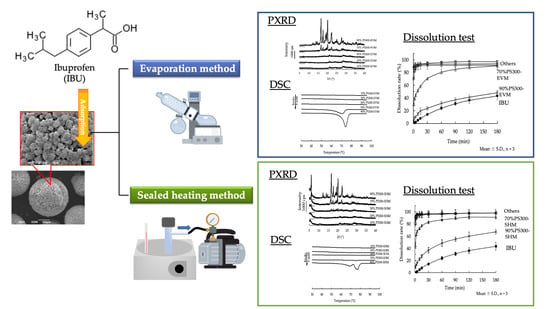
Solubility Enhancement of Ibuprofen by Adsorption onto Spherical Porous Calcium Silicate
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of Samples
2.2.1. Preparation of Physical Mixtures
2.2.2. Adsorption of IBU onto Drug Carriers
EV Method
SH Method
2.2.3. Preparation of IBU Calcium
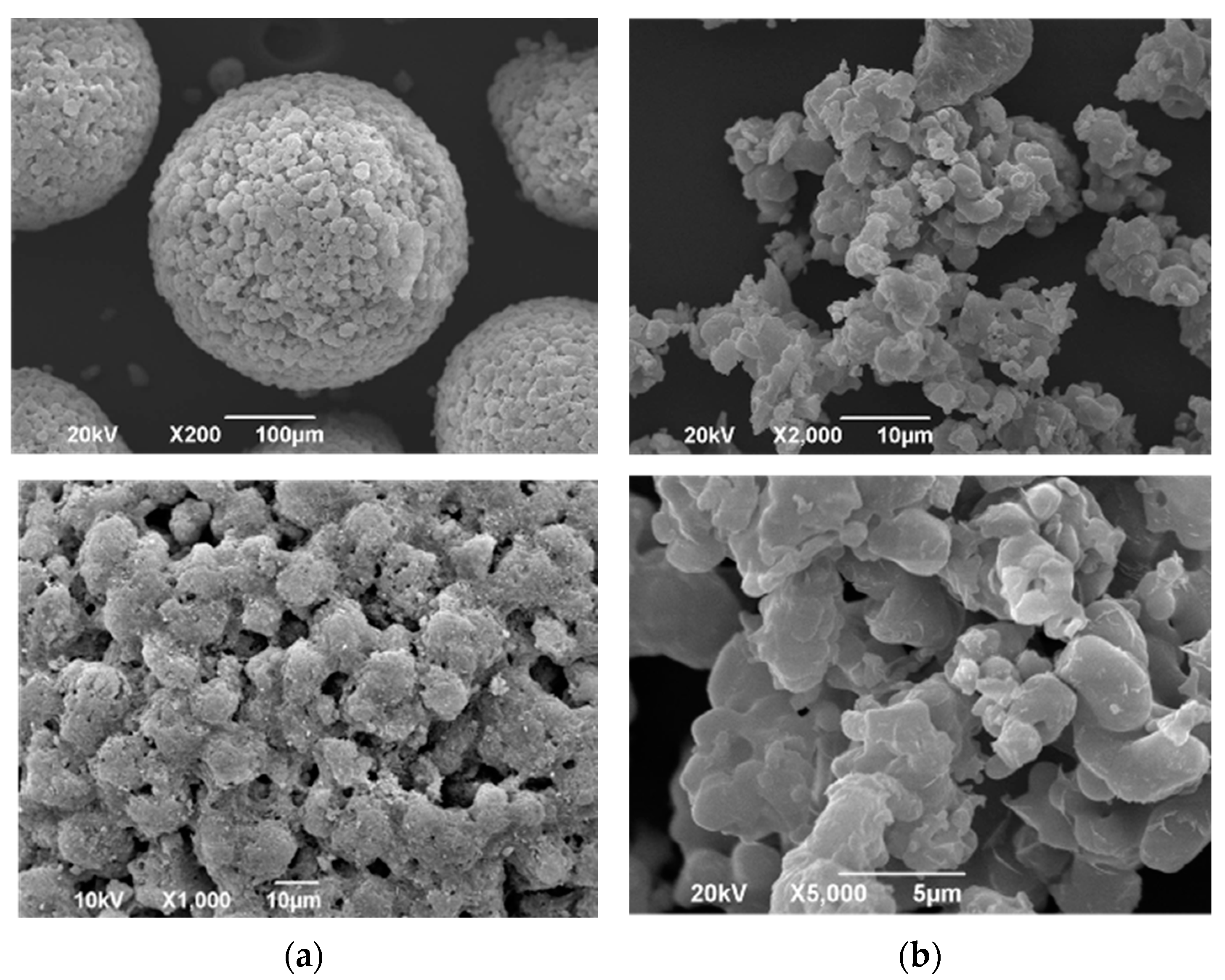
2.3. Physicochemical Properties of Various Samples
2.4. Powder X-ray Diffractometry (PXRD)
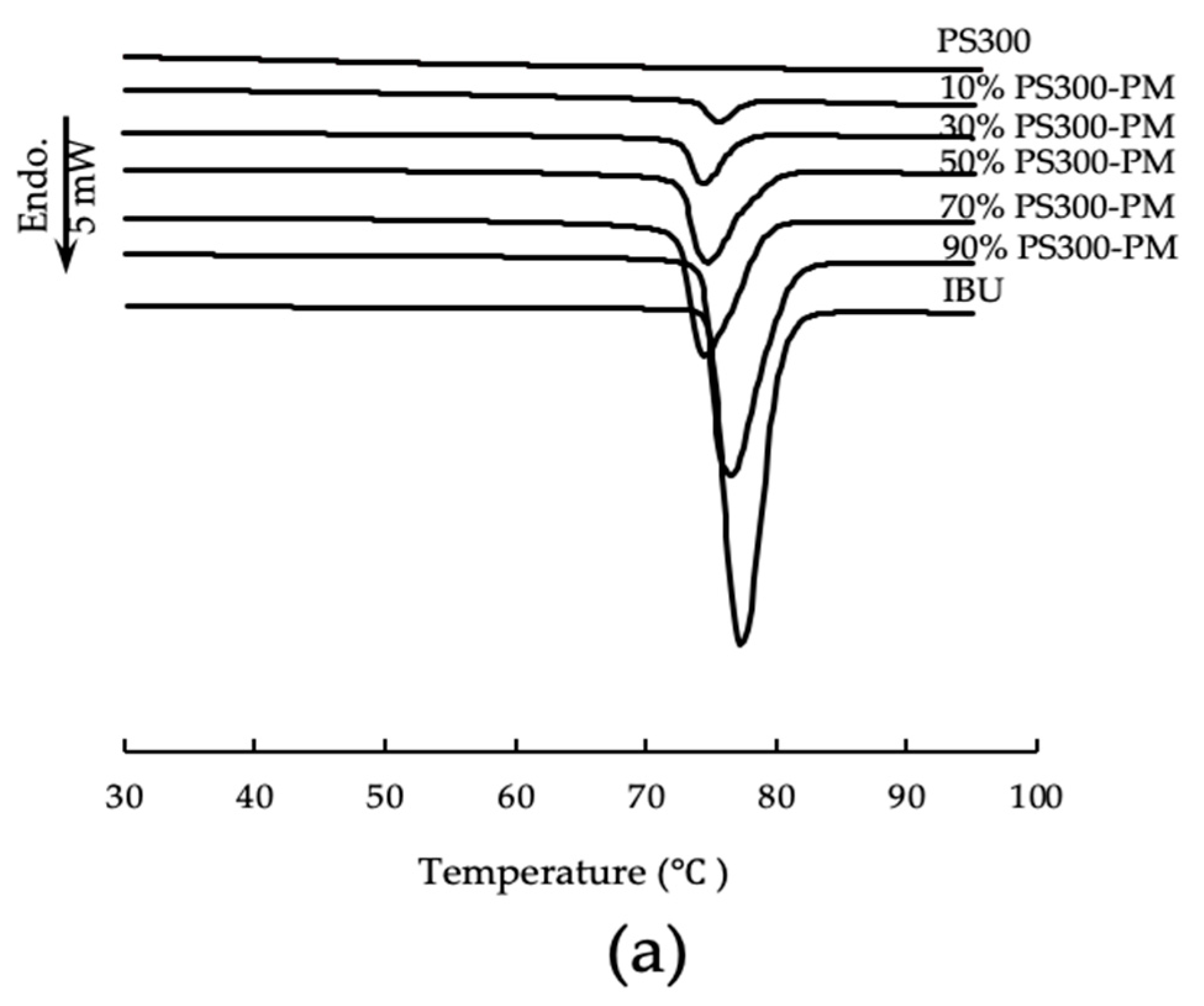
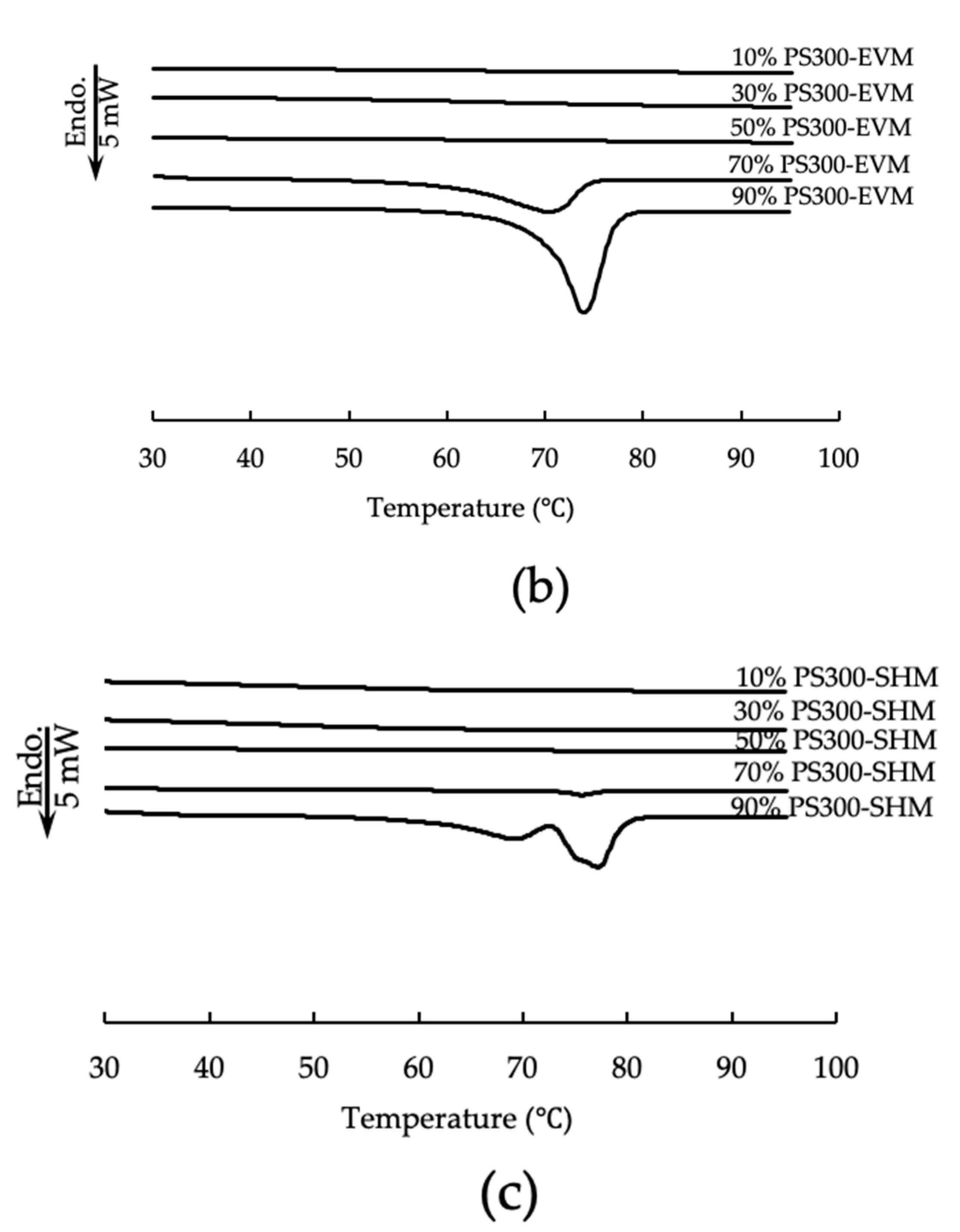
2.5. Differential Scanning Calorimetry (DSC)
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7. Dissolution Study
3. Results and Discussion
3.1. Physicochemical Properties of Various Samples
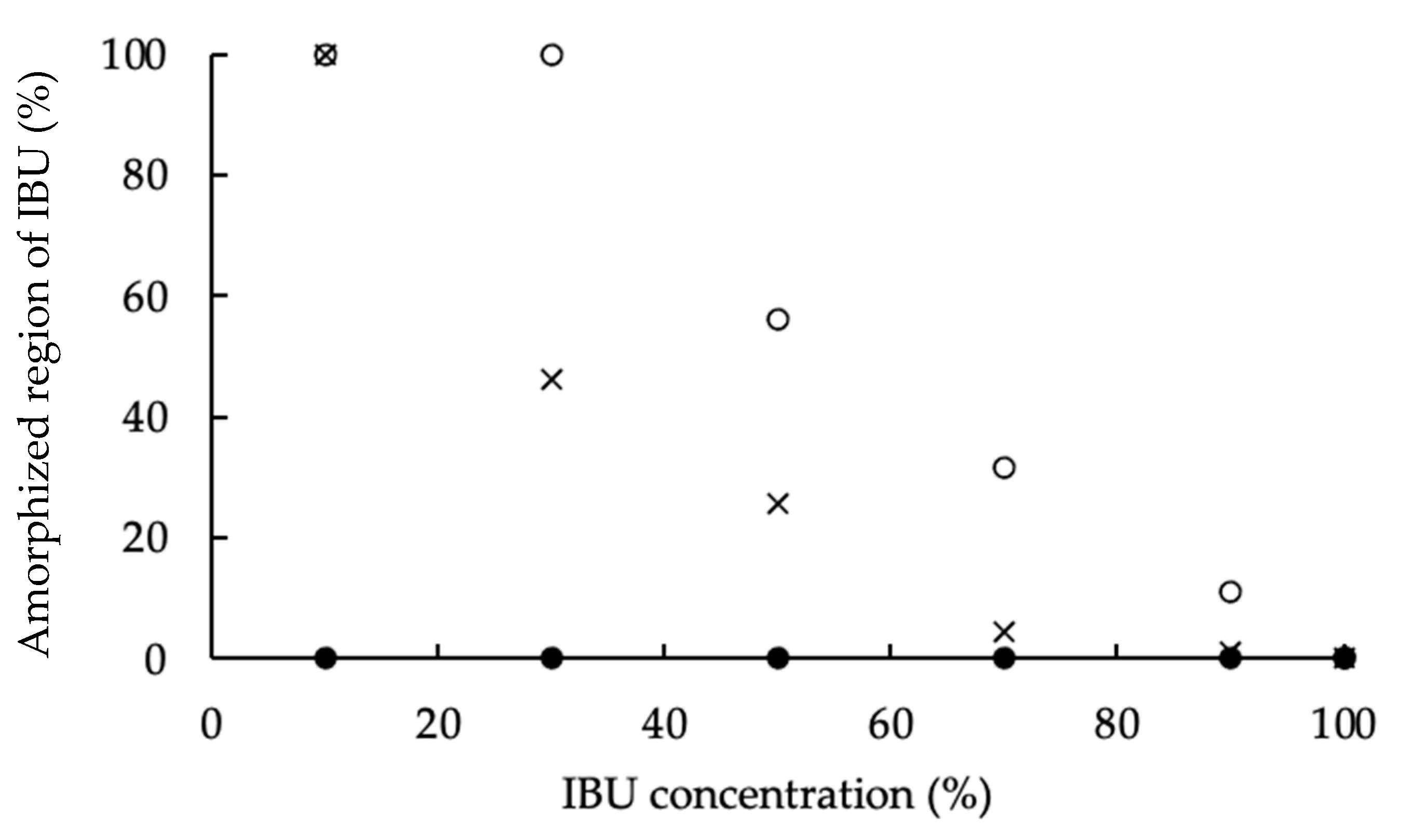
3.2. Powder X-ray Diffractometry (PXRD)
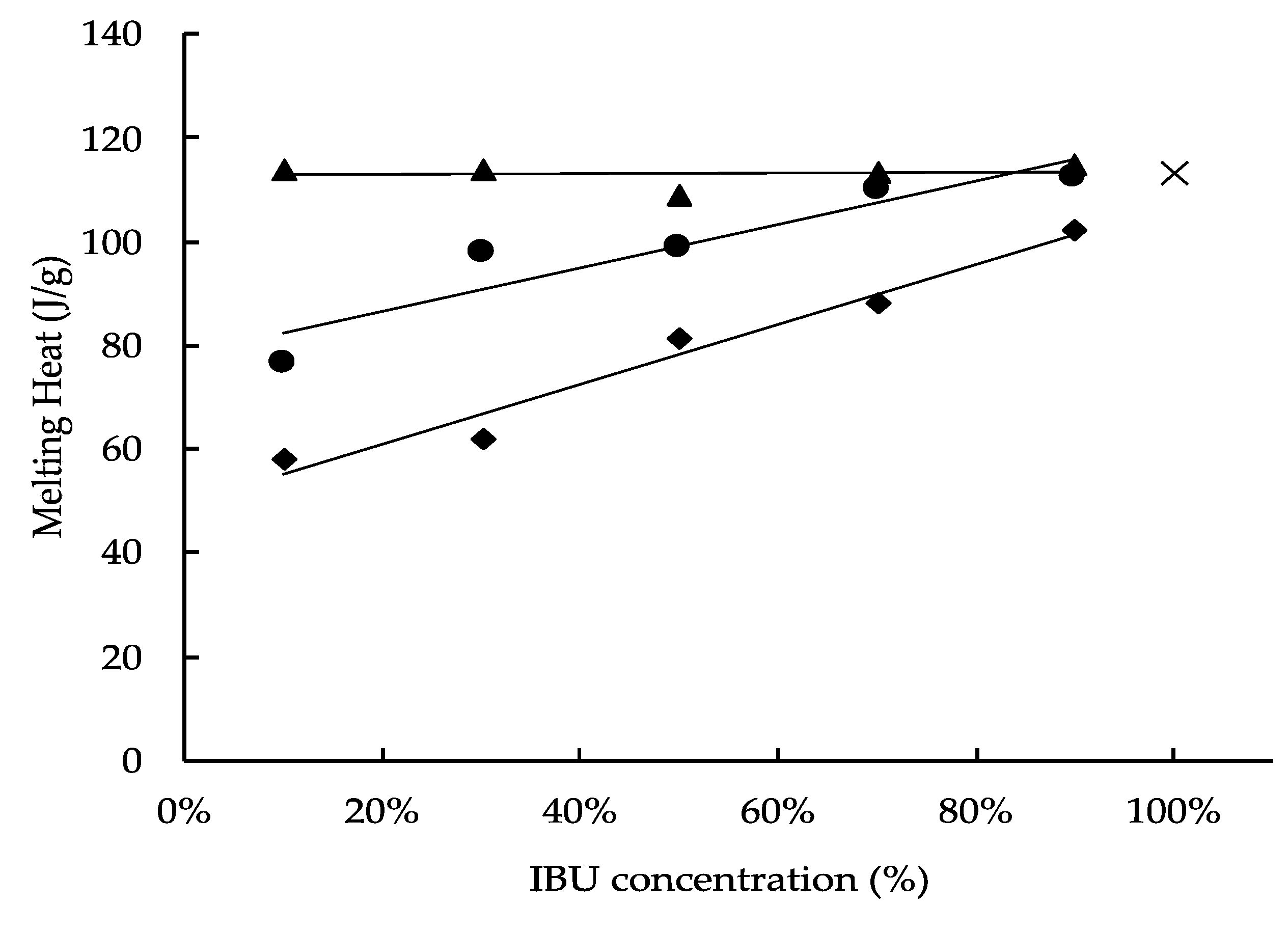
3.3. Differential Scanning Calorimetry (DSC)
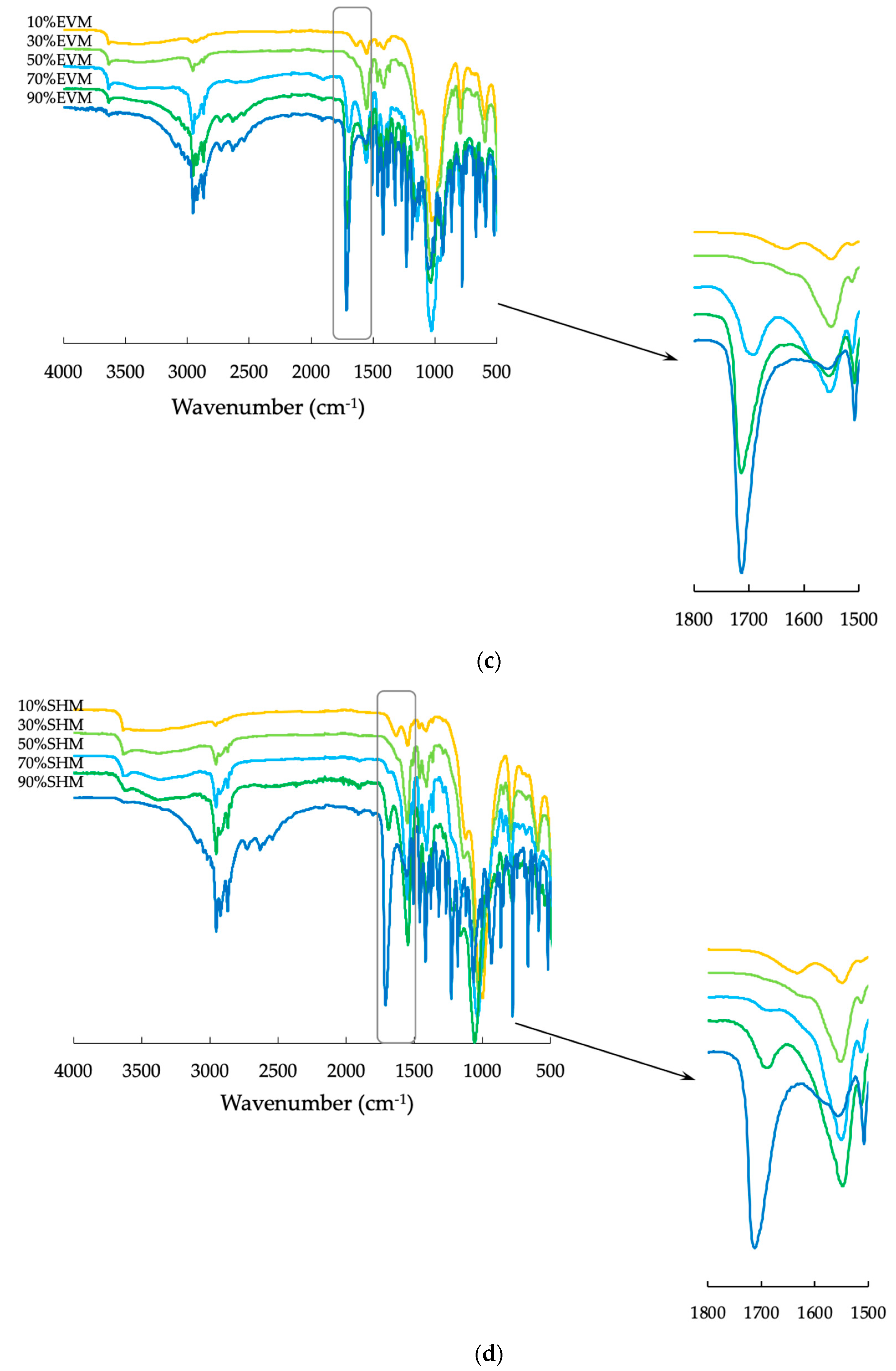
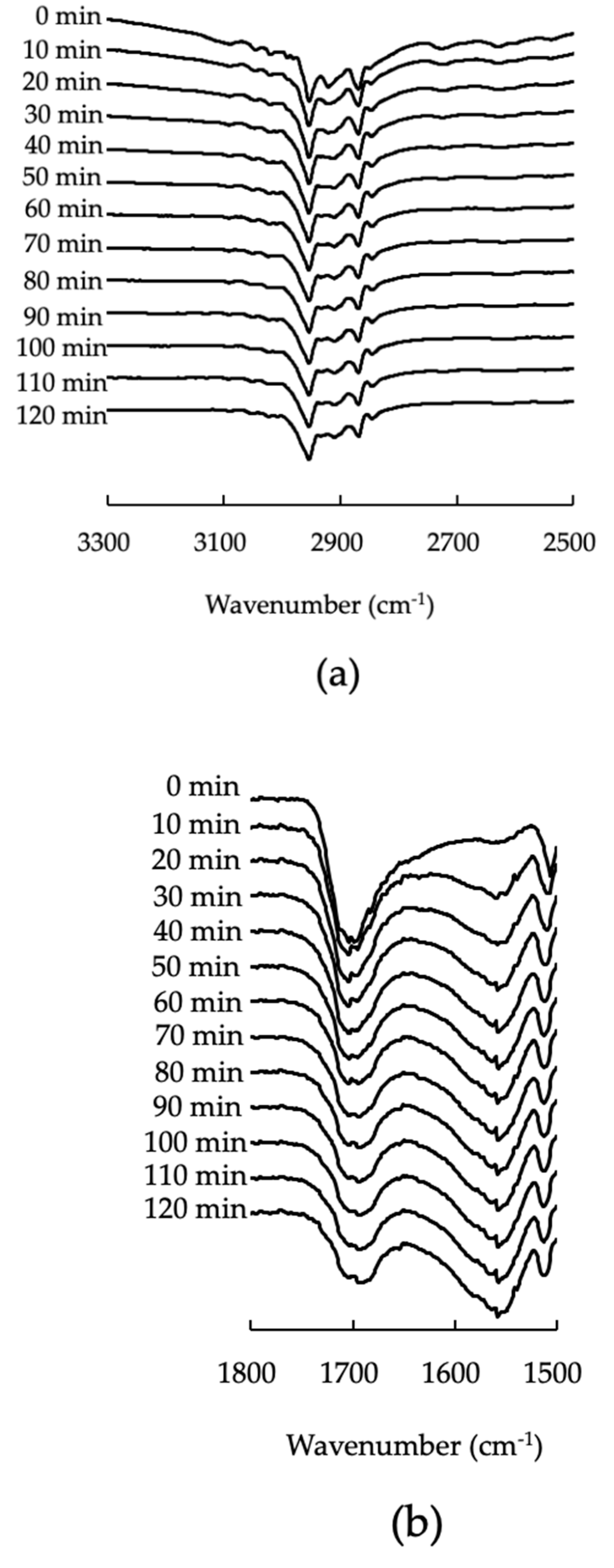
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
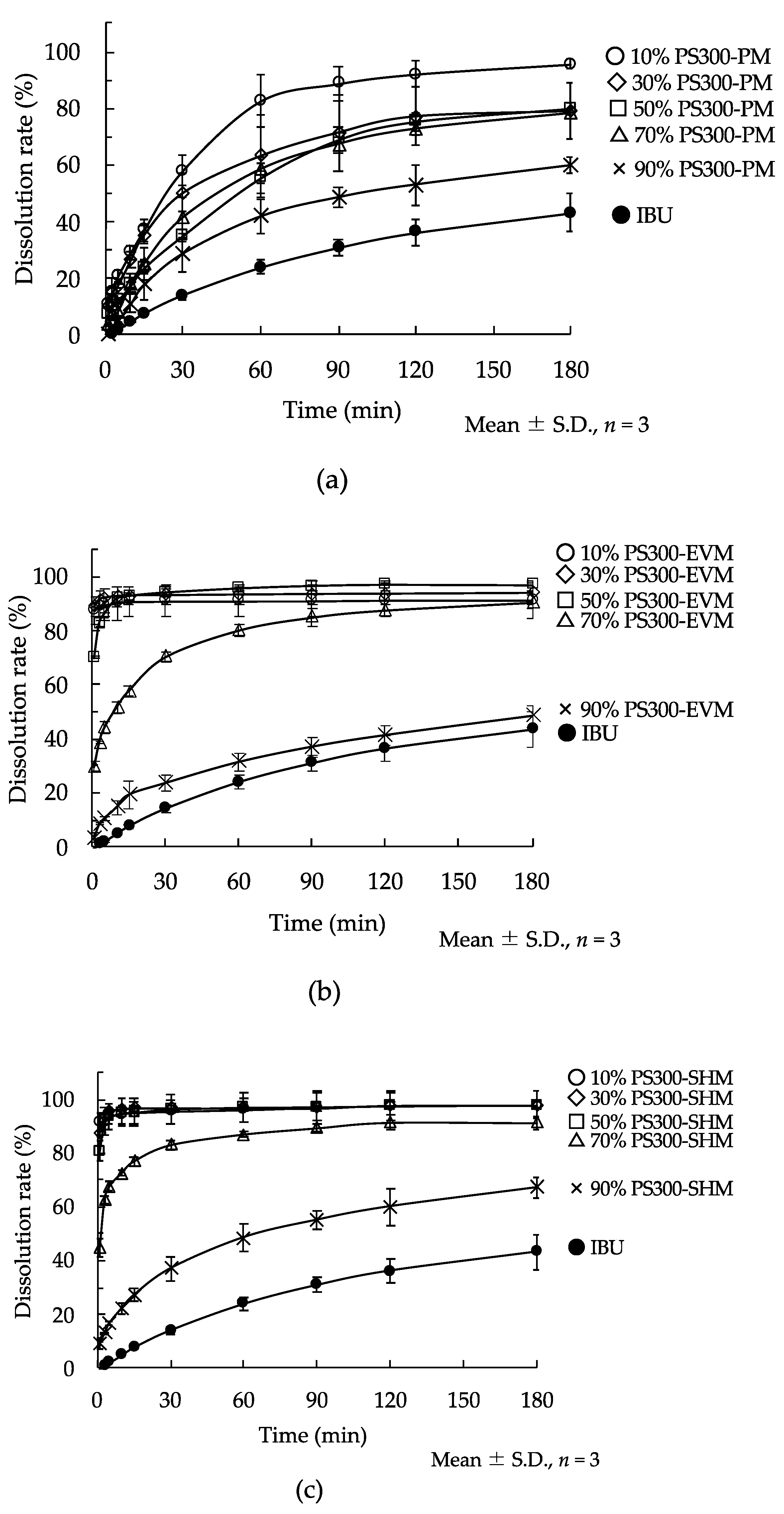
3.5. Dissolution Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jermain, S.V.; Brough, C.; Williams, R.O., III. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Giliyar, C.; Fickstad, D.T.; Tyavanagimatt, S. Challenges and opportunities in oral delivery of poorly water-soluble drugs. Drug Deliv. Technol. 2006, 6, 57–63. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M.; Muller, R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenization. Eur. J. Pharm. Biopharm. 2006, 6, 3–16. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Muller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Hickey, M.B.; Peterson, M.L.; Scoppettuolo, L.A.; Morrisette, S.L.; Vetter, A.; Guzman, H.; Remenar, J.F.; Zhang, Z.; Tawa, M.D.; Heley, S.; et al. Performance comparison of a co-crystal of carbamazepine with marketed product. Eur. J. Pharm. Biopharm. 2007, 67, 112–119. [Google Scholar] [CrossRef]
- Childs, S.L.; Chyall, L.J.; Dunlap, J.T.; Smolenskaya, V.N.; Stahly, B.C.; Stahly, G.P. Crystal engineering approach to forming cocrystals of amine hydrochlorides with organic acids. Molecular complexes of fluoxetine hydrochloride with benzoic, succinic, and fumaric acids. J. Am. Chem. Soc. 2004, 126, 13335–13342. [Google Scholar] [CrossRef]
- Remenar, J.F.; Morissette, S.L.; Peterson, M.L.; Moulton, B.; MacPhee, J.M.; Guzman, H.G.; Almarsson, O. Crystal engineering of novel cocrystals of a triazole drug with 1,4-dicarboxylic acids. J. Am. Chem. Soc. 2003, 125, 8456–8457. [Google Scholar] [CrossRef]
- Fresta, M.; Villari, A.; Puglisi, G.; Cavallaro, G. 5-fluorouracil-various kinds of loaded liposomes-encapsulation efficiency, storage stability and fusogenic propertie. Int. J. Pharm. 1993, 99, 145–156. [Google Scholar] [CrossRef]
- Dupont, B. Overview of the lipid formulations of amphotericin B. J. Antimicrob. Chemother. 2002, 49, 31–36. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Weston, N.; Coombes, A.G.A.; Fitzgerald, M.; Perrie, Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.G.; Choi, H.G.; Shin, H.J.; Park, K.M.; Lim, S.J.; Hwang, K.J.; Kim, C.K. Physicochemical characterization and evaluation of a microemulsion system for oral delivery of cyclosporin A. Int. J. Pharm. 1998, 161, 75–86. [Google Scholar] [CrossRef]
- Yin, Y.M.; Cui, F.D.; Mu, C.F.; Choi, M.K.; Kim, J.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Docetaxel microemulsion for enhanced oral boavailability: Preparation and in vitro and in vivo evaluation. J. Control. Release 2009, 140, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.F.; Lu, C.H.; Zhou, A.; Min, Z.W.; Zhang, Y.L. Enhanced oral bioavailability of puerarin using microemulsion vehicle. Drug Dev. Ind. Pharm. 2009, 35, 138–144. [Google Scholar] [CrossRef]
- Shah, N.H.; Carvajal, M.T.; Patel, C.I.; Infeld, M.H.; Malick, A.W. Self-emulsifying drug-delivery systems (Sedds) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int. J. Pharm. 1994, 106, 15–23. [Google Scholar] [CrossRef]
- Arida, A.I.; Al-Tabakha, M.M.; Hamoury, H.A.J. Improving the high variable bioavailability of griseofulvin by SEDDS. Chem. Pharm. Bull. 2007, 55, 1713–1719. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Lee, B.J.; Oh, D.H.; Kim, J.O.; Hong, M.J.; Jee, J.P.; Kim, J.A.; Yoo, B.K.; Woo, J.S.; Ypng, C.S.; et al. Enhanced oral bioavailability of dexibuprofen by a novel solid Self-emulsifying drug delivery system (SEDDS). Eur. J. Pharm. Biopharm. 2009, 72, 539–545. [Google Scholar] [CrossRef]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef]
- Patel, D.; Sawant, K.K. Oral bioavailability enhancement of acyclovir by self-microemulsifying drug delivery systems (SMEDDS). Drug Dev. Ind. Pharm. 2007, 33, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Ranpise, N.S.; Kulkarni, N.S.; Mair, P.D.; Ranade, A.N. Improvement of water solubility and in vitro dissolution rate of aceclofenac by complexation with beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin. Pharm. Dev. Technol. 2010, 15, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Formulation and biological evaluation of glimepiride-cyclodextrin-polymer systems. Int. J. Pharm. 2006, 309, 129–138. [Google Scholar] [CrossRef]
- Sencar-Bozic, P.; Srcic, S.; Knez, Z.; Kerc, J. Improvement of nifedipine dis- solution characteristics using supercritical CO2. Int. J. Pharm. 1997, 148, 123–130. [Google Scholar] [CrossRef]
- Kerc, J.; Srcic, S.; Knez, Z.; Sencar-Bozic, P. Micronization of drug using supercritical carbon dioxide. Int. J. Pharm. 1999, 102, 33–39. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, M.S.; Park, H.J.; Jin, S.J.; Lee, S.; Hwang, S.J. Physicochemical properties and oral bioavailability of amorphous atorvastatin hemi-calcium using spray-drying and SAS process. Int. J. Pharm. 2008, 359, 211–219. [Google Scholar] [CrossRef]
- Lee, S.; Nam, K.; Kim, M.S.; Jun, S.W.; Park, J.-S.; Woo, J.S.; Hwang, S.-J. Preparation and characterization of solid dispersions of itraconazole by using aerosol solvent extraction system for improvement in drug solubility and bioavailability. Arch. Oharm. Res. 2005, 28, 866–874. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, L.L.; Wei, L.L.; Hong, G.; Nie, S.F.; Yang, X.G.; Tang, R.; Pan, W.S. Preparation of budesonide-poly (ethylene oxide) solid dispersions using supercritical fluid technology. Drug Dev. Ind. Pharm. 2007, 33, 959–966. [Google Scholar] [CrossRef]
- Rogers, T.L.; Nelsen, A.C.; Sarkari, M.; Young, T.J.; Johnston, K.P.; Williams, R.O., III. Enhanced aqueous dissolution of a poorly water soluble drug by novel particle engineering technology: Spray-freezing into liquid with atmospheric freeze-drying. Pharm. Res. 2003, 20, 485–493. [Google Scholar] [CrossRef]
- Yonemichi, E.; Oguchi, T.; Terada, K.; Yamamoto, K.; Nakai, Y. Acceleration of the addition reaction of succinic anhydride and p-nitroaniline in controlled glass solid dispersions. Chem. Pharm. Bull. 1989, 37, 3083–3087. [Google Scholar] [CrossRef][Green Version]
- Yuasa, H.; Asahi, D.; Takashima, Y.; Kanaya, Y.; Shinozawa, K. Application of calcium silicate for medicinal preparation. I. Solid preparation adsorbing an oily medicine to calcium silicate. Chem. Pharm. Bull. 1994, 42, 2327–2331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kinoshita, M.; Baba, K.; Nagayasu, A.; Yamabe, K.; Shimooka, T.; Takeichi, Y.; Azuma, M.; Houchi, H.; Minakuchi, K. Improvement of solubility and oral bioavailability of a poorly water-soluble drug, TAS-301, by its melt-adsorption on a porous calcium silicate. J. Pharm. Sci. 2002, 91, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sher, P.; Badve, S.; Pawar, A.P. Adsorption of Meloxicam on Porous Calcium Silicate: Characterization and Tablet Formulation. AAPS Pharm. Sci. Tech. 2005, 6, E618–E625. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Hirai, N.; Takatani-Nakase, T.; Takahashi, K. Photostable solid dispersion of nifedipine by porous calcium silicate. Cham. Pharm. Bull. 2016, 64, 1218–1221. [Google Scholar] [CrossRef]
- Mehrabifar, A.; Rahmati, M. Significany increasing of gastrointestinal dissolution of aceclofenac via Florite. MedBioTech J. 2017, 1, 9–14. [Google Scholar] [CrossRef]
- Ali, A.S.; Yamamoto, K.; El-Sayed, A.M.; Habib, F.S.; Nakai, Y. Molecular behavior of flufenamic acid in physical and ground mixtures with florite. Cham. Pharm. Bull. 1992, 40, 1289–1294. [Google Scholar] [CrossRef][Green Version]
- Grodowska, K.; Parczewski, A. Organic Solvents in the pharmaceutical industry. Acta Pol. Pharm. Drug Res. 2010, 67, 3–12. [Google Scholar]
- Kawano, Y.; Chen, S.; Hanawa, T. Adsorption of a poorly water-soluble drug onto porous calcium silicate by the sealed heating method. Int. J. Pharm. 2020, 587, 119637. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Hirai, N.; Takatani-Nakase, T.; Takahashi, K. Preparation and evaluation of solid dispersion tablets by a simple and manufacturable wet granulation method using porous calcium silicate. Cham. Pharm. Bull. 2016, 64, 311–318. [Google Scholar] [CrossRef][Green Version]
- Fujimoto, Y.; Hirai, N.; Takatani-Nakase, T.; Takahashi, K. Novel tablet formulation of amorphous indomethacin using wet granulation with a high-speed mixer granulator combined with porous calcium silicate. J. Drug. Deliv. Sci. Technol. 2016, 33, 51–57. [Google Scholar] [CrossRef]
- Hirai, N.; Takatani-Nakase, T.; Takahashi, K. Preparation and evaluation of Ibuprofen solid dispersion tablets with improved dissolution and less sticking using porous calcium silicate. J. Pharm. Pharmacol. 2018, 6, 8. [Google Scholar]
- Pan, X.; Julian, T.; Augsburger, L. Increasing the dissolution rate of a low-solubility drug through a crystalline-amorphous transition: A case study with indomethacin. Drug Dev. Ind. Pharm. 2008, 34, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Hugo, M.; Kunath, K.; Dressman, J. Selection of excipient, solvent and packaging to optimize the performance of spray-dried formulations: Case example fenofibrate. Drug Dev. Ind. Pharm. 2013, 39, 402–412. [Google Scholar] [CrossRef]
- Osawa, M.; Higashi, K.; Yamashita, J.; Moribe, K.; Yamamoto, K. Characterization of sulindac solid dispersion with hydroxypropyl cellulose prepared by hot melt extrusion. J. Pharm. Sci. Technol. Jpn. 2014, 74, 160–169. [Google Scholar] [CrossRef]
- Zhang, G.G.; Paspal, S.Y.; Suryanarayanan, R.; Grant, D.J. Racemic species of sodium ibuprofen: Characterization and polymorphic relationships. J. Pharm. Sci. 2003, 92, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Yamamoto, K.; Tarada, K.; Ichikawa, J. Interaction of medicinals and porous powder. I. Anomalous thermal behavior of porous glass mixture. Chem. Pharm. Bull. 1984, 32, 4566–4571. [Google Scholar] [CrossRef]
- Nakai, Y.; Yamamoto, K.; Izumikawa, S. Interaction of medicinals and porous powder. III. Effect of pore diameter of porous glass powder on crystalline properties. Chem. Pharm. Bull. 1989, 37, 435–438. [Google Scholar] [CrossRef][Green Version]


| Content of IBU | |||||
|---|---|---|---|---|---|
| wt/wt% | 10 | 30 | 50 | 70 | 90 |
| Weight Mixing Ratio | |||||
| PS300 | 9 | 7 | 1 | 3 | 1 |
| IBU | 1 | 3 | 1 | 7 | 9 |
| Carrier | Average Particle Diameter (μm) | Angle of Repose (°) | Density (g/cm3) | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|---|
| PS300 | 280.20 | 18.0 | 2.6085 | 177.20 | 0.723 |
| CaSiO3 | 14.82 | 47.9 | 2.5810 | 1.33 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawano, Y.; Chen, S.; Hanawa, T. Solubility Enhancement of Ibuprofen by Adsorption onto Spherical Porous Calcium Silicate. Pharmaceutics 2021, 13, 767. https://doi.org/10.3390/pharmaceutics13060767
Kawano Y, Chen S, Hanawa T. Solubility Enhancement of Ibuprofen by Adsorption onto Spherical Porous Calcium Silicate. Pharmaceutics. 2021; 13(6):767. https://doi.org/10.3390/pharmaceutics13060767
Chicago/Turabian StyleKawano, Yayoi, Shiyang Chen, and Takehisa Hanawa. 2021. "Solubility Enhancement of Ibuprofen by Adsorption onto Spherical Porous Calcium Silicate" Pharmaceutics 13, no. 6: 767. https://doi.org/10.3390/pharmaceutics13060767
APA StyleKawano, Y., Chen, S., & Hanawa, T. (2021). Solubility Enhancement of Ibuprofen by Adsorption onto Spherical Porous Calcium Silicate. Pharmaceutics, 13(6), 767. https://doi.org/10.3390/pharmaceutics13060767