Nobiletin Inhibits Inflammatory Reaction in Interleukin-1β-Stimulated Human Periodontal Ligament Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. The Release of Cytokines and MMPs from HPDLCs
2.4. Protein Extraction and Western Blot Analysis
2.5. Statistical Analysis
3. Results
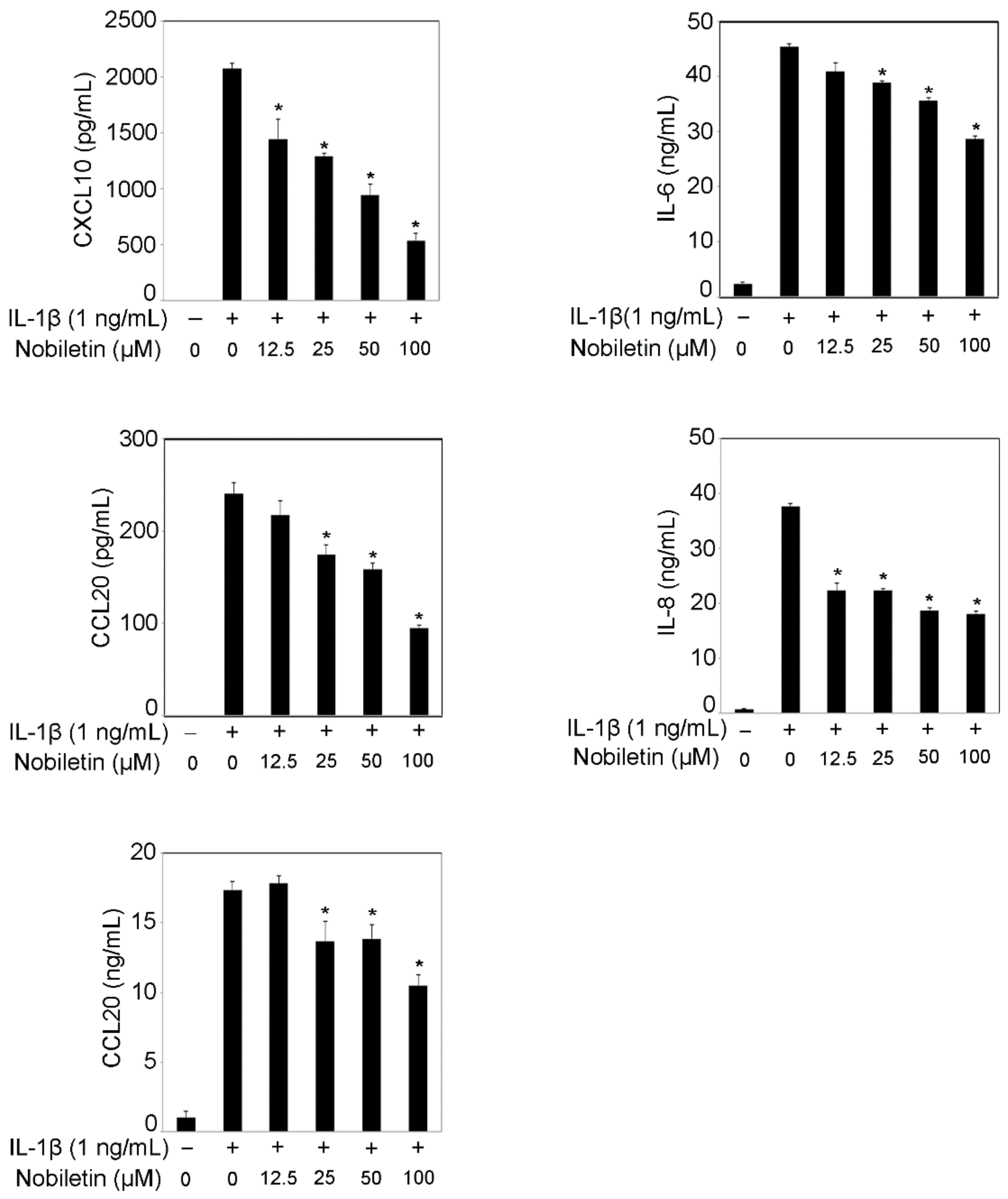
3.1. Nobiletin Could Inhibit Inflammatory Cytokine Production in IL-1β-Stimulated HPDLCs
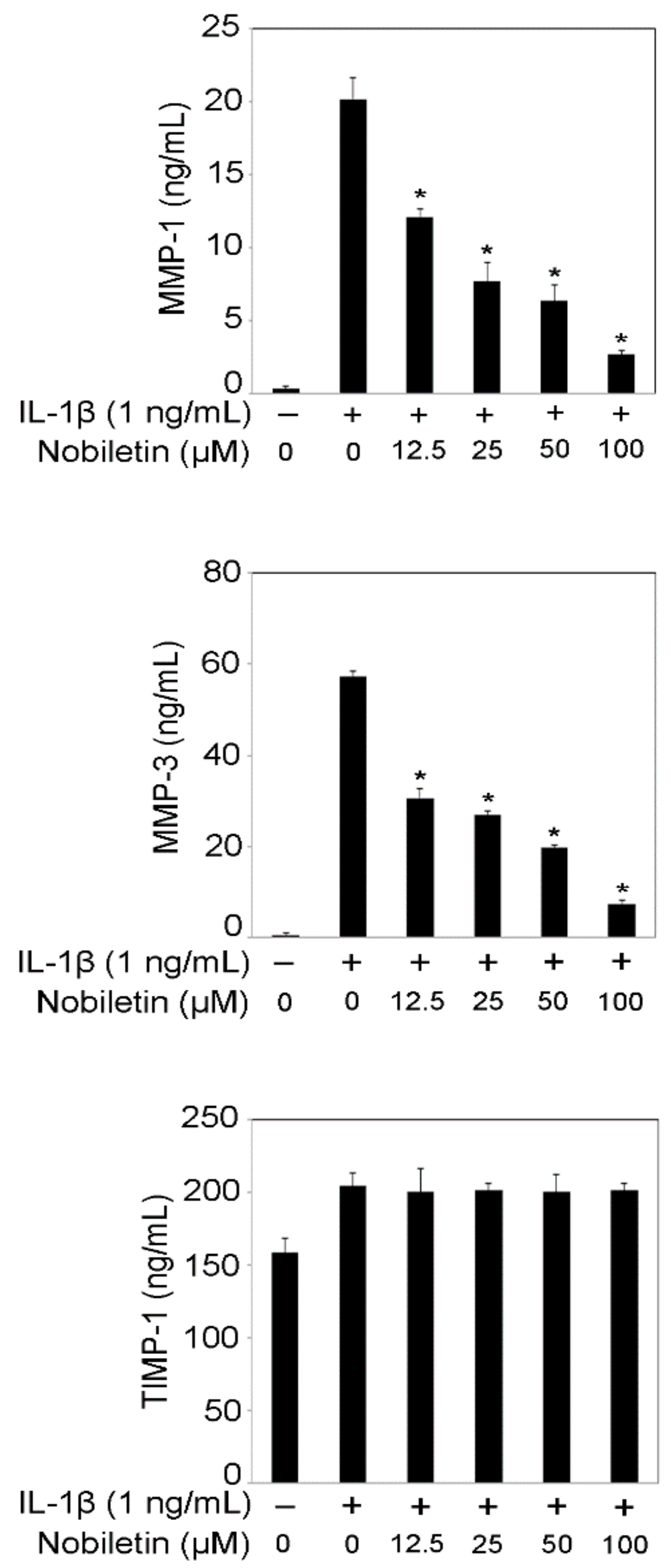
3.2. Nobiletin Inhibited MMP-1 and MMP-3 Production in IL-1β-Stimulated HPDLCs
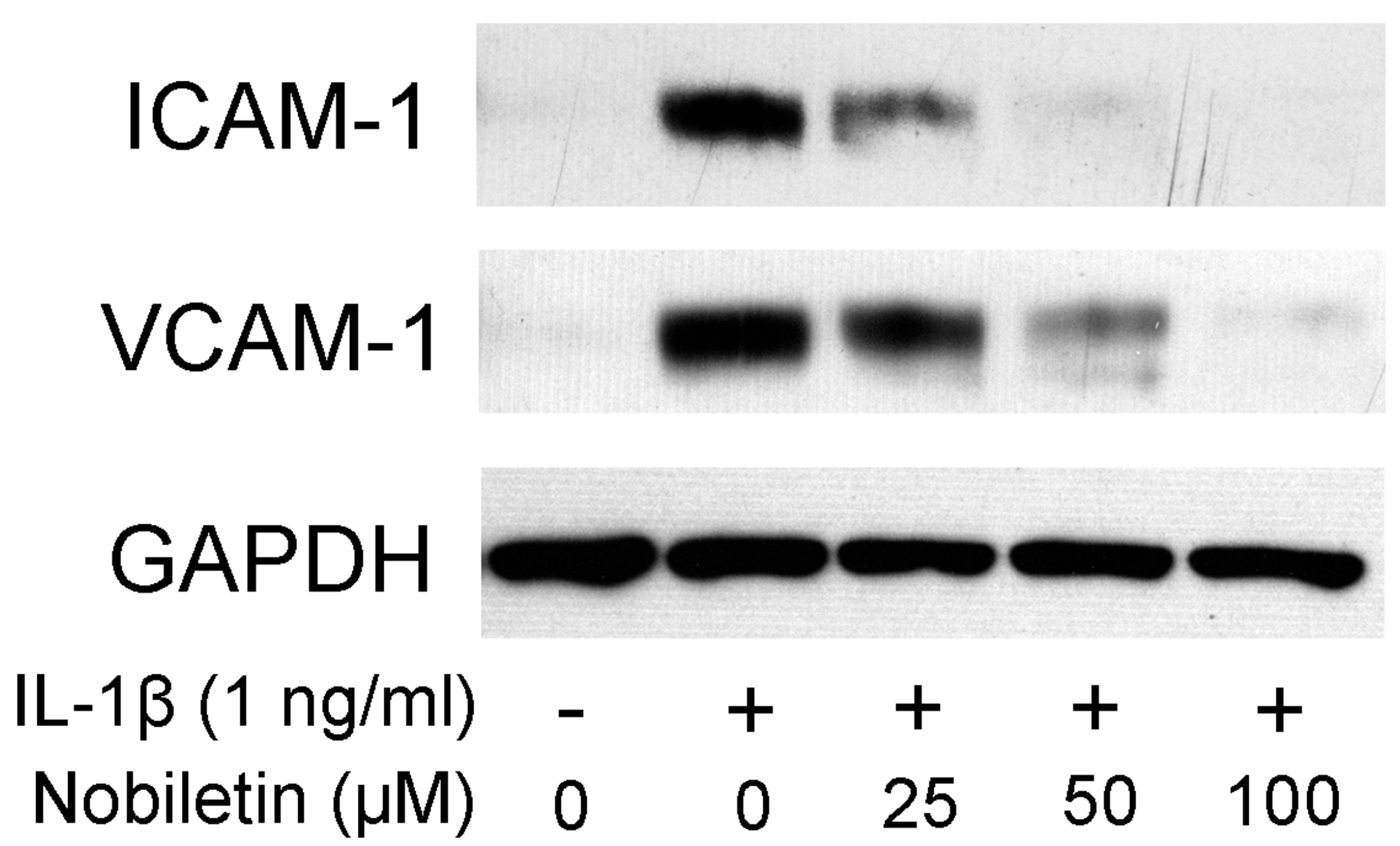
3.3. Nobiletin Decreased ICAM-1 and VCAM-1 Expression in IL-1β-Stimulated HPDLCs
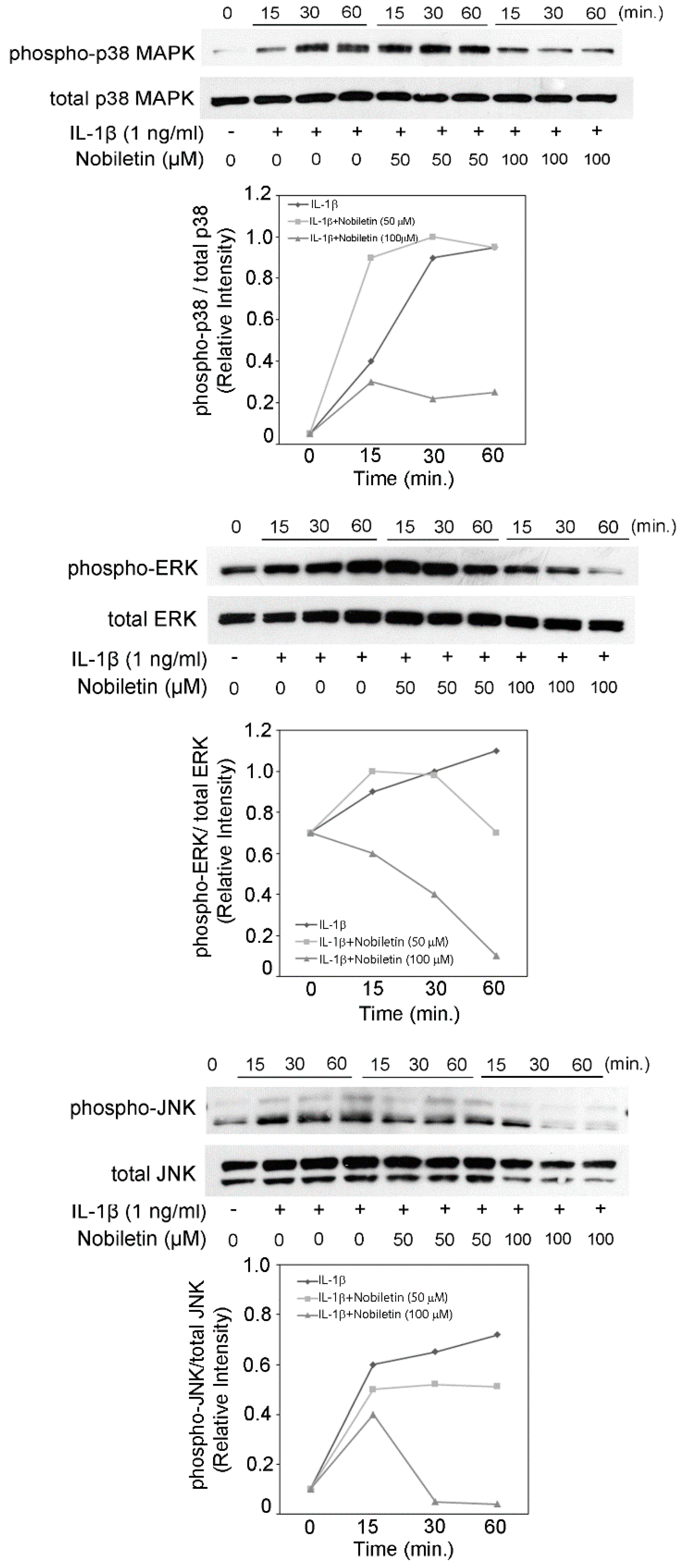
3.4. Nobiletin Decreased the Level of p38 MAPK, ERK, and JNK Phosphorylation in IL-1β-Stimulated HPDLCs
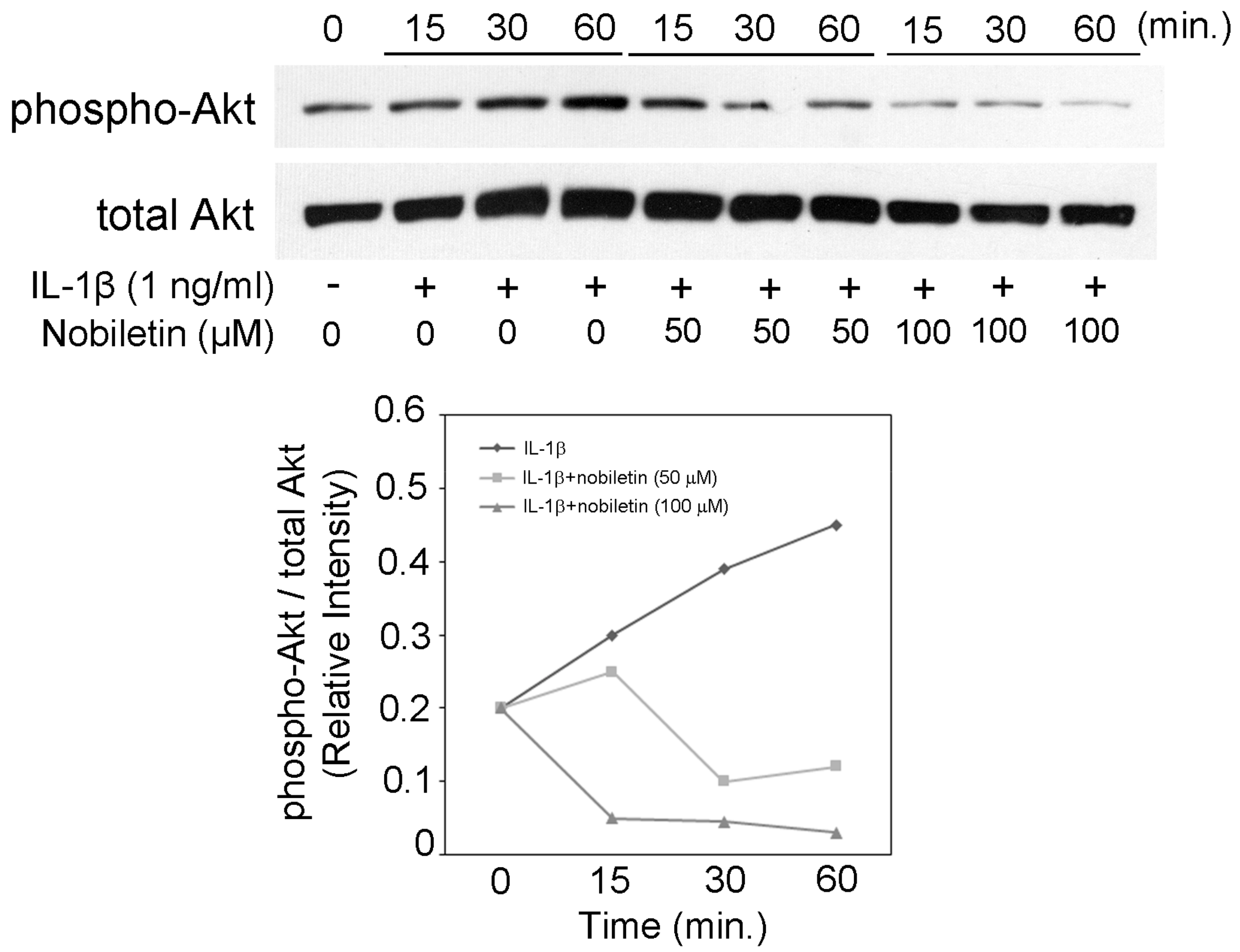
3.5. Nobiletin Could Inhibit Akt Phosphorylation in IL-1β-Stimulated HPDLCs
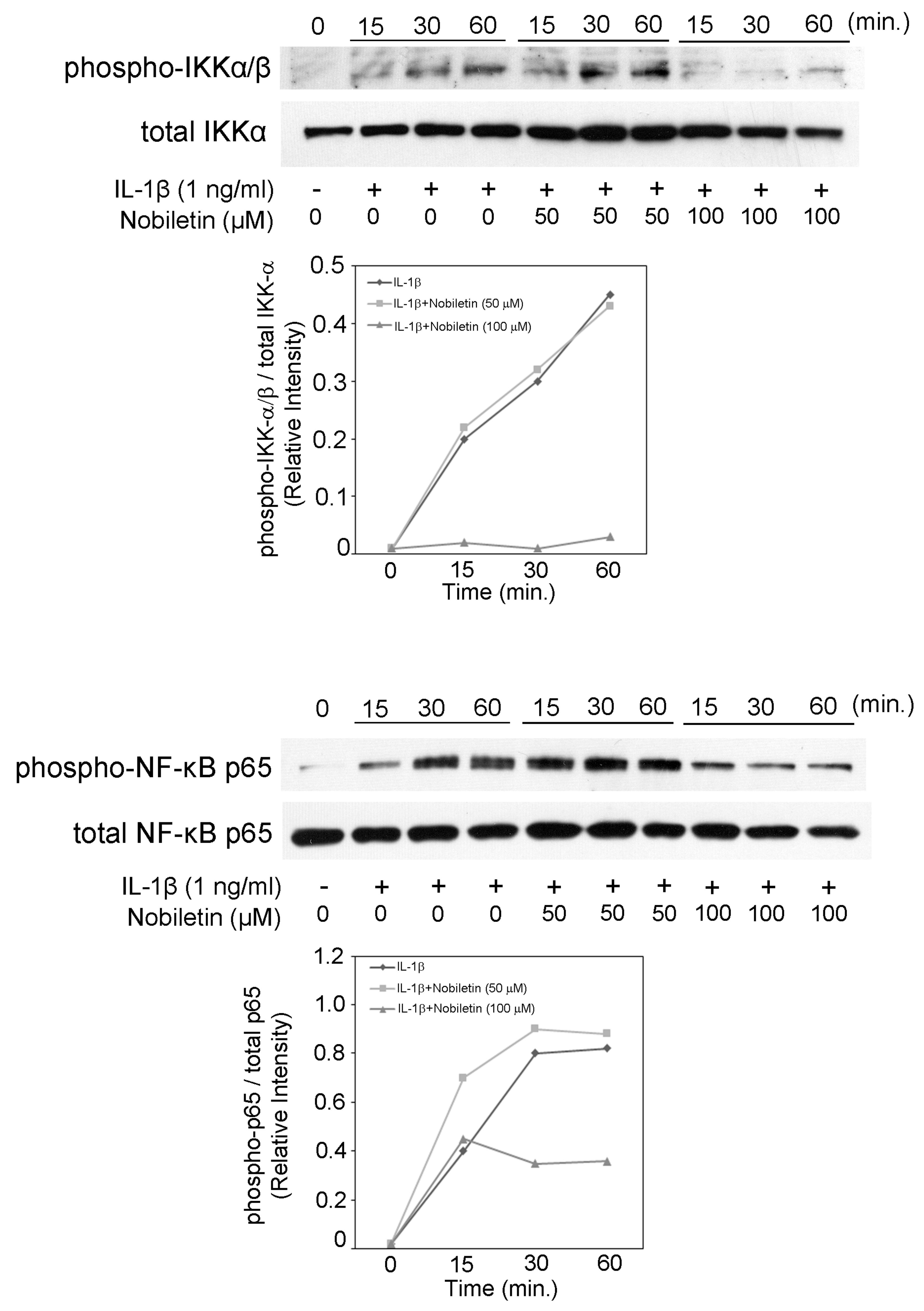
3.6. Nobiletin Could Inhibit NF-κB Activation in IL-1β-Stimulated HPDLCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernández, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Birkedal-Hansen, H. Role of Matrix Metalloproteinases in Human Periodontal Diseases. J. Periodontol. 1993, 64, 474–484. [Google Scholar] [PubMed]
- Sahingur, S.E.; Yeudall, W.A. Chemokine function in periodontal disease and oral cavity cancer. Front. Immunol. 2015, 6, 214. [Google Scholar] [CrossRef]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The Multifunctional Effects of Nobiletin and Its Metabolites In Vivo and In Vitro. Evid. Based Complement. Altern. Med. 2016, 2016, 2918796. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Creed, A.; Chen, A.Y.; Huang, H.; Li, Z.; Rankin, G.O.; Ye, X.; Xu, G.; Chen, Y.C. Nobiletin Supresses Cell Viability through AKT Pathways in PC-3 and DU-145 Prostate Cancer Cells. BMC Pharmacol. Toxicol. 2014, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Chen, M.C.; Li, S.; Lin, C.C.; Wang, T.T. Antiviral activity of nobiletin against chikungunya virus in vitro. Antivir. Ther. 2017, 22, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Liu, Z.; Zhang, T.; Sun, S.; Ye, J.; Li, Z.; Mao, L.; Ren, J. Enhancement of Anti-Inflammatory Properties of Nobiletin in Macrophages by a Nano-Emulsion Preparation. J. Agric. Food Chem. 2018, 66, 91–98. [Google Scholar] [CrossRef]
- Kaneda, H.; Otomo, R.; Sasaki, N.; Omi, T.; Sato, T.; Kaneda, T. Endothelium-independent vasodilator effects of nobiletin in rat aorta. J. Pharmacol. Sci. 2019, 140, 48–53. [Google Scholar] [CrossRef]
- Tominari, T.; Hirata, M.; Matsumoto, C.; Inada, M.; Miyaura, C. Polymethoxy flavonoids, nobiletin and tangeretin, prevent lipopolysaccharide-induced inflammatory bone loss in an experimental model for periodontitis. J. Pharmacol. Sci. 2012, 119, 390–394. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Hosokawa, I.; Shindo, S.; Ozaki, K.; Matsuo, T. Calcitriol Suppressed Inflammatory Reactions in IL-1β-Stimulated Human Periodontal Ligament Cells. Inflammation 2015, 38, 2252–2258. [Google Scholar] [CrossRef]
- Hosokawa, I.; Hosokawa, Y.; Shindo, S.; Ozaki, K.; Matsuo, T. Melatonin Inhibits CXCL10 and MMP-1 Production in IL-1β-Stimulated Human Periodontal Ligament Cells. Inflammation 2016, 39, 1520–1526. [Google Scholar] [CrossRef]
- Silva, M.T. Neutrophils and macrophages work in concert as inducer and effectors of adaptive immnity against extracellular and intracellular microbial pathogens. J. Leukoc Biol. 2010, 87, 805–813. [Google Scholar] [CrossRef]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front. Endocrinol. 2019, 9, 788. [Google Scholar] [CrossRef]
- Joe, B.H.; Borke, J.L.; Keskintepe, M.; Hanes, P.J.; Mailhot, J.M.; Singh, B.B. Interleukin-1beta regulation of adhesion molecules on human gingival and periodontal ligament fibroblasts. J. Periodontol. 2001, 72, 865–870. [Google Scholar] [CrossRef]
- Shindo, S.; Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Matsuo, T. Genipin inhibits IL-1β-induced CCL20 and IL-6 production from human periodontal ligament cells. Cell Physiol. Biochem. 2014, 33, 357–364. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Hosokawa, I.; Shindo, S.; Ozaki, K.; Matsuo, T. Gomisin N Decreases Inflammatory Cytokine Production in Human Periodontal Ligament Cells. Inflammation 2017, 40, 360–365. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, J.W.; Kim, S.C.; Kang, K.H.; Lee, S.K.; Pi, S.H.; Lee, S.K.; Kim, E.C. Roles of p38 and ERK MAP kinases in IL-8 expression in TNF-alpha- and dexamethasone-stimulated human periodontal ligament cells. Cytokine 2006, 35, 67–76. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ouyang, X. Beyond toll-like receptors: Porphyromonas gingivalis induces IL-6, IL-8, and VCAM-1 expression through NOD-mediated NF-kappaB and ERK signaling pathways in periodontal fibroblasts. Inflammation 2014, 37, 522–533. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Matsuo, T. Sudachitin Inhibits Matrix Metalloproteinase-1 and -3 Production in Tumor Necrosis Factor-alpha-Stimulated Human Periodontal Ligament Cells. Inflammation 2019, 42, 1456–1462. [Google Scholar] [CrossRef]
- Guan, S.M.; Zhang, M.; He, J.J.; Wu, J.Z. Mitogen-activated protein kinases and phosphatidylinositol 3-kinase are involved in Prevotella intermedia-induced proinflammatory cytokines expression in human periodontal ligament cells. Biochem. Biophys. Res. Commun. 2009, 386, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Park, K.H.; Kim, S.J.; Kang, Y.G.; Lee, Y.M.; Kim, E.C. Mechanical stress-activated immune response genes via Sirtuin 1 expression in human periodontal ligament cells. Clin. Exp. Immunol. 2012, 168, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Arora, P.D.; Rajshankar, D.; McCulloch, C.A. Cell adhesion proteins: Roles in periodontal physiology and discovery by proteomics. Periodontol. 2000 2013, 63, 48–58. [Google Scholar] [CrossRef]
- Kim, J.J.; Korm, S.; Kim, W.S.; Kim, O.S.; Lee, J.S.; Min, H.G.; Chin, Y.W.; Cha, H.J. Nobiletin suppresses MMP-9 expression through modulation of p38 MAPK activity in human dermal fibrobalsts. Biol. Pharm. Bull. 2014, 37, 158–163. [Google Scholar] [CrossRef]
- Zhou, C.H.; Wu, X.H.; Wu, Y.Q. Nobiletin, a dietary phytochemical, inhibits vascular smooth muscle cells proliferation via calcium-mediated c-Jun N-terminal kinases pathway. Eur. J. Pharmacol. 2009, 615, 55–60. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, D.; Huang, L.; Jiang, C.; Pan, T.; Kang, X.; Pan, J. Nobiletin Inhibits IL-1β-Induced Inflammation in Chondrocytes via Suppression of NF-κB Signaling and Attenuates Osteoarthritis in Mice. Front. Pharmacol. 2019, 10, 570. [Google Scholar] [CrossRef]
- Li, W.; Zhao, R.; Wang, X.; Liu, F.; Zhao, J.; Yao, Q.; Zhi, W.; He, Z.; Niu, X. Nobiletin-Ameliorated Lipopolysaccharide-Induced Inflammation in Acute Lung Injury by Suppression of NF-κB Pathway In Vivo and Vitro. Inflammation 2018, 41, 996–1007. [Google Scholar] [CrossRef]
- Xie, L.; Xie, H.; Chen, C.; Tao, Z.; Zhang, C.; Cai, L. Inhibiting the PI3K/AKT/NF-kappaB signal pathway with nobiletin for attenuating the development of osteoarthritis: In vitro and in vivo studies. Food Funct. 2019, 10, 2161–2175. [Google Scholar] [CrossRef]
- Qi, G.; Mi, Y.; Fan, R.; Li, R.; Liu, Z.; Liu, X. Nobiletin Protects against Systemic Inflammation-Stimulated Memory Impairment via MAPK and NF-kappaB Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5122–5134. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Matsuo, T. Nobiletin Inhibits Inflammatory Reaction in Interleukin-1β-Stimulated Human Periodontal Ligament Cells. Pharmaceutics 2021, 13, 667. https://doi.org/10.3390/pharmaceutics13050667
Hosokawa Y, Hosokawa I, Ozaki K, Matsuo T. Nobiletin Inhibits Inflammatory Reaction in Interleukin-1β-Stimulated Human Periodontal Ligament Cells. Pharmaceutics. 2021; 13(5):667. https://doi.org/10.3390/pharmaceutics13050667
Chicago/Turabian StyleHosokawa, Yoshitaka, Ikuko Hosokawa, Kazumi Ozaki, and Takashi Matsuo. 2021. "Nobiletin Inhibits Inflammatory Reaction in Interleukin-1β-Stimulated Human Periodontal Ligament Cells" Pharmaceutics 13, no. 5: 667. https://doi.org/10.3390/pharmaceutics13050667
APA StyleHosokawa, Y., Hosokawa, I., Ozaki, K., & Matsuo, T. (2021). Nobiletin Inhibits Inflammatory Reaction in Interleukin-1β-Stimulated Human Periodontal Ligament Cells. Pharmaceutics, 13(5), 667. https://doi.org/10.3390/pharmaceutics13050667





