RETRACTED: Investigation of the Potential of Nebivolol Hydrochloride-Loaded Chitosomal Systems for Tissue Regeneration: In Vitro Characterization and In Vivo Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NVH-Loaded Chitosomes Using a Full Factorial Design
2.3. Characterization of NVH-Loaded Chitosomes
2.3.1. Determination of NVH Entrapment Efficiency Percentage (EE%)
2.3.2. Determination of Particle Size Distribution
2.3.3. In Vitro Release of the Selected NVH-Loaded Chitosomes
2.3.4. Zeta Potential of the Selected NVH-Loaded Chitosomes
2.3.5. Transmission Electron Microscopy (TEM) of the Selected NVH-Loaded Chitosomes
2.3.6. Differential Scanning Calorimetry (DSC) of the Selected NVH-Loaded Chitosomes
2.3.7. X-ray Diffractometry (XRD) of the Selected NVH-Loaded Chitosomes
2.3.8. Effect of Storage on the Selected NVH-Loaded Chitosomes
2.4. Cell Culture Study and In Vitro Evaluation of Human Fibroblast Cell Proliferation
2.4.1. Sterilization of the Samples by Gamma Radiation
2.4.2. HDFa Cells Culture
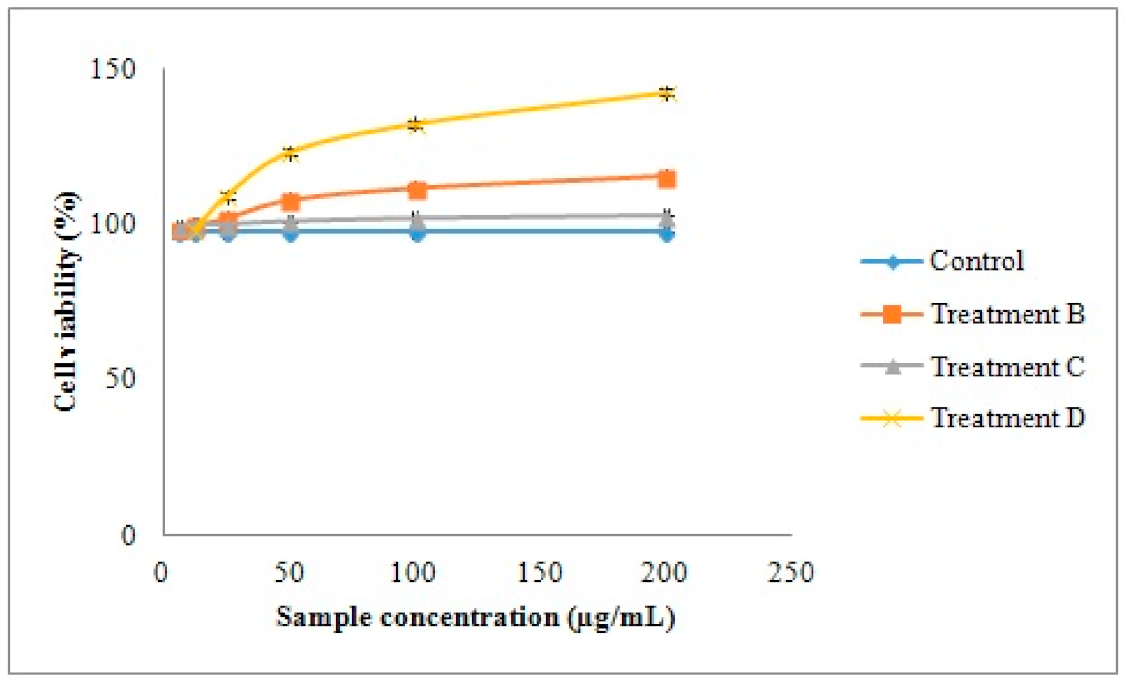
2.4.3. Effects of Samples on the HDFa Cell Viability
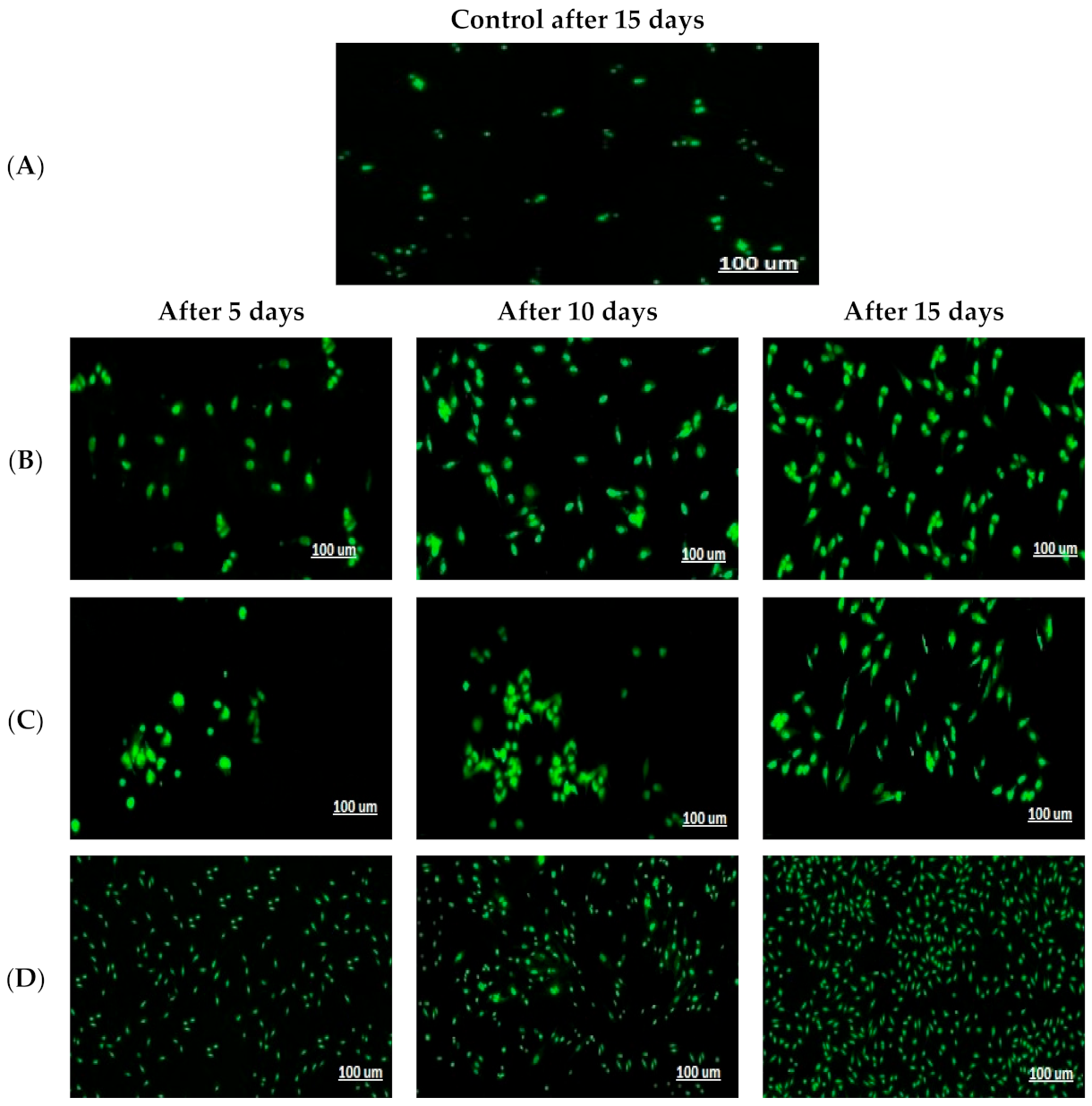
2.4.4. Pattern of HDFa Cell Proliferation
2.5. In Vivo Animal Study
2.5.1. Animal Model
2.5.2. Wound Induction Protocol
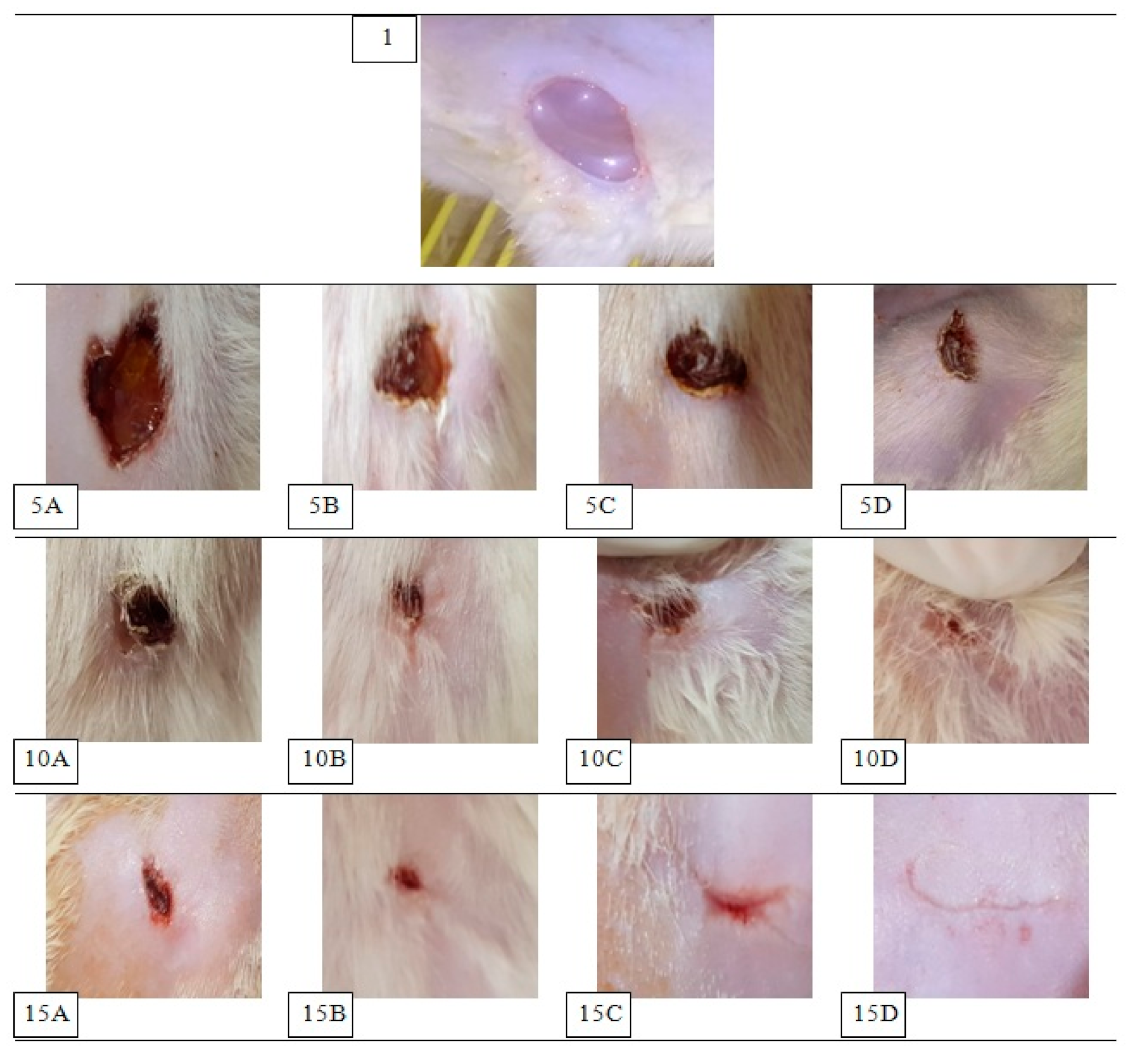
2.5.3. Evaluation of Wound Healing Process
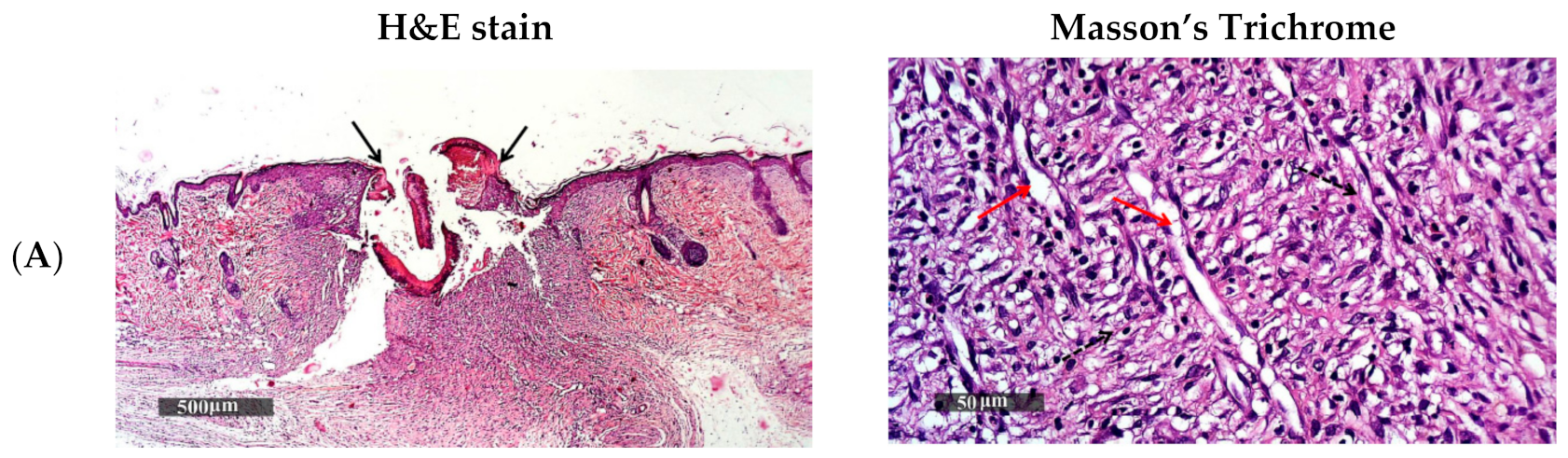
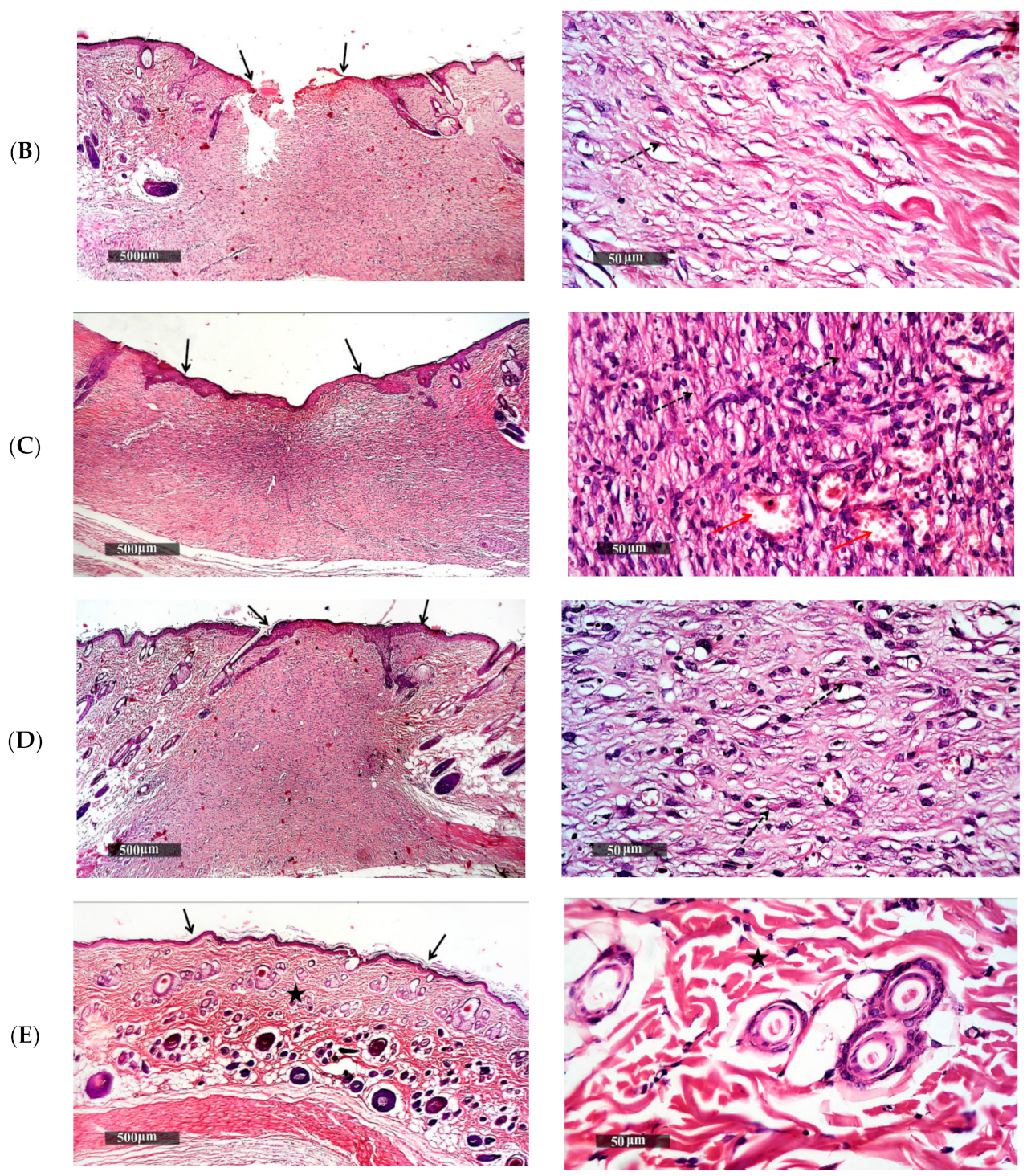
2.5.4. Histopathological Examination of Wound Granulating Tissue
2.5.5. Statistical Analysis
3. Results and Discussion
3.1. Preparation of NVH-Loaded Chitosomes
3.2. Characterization of NVH-Loaded Chitosomes
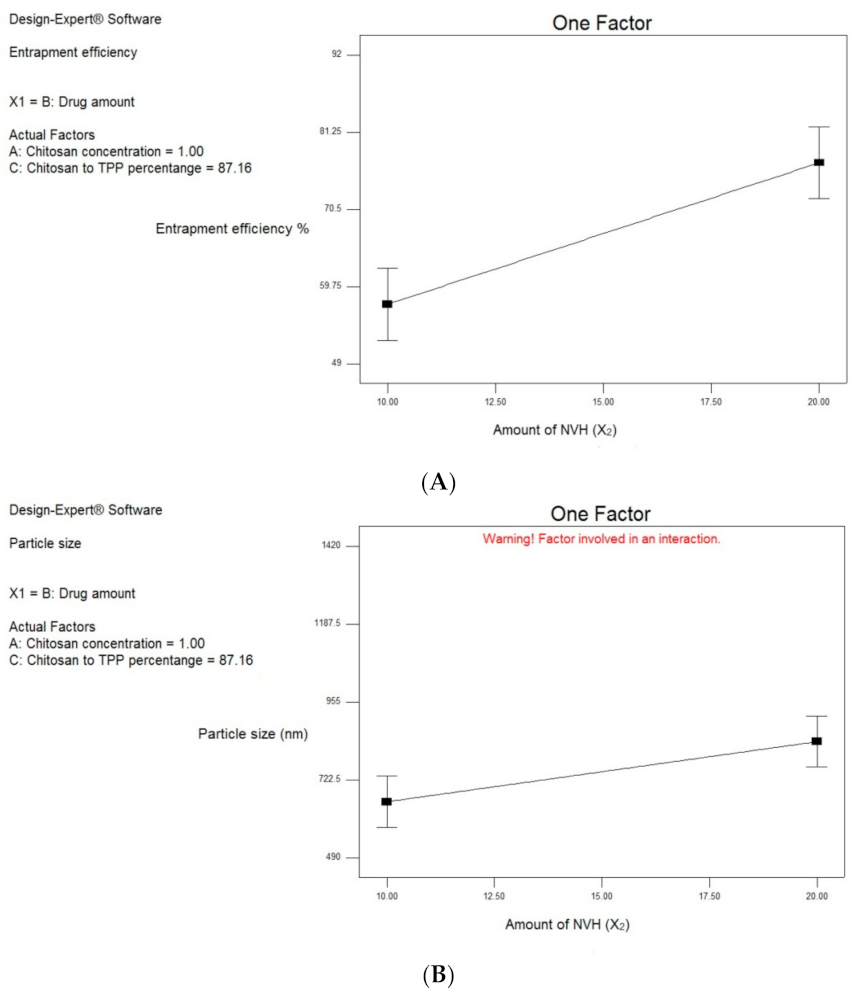
3.2.1. Effect of Formulation and Process Variables on EE% (Y1)
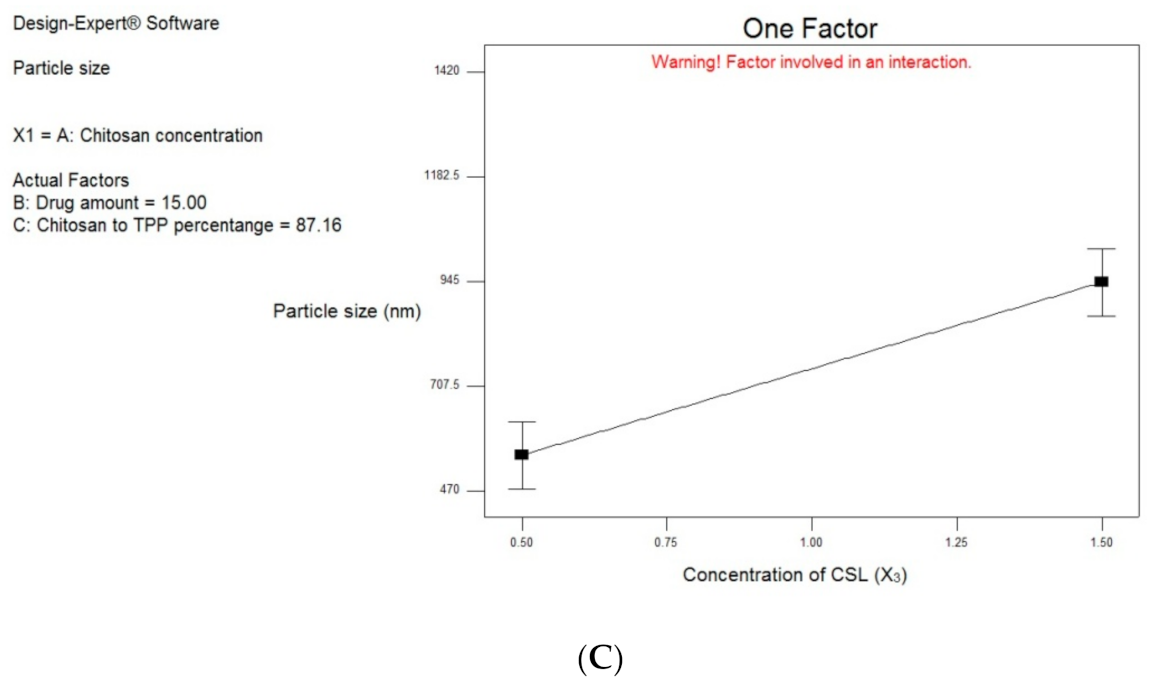
3.2.2. Effect of Formulation and Process Variables on Particle Size Distribution
3.3. Characterization of the Best Achieved NVH-Loaded Chitosomes
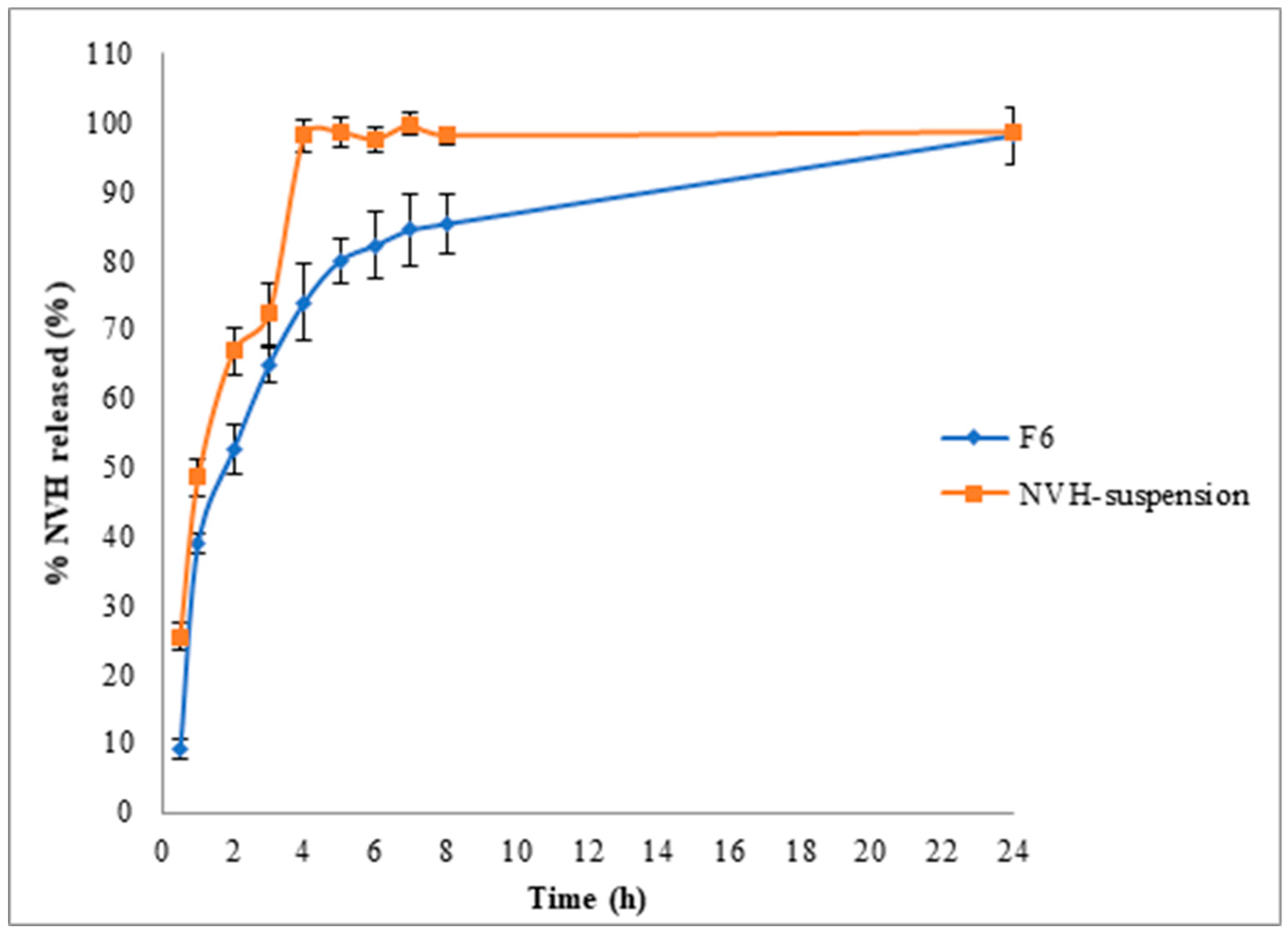
3.3.1. In Vitro Release Study
3.3.2. Zeta Potential Determination
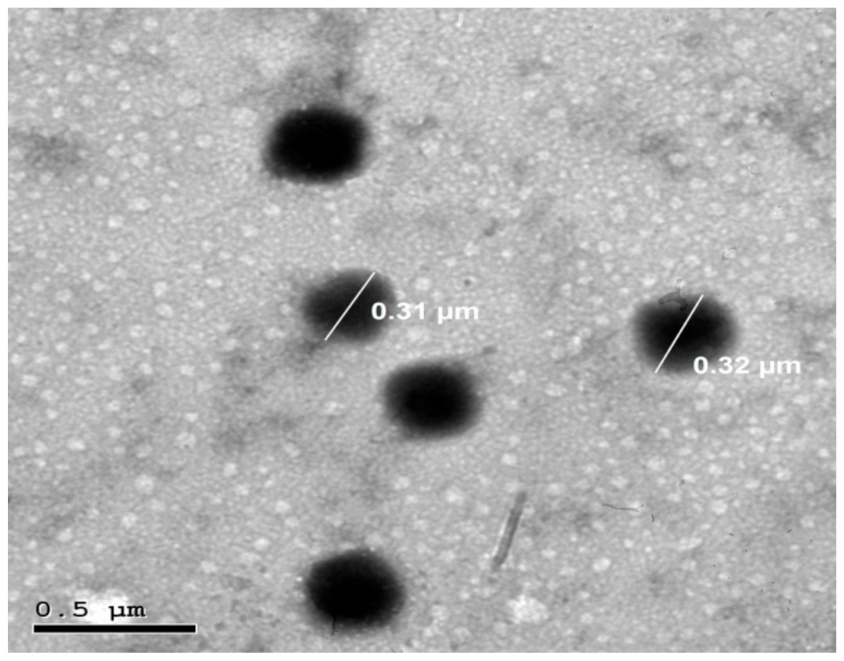
3.3.3. Transmission Electron Microscopy (TEM)
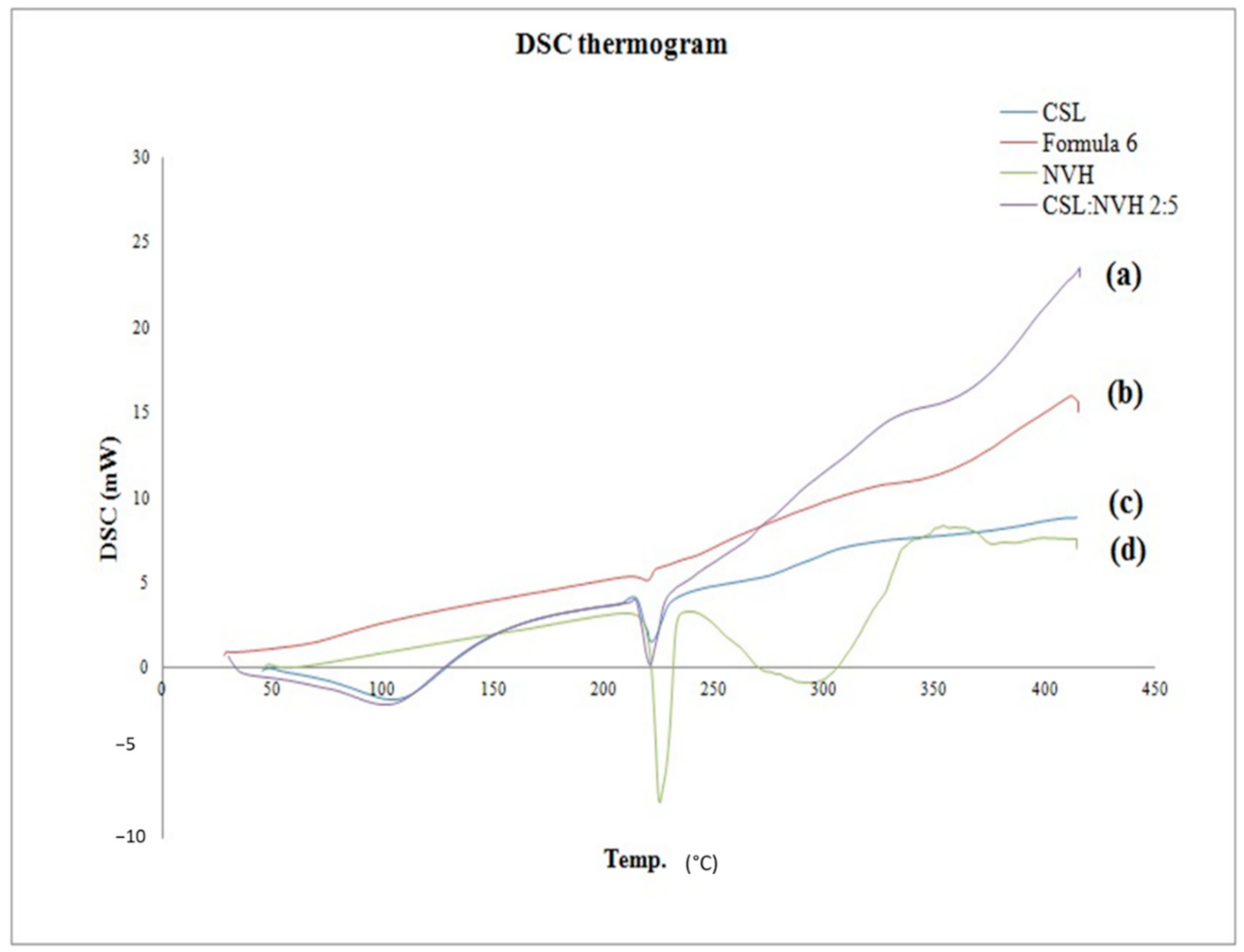
3.3.4. Differential Scanning Calorimetry (DSC)
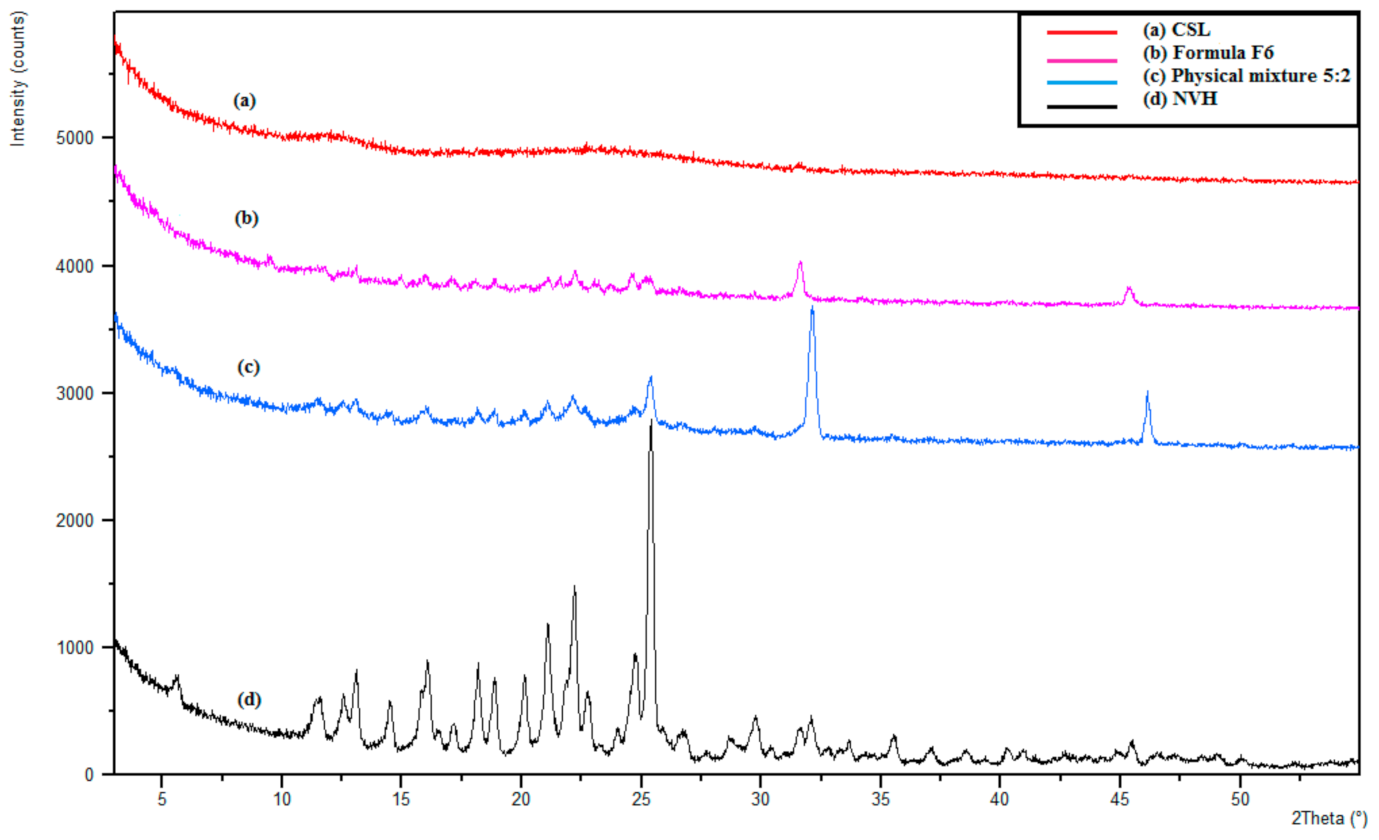
3.3.5. X-Ray Diffractometry (XRD)
3.3.6. Effect of Storage
3.4. Cell Culture Study and In Vitro Evaluation of Human Fibroblast Cell Proliferation
3.5. In Vivo Animal Studies
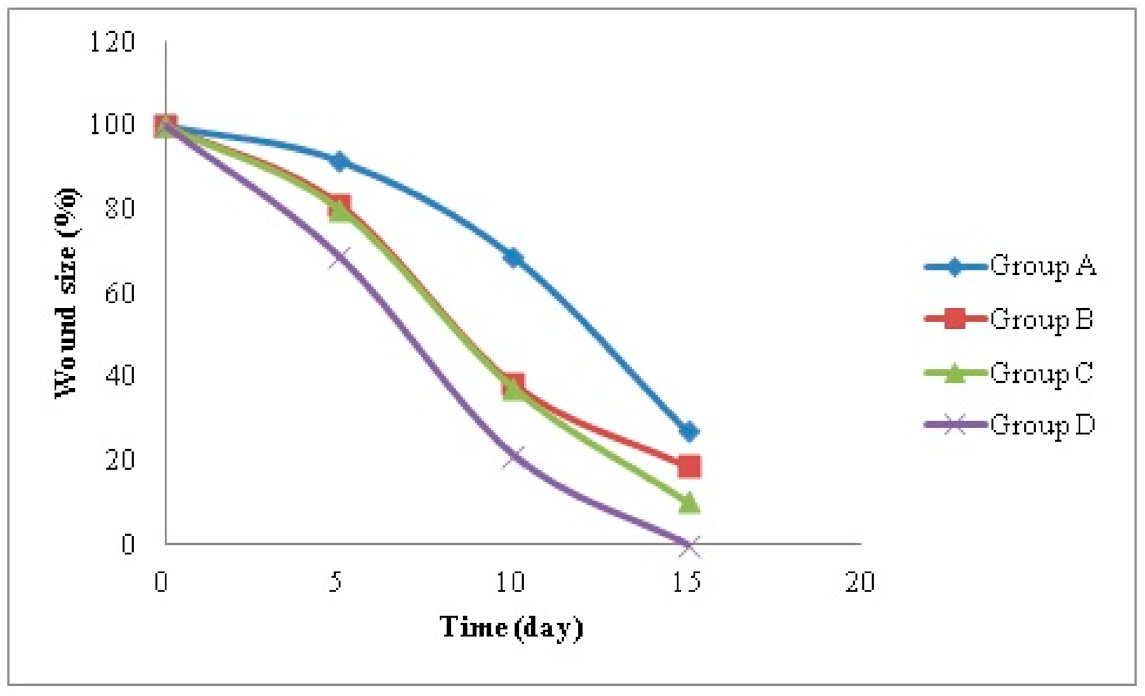
3.5.1. Assessment of Wound Healing
3.5.2. Histopathological Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frank, S.; Kämpfer, H.; Wetzler, C.; Pfeilschifter, J. Nitric oxide drives skin repair: Novel functions of an established mediator. Kidney Int. 2002, 61, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-D.; Chen, A.F. Nitric oxide: A newly discovered function on wound healing. Acta Pharmacol. Sin. 2005, 26, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, M.R.; Tantry, U.; Gross, S.S.; Wasserburg, H.L.; Barbul, A. Nitric oxide regulates wound healing. J. Surg. Res. 1996, 63, 237–240. [Google Scholar] [CrossRef]
- Schäffer, M.R.; Tantry, U.; Ahrendt, G.M.; Wasserkrug, H.L.; Barbul, A. Acute protein-calorie malnutrition impairs wound healing: A possible role of decreased wound nitric oxide synthesis. J. Am. Coll. Surg. 1997, 184, 37–43. [Google Scholar] [PubMed]
- Ulger, B.V.; Kapan, M.; Uslukaya, O.; Bozdag, Z.; Turkoglu, A.; Alabalık, U.; Onder, A. Comparing the effects of nebivolol and dexpanthenol on wound healing: An experimental study. Int. Wound J. 2014, 13, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R. Nebivolol: A novel beta-blocker with nitric oxide-induced vasodilatation. Vasc. Health Risk Manag. 2006, 2, 303–308. [Google Scholar] [CrossRef]
- Jatav, V.S.; Saggu, J.S.; Sharma, A.K.; Sharma, A.; Jat, R.K. Design, development and permeation studies of nebivolol hydrochloride from novel matrix type transdermal patches. Adv. Biomed. Res. 2013, 2, 62. [Google Scholar] [CrossRef]
- Hilas, O.; Ezzo, D. Nebivolol (bystolic), a novel Beta blocker for hypertension. Pharm. Ther. 2009, 34, 188–192. [Google Scholar]
- Toker, A.; Gulcan, E.; Toker, S.; Erbilen, E.; Aksakalli, E. Nebivolol might be Beneficial in Osteoporosis Treatment: A Hypothesis. Trop. J. Pharm. Res. 2009, 8, 8. [Google Scholar] [CrossRef]
- Weber, M. The role of the new beta-blockers in treating cardiovascular disease. Am. J. Hypertens. 2005, 18, 169S–176S. [Google Scholar]
- Pandit, A.; Patel, S.A.; Bhanushali, V.P.; Kulkarni, V.S.; Kakad, V.D. Nebivolol-Loaded Microsponge Gel for Healing of Diabetic Wound. AAPS PharmSciTech 2017, 18, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, L.; Dobrucki, L.W.; Szczepanska-Konkel, M.; Jankowski, M.; Martyniec, L.; Angielski, S.; Malinski, T. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: A novel mechanism for antihypertensive action. Circulation 2003, 107, 2747–2752. [Google Scholar] [CrossRef]
- Thadkala, K.; Sailu, C.; Aukunuru, J. Formulation, optimization and evaluation of oral nanosuspension tablets of nebivolol hydrochloride for enhancement of dissoluton rate. Pharm. Lett. 2015, 7, 71–84. [Google Scholar]
- Al-Dhubiab, B.; Nair, A.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of nebivolol hydrochloride nanocrystals impregnated buccal film. Farmacia 2019, 67, 282–289. [Google Scholar] [CrossRef]
- Gulcan, E.; Küçük, A.; Çayci, K.; Tosun, M.; Emre, H.; Koral, L.; Aktan, Y.; Avsar, U.; Aktan, Y. Topical effects of nebivolol on wounds in diabetic rats. Eur. J. Pharm. Sci. 2012, 47, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Mollnau, H.; Schulz, E.; Daiber, A.; Baldus, S.; Oelze, M.; August, M.; Wendt, M.; Walter, U.; Geiger, C.; Agrawal, R.; et al. Nebivolol Prevents Vascular NOS III Uncoupling in Experimental Hyperlipidemia and Inhibits NADPH Oxidase Activity in Inflammatory Cells. Arter. Thromb. Vasc. Biol. 2003, 23, 615–621. [Google Scholar] [CrossRef]
- Cosentino, F.; Bonetti, S.; Rehorik, R.; Eto, M.; Werner-Felmayer, G.; Volpe, M.; Lüscher, T.F. Nitric-oxide-mediated relaxations in salt-induced hypertension: Effect of chronic β1-selective receptor blockade. J. Hypertens. 2002, 20, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.A.M.; Corrales, F.Z. Chitosan, Chitosan Derivatives and their Biomedical Applications. In Biological Activities and Application of Marine Polysaccharides; Shalaby, E.A., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 87–106. [Google Scholar] [CrossRef]
- Francesko, A.; Tzanov, T. Chitin, chitosan and derivatives for wound healing and tissue engineering. Adv. Biochem. Eng. Biotechnol. 2011, 125, 1–27. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The Extracellular Matrix: Not. Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Ulubayram, K.; Nur Cakar, A.; Korkusuz, P.; Ertan, C.; Hasirci, N. EGF containing gelatin-based wound dressings. Biomaterials 2001, 22, 1345–1356. [Google Scholar] [CrossRef]
- Estes, J.M.; Adzick, N.S.; Harrison, M.R.; Longaker, M.T.; Stern, R. Hyaluronate metabolism undergoes and ontogenic transition during fetal development: Implications for Scar-free wound healing. J. Pediatr. Surg. 1993, 28, 1227–1231. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Watanabe, J.; Iwamoto, S.; Ichikawa, S. Entrapment of some compounds into biocompatible nano-sized particles and their releasing properties. Colloids Surf. B Biointerfaces 2005, 42, 141–146. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Kavya, K.; Jayakumar, R.; Nair, S.; Chennazhi, K.P. Fabrication and characterization of chitosan/gelatin/nSiO2 composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2013, 59, 255–263. [Google Scholar] [CrossRef]
- Mezzana, P. Clinical efficacy of a new chitin nanofibrils-based gel in wound healing. Acta Chir. Plast. 2008, 50, 81–84. [Google Scholar]
- Muzzarelli, R.A.A.; Mattioli-Belmonte, M.; Pugnaloni, A.; Biagini, G. Biochemistry, histology and clinical uses of chitins and chitosans in wound healing. Chitin Chitinases 1999, 87, 251–264. [Google Scholar] [CrossRef]
- Sashiwa, H.; Saimoto, H.; Shigemasa, Y.; Ogawa, R.; Tokura, S. Lysozyme susceptibility of partially deacetylated chitin. Int. J. Biol. Macromol. 1990, 12, 295–296. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K.; Stevens, W.F. Chitosan–alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm. 2005, 290, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K.; Tobiassen, A.; Flanagan, J.; Singh, H. Preparation and characterization of nanoparticles formed by chitosan–caseinate interactions. Colloids Surf. B Biointerfaces 2008, 64, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Morsi, N.M.; Shamma, R.N.; El-Adawy, N.O.; Abdelkhalek, A.A. Bioactive injectable triple acting thermosensitive hydrogel enriched with nano-hydroxyapatite for bone regeneration: In-vitro characterization, Saos-2 cell line cell viability and osteogenic markers evaluation. Drug Dev. Ind. Pharm. 2019, 45, 787–804. [Google Scholar] [CrossRef]
- Maged, A.; Abdelkhalek, A.A.; Mahmoud, A.A.; Salah, S.; Ammar, M.M.; Ghorab, M.M. Mesenchymal stem cells associated with chitosan scaffolds loaded with rosuvastatin to improve wound healing. Eur. J. Pharm. Sci. 2019, 127, 185–198. [Google Scholar] [CrossRef]
- Shamma, R.; Basalious, E.B.; Shoukri, R. Development of novel sustained release matrix pellets of betahistine dihydrochloride: Effect of lipophilic surfactants and co-surfactants. Pharm. Dev. Technol. 2011, 17, 583–593. [Google Scholar] [CrossRef]
- Berthold, A.; Cremer, K.; Kreuter, J. Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for anti-inflammatory drugs. J. Control Release 1996, 39, 17–25. [Google Scholar] [CrossRef]
- Hashad, R.A.; Ishak, R.A.; Geneidi, A.S.; Mansour, S. Methotrexate loading in chitosan nanoparticles at a novel pH: Response surface modeling, optimization and characterization. Int. J. Biol. Macromol. 2016, 91, 630–639. [Google Scholar] [CrossRef]
- Rao, A.L.; Rajeswari, K.R.; Sankar, G.G. Spectrophotometric Method for the Determination of Nebivolol Hydrochloride in Bulk and Pharmaceutical Formulations. E-J. Chem. 2010, 7, 445–448. [Google Scholar] [CrossRef]
- Meyyanathan, S.; Birajdar, A.; Suresh, B. Simultaneous Estimation of Nebivolol Hydrochloride and Valsartan and Nebivolol Hydrochloride and Hydrochlorothiazide in Pharmaceutical Formulations by UV Spectrophotometric Methods. Indian J. Pharm. Educ. Res. 2010, 44, 156–159. [Google Scholar]
- Albash, R.; Al-Mahallawi, A.M.; Hassan, M.; Alaa-Eldin, A.A. Development and Optimization of Terpene-Enriched Vesicles (Terpesomes) for Effective Ocular Delivery of Fenticonazole Nitrate: In vitro Characterization and in vivo Assessment. Int. J. Nanomed. 2021, 16, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Yousry, C.; Al-Mahallawi, A.M.; Alaa-Eldin, A.A. Utilization of PEGylated cerosomes for effective topical delivery of fenticonazole nitrate: In-vitro characterization, statistical optimization, and in-vivo assessment. Drug Deliv. 2021, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of levofloxacin polyethylene glycol decorated nanoliposomes for enhanced management of acute otitis media: Statistical optimization, trans-tympanic permeation and in vivo evaluation. Int. J. Pharm. 2019, 559, 201–209. [Google Scholar] [CrossRef]
- Kumbhar, D.D.; Pokharkar, V.B. Engineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: Physicochemical investigations. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 32–42. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Ahmed, D.; Hassan, M.; El-Setouhy, D.A. Enhanced ocular delivery of clotrimazole via loading into mucoadhesive microemulsion system: In vitro characterization and in vivo assessment. J. Drug Deliv. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Al-Mahallawi, A.M.; El-Helaly, S.N.; Abd-Elsalam, W.H. The effect of the saturation degree of phospholipid on the formation of a novel self-assembled nano-micellar complex carrier with enhanced intestinal permeability. Int. J. Pharm. 2019, 569, 118567. [Google Scholar] [CrossRef] [PubMed]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Duodenum-triggered delivery of pravastatin sodium via enteric surface-coated nanovesicular spanlastic dispersions: Development, characterization and pharmacokinetic assessments. Int. J. Pharm. 2015, 483, 77–88. [Google Scholar] [CrossRef]
- Wang, J.; Plourde, N.M.; Iverson, N.; Moghe, P.V.; Uhrich, E.K. Nanoscale amphiphilic macromolecules as lipoprotein inhibitors: The role of charge and architecture. Int. J. Nanomed. 2007, 2, 697–705. [Google Scholar]
- Fahmy, A.M.; Hassan, M.; El-Setouhy, D.A.; Tayel, S.A.; Al-Mahallawi, A.M. Voriconazole Ternary Micellar Systems for the Treatment of Ocular Mycosis: Statistical Optimization and In Vivo Evaluation. J. Pharm. Sci. 2021, 110, 2130–2138. [Google Scholar] [CrossRef]
- Fahmy, A.M.; Hassan, M.; El-Setouhy, D.A.; Tayel, S.A.; Al-Mahallawi, A.M. Statistical optimization of hyaluronic acid enriched ultradeformable elastosomes for ocular delivery of voriconazole via Box-Behnken design: In vitro characterization and in vivo evaluation. Drug Deliv. 2021, 28, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, R.; Weng, Y.; Tang, X. Preparation and evaluation of lyophilized liposome-encapsulated bufadienolides. Drug Dev. Ind. Pharm. 2009, 35, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.; Ouyang, M.; Xia, D.; Quan, P.; Xiao, W.; Song, Y.; Cui, F. In vitro–in vivo study of CoQ10-loaded lipid nanoparticles in comparison with nanocrystals. Int. J. Pharm. 2011, 419, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Bektas, N.; Şenel, B.; Yenilmez, E.; Özatik, O.; Arslan, R. Evaluation of wound healing effect of chitosan-based gel formulation containing vitexin. Saudi Pharm. J. 2020, 28, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Riyadh, S.; Gomha, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and Anticancer Activities of Thiazoles, 1,3 Thiazines, and Thiazolidine Using ChitosaŽ Grafted Poly(vinylpyridine) as Basic Catalyst. ChemInform 2015, 46, 3149–3157. [Google Scholar] [CrossRef]
- Gohel, A.; McCarthy, M.-B.; Gronowicz, G. Estrogen Prevents Glucocorticoid-Induced Apoptosis in Osteoblasts in Vivo and in Vitro1. Endocrinology 1999, 140, 5339–5347. [Google Scholar] [CrossRef]
- Nagar, H.K.; Srivastava, A.K.; Srivastava, R.; Kurmi, M.L.; Chandel, H.S.; Ranawat, M.S. Pharmacological Investigation of the Wound Healing Activity of Cestrum nocturnum (L.) Ointment in Wistar Albino Rats. J. Pharm. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Sirohi, B.; Sagar, R. Effect of Hydroalcoholic Extract of Dactylorhiza Hatagirea Roots & Lavandula Stoechas Flower on Thiopental Sodium Induced Hypnosis in Mice. J. Drug Deliv. Ther. 2019, 9, 414–417. [Google Scholar] [CrossRef]
- El-Bahy, A.A.Z.; Aboulmagd, Y.M.; Zaki, M. Diabetex: A novel approach for diabetic wound healing. Life Sci. 2018, 207, 332–339. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Culling, C. Handbook of Histopathological and Histochemical Techniques; Butterworth-Heinemann: Oxford, UK, 1974. [Google Scholar]
- Amrutiya, N.; Bajaj, A.; Madan, M. Development of Microsponges for Topical Delivery of Mupirocin. AAPS PharmSciTech 2009, 10, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Haylor, J. Experimental rat models of chronic allograft nephropathy: A review. Int. J. Nephrol. Renov. Dis. 2014, 7, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Hashad, R.A.; Ishak, R.A.; Fahmy, S.; Mansour, S.; Geneidi, A.S. Chitosan-tripolyphosphate nanoparticles: Optimization of formulation parameters for improving process yield at a novel pH using artificial neural networks. Int. J. Biol. Macromol. 2016, 86, 50–58. [Google Scholar] [CrossRef]
- Fàbregas, A.; Miñarro, M.; García-Montoya, E.; Pérez-Lozano, P.; Carrillo, C.; Sarrate, R.; Sánchez, N.; Ticó, J.; Suñé-Negre, J. Impact of physical parameters on particle size and reaction yield when using the ionic gelation method to obtain cationic polymeric chitosan–tripolyphosphate nanoparticles. Int. J. Pharm. 2013, 446, 199–204. [Google Scholar] [CrossRef]
- Debnath, S.; Kumar, R.; Babu, M. Ionotropic gelation—A novel method to prepare chitosan nanoparticles. Res. J. Pharm. Technol. 2011, 4, 492–495. [Google Scholar]
- Alarcón-Payán, D.A.; Koyani, R.D.; Vazquez-Duhalt, R. Chitosan-based biocatalytic nanoparticles for pollutant removal from wastewater. Enzym. Microb. Technol. 2017, 100, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Pande, V.; Sonawane, R. Nano and Microparticulate Chitosan Based System for Formulation of Carvedilol Rapid Melt Tablet. Adv. Pharm. Bull. 2015, 5, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Ramay, H.R.; Chou, S.-H.; Zhang, M. Chitosan and lactic acid-grafted chitosan nanoparticles as carriers for prolonged drug delivery. Int. J. Nanomed. 2006, 1, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Woraphatphadung, T.; Sajomsang, W.; Rojanarata, T.; Ngawhirunpat, T.; Tonglairoum, P.; Opanasopit, P. Development of Chitosan-Based pH-Sensitive Polymeric Micelles Containing Curcumin for Colon-Targeted Drug Delivery. AAPS PharmSciTech 2018, 19, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ng, W.K.; Tan, R.B. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Chavda, K.; Vyas, B.; Patel, S. Formulation development of linagliptin solid lipid nanoparticles for oral bioavailability enhancement: Role of P-gp inhibition. Drug Deliv. Transl. Res. 2021, 11, 1166–1185. [Google Scholar] [CrossRef]
- El-Meshad, A.N.; Mohsen, A.M. Enhanced corneal permeation and antimycotic activity of itraconazole against Candida albicans via a novel nanosystem vesicle. Drug Deliv. 2014, 23, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A.; El-Feky, G.S.; Kamel, R.; Awad, G.E. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for ocular drug delivery. Int. J. Pharm. 2011, 413, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ-Okur, N.; Yurdasiper, A.; Gündoğdu, E.; Gökçe, E.H. Modification of solid lipid nanoparticles loaded with nebivolol hydrochloride for improvement of oral bioavailability in treatment of hypertension: Polyethylene glycol versus chitosan oligosaccharide lactate. J. Microencapsul. 2015, 33, 1–13. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters influencing the size of chitosan-TPP nano- and microparticles. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jana, U.; Mohanty, A.K.; Manna, P.K.; Mohanta, G.P. Preparation and characterization of nebivolol nanoparticles using Eudragit® RS100. Colloids Surf. B Biointerfaces 2014, 113, 269–275. [Google Scholar] [CrossRef]
- Abdelbary, A.; AbouGhaly, M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: Application of Box-Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015, 485, 235–243. [Google Scholar] [CrossRef]
- Greenwood, R. Review of the measurement of zeta potentials in concentrated aqueous suspensions using electroacoustics. Adv. Colloid Interface Sci. 2003, 106, 55–81. [Google Scholar] [CrossRef]
- Müller, R.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Eulalio, C.H.; Rodrigues, J.F.B.; Santos, K.; Peniche, C.; LiaFook, M.V. Characterization and thermal properties of chitosan films prepared with different acid solvents. Rev. Cuba. Química 2019, 31, 309–323. [Google Scholar]
- Luo, Q.; Zhao, J.; Zhang, X.; Pan, W. Nanostructured lipid carrier (NLC) coated with Chitosan Oligosaccharides and its potential use in ocular drug delivery system. Int. J. Pharm. 2011, 403, 185–191. [Google Scholar] [CrossRef]
- Basha, M.; Abd El-Alim, S.H.; Shamma, R.N.; Awad, G.E. Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole. J. Liposome Res. 2013, 23, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Vijayanand, P.; Patil, J.; Reddy, M.V. Formulation, characterization and in vivo evaluation of novel edible dosage form containing nebivolol HCl. Braz. J. Pharm. Sci. 2016, 52, 179–190. [Google Scholar] [CrossRef]
- Sipos, E.; Szabó, Z.I.; Redai, E.; Szabó, P.; Sebe, I.; Zelko, R. Preparation and characterization of nanofibrous sheets for enhanced oral dissolution of nebivolol hydrochloride. J. Pharm. Biomed. Anal. 2016, 129, 224–228. [Google Scholar] [CrossRef]
- Al-Bakain, R.; Abulateefeh, S.R.; Taha, M.O. Synthesis and characterization of chitosan-lactate–phthalate and evaluation of the corresponding zinc- and aluminum-crosslinked beads as potential controlled release matrices. Eur. Polym. J. 2015, 73, 402–412. [Google Scholar] [CrossRef]
- Cervera, M.; Heinämäki, J.; de la Paz, N.; López, O.; Maunu, S.L.; Virtanen, T.; Hatanpää, T.; Antikainen, O.; Nogueira, A.; Fundora, J.; et al. Effects of spray drying on physicochemical properties of chitosan acid salts. AAPS PharmSciTech 2011, 12, 637–649. [Google Scholar] [CrossRef]
- Parize, A.; Cristina, T.; Souza, R.; Brighente, I.I.; Fávere, V.; Spinelli, A.; Longo, E. Microencapsulation of the natural urucum pigment with chitosan by spray drying in different solvents. Afr. J. Biotechnol. 2010, 7, 3107–3114. [Google Scholar]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine Hydrochloride Trans-ungual Delivery via Nanovesicular Systems: In Vitro Characterization and Ex Vivo Evaluation. AAPS PharmSciTech 2016, 18, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Pravala, K.; Nagabandi, V.K.; Divya, A. Enhancement of bioavailability of nebivolol hydrochloride through liquisolid formulations: In Vitro and In Vivo evaluation. Der. Pharm. Lett. 2013, 5, 151–163. [Google Scholar]
- Hanif, M.; Khan, H.; Afzal, S.; Mahmood, D.; Maheen, S.; Afzal, K.; Iqbal, N.; Andleeb, M.; Abbas, N. Sustained release biodegradable solid lipid microparticles: Formulation, evaluation and statistical optimization by response surface methodology. Acta Pharm. 2018, 67, 441–461. [Google Scholar] [CrossRef]
- Linga Reddy, B. X-Ray powder diffraction and crystallographic data of Nebivolol Hydrochloride. Int. J. Mater. Sci. 2019, 14, 31–35. [Google Scholar]
- Shah, H.; Shah, V.; Butani, S.; Parikh, D.; Mehta, T. Dissolution improvement of nebivolol hydrochloride using solid dispersion adsorbate technique. Asian J. Pharm. 2015, 9, 49–55. [Google Scholar] [CrossRef]
- Silindir Gunay, M.; Ozer, Y. Sterilization methods and the comparison of E-Beam sterilization with gamma radiation sterilization. FABAD J. Pharm. Sci. 2009, 34, 43–53. [Google Scholar]
- Fairand, B. Radiation Sterilization for Health Care Products: X-ray, Gamma, and Electron Beam; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Desai, K.; Park, H.J. Study of gamma-irradiation effects on chitosan microparticles. Drug Deliv. 2006, 13, 39–50. [Google Scholar] [CrossRef]
- Yang, F.; Li, X.; Cheng, M.; Gong, Y.; Zhao, N.; Zhang, X.; Yang, Y. Performance modification of chitosan membranes induced by gamma irradiation. J. Biomater. Appl. 2002, 16, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Yoo, M.; Seo, J.; Park, S.; Na, H.; Lee, H.; Kim, S.; Cho, C. Evaluation of semi-interpenetrating polymer networks composed of chitosan and poloxamer for wound dressing application. Int. J. Pharm. 2007, 341, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.; Hardikar, A.; Bhonde, R. Growth modulation of fibroblasts by chitosan-polyvinyl pyrrolidone hydrogel: Implications for wound management? J. Biosci. 2000, 25, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Kutty, N.G.; Rao, C.M. Wound healing activity of NOE-aspirin: A pre-clinical study. Nitric Oxide 2007, 16, 150–156. [Google Scholar] [CrossRef]
- Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Longaker, M.T.; Gurtner, G.C. Surgical Approaches to Create Murine Models of Human Wound Healing. J. Biomed. Biotechnol. 2010, 2011, 1–8. [Google Scholar] [CrossRef]
- Peplow, P.V.; Chung, T.-Y.; Baxter, G.D. Laser Photobiomodulation of Proliferation of Cells in Culture: A Review of Human and Animal Studies. Photomed. Laser Surg. 2010, 28, S3. [Google Scholar] [CrossRef] [PubMed]
- Papier, A.; Peres, M.R.; Bobrow, M.; Bhatia, A. The digital imaging system and dermatology. Int. J. Dermatol. 2000, 39, 561–575. [Google Scholar] [CrossRef]
- Geer, D.; Swartz, D.D.; Andreadis, S.T. In vivo model of wound healing based on transplanted tissue-engineered skin. Tissue Eng. 2004, 10, 1006–1017. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Rivera-González, T.I.; Franco-Molina, M.A.; Bollain, Y.G.J.J.; Martínez-Sanmiguel, J.J.; Zárate-Triviño, D.G.; Rodríguez-Padilla, C. A Novel Gold Calreticulin Nanocomposite Based on Chitosan for Wound Healing in a Diabetic Mice Model. Nanomaterials 2019, 9, 75. [Google Scholar] [CrossRef]
- Du, Y.; Luo, X.-L.; Xu, J.-J.; Chen, H.-Y. A simple method to fabricate a chitosan-gold nanoparticles film and its application in glucose biosensor. Bioelectrochemistry 2007, 70, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An. Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Djekic, L.; Martinović, M.; Ćirić, A.; Fraj, J. Composite chitosan hydrogels as advanced wound dressings with sustained ibuprofen release and suitable application characteristics. Pharm. Dev. Technol. 2019, 25, 332–339. [Google Scholar] [CrossRef]
- Archana, D.; Dutta, J.; Dutta, P. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
| Factors (Independent Variables) | Levels | |
|---|---|---|
| X1: Ratio of the volume of CSL to TPP solutions | 5:1 | 10:1 |
| X2: Amount of NVH (mg) | 10 | 20 |
| X3: Concentration of CSL solution used (%) | 0.5 | 1.5 |
| Responses (Dependent Variables) | Constraints | |
| Y1: Entrapment efficiency (%) | Maximum | |
| Y2: Particle size (nm) | Minimum | |
| Formula | Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|
| X1: Ratio of the Volume of CSL Solution to TPP Solution | X2: Amount of NVH (mg) | X3: Concentration of CSL Solution Used (%) | Y1: EE% * | Y2: PS (nm) * | PDI * | |
| F1 | 5:1 | 10 | 0.5 | 60.69 ± 3.64 | 640.20 ± 9.89 | 0.555 ± 0.043 |
| F2 | 5:1 | 20 | 1.5 | 77.50 ± 5.22 | 1262.5 ± 68.59 | 0.451 ± 0.017 |
| F3 | 10:1 | 10 | 0.5 | 51.20 ± 2.33 | 508.00 ± 18.24 | 0.406 ± 0.058 |
| F4 | 10:1 | 20 | 1.5 | 65.17 ± 4.67 | 918.20 ± 59.84 | 0.419 ± 0.069 |
| F5 | 5:1 | 10 | 1.5 | 49.13 ± 0.84 | 611.15 ± 42.35 | 0.361 ± 0.067 |
| F6 | 5:1 | 20 | 0.5 | 73.68 ± 3.61 | 404.05 ± 11.24 | 0.479 ± 0.055 |
| F7 | 10:1 | 10 | 1.5 | 68.61 ± 2.55 | 858.20 ± 58.68 | 0.305 ± 0.047 |
| F8 | 10:1 | 20 | 0.5 | 91.50 ± 1.36 | 549.70 ± 12.44 | 0.511 ± 0.027 |
| Parameter | At 0 Time | After 6 Months at 4 ± 2 °C | After 6 Months at 25 ± 2 °C |
|---|---|---|---|
| EE% * | 73.68 ± 3.61% | 72.12 ± 4.65% | 72.62 ± 0.14% |
| PS * | 404.5 ± 11.24 nm | 440.3 ± 19.31 nm | 425.6 ± 21.60 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsherif, N.I.; Al-Mahallawi, A.M.; Abdelkhalek, A.A.; Shamma, R.N. RETRACTED: Investigation of the Potential of Nebivolol Hydrochloride-Loaded Chitosomal Systems for Tissue Regeneration: In Vitro Characterization and In Vivo Assessment. Pharmaceutics 2021, 13, 700. https://doi.org/10.3390/pharmaceutics13050700
Elsherif NI, Al-Mahallawi AM, Abdelkhalek AA, Shamma RN. RETRACTED: Investigation of the Potential of Nebivolol Hydrochloride-Loaded Chitosomal Systems for Tissue Regeneration: In Vitro Characterization and In Vivo Assessment. Pharmaceutics. 2021; 13(5):700. https://doi.org/10.3390/pharmaceutics13050700
Chicago/Turabian StyleElsherif, Noha Ibrahim, Abdulaziz Mohsen Al-Mahallawi, Abdelfattah Ahmed Abdelkhalek, and Rehab Nabil Shamma. 2021. "RETRACTED: Investigation of the Potential of Nebivolol Hydrochloride-Loaded Chitosomal Systems for Tissue Regeneration: In Vitro Characterization and In Vivo Assessment" Pharmaceutics 13, no. 5: 700. https://doi.org/10.3390/pharmaceutics13050700
APA StyleElsherif, N. I., Al-Mahallawi, A. M., Abdelkhalek, A. A., & Shamma, R. N. (2021). RETRACTED: Investigation of the Potential of Nebivolol Hydrochloride-Loaded Chitosomal Systems for Tissue Regeneration: In Vitro Characterization and In Vivo Assessment. Pharmaceutics, 13(5), 700. https://doi.org/10.3390/pharmaceutics13050700








