Stability and In Vitro Aerodynamic Studies of Inhalation Powders Containing Ciprofloxacin Hydrochloride Applying Different DPI Capsule Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
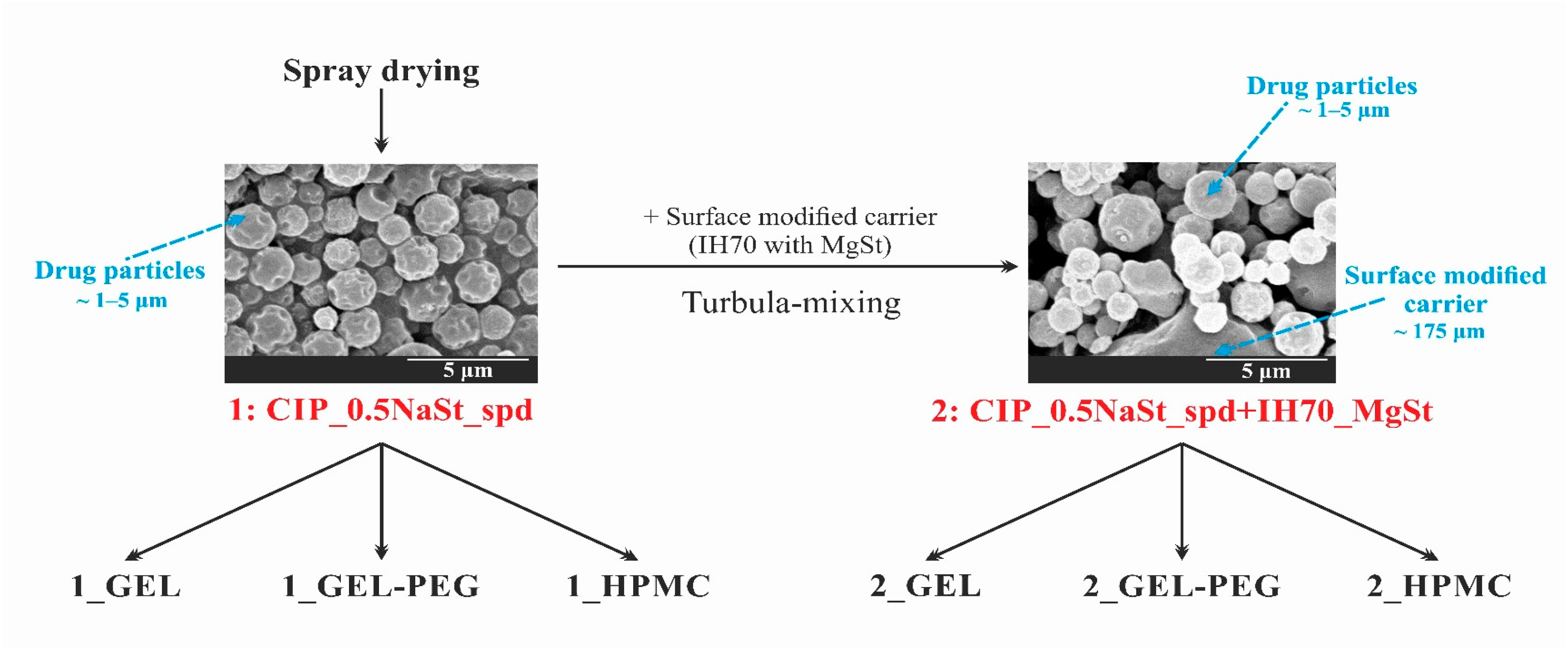
2.2.1. Preparation of the Samples
2.2.2. Homogeneity and Drug Content Test
2.2.3. Investigation of the Stability of the Formulations and the Capsules
2.2.4. Light Microscopic Examination
2.2.5. Thermoanalytical Test
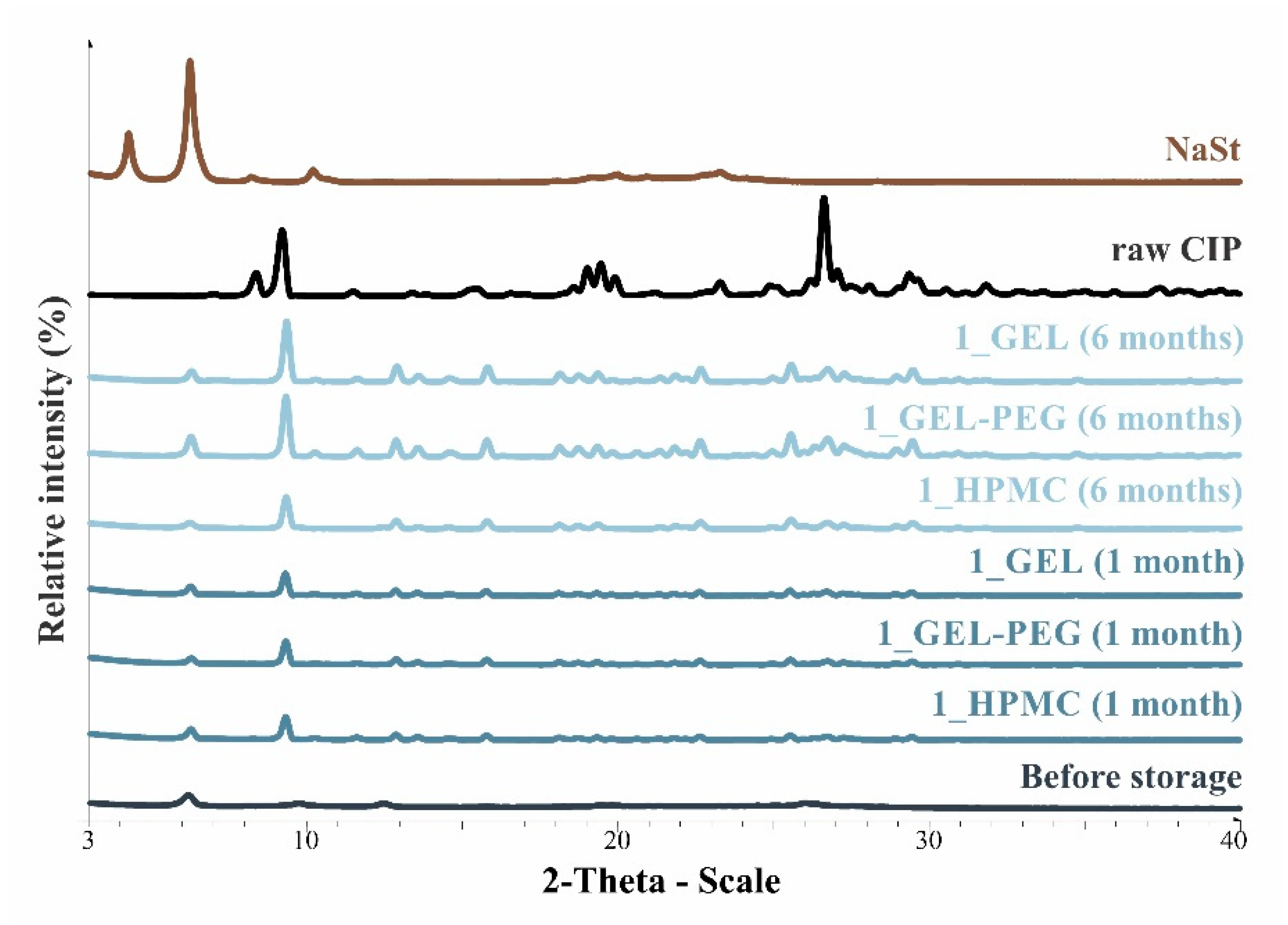
2.2.6. X-ray Powder Diffraction (XRPD)
2.2.7. Particle Size Distribution
2.2.8. Scanning Electron Microscopy (SEM)
2.2.9. In Vitro Aerodynamic Investigation
2.2.10. Statistical Analyses
3. Results and Discussion
3.1. Blend Uniformity and Drug Content
3.2. Stability of the Capsules
3.3. Residual Solvent Content of the Samples
3.4. Structural Investigations
3.5. Particle Size Analysis and Scanning Electron Microscopy (SEM) of the Samples
3.6. In Vitro Aerodynamic Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Contreras, L.; Ibrahim, M.; Verma, R. Inhalation Drug Delivery Devices: Technology Update. Med. Devices Evid. Res. 2015, 8, 131–139. [Google Scholar] [CrossRef]
- Patil, J.S.; Sarasija, S. Pulmonary Drug Delivery Strategies: A Concise, Systematic Review. Lung India 2012, 29, 44. [Google Scholar] [CrossRef] [PubMed]
- Dua, K.; Bebawy, M.; Awasthi, R.; Tekade, R.K.; Tekade, M.; Gupta, G.; De Jesus Andreoli Pinto, T.; Hansbro, P.M. Application of Chitosan and Its Derivatives in Nanocarrier Based Pulmonary Drug Delivery Systems. Pharm. Nanotechnol. 2018, 5, 243–249. [Google Scholar] [CrossRef]
- Courrier, H.M.; Butz, N.; Vandamme, T.F. Pulmonary Drug Delivery Systems: Recent Developments and Prospects. Crit. Rev. Ther. Drug Carrier Syst. 2002, 19, 425–498. [Google Scholar] [CrossRef]
- Lu, D.; Hickey, A.J. Pulmonary Vaccine Delivery. Expert Rev. Vaccines 2007, 6, 213–226. [Google Scholar] [CrossRef]
- Tan, M.; Reyes-Ortega, F.; Schneider-Futschik, E.K. Successes and Challenges: Inhaled Treatment Approaches Using Magnetic Nanoparticles in Cystic Fibrosis. Magnetochemistry 2020, 6, 25. [Google Scholar] [CrossRef]
- Hamed, K.; Debonnett, L. Tobramycin Inhalation Powder for the Treatment of Pulmonary Pseudomonas Aeruginosa Infection in Patients with Cystic Fibrosis: A Review Based on Clinical Evidence. Ther. Adv. Respir. Dis. 2017, 11, 193–209. [Google Scholar] [CrossRef]
- ARIKAYCE, an FDA-Approved Treatment. Available online: https://www.arikayce.com/ (accessed on 1 March 2021).
- Banaschewski, B.; Hofmann, T. Inhaled Antibiotics for Mycobacterial Lung Disease. Pharmaceutics 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Polyphor Receives Approval to Start First-in-Human Clinical Trial of Inhaled Antibiotic Murepavadin. Available online: https://www.polyphor.com/news/corporate-news-details/ (accessed on 1 March 2021).
- Cazzola, M.; Cavalli, F.; Usmani, O.S.; Rogliani, P. Advances in Pulmonary Drug Delivery Devices for the Treatment of Chronic Obstructive Pulmonary Disease. Expert Opin. Drug Deliv. 2020, 17, 635–646. [Google Scholar] [CrossRef]
- Adlakha, S.; Vaghasiya, K.; Sharma, A.; Ray, E.; Verma, R.K. Inhalable polymeric dry powders for antituberculosis drug delivery. In Nanotechnology Based Approaches for Tuberculosis Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–105. ISBN 978-0-12-819811-7. [Google Scholar]
- Liang, W.; Pan, H.W.; Vllasaliu, D.; Lam, J.K.W. Pulmonary Delivery of Biological Drugs. Pharmaceutics 2020, 12, 1025. [Google Scholar] [CrossRef]
- Benke, E.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P.; Ambrus, R. The Actuality of Devices for the Delivery of Dry Powder Inhalation, Formulations and Modern Assemblies I. Gyógyszerészet/Pharmacy 2018, 62, 131–139. [Google Scholar]
- Capsule Based Inhalers Market—Global Industry Trend Analysis 2013 to 2017 and Forecast 2018–2028. Available online: http://www.persistencemarketresearch.com/market-research/capsule-based-inhalers-market.asp (accessed on 18 March 2021).
- Wauthoz, N.; Hennia, I.; Ecenarro, S.; Amighi, K. Impact of Capsule Type on Aerodynamic Performance of Inhalation Products: A Case Study Using a Formoterol-Lactose Binary or Ternary Blend. Int. J. Pharm. 2018, 553, 47–56. [Google Scholar] [CrossRef]
- Pinto, J.T.; Wutscher, T.; Stankovic-Brandl, M.; Zellnitz, S.; Biserni, S.; Mercandelli, A.; Kobler, M.; Buttini, F.; Andrade, L.; Daza, V.; et al. Evaluation of the Physico-Mechanical Properties and Electrostatic Charging Behavior of Different Capsule Types for Inhalation Under Distinct Environmental Conditions. AAPS PharmSciTech 2020, 21, 128. [Google Scholar] [CrossRef]
- Martinelli, F.; Balducci, A.G.; Rossi, A.; Sonvico, F.; Colombo, P.; Buttini, F. “Pierce and Inhale” Design in Capsule Based Dry Powder Inhalers: Effect of Capsule Piercing and Motion on Aerodynamic Performance of Drugs. Int. J. Pharm. 2015, 487, 197–204. [Google Scholar] [CrossRef]
- Schoubben, A.; Blasi, P.; Giontella, A.; Giovagnoli, S.; Ricci, M. Powder, Capsule and Device: An Imperative Ménage à Trois for Respirable Dry Powders. Int. J. Pharm. 2015, 494, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, B.M.; Birchall, J.C.; Jones, B.E.; Díez, F.; Coulman, S.A. The Development of a Sensitive Methodology to Characterise Hard Shell Capsule Puncture by Dry Powder Inhaler Pins. Int. J. Pharm. 2013, 456, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Developing Capsules for DPIs. Available online: https://www.pharmtech.com/view/developing-capsules-dpis (accessed on 19 March 2021).
- Coates, M.S.; Fletcher, D.F.; Chan, H.-K.; Raper, J.A. The Role of Capsule on the Performance of a Dry Powder Inhaler Using Computational and Experimental Analyses. Pharm. Res. 2005, 22, 923–932. [Google Scholar] [CrossRef]
- Probst, S.E. The Capsule-Based DPI, an Environmentally Friendly and Efficient Drug Delivery Dosage Form. Available online: https://www.researchgate.net/publication/312228321_The_capsule-based_DPI_an_environmentally_friendly_and_efficient_drug_delivery_dosage_form (accessed on 6 February 2020).
- Díez, F.; Kalafat, J.; Bhat, J. The Science behind Capsule Based Dry Powder Inhalation Technology. ONdrugDelivery 2017. Available online: https://www.ondrugdelivery.com/wp-content/uploads/2017/11/ONdrugDel-PULMONARY-NASAL-DEL-80-Nov-2017-ACG-2.pdf (accessed on 5 January 2021).
- Lavorini, F.; Pistolesi, M.; Usmani, O.S. Recent Advances in Capsule-Based Dry Powder Inhaler Technology. Multidiscip. Respir. Med. 2017, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Capsules|Films & Foils|Engineering|Inspection|Track & Trace Systems|ACG. Available online: https://www.acg-world.com/ (accessed on 18 March 2021).
- Capsugel Capsugel® ZephyrTM—Dry-Powder Inhalation Capsule Portfolio. Available online: https://www.capsugel.com/biopharmaceutical-products/capsugel-zephyr-dry-powder-inhalation-capsule-portfolio (accessed on 18 March 2021).
- Qualicaps. Available online: https://qualicaps.com/Capsules/pharma (accessed on 18 March 2021).
- Pharmaceutical Empty Capsules|Hard Gelatin Capsules Manufacturer|ACG. Available online: https://www.acg-world.com/capsules (accessed on 18 March 2021).
- Ambrus, R.; Benke, E.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P. Novel Dry Powder Inhaler Formulation Containing Antibiotic Using Combined Technology to Improve Aerodynamic Properties. Eur. J. Pharm. Sci. 2018, 123, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Benke, E.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P.; Ambrus, R. Stability Test of Novel Combined Formulated Dry Powder Inhalation System Containing Antibiotic: Physical Characterization and in Vitro—In Silico Lung Deposition Results. Drug Dev. Ind. Pharm. 2019, 45, 1369–1378. [Google Scholar] [CrossRef]
- Cocconi, D.; Dagli Alberi, M.; Busca, A.; Schiaretti, F. Use of Magnesium Stearate in Dry Powder Formulations for Inhalation. U.S. Patent 20120082727A1, 5 April 2012. [Google Scholar]
- Lau, M.; Young, P.M.; Traini, D. Co-Milled API-Lactose Systems for Inhalation Therapy: Impact of Magnesium Stearate on Physico-Chemical Stability and Aerosolization Performance. Drug Dev. Ind. Pharm. 2017, 43, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Hazare, S.; Menon, M. Improvement of Inhalation Profile of DPI Formulations by Carrier Treatment with Magnesium Stearate. Indian J. Pharm. Sci. 2009, 71, 725–727. [Google Scholar]
- Buttini, F.; Cuoghi, E.; Miozzi, M.; Rossi, A.; Sonvico, F.; Colombo, P. Insulin Spray-Dried Powder and Smoothed Lactose: A New Formulation Strategy for Nasal and Pulmonary Delivery. Available online: https://www.researchgate.net/publication/284045495_Insulin_spray-dried_powder_and_smoothed_lactose_a_new_formulation_strategy_for_nasal_and_pulmonary_delivery (accessed on 11 April 2018).
- Plastira, M. The Influence of Magnesium Stearate and Carrier Surface on the Deposition Performace of Carrier Based Dry Powder Inhaler Formulations. Ph.D. Thesis, University of Bath, Bath, UK, 2008. [Google Scholar]
- Benke, E.; Szabó-Révész, P.; Ambrus, R. Development of Ciprofloxacin Hydrochloride Containing Dry Powder Inhalation System with an Innovative Technology. Acta Pharm. Hung. 2017, 87, 49–58. [Google Scholar]
- Pharmaceutical Inhalation Aerosol Technology, 3rd ed.; Hickey, A.J., Rocha, S.R.P.D., Eds.; Drugs and the Pharmaceutical Sciences; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; ISBN 978-1-138-06307-5. [Google Scholar]
- Kaialy, W.; Nokhodchi, A. Engineered Mannitol Ternary Additives Improve Dispersion of Lactose–Salbutamol Sulphate Dry Powder Inhalations. AAPS J. 2013, 15, 728–743. [Google Scholar] [CrossRef]
- Inhaler Testing Brochure 2021. Available online: https://www.copleyscientific.com/downloads/inhaler-testing-brochure-2021/ (accessed on 18 March 2021).
- Li, X.; Vogt, F.G.; Hayes, D.; Mansour, H.M. Physicochemical Characterization and Aerosol Dispersion Performance of Organic Solution Advanced Spray-Dried Microparticulate/Nanoparticulate Antibiotic Dry Powders of Tobramycin and Azithromycin for Pulmonary Inhalation Aerosol Delivery. Eur. J. Pharm. Sci. 2014, 52, 191–205. [Google Scholar] [CrossRef]
- Benke, E.; Farkas, Á.; Szabó-Révész, P.; Ambrus, R. Development of an Innovative, Carrier-Based Dry Powder Inhalation Formulation Containing Spray-Dried Meloxicam Potassium to Improve the In Vitro and In Silico Aerodynamic Properties. Pharmaceutics 2020, 12, 535. [Google Scholar] [CrossRef]
- Cunha, L.; Rodrigues, S.; Rosa da Costa, A.; Faleiro, M.; Buttini, F.; Grenha, A. Inhalable Fucoidan Microparticles Combining Two Antitubercular Drugs with Potential Application in Pulmonary Tuberculosis Therapy. Polymers 2018, 10, 636. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Cabral, L.M.; Healy, A.M.; de Sousa, V.P. Development of a Novel Dry Powder Inhalation Formulation for the Delivery of Rivastigmine Hydrogen Tartrate. Int. J. Pharm. 2016, 501, 124–138. [Google Scholar] [CrossRef]
- Ceschan, N.E.; Bucalá, V.; Mateos, M.V.; Smyth, H.D.C.; Ramírez-Rigo, M.V. Carrier Free Indomethacin Microparticles for Dry Powder Inhalation. Int. J. Pharm. 2018, 549, 169–178. [Google Scholar] [CrossRef]
- Hoffelder, T.; Wellek, S. Equivalence Testing With Particle Size Distribution Data: Methods and Applications in the Development of Inhalative Drugs. Stat. Biopharm. Res. 2017, 9, 12–24. [Google Scholar] [CrossRef]
- Laube, B.L.; Janssens, H.M.; de Jongh, F.H.C.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the Pulmonary Specialist Should Know about the New Inhalation Therapies. Eur. Respir. J. 2011, 37, 1308–1417. [Google Scholar] [CrossRef]
- Social Science Statistic Online. Available online: https://www.socscistatistics.com/tests/studentttest/default2.aspx (accessed on 6 April 2020).
- Della Bella, A.; Müller, M.; Danani, A.; Soldati, L.; Bettini, R. Effect of Lactose Pseudopolymorphic Transition on the Aerosolization Performance of Drug/Carrier Mixtures. Pharmaceutics 2019, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Hohenheim, U. Determination of Water Content in Lactose by Karl Fischer Titration—Interlaboratory Collaborative Study. Available online: https://www.uni-hohenheim.de/organisation/publikation/determination-of-water-content-in-lactose-by-karl-fischer-titration-interlaboratory-collaborative-study (accessed on 18 March 2021).
- Guchardi, R.; Frei, M.; John, E.; Kaerger, J. Influence of Fine Lactose and Magnesium Stearate on Low Dose Dry Powder Inhaler Formulations. Int. J. Pharm. 2008, 348, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.; Rouse, T.; Singh, D.; Edge, S. Defining the ‘Dose’ for Dry Powder Inhalers: The Challenge of Correlating In-Vitro Dose Delivery Results with Clinical Efficacy. Am. Pharm. Rev. 2017, 20, 54–62. [Google Scholar]
| Samples | Compositions of the DPI Formulations | Applied DPI Capsule Types | |||||
|---|---|---|---|---|---|---|---|
| CIP (w/w %) | NaSt (w/w %) | IH 70 (w/w %) | MgSt (w/w %) | GEL | GEL-PEG | HPMC | |
| 1_GEL | 99.50 | 0.500 | – | – | + | – | – |
| 1_GEL-PEG | 99.50 | 0.500 | – | – | – | + | – |
| 1_HPMC | 99.50 | 0.500 | – | – | – | – | + |
| 2_GEL | 9.045 | 0.045 | 88.91 | 2.000 | + | – | – |
| 2_GEL-PEG | 9.045 | 0.045 | 88.91 | 2.000 | – | + | – |
| 2_HPMC | 9.045 | 0.045 | 88.91 | 2.000 | – | – | + |
| Capsule Type | Before Storage | 1 Month | 3 Months | 6 Months |
|---|---|---|---|---|
| GEL |  | 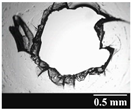 | 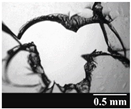 | 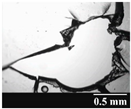 |
| GEL-PEG |  |  |  |  |
| HPMC |  |  |  |  |
| Capsule Type | Time | RSC (%) | Area of Capsule Puncture (mm2) |
|---|---|---|---|
| GEL | Before storage | 15.26 ± 0.18 | 0.60 ± 0.16 |
| 1 month | 10.31 ± 0.21 | 0.74 ± 0.11 | |
| 3 months | 7.23 ± 0.28 | 1.01 ± 0.28 | |
| 6 months | 6.68 ± 0.12 | 1.14 ± 0.38 | |
| GEL-PEG | Before storage | 11.87 ± 0.09 | 0.54 ± 0.10 |
| 1 month | 10.68 ± 0.32 | 0.84 ± 0.12 | |
| 3 months | 8.74 ± 0.15 | 0.89 ± 0.14 | |
| 6 months | 7.12 ± 0.12 | 0.92 ± 0.07 | |
| HPMC | Before storage | 5.98 ± 0.11 | 0.79 ± 0.05 |
| 1 month | 5.45 ± 0.09 | 0.79 ± 0.04 | |
| 3 months | 4.84 ± 0.13 | 0.86 ± 0.08 | |
| 6 months | 4.62 ± 0.02 | 0.88 ± 0.03 |
| Samples | RSC (%) | |||
|---|---|---|---|---|
| Before Storage | 1 Month | 3 Months | 6 Months | |
| Formulation (1) | 3.76 ± 0.07 | — | — | — |
| Formulation (2) | 4.61 ± 0.12 | — | — | — |
| 1_GEL | — | 3.99 ± 0.06 | 4.62 ± 0.08 | 5.21 ± 0.08 |
| 1_GEL-PEG | — | 3.92 ± 0.03 | 4.48 ± 0.06 | 5.03 ± 0.09 |
| 1_HPMC | — | 3.85 ± 0.10 | 4.26 ± 0.13 | 4.72 ± 0.04 |
| 2_GEL | — | 4.93 ± 0.11 | 5.13 ± 0.09 | 5.64 ± 0.13 |
| 2_GEL-PEG | — | 4.85 ± 0.06 | 5.04 ± 0.03 | 5.45 ± 0.06 |
| 2_HPMC | — | 4.76 ± 0.07 | 4.91 ± 0.06 | 5.16 ± 0.04 |
| Formulation | 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before storage |  | ||||||||
| D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | |||||||
| 1.167 ± 0.07 | 2.167 ± 0.10 | 3.715 ± 0.12 | |||||||
| Samples | 1_GEL | 1_GEL-PEG | 1_HPMC | ||||||
| 1 month |  |  |  | ||||||
| D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | |
| 1.232 ± 0.08 | 2.264 ± 0.11 | 4.022 ± 0.11 | 1.313 ± 0.02 | 2.231 ± 0.03 | 3.788 ± 0.15 | 1.247 ± 0.08 | 2.230 ± 0.10 | 3.804 ± 0.13 | |
| 3 months |  |  |  | ||||||
| D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | |
| 1.244 ± 0.03 | 2.828 ± 0.14 | 4.468 ± 0.13 | 1.210 ± 0.03 | 2.769 ± 0.12 | 4.662 ± 0.18 | 1.442 ± 0.06 | 2.711 ± 0.07 | 4.454 ± 0.14 | |
| 6 months |  |  |  | ||||||
| D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | |
| 1.329 ± 0.06 | 3.615 ± 0.17 | 7.929 ± 0.21 | 1.262 ± 0.11 | 3.452 ± 0.05 | 6.731 ± 0.13 | 1.358 ± 0.02 | 3.460 ± 0.15 | 6.371 ± 0.09 | |
| Formulation | 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before storage |  | ||||||||
| D (0.1) (µm) | D (0.5) (µm) | D (0.9)(µm) | |||||||
| 3.675 ± 0.12 | 130.459 ± 0.18 | 235.25 ± 1.15 | |||||||
| Samples | 2_GEL | 2_GEL-PEG | 2_HPMC | ||||||
| 1 month |  |  |  | ||||||
| D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | |
| 14.389 ± 0.23 | 160.591 ± 1.09 | 317.334 ± 1.76 | 9.128 ± 0.21 | 158.867 ± 0.54 | 280.981 ± 1.18 | 3.913 ± 0.11 | 155.349 ± 0.71 | 273.114 ± 1.42 | |
| 3 months |  |  |  | ||||||
| D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | |
| 29.426 ± 0.19 | 168.583 ± 0.71 | 305.176 ± 1.81 | 22.836 ± 0.13 | 166.571 ± 0.86 | 303.715 ± 0.96 | 22.315 ± 0.31 | 164.727 ± 0.38 | 291.028 ± 1.23 | |
| 6 months | 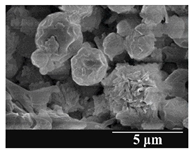 | 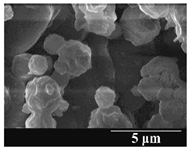 | 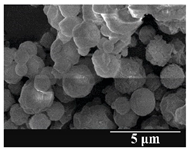 | ||||||
| D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | D (0.1) (µm) | D (0.5) (µm) | D (0.9) (µm) | |
| 27.381 ± 0.08 | 172.772 ± 0.36 | 331.195 ± 1.39 | 29.003 ± 0.15 | 170.503 ± 0.37 | 328.693 ± 1.41 | 26.122 ± 0.18 | 168.635 ± 0.89 | 305.315 ± 1.72 | |
| Samples | Time | FPF (%) <5 μm | MMAD (μm) | EF (%) |
|---|---|---|---|---|
| 1_GEL | Before storage | 53.42 ± 1.23 | 3.98 ± 0.15 | 77.04 ± 1.03 |
| 1 month | 31.87 ± 0.11 | 4.43 ± 0.14 | 85.75 ± 0.16 | |
| 3 months | 29.94 ± 0.25 | 4.86 ± 0.17 | 86.14 ± 0.81 | |
| 6 months | 28.83 ± 0.65 | 5.02 ± 0.22 | 87.70 ± 0.64 | |
| 1_GEL-PEG | Before storage | 54.13 ± 0.89 | 3.81 ± 0.06 | 72.72 ± 0.76 |
| 1 month | 42.25 ± 0.38 | 4.31 ± 0.21 | 86.54 ± 0.54 | |
| 3 months | 36.31 ± 0.43 | 4.62 ± 0.15 | 86.85 ± 0.85 | |
| 6 months | 31.67 ± 0.07 | 4.93 ± 0.12 | 87.80 ± 0.73 | |
| 1_HPMC | Before storage | 53.97 ± 1.08 | 3.78 ± 0.26 | 86.44 ± 0.99 |
| 1 month | 44.71 ± 0.94 | 4.16 ± 0.14 | 86.96 ± 0.36 | |
| 3 months | 39.18 ± 0.27 | 4.32 ± 0.08 | 87.55 ± 0.49 | |
| 6 months | 38.59 ± 0.44 | 4.40 ± 0.11 | 90.16 ± 0.34 |
| Samples | Time | FPF (%) <5 μm | MMAD (μm) | EF (%) |
|---|---|---|---|---|
| 2_GEL | Before storage | 62.91 ± 1.02 | 3.51 ± 0.09 | 90.31 ± 0.95 |
| 1 month | 43.89 ± 1.28 | 3.84 ± 0.13 | 91.15 ± 0.12 | |
| 3 months | 35.03 ± 0.23 | 3.93 ± 0.07 | 91.27 ± 0.36 | |
| 6 months | 31.71 ± 0.64 | 4.10 ± 0.16 | 92.89 ± 0.41 | |
| 2_GEL-PEG | Before storage | 62.53 ± 0.48 | 3.45 ± 0.12 | 90.21 ± 0.83 |
| 1 month | 46.11 ± 1.32 | 3.72 ± 0.05 | 89.75 ± 0.45 | |
| 3 months | 38.66 ± 0.96 | 3.87 ± 0.09 | 91.39 ± 0.21 | |
| 6 months | 36.26 ± 0.39 | 4.03 ± 0.13 | 92.56 ± 0.66 | |
| 2_HPMC | Before storage | 63.15 ± 0.41 | 3.47 ± 0.08 | 89.55 ± 0.26 |
| 1 month | 53.29 ± 0.72 | 3.68 ± 0.21 | 91.42 ± 0.52 | |
| 3 months | 45.23 ± 1.12 | 3.84 ± 0.04 | 94.39 ± 0.74 | |
| 6 months | 43.40 ± 0.57 | 3.91 ± 0.15 | 96.98 ± 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benke, E.; Varga, P.; Szabó-Révész, P.; Ambrus, R. Stability and In Vitro Aerodynamic Studies of Inhalation Powders Containing Ciprofloxacin Hydrochloride Applying Different DPI Capsule Types. Pharmaceutics 2021, 13, 689. https://doi.org/10.3390/pharmaceutics13050689
Benke E, Varga P, Szabó-Révész P, Ambrus R. Stability and In Vitro Aerodynamic Studies of Inhalation Powders Containing Ciprofloxacin Hydrochloride Applying Different DPI Capsule Types. Pharmaceutics. 2021; 13(5):689. https://doi.org/10.3390/pharmaceutics13050689
Chicago/Turabian StyleBenke, Edit, Patrícia Varga, Piroska Szabó-Révész, and Rita Ambrus. 2021. "Stability and In Vitro Aerodynamic Studies of Inhalation Powders Containing Ciprofloxacin Hydrochloride Applying Different DPI Capsule Types" Pharmaceutics 13, no. 5: 689. https://doi.org/10.3390/pharmaceutics13050689
APA StyleBenke, E., Varga, P., Szabó-Révész, P., & Ambrus, R. (2021). Stability and In Vitro Aerodynamic Studies of Inhalation Powders Containing Ciprofloxacin Hydrochloride Applying Different DPI Capsule Types. Pharmaceutics, 13(5), 689. https://doi.org/10.3390/pharmaceutics13050689







