Roflumilast Powders for Chronic Obstructive Pulmonary Disease: Formulation Design and the Influence of Device, Inhalation Flow Rate, and Storage Relative Humidity on Aerosolization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quantification by HPLC Analysis
2.3. Preparation of Powders, Study Design, and Optimization
2.4. Particle Size and Size Distribution by Laser Light Diffraction
2.5. Crystallinity by X-ray Powder Diffraction (XRPD)
2.6. Residual Solvent Content by Thermogravimetric Analysis (TGA)
2.7. Phase Transition by Differential Scanning Calorimetry (DSC)
2.8. Powder Density and Flow Property
2.9. Particle Morphology by Scanning Electron Microscopy (SEM)
2.10. In Vitro Aerosolization Performance by Next-Generation Impactor (NGI)
2.11. Stability Studies
2.12. Effect of Inhaler Device and Flow Rate on Aerosolization
2.13. Cytotoxicity Studies by MTT Assay
2.14. Statistical Analysis
3. Results and Discussions
3.1. Powder Production and Optimization
3.2. Process Yield, Particle Size, and Drug Content of the Optimized Powder
3.3. Crystallinity
3.4. Moisture Content
3.5. Differential Scanning Calorimetry (DSC)
3.6. Powder Density
3.7. Surface Morphology
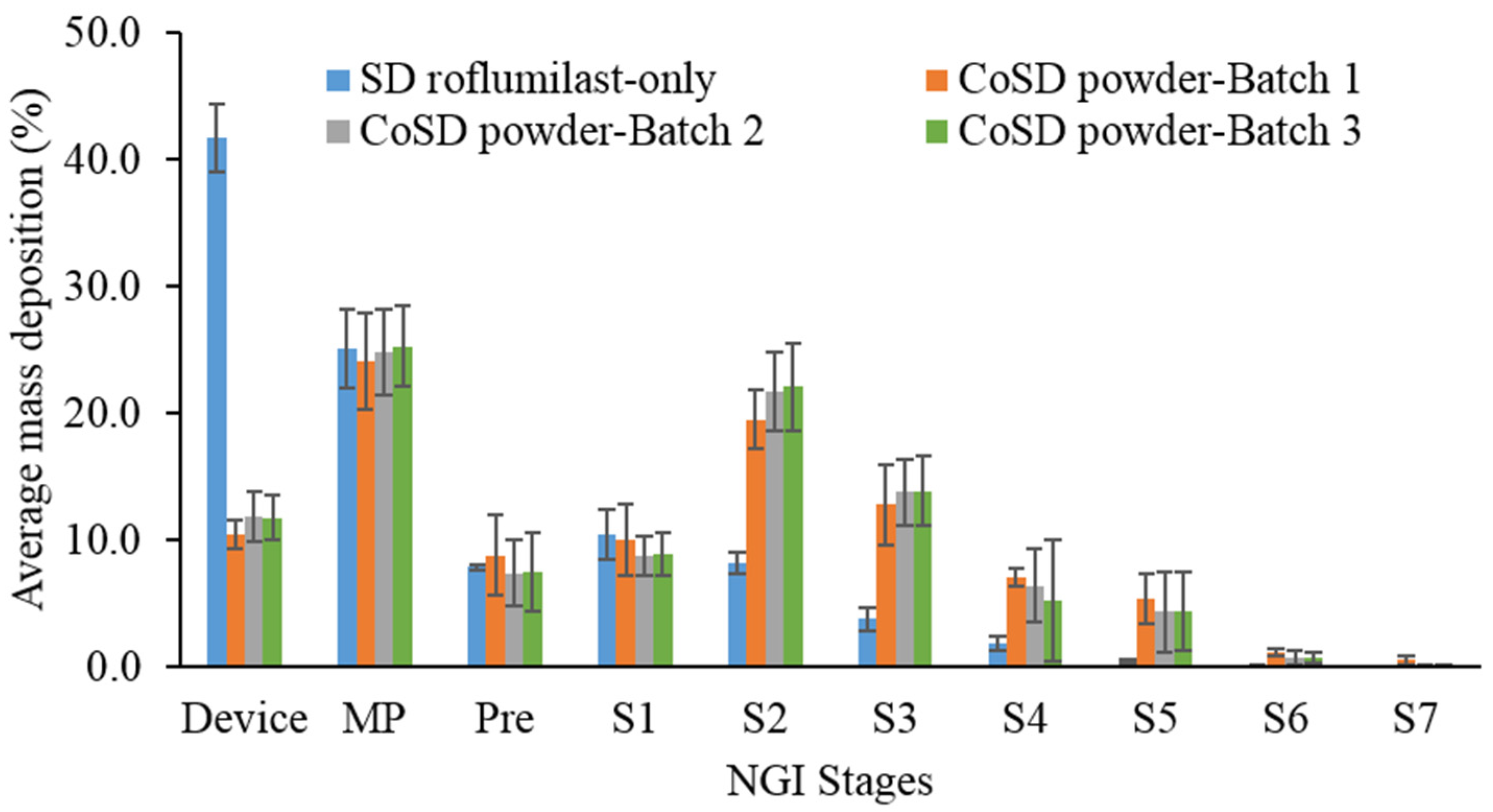
3.8. In Vitro Aerosolization Performance
3.9. Stability Studies
3.10. Effects of Device and Flow Rate on In Vitro Aerosolization
3.11. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, P.W.; Agusti, A.G. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur. Respir. J. 2006, 27, 822–832. [Google Scholar] [CrossRef]
- Chapman, K.R.; Mannino, D.M.; Soriano, J.B.; Vermeire, P.A.; Buist, A.S.; Thun, M.J.; Connell, C.; Jemal, A.; Lee, T.A.; Miravitlles, M.; et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur. Respir. J. 2006, 27, 188–207. [Google Scholar] [CrossRef] [Green Version]
- Pocket Guide to COPD Diagnosis, Management, and Preventation 2019 Report. Global Initiative for Chronic Obstructive Lung Disease. Available online: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-POCKET-GUIDE-DRAFT-v1.7-14Nov2018-WMS.pdf (accessed on 15 December 2020).
- Abbott-Banner, K.H.; Page, C.P. Dual PDE3/4 and PDE4 inhibitors: Novel treatments for COPD and other inflammatory airway diseases. Basic Clin. Pharmacol. Toxicol. 2014, 114, 365–376. [Google Scholar] [CrossRef]
- Yuan, L.; Dai, X.; Yang, M.; Cai, Q.; Shao, N. Potential treatment benefits and safety of roflumilast in COPD: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmoary Dis. 2016, 11, 1477–1483. [Google Scholar] [CrossRef] [Green Version]
- Baye, J. Roflumilast (daliresp): A novel phosphodiesterase-4 inhibitor for the treatment of severe chronic obstructive pulmonary disease. Pharm. Ther. 2012, 37, 149–161. [Google Scholar]
- Chapman, R.W.; House, A.; Jones, H.; Richard, J.; Celly, C.; Prelusky, D.; Ting, P.; Hunter, J.C.; Lamca, J.; Phillips, J.E. Effect of inhaled roflumilast on the prevention and resolution of allergen-induced late phase airflow obstruction in Brown Norway rats. Eur. J. Pharmacol. 2007, 571, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, É.Y.; Amaro, M.I.; de Almeida, G.S.; Cabral, L.M.; Healy, A.M.; de Sousa, V.P. Development of a new formulation of roflumilast for pulmonary drug delivery to treat inflammatory lung conditions. Int. J. Pharm. 2018, 550, 89–99. [Google Scholar] [CrossRef]
- Evrard, B.; Bertholet, P.; Gueders, M.; Flament, M.P.; Piel, G.; Delattre, L.; Gayot, A.; Leterme, P.; Foidart, J.M.; Cataldo, D. Cyclodextrins as a potential carrier in drug nebulization. J. Control. Release 2004, 96, 403–410. [Google Scholar] [CrossRef]
- Clark, A.R.; Hollingworth, A.M. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers--Implications for in vitro testing. J. Aerosol Med. 1993, 6, 99–110. [Google Scholar] [CrossRef]
- Dal Negro, R.W. Dry powder inhalers and the right things to remember: A concept review. Multidiscip. Respir. Med. 2015, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Laube, B.L.; Janssens, H.M.; de Jongh, F.H.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 2011, 37, 1308–1331. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.Y.; Huang, C.K.; Peng, H.C.; Yu, C.J.; Chien, J.Y. Inappropriate peak inspiratory flow rate with dry powder inhaler in chronic obstructive pulmonary disease. Sci. Rep. 2020, 10, 7271. [Google Scholar] [CrossRef]
- Zervas, E.; Petrocheilou, K.; Tsopa, P.; Evangeliou, M.; Athanassiou, A. Peak inspiratory flow evaluation in different stages’ COPD patients using in-check method (INSPIRE STUDY). Eur. Respir. J. 2016, 48, PA3962. [Google Scholar]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Rahimpour, Y.; Kouhsoltani, M.; Hamishehkar, H. Alternative carriers in dry powder inhaler formulations. Drug Discov. Today 2014, 19, 618–626. [Google Scholar] [CrossRef]
- Momin, M.A.M.; Sinha, S.; Tucker, I.G.; Doyle, C.; Das, S.C. Dry powder formulation of kanamycin with enhanced aerosolization efficiency for drug-resistant tuberculosis. Int. J. Pharm. 2017, 528, 107–117. [Google Scholar] [CrossRef]
- Adhikari, B.R.; Bērziņš, K.; Fraser-Miller, S.J.; Gordon, K.C.; Das, S.C. Co-Amorphization of Kanamycin with Amino Acids Improves Aerosolization. Pharmaceutics 2020, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Belal, T.S.; Ahmed, H.M.; Mahrous, M.S.; Daabees, H.G.; Baker, M.M. Validated stability-indicating HPLC-DAD method for determination of the phosphodiesterase (PDE-4) inhibitor roflumilast. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 79–89. [Google Scholar] [CrossRef] [Green Version]
- de Boer, A.H.; Chan, H.K.; Price, R. A critical view on lactose-based drug formulation and device studies for dry powder inhalation: Which are relevant and what interactions to expect? Adv. Drug Deliv. Rev. 2012, 64, 257–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, M.J. Guiding inspiratory flow: Development of the in-check DIAL G16, a tool for improving inhaler technique. Pulm. Med. 2017, 2017, 1495867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, S.A.; Madhuri, V.; Padmalatha, K. Design and evaluation of fast dissilving tablets of roflumilast solid dispersion. Int. J. Pharm. Sci. Res. 2019, 10, 559–611. [Google Scholar]
- Pritchard, J.N. The Influence of Lung Deposition on Clinical Response. J. Aerosol Med. 2001, 14, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Heyder, J.; Gebhart, J.; Rudolf, G.; Schiller, C.F.; Stahlhofen, W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986, 17, 811–825. [Google Scholar] [CrossRef]
- British Pharmacopoeia; British Pharmacopoeia Commission: London, UK, 2015.
- Focaroli, S.; Mah, P.T.; Hastedt, J.E.; Gitlin, I.; Oscarson, S.; Fahy, J.V.; Healy, A.M. A design of experiment (DoE) approach to optimise spray drying process conditions for the production of trehalose/leucine formulations with application in pulmonary delivery. Int. J. Pharm. 2019, 562, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Price, R.; Young, P.M.; Edge, S.; Staniforth, J.N. The influence of relative humidity on particulate interactions in carrier-based dry powder inhaler formulations. Int. J. Pharm. 2002, 246, 47–59. [Google Scholar] [CrossRef]
- Tan, S.B.; Newton, J.M. Powder flowability as an indication of capsule filling performance. Int. J. Pharm. 1990, 61, 145–155. [Google Scholar] [CrossRef]
- Adler, M.; Unger, M.; Lee, G. Surface composition of spray-dried particles of bovine serum albumin/trehalose/surfactant. Pharm. Res. 2000, 17, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.J. Lung Deposition and Clearance of Pharmaceutical Aerosols: What Can Be Learned from Inhalation Toxicology and Industrial Hygiene? Aerosol Sci. Technol. 1993, 18, 290–304. [Google Scholar] [CrossRef]
- Young, P.M.; Sung, A.; Traini, D.; Kwok, P.; Chiou, H.; Chan, H.K. Influence of humidity on the electrostatic charge and aerosol performance of dry powder inhaler carrier based systems. Pharm. Res. 2007, 24, 963–970. [Google Scholar] [CrossRef]
- Clark, A.R.; Weers, J.G.; Dhand, R. The Confusing World of Dry Powder Inhalers: It Is All About Inspiratory Pressures, Not Inspiratory Flow Rates. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 1–11. [Google Scholar] [CrossRef] [Green Version]








| Factors | Levels | ||
|---|---|---|---|
| Low (1) | Medium (2) | High (3) | |
| A: Feed concentration (% w/v) | 0.25 | 0.50 | 0.75 |
| B: Spray-gas flow rate (L/h) | 601 | 670 | 742 |
| Formulation | Level of Factor in the Experiment | |
|---|---|---|
| A | B | |
| F1 | 1 | 1 |
| F2 | 2 | 1 |
| F3 | 2 | 2 |
| F4 | 2 | 3 |
| F5 | 3 | 1 |
| F6 | 3 | 2 |
| F7 | 1 | 2 |
| F8 | 3 | 3 |
| F9 | 1 | 3 |
| Formulation | Process Yield (%) | Volumetric Particle Diameter | |||
|---|---|---|---|---|---|
| D10 (µm) | D50 (µm) | D90 (µm) | Span | ||
| F1 | 21.9 | 4.8 | 7.9 | 13.7 | 1.1 |
| F2 | 56.7 | 7.2 | 8.7 | 10.4 | 0.4 |
| F3 | 34.0 | 3.3 | 7.3 | 13.3 | 1.4 |
| F4 | 62.0 | 5.3 | 8.5 | 13.2 | 0.9 |
| F5 | 57.9 | 1.9 | 4.3 | 8.9 | 1.6 |
| F6 | 48.8 | 4.2 | 6.7 | 10.1 | 0.9 |
| F7 | 8.0 | 3.4 | 7.8 | 12.6 | 1.2 |
| F8 | 39.6 | 5.5 | 6.5 | 7.6 | 0.3 |
| F9 | 18.0 | 6.5 | 11.8 | 18 | 1.0 |
| Formulation | Yield (%) | Particle Size (D50) | Moisture Content (%) | Bulk Density (g/mL) | Tapped Density (g/mL) | Carr’s Index (%) |
|---|---|---|---|---|---|---|
| SD roflumilast-only | 47.7 | 5.5 ± 0.4 | 1.1 ± 0.2 | 0.363 | 0.539 | 32.7 |
| CoSD powder-Batch 1 | 62.3 | 4.9 ± 0.3 | 1.9 ± 0.7 | 0.345 | 0.489 | 29.6 |
| CoSD powder-Batch 2 | 62.5 | 5.4 ± 0.2 | 1.4 ± 0.1 | 0.356 | 0.495 | 28.1 |
| CoSD powder-Batch 3 | 64.7 | 5.2 ± 0.1 | 1.4 ± 0.1 | 0.352 | 0.514 | 31.5 |
| Formulation | ED (%) | FPF (%) | MMAD (µm) | GSD |
|---|---|---|---|---|
| SD roflumilast-only | 58.3 ± 2.7 | 25.5 ± 5.3 | 5.3 ± 0.7 | 2.0 ± 0.0 |
| CoSD powder—Batch 1 | 89.6 ± 1.1 | 52.0 ± 5.4 | 3.5 ± 0.2 | 2.0 ± 0.3 |
| CoSD powder—Batch 2 | 88.1 ± 2.0 | 53.5 ± 4.5 | 3.6 ± 0.2 | 1.7 ± 0.0 |
| CoSD powder—Batch 3 | 88.2 ± 1.8 | 52.7 ± 5.3 | 3.7 ± 0.3 | 1.6 ± 0.1 |
| Formulation | ED (%) | FPF (%) | ||||
|---|---|---|---|---|---|---|
| Initial | 15% RH | 75% RH | Initial | 15% RH | 75% RH | |
| SD roflumilast-only | 58.3 ± 2.7 | 89.5 ± 0.8 * | 85.8 ± 1.1 * | 25.5 ± 5.3 | 23.3 ± 0.3 | 14.1 ± 0.4 * |
| CoSD powder | 88.1 ± 2.0 | 87.9 ± 2.1 | 85.1 ± 1.2 | 53.5 ± 4.5 | 59.0 ± 1.2 | 31.3 ± 1.5 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momin, M.A.M.; Adhikari, B.R.; Sinha, S.; Larson, I.; Das, S.C. Roflumilast Powders for Chronic Obstructive Pulmonary Disease: Formulation Design and the Influence of Device, Inhalation Flow Rate, and Storage Relative Humidity on Aerosolization. Pharmaceutics 2021, 13, 1254. https://doi.org/10.3390/pharmaceutics13081254
Momin MAM, Adhikari BR, Sinha S, Larson I, Das SC. Roflumilast Powders for Chronic Obstructive Pulmonary Disease: Formulation Design and the Influence of Device, Inhalation Flow Rate, and Storage Relative Humidity on Aerosolization. Pharmaceutics. 2021; 13(8):1254. https://doi.org/10.3390/pharmaceutics13081254
Chicago/Turabian StyleMomin, Mohammad A. M., Bishal Raj Adhikari, Shubhra Sinha, Ian Larson, and Shyamal C. Das. 2021. "Roflumilast Powders for Chronic Obstructive Pulmonary Disease: Formulation Design and the Influence of Device, Inhalation Flow Rate, and Storage Relative Humidity on Aerosolization" Pharmaceutics 13, no. 8: 1254. https://doi.org/10.3390/pharmaceutics13081254
APA StyleMomin, M. A. M., Adhikari, B. R., Sinha, S., Larson, I., & Das, S. C. (2021). Roflumilast Powders for Chronic Obstructive Pulmonary Disease: Formulation Design and the Influence of Device, Inhalation Flow Rate, and Storage Relative Humidity on Aerosolization. Pharmaceutics, 13(8), 1254. https://doi.org/10.3390/pharmaceutics13081254