.08 | SM-RCT | CP (PD > 5 mm) | 60 sites | NI | NI | SRP+ sham procedure (Hand and ultrasonic instruments) | SRP+aPDT | Baseline, 1 and 3 months | PI, GI, PD, RAL | Mean baseline values for PI, GI, PPD and RAL were not different in the test group and control group. Statistically significant difference in PPD and RAL, 3 months after treatment was seen in test group as compared to the control group (p < 0.05). | ||
| Sigusch et al., 2010 (Germany) [94] | Journal of Periodontology IF 2020: 3.742 IF 2010: 2.946 | PG-RCT | CP (<30% of sites with PPD >3.5 mm) | 24 (12 in each group) | PDT group: 4/8 Control group: 3/9 | PDT group F: 39.75 M: 45 Control group: F: 44.22 M:42.67 | SRP+ sham procedure (Type of instruments utilized- NI) | SRP+aPDT | Baseline, 1, 4 and 12 weeks. | PI, reddening, PD, BOP, CAL, GR Quantitative analysis for F.n. | In patients with localized CP who received aPDT treatment, significant reductions in reddening, BOP, and mean PD and CAL were observed during the observation period and with respect to controls (p < 0.001). Four and 12 weeks after aPDT, the mean PD and CAL showed significant differences from baseline values and from those of the control group. In the aPDT group, 12 weeks after treatment, the F.n. DNA concentration was found to be significantly reduced compared to the baseline level (p < 0.001) compared to control group. | ||
| Theodoro et al., 2012 (Brazil) [95] | Lasers in Medical Science IF 2019: 2.574 IF 2012: 2.645 | SM-RCT | CP (At least three non-adjacent sites with BOP and a PD of 5–9 mm at least 20 teeth in the oral cavity) | 33 | 12/21 | 43.12 ± 8.2 | SRP (Hand instruments) | SRP+ PS (TBO) only | SRP+aPDT | Baseline, 60, 90 and 180 days | VPI, GI, BOP, PD, CAL, GR, microbiological analysis | All treatment groups showed an improvement in all clinical parameters, and a significant reduction in the proportion of sites positive for periodontopathogens at 60, 90 and 180 days compared to baseline (p < 0.05). None of the periodontal parameters showed a significant difference among the groups (p > 0.05). At 180 days, PDT treatment led to a significant reduction in the percentage of sites positive for all bacteria compared to SRP alone (p < 0.05). | |
| Theodoro et al., 2017 (Brazil) [96] | Journal of Photochemistry and Photobiology B IF 2020: 4.383 IF 2017: 3.438 | PG-RCT | CP (Severe generalized CP in at least 6 teeth and with one or several sites with PD ≥ 5 mm; a loss of CAL ≥ 5 mm; a minimum of 30% of the sites with PD and CAL ≥ 4 mm and BOP; and the presence of at least 15 teeth) | 34 | AB group: 7/7 aPDT group: 9/5 | AB group: 46.3 ± 6.8 aPDT group: 48.8 ± 8.3 | SRP+ (MTZ+ AMX) MTZ dose: 400mg TDS-7 days AMX dose: 500mg TDS-7 days (Type of instruments utilized for SRP-NI) | SRP +aPDT+ placebo pills | Baseline and 90 days | BOP, PD, CAL | There was a significant improvement in CAL only in the intermediate pocket in the aPDT group com- pared to the MTZ + AMX group between baseline and 90 days post-treatment (p = 0.01). There was a reduction of both BOP and the percentage of residual pockets at 90 days after treatment compared with baseline in both groups (p < 0.05). | ||
| Study, Year, Origin and Citation | Photosensitizer (PS) Used and Its Concentration | Pre-Irradiation Exposure Time to PS (min) | Laser Wavelength Utilized | Emission Mode Contact/No Contact Tip Initiation | Energy (J) | Power Output (W) | Pulse Length (Duration), Pulse Interval | Use of Power Meter | Distance from Target | Spot Size/Fibre-Tip Diameter/Spot Diameter | Energy Density [Fluence] (J/cm2) | Power Density [Irradiance] (W/cm2) | Exposure Time to Laser Irradiation [Minute (min)/Second (s)] | No. of aPDT Applications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Oliveira et al., 2009 (Brazil) [2] | Phenothiazine chloride (10 mg/mL) | 1 min | 660 nm | Contact mode, fibre tip was place at the entrance of the gingival sulcus | NI | NI | NI | NI | NA | Tip diameter: 600 µm | NI | 60 mW/cm2 | 10 s/site (6 sites = 1 min/tooth) | 1 |
| De Oliveira et al., 2007 (Brazil) [3] | Phenothiazine chloride (10 mg/mL) | 1 min | 660 nm | Contact mode, fibre tip was place at the entrance of the gingival sulcus | NI | NI | NI | NI | NI | Tip diameter: 600 µm | NI | 60 mW/cm2 | 10 s/site (6 sites = 1 min/tooth) | 1 |
| Novaes et al., 2012 (Brazil) [5] | Phenothiazine chloride | NI | 660 nm | Contact mode, fibre tip was place at the entrance of the gingival sulcus | NI | NI | NI | NI | NI | Tip diameter: 600 µm [8.5 cm long optic fibre with 60° angulated tip] Spot size: 0.06 | 212.23 J/cm2 | 60 mW/cm2 | 10 s/site (6 sites/tooth) 60 s/tooth | 1 |
| Franco et al., 2014 (Brazil) [17] | Methylene blue (0.01%) | 5 min | 660 nm | NI | NI | NI | NI | NI | NI | NI | 5.4 J/cm2 | 60 mW/cm2 | 5 s/site (6 sites/tooth) 90 s/tooth | 4 |
| Pourabbas et al., 2014 (Iran) [70] | Toluidine blue | 60 s | 638 nm | NI | NI | NI | NI | NI | NI | NI | 8–10 J/cm2 | NI | 120 s | 1 |
| Moreira et al., 2015 (Brazil) [71] | Phenothiazine chloride (10 mg/mL) | 1 min | 670 nm | NI | NI | 75 mW | NI | NI | NI | Tip diameter: 600 µm | Fluence/site: 2.49 J/cm2 Fluence/tooth: 14.94 J/cm2 | 0.25 W/cm2 | 10 s /site | 4 (0, 2nd, 7th and 14th day) |
| Skurska et al., 2015 (Poland) [72] | Phenothiazine chloride | 3 min | 660 nm | NI | NI | NI | NI | NI | NI | NI | 120 J/cm2 | 60 mw/cm2 | 60 s/site | 2 (0 and 7th day) |
| Arweiler et al., 2014 (Poland) [73] | Phenothiazine chloride | 3 min | 660 nm | NI | NI | NI | NI | NI | NI | NI | 120 J/cm2 | 60 mw/cm2 | 60 s/site | 2 (0 and 7th day) |
| Arweiler et al., 2013 (Poland) [74] | Phenothiazine chloride | 3 min | 660 nm | NI | NI | NI | NI | NI | NI | NI | 120 J/cm2 | 60 mw/cm2 | 60 s/site | 2 (0 and 7th day) |
| Vidal et al., 2017 (Spain) [75] | Methylene blue (0.005%) | NI | 670 nm | NI | NI | 150 mW | NI | NI | NI | NI | NI | NI | 60 s/pocket | 3 (1, 5 and 13 weeks) |
| Braun et al., 2008 (Germany) [76] | Phenothiazine chloride | 3 min | 660 nm | NI | NI | 100 mW | NI | NI | NI | NI | NI | NI | 10 s/site (6 sites = 1 min /tooth) | 1 |
| Berakdar et al., 2012 (Germany) [77] | Methylene blue 0.005% | NI | 670 nm | NI | NI | 150 mW | NI | NI | NI | NI | NI | NI | 1 min | 1 |
| Raut et al., 2018 (India) [78] | Indocyanine green (5 mg/mL) | 60 s | 810 nm | CW, contact mode | NI | 80 mW | NI | NI | NA | NI | 5.4 J/cm2 | NI | 60 s | 1 |
| Hokari et al., 2018 (Japan) [79] | Methylene blue dye 0.01% | 1 min | 670 nm | NI, contact mode | NI | 140 mW | NI | NI | NA | NI | 21 J/cm2 | NI | 60 s | 2 (0 and 7th day) |
| Hill et al., 2019 (Germany) [80] | Indocyanine green (0.1 mg/mL) | 60 s | 808 nm | NI | NI | 100 mW | NI | NI | NI | Tip diameter: 300 µm | 2829 J/cm2 | NI | NI | 1 |
| Ahad et al., 2016 (India) [81] | Phenothiazine chloride | 3 min | 660 nm | Contact mode | NI | NI | NI | NI | NA | Tip diameter: 0.6 µm | NI | 100 mW/cm2 | 10 s/site (6 sites, 1 min/tooth) | 1 |
| Balata et al., 2013 (Brazil) [82] | Methylene blue 0.005% | 2 min | 660 nm | 90° angle with the gingival surface and with no contact with the tissues | 9 J | 100 mW | NI | NI | NI | Tip diameter: 600 µm tip | 320 J/cm2 | NI | 90 s/site | 1 |
| Bechara et al., 2018 (Brazil) [83] | Methylene Blue (10 mg/mL) | 1 min | 660 nm | NI | NI | 60 mW | NI | NI | NI | NI | 129 J/cm2 | NI | 60 s/tooth (2 sites/tooth) | 1 |
| Bundidpun et al., 2017 (Thailand) [84] | Phenothiazine chloride | 1 min | 660 nm | Contact mode | NI | 100 mW | NI | NI | NA | NI | NI | NI | 10 s/site (6 sites) 1 min/tooth | 1 |
| Chitsazi et al., 2014 (Iran) [85] | Toluidine Blue | 1 min | 670–690 nm | Contact mode | NI | 75 mW | NI | NI | NA | NI | NI | NI | 120 s/site | 1 |
| Chitsazi et al., 2014 (Iran) [86] | Tolonium chloride (Toluidine Blue O) | 60 s | 638 nm | Contact mode | NI | NI | NI | NI | NA | NI | 8–10 J/cm2 | NI | 120 s | 1 |
| Garcia et al., 2011 (Brazil) [87] | Methylene blue (0.005%) | 5 min | 660 nm | NI | NI | 40 mW | NI | NI | NI | NI | 120 J/cm2 | NI | 120 s/site | 1 |
| Joseph et al., 2014 (India) [88] | Methylene blue (10 mg/mL) | 3 min | 655 nm | CW, contact mode, tip was inserted into the gingival sulcus | NI | NI | NI | NI | NA | Tip diameter: 200 µm Probe tip diameter: 0.5 mm | NI | 60 mW/ cm2 | 60 s/site (4 sites/ tooth) | 1 |
| Malgikar et al., 2015 (India) [89] | Methylene blue 1% | 3 min | 980 nm | Contact mode, tip was initiated | NI | Peak Power: 5 W Average power 1 W | Pulse length: 200 µs, Pulse interval: 200 | NI | NA | Tip diameter: 400 µm | NI | NI | 30–45 s/site | 1 |
| Monzavi et al., 2016 (Iran) [90] | Indocyanine green (1 mg/mL) | NI | 810 nm | CW, contact mode | PBM tip: 6 J Bulb tip: 4 J | 200 mW | NI | NI | NA | Use of two types of tips: PBM tip was placed on papilla and then the bulb tip was inserted inside the pocket from each buccal or lingual/palatal side, moving from the bottom of the pocket to the coronal aspect. | NI | NI | PBM tip: 30 s Bulb tip: 10 s | 4 (0, 7th, 17th and 27th days) |
| Raj et al., 2016 (India) [91] | Toluidine blue | 1 min | 635 nm | Contact mode | NI | 500 W | NI | NI | NA | NI | NI | NI | 60 s | 1 |
| Sena et al., 2019 (Brazil) [92] | Chloro-aluminum pthalocyanine (AlClFc) 5 µM | 5 min | 660 nm | CW, laser optical fiber tip was positioned parallel to the tooth axis in contact with the gingival margin (without penetrating the pocket) | 1.5 J | 100 mW | NI | NI | NA | Spot size: 0.028 cm2 | 54 J/cm2 | 4 W/cm2 | 15 s | 1 |
| Shingnapurkar et al., 2016 (India) [93] | Indocyanine green (1 mg/mL) | 3 min | 810 nm | Gated CW, Contact mode | 3 J | 200 mW | Pulse duration: 25 µm Duty cycle 50% | NI | NA | Tip diameter: 400 µm | 0.0125 J/cm2 | NI | 30 s/site | 1 |
| Sigusch et al., 2010 (Germany) [94] | Phenothiazine chloride | 1 min | 660 nm | Contact mode | NI | NI | NI | NI | NA | Tip diameter: 600 µm tip | NI | 60 mW/cm2 | 10 s/site (6 sites =1 min /tooth) | 1 |
| Theodoro et al., 2012 (Brazil) [95] | Toluidine blue O 100 µg/mL | 1 min | 660 nm | The laser optical fiber tip was positioned parallel to and in contact with the selected site | 4.5 J | 30 mW | NI | NI | NA | Spot size: 0.07 cm2 | 64.28 J/cm2 | 0.4 W/cm2 | 150 s | 1 |
| Theodoro et al., 2017 (Brazil) [96] | Methylene blue (10 mg/mL) | 1 min | 660 nm | Contact mode | 4.8 J | 100 mW | NI | NI | NA | Spot size 0.03 cm2 | 160 J/cm2 | NI | 48 s | 3 (0, 48 h, 96 h) |
| Overall PPD Reduction for SM Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.463 | 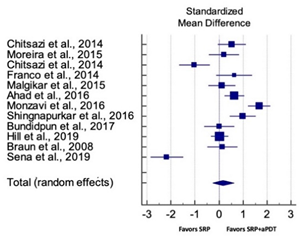 |
| Chitsazi et al., 2014 | 0.525 | 0.289 | −0.056 to 1.106 | 8.28 | ||
| Moreira et al., 2015 | 0.205 | 0.311 | −0.425 to 0.834 | 8.11 | ||
| Chitsazi et al., 2014 | −1.023 | 0.316 | −1.659 to −0.386 | 8.07 | ||
| Franco et al., 2014 | 0.631 | 0.364 | −0.115 to 1.378 | 7.67 | ||
| Malgikar et al., 2015 | 0.119 | 0.284 | −0.453 to 0.691 | 8.31 | ||
| Ahad et al., 2016 | 0.639 | 0.204 | 0.235 to 1.043 | 8.88 | ||
| Monzavi et al., 2016 | 1.669 | 0.231 | 1.211 to 2.127 | 8.70 | ||
| Shingnapurkar et al., 2016 | 0.995 | 0.271 | 0.454 to 1.537 | 8.42 | ||
| Bundidpun et al., 2017 | 0.007 | 0.310 | −0.620 to 0.635 | 8.11 | ||
| Hill et al., 2019 | 0.039 | 0.072 | −0.103 to 0.181 | 9.47 | ||
| Braun et al., 2008 | 0.139 | 0.310 | −0.490 to 0.767 | 8.11 | ||
| Sena et al., 2019 | −2.169 | 0.340 | −2.851 to −1.487 | 7.87 | ||
| Total (random effects) | 0.166 | 0.227 | −0.278 to 0.611 | 100.00 | ||
| Heterogeneity: Q = 15.81; DF = 11; P = 0.0001; I2 = 91.21% | ||||||
| Overall PPD Reduction for PG Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.763 | 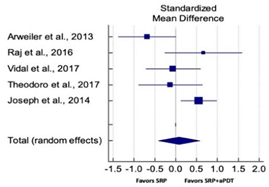 |
| Arweiler et al., 2013 | −0.681 | 0.340 | −1.374 to 0.011 | 19.80 | ||
| Raj et al., 2016 | 0.669 | 0.441 | −0.258 to 1.595 | 15.89 | ||
| Vidal et al., 2017 | −0.060 | 0.322 | −0.714 to 0.593 | 20.59 | ||
| Theodoro et al., 2017 | −0.127 | 0.374 | −0.897 to 0.643 | 18.43 | ||
| Joseph et al., 2014 | 0.556 | 0.215 | 0.127 to 0.984 | 25.28 | ||
| Total (random effects) | 0.076 | 0.252 | −0.420 to 0.573 | 100.00 | ||
| Heterogeneity: Q = 11.87; DF = 4; P = 0.018; I2 = 66.31% | ||||||
| Overall CAL Gain for SM Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.088 | 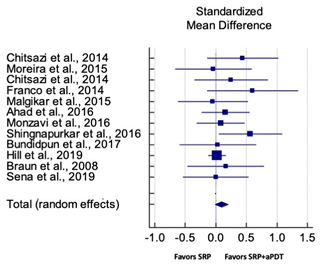 |
| Chitsazi et al., 2014 | 0.439 | 0.287 | −0.140 to 1.017 | 3.52 | ||
| Moreira et al., 2015 | −0.040 | 0.310 | −0.667 to 0.588 | 3.02 | ||
| Chitsazi et al., 2014 | 0.249 | 0.297 | −0.351 to 0.849 | 3.29 | ||
| Franco et al., 2014 | 0.601 | 0.364 | −0.144 to 1.346 | 2.20 | ||
| Malgikar et al., 2015 | −0.048 | 0.284 | −0.620 to 0.523 | 3.60 | ||
| Ahad et al., 2016 | 0.158 | 0.199 | −0.237 to 0.552 | 7.35 | ||
| Monzavi et al., 2016 | 0.080 | 0.199 | −0.314 to 0.474 | 7.37 | ||
| Shingnapurkar et al., 2016 | 0.564 | 0.260 | 0.043 to 1.084 | 4.30 | ||
| Bundidpun et al., 2017 | 0.032 | 0.310 | −0.595 to 0.660 | 3.02 | ||
| Hill et al., 2019 | 0.019 | 0.072 | −0.123 to 0.161 | 55.29 | ||
| Braun et al., 2008 | 0.161 | 0.310 | −0.468 to 0.789 | 3.01 | ||
| Sena et al., 2019 | 0.000 | 0.268 | −0.538 to 0.538 | 4.04 | ||
| Total (random effects) | 0.092 | 0.233 | −0.013 to 0.198 | 100.00 | ||
| Heterogeneity: Q = 8.74; DF = 11; P = 0.655; I2 = 0.00% | ||||||
| Overall CAL Gain for PG Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.745 |  |
| Arweiler et al., 2013 | −0.662 | 0.340 | −1.356 to 0.010 | 19.80 | ||
| Raj et al., 2016 | 0.669 | 0.441 | −0.258 to 1.595 | 15.89 | ||
| Vidal et al., 2017 | −0.102 | 0.372 | −0.514 to 0.793 | 20.59 | ||
| Theodoro et al., 2017 | −0.106 | 0.374 | −0.997 to 0.743 | 18.43 | ||
| Joseph et al., 2014 | 0.456 | 0.255 | −0.120 to 0.673 | 25.28 | ||
| Total (random effects) | 0.056 | 0.358 | −0.408 to 0.552 | 100.00 | ||
| Heterogeneity: Q = 8.95; DF = 4; P = 0.028; I2 = 70.31% | ||||||
| Overall PPD Reduction for SM Studies at 6 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.935 |  |
| Berakdar et al., 2012 | 0.040 | 0.296 | −0.598 to 0.598 | 5.08 | ||
| Malgikar et al., 2015 | 0.037 | 0.284 | −0.535 to 0.609 | 5.52 | ||
| Bundidpun et al., 2017 | 0.072 | 0.310 | −0.555 to 0.701 | 4.63 | ||
| Hill et al., 2019 | 0.060 | 0.072 | −0.142 to 0.142 | 84.77 | ||
| Total (random effects) | 0.005 | 0.066 | −0.126 to 0.136 | 100.00 | ||
| Heterogeneity: Q = 0.06; DF = 3; P = 0.99; I2 = 0.00% | ||||||
| Overall PPD Reduction for PG Studies at 6 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.809 | 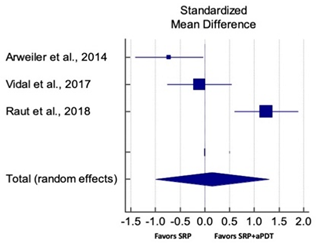 |
| Arweiler et al., 2014 | −0.722 | 0.342 | −1.417 to −0.027 | 33.04 | ||
| Vidal et al., 2017 | −0.109 | 0.322 | −0.763 to 0.545 | 33.47 | ||
| Raut et al., 2018 | 1.241 | 0.321 | 0.594 to 1.888 | 33.49 | ||
| Total (random effects) | 0.141 | 0.579 | −1.007 to 1.288 | 100.00 | ||
| Heterogeneity: Q = 18.71; DF = 2; P = 0.0001; I2 = 89.31% | ||||||
| Overall CAL Gain for SM Studies at 6 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.846 | 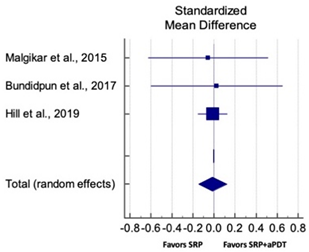 |
| Malgikar et al., 2015 | −0.057 | 0.284 | −0.629 to 0.515 | 5.82 | ||
| Bundidpun et al., 2017 | 0.025 | 0.310 | −0.602 to 0.653 | 4.88 | ||
| Hill et al., 2019 | −0.012 | 0.072 | −0.155 to 0.130 | 89.30 | ||
| Total (random effects) | −0.013 | 0.068 | −0.148 to 0.121 | 100.00 | ||
| Heterogeneity: Q = 0.03; DF = 2; P = 0.984; I2 = 0.00% | ||||||
| Overall CAL Gain for PG Studies at 6 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.018 | 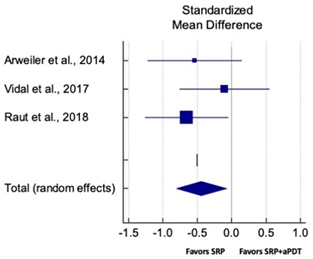 |
| Arweiler et al., 2014 | −0.539 | 0.337 | −1.224 to 0.146 | 29.91 | ||
| Vidal et al., 2017 | −0.103 | 0.322 | −0.756 to 0.551 | 32.69 | ||
| Raut et al., 2018 | −0.658 | 0.301 | −1.265 to −0.050 | 37.40 | ||
| Total (random effects) | −0.441 | 0.184 | −0.805 to −0.075 | 100.00 | ||
| Heterogeneity: Q = 1.70; DF = 2; P = 0.42; I2 = 0.00% | ||||||
| Study, Year, Origin and Citation | PPD | CAL | BOP/SBI | PI | GI | GR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistically Significant Y/N/NI/NS | Not Statistically Significant Y/N/NI/NS | Statistically Significant Y/N/NI/NS | Not Statistically Significant Y/N/NI/NS | Statistically Significant Y/N/NI/NS | Not Statistically Significant Y/N/NI/NS | Statistically Significant Y/N/NI/NS | Not Statistically Significant Y/N/NI/NS | Statistically Significant Y/N/NI/NS | Not Statistically Significant Y/N/NI/NS | Statistically Significant Y/N/NI/NS | Not Statistically Significant Y/N/NI/NS | |
| De Oliveira et al., 2007 (Brazil) [3] | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y |
| Franco et al., 2014 (Brazil) [17] | N | Y | N | Y | Y | N | N | Y | NS | NS | NS | NS |
| Pourabbas et al., 2014 (Iran) [70] | N | Y | N | Y | N | Y | NS | NS | NS | NS | N | Y |
| Moreira et al., 2015 (Brazil) [71] | Y | N | Y | N | Y | N | Y | N | NS | NS | Y | N |
| Arweiler et al., 2014 (Poland) [73] | N | Y | N | Y | N | Y | N | Y | NS | NS | N | Y |
| Arweiler et al., 2013 (Poland) [74] | N | Y | N | Y | N | Y | N | Y | NS | NS | N | Y |
| Vidal et al., 2017 (Spain) [75] | N | Y | N | Y | N | Y | N | Y | NS | NS | N | Y |
| Braun et al., 2008 (Germany) [76] | Y | N | Y | N | Y | N | NS | NS | NS | NS | N | Y |
| Berakdar et al., 2012 (Germany) [77] | Y | N | Y | N | N | Y | N | Y | NS | NS | NS | NS |
| Raut et al., 2018 (India) [78] | Y | N | Y | N | Y | N | N | Y | NS | NS | NS | NS |
| Hokari et al., 2018 (Japan) [79] | N | Y | N | Y | N | Y | N | Y | N | Y | NS | NS |
| Hill et al., 2019 (Germany) [80] | N | Y | N | Y | N | Y | NS | NS | NS | NS | N | Y |
| Ahad et al., 2016 (India) [81] | N | Y | N | Y | Y | N | N | Y | NS | NS | NS | NS |
| Balata et al., 2013 (Brazil) [82] | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y |
| Bechara et al., 2018 (Brazil) [83] | Y | N | Y | N | Y | N | NS | NS | NS | NS | Y | N |
| Bundidpun et al., 2017 (Thailand) [84] | N | Y | N | Y | Y | N | N | Y | Y | N | NS | NS |
| Chitsazi et al., 2014 (Iran) [85] | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y |
| Chitsazi et al., 2014 (Iran) [86] | N | Y | N | Y | N | Y | NS | NS | NS | NS | N | Y |
| Garcia et al., 2011 (Brazil) [87] | N | Y | N | Y | NS | NS | NS | NS | NS | NS | NS | NS |
| Joseph et al., 2014 (India) [88] | Y | N | Y | N | N | Y | N | Y | N | Y | NS | NS |
| Malgikar et al., 2015 (India) [89] | Y | N | Y | N | Y | N | Y | N | Y | N | NS | NS |
| Monzavi et al., 2016 (Iran) [90] | Y | N | N | Y | Y | N | N | Y | NS | NS | NS | NS |
| Raj et al., 2016 (India) [91] | Y | N | N | Y | NS | NS | Y | N | Y | N | NS | NS |
| Sena et al., 2019 (Brazil) [92] | N | Y | N | Y | N | Y | N | Y | NS | NS | NS | NS |
| Shingnapurkar et al., 2016 (India) [93] | Y | N | Y | N | NS | NS | N | Y | N | Y | NS | NS |
| Sigusch et al., 2010 (Germany) [94] | Y | N | Y | N | Y | N | Y | N | NS | NS | Y | N |
| Theodoro et al., 2012 (Brazil) [95] | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y |
| Theodoro et al., 2017 (Brazil) [96] | N | Y | N | Y | N | Y | NS | NS | NS | NS | NS | NS |
| Overall PPD Reduction for SM Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.153 | 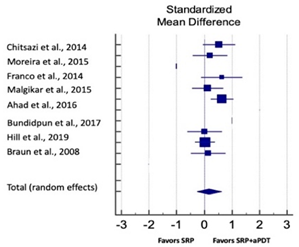 |
| Chitsazi et al., 2014 | 0.525 | 0.289 | −0.056 to 1.106 | 12.28 | ||
| Moreira et al., 2015 | 0.205 | 0.311 | −0.425 to 0.834 | 12.11 | ||
| Franco et al., 2014 | 0.631 | 0.364 | −0.115 to 1.378 | 11.67 | ||
| Malgikar et al., 2015 | 0.119 | 0.284 | −0.453 to 0.691 | 12.31 | ||
| Ahad et al., 2016 | 0.639 | 0.204 | 0.235 to 1.043 | 12.88 | ||
| Bundidpun et al., 2017 | 0.007 | 0.310 | −0.620 to 0.635 | 12.11 | ||
| Hill et al., 2019 | 0.039 | 0.072 | −0.103 to 0.181 | 14.47 | ||
| Braun et al., 2008 | 0.139 | 0.310 | −0.490 to 0.767 | 12.11 | ||
| Total (random effects) | 0.282 | 0.234 | −0.286 to 0.624 | 100.00 | ||
| Heterogeneity: Q = 9.14; DF = 7; P = 0.71; I2 = 0.00% | ||||||
| Overall PPD Reduction for PG Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.361 |  |
| Raj et al., 2016 | 0.669 | 0.441 | −0.258 to 1.595 | 18.89 | ||
| Vidal et al., 2017 | −0.060 | 0.322 | −0.714 to 0.593 | 25.59 | ||
| Theodoro et al., 2017 | −0.127 | 0.374 | −0.897 to 0.643 | 22.43 | ||
| Joseph et al., 2014 | 0.556 | 0.215 | 0.127 to 0.984 | 33.19 | ||
| Total (random effects) | 0.257 | 0.278 | −0.230 to 0.683 | 100.00 | ||
| Heterogeneity: Q = 8.87; DF = 3; P = 0.22; I2 = 0.00% | ||||||
| Overall CAL Gain for SM Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.166 | 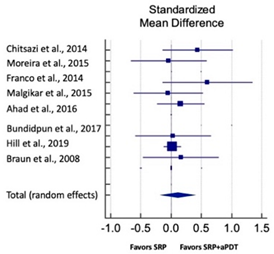 |
| Chitsazi et al., 2014 | 0.439 | 0.287 | −0.140 to 1.017 | 5.52 | ||
| Moreira et al., 2015 | −0.040 | 0.310 | −0.667 to 0.588 | 5.02 | ||
| Franco et al., 2014 | 0.601 | 0.364 | −0.144 to 1.346 | 4.20 | ||
| Malgikar et al., 2015 | −0.048 | 0.284 | −0.620 to 0.523 | 5.60 | ||
| Ahad et al., 2016 | 0.158 | 0.199 | −0.237 to 0.552 | 10.35 | ||
| Bundidpun et al., 2017 | 0.032 | 0.310 | −0.595 to 0.660 | 5.02 | ||
| Hill et al., 2019 | 0.019 | 0.072 | −0.123 to 0.161 | 59.29 | ||
| Braun et al., 2008 | 0.161 | 0.310 | −0.468 to 0.789 | 5.01 | ||
| Total (random effects) | 0.162 | 0.253 | −0.326 to 0.406 | 100.00 | ||
| Heterogeneity: Q = 8.40; DF = 7; P = 0.625; I2 = 0.00% | ||||||
| Overall CAL Gain for PG Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.234 | 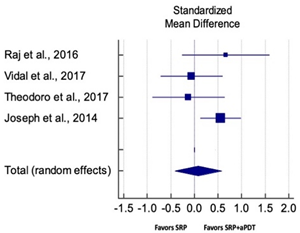 |
| Raj et al., 2016 | 0.669 | 0.441 | −0.258 to 1.595 | 18.89 | ||
| Vidal et al., 2017 | −0.102 | 0.372 | −0.514 to 0.793 | 25.59 | ||
| Theodoro et al., 2017 | −0.106 | 0.374 | −0.997 to 0.743 | 22.43 | ||
| Joseph et al., 2014 | 0.456 | 0.255 | −0.120 to 0.673 | 33.19 | ||
| Total (random effects) | 0.227 | 0.352 | −0.420 to 0.673 | 100.00 | ||
| Heterogeneity: Q = 9.7; DF = 3; P = 0.22; I2 = 0.00% | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalvi, S.; Benedicenti, S.; Sălăgean, T.; Bordea, I.R.; Hanna, R. Effectiveness of Antimicrobial Photodynamic Therapy in the Treatment of Periodontitis: A Systematic Review and Meta-Analysis of In Vivo Human Randomized Controlled Clinical Trials. Pharmaceutics 2021, 13, 836. https://doi.org/10.3390/pharmaceutics13060836
Dalvi S, Benedicenti S, Sălăgean T, Bordea IR, Hanna R. Effectiveness of Antimicrobial Photodynamic Therapy in the Treatment of Periodontitis: A Systematic Review and Meta-Analysis of In Vivo Human Randomized Controlled Clinical Trials. Pharmaceutics. 2021; 13(6):836. https://doi.org/10.3390/pharmaceutics13060836
Chicago/Turabian StyleDalvi, Snehal, Stefano Benedicenti, Tudor Sălăgean, Ioana Roxana Bordea, and Reem Hanna. 2021. "Effectiveness of Antimicrobial Photodynamic Therapy in the Treatment of Periodontitis: A Systematic Review and Meta-Analysis of In Vivo Human Randomized Controlled Clinical Trials" Pharmaceutics 13, no. 6: 836. https://doi.org/10.3390/pharmaceutics13060836
APA StyleDalvi, S., Benedicenti, S., Sălăgean, T., Bordea, I. R., & Hanna, R. (2021). Effectiveness of Antimicrobial Photodynamic Therapy in the Treatment of Periodontitis: A Systematic Review and Meta-Analysis of In Vivo Human Randomized Controlled Clinical Trials. Pharmaceutics, 13(6), 836. https://doi.org/10.3390/pharmaceutics13060836