Liposomal Encapsulated FSC231, a PICK1 Inhibitor, Prevents the Ischemia/Reperfusion-Induced Degradation of GluA2-Containing AMPA Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Liposomal Formulation of FSC231
2.2. LC/MS
2.3. Size Distribution and Surface Potential Measurements
2.4. Encapsulation Efficiency
2.5. Animals
2.6. Preparation of Acute Rat Hippocampal Slices
2.7. OGD/R Procedure
2.8. Lysate Preparation
2.9. Western Blotting
2.10. Immunoprecipitation
2.11. Statistical Analyses
2.12. Data Availability
3. Results
3.1. Characterization of FSC231-Containing Liposomes
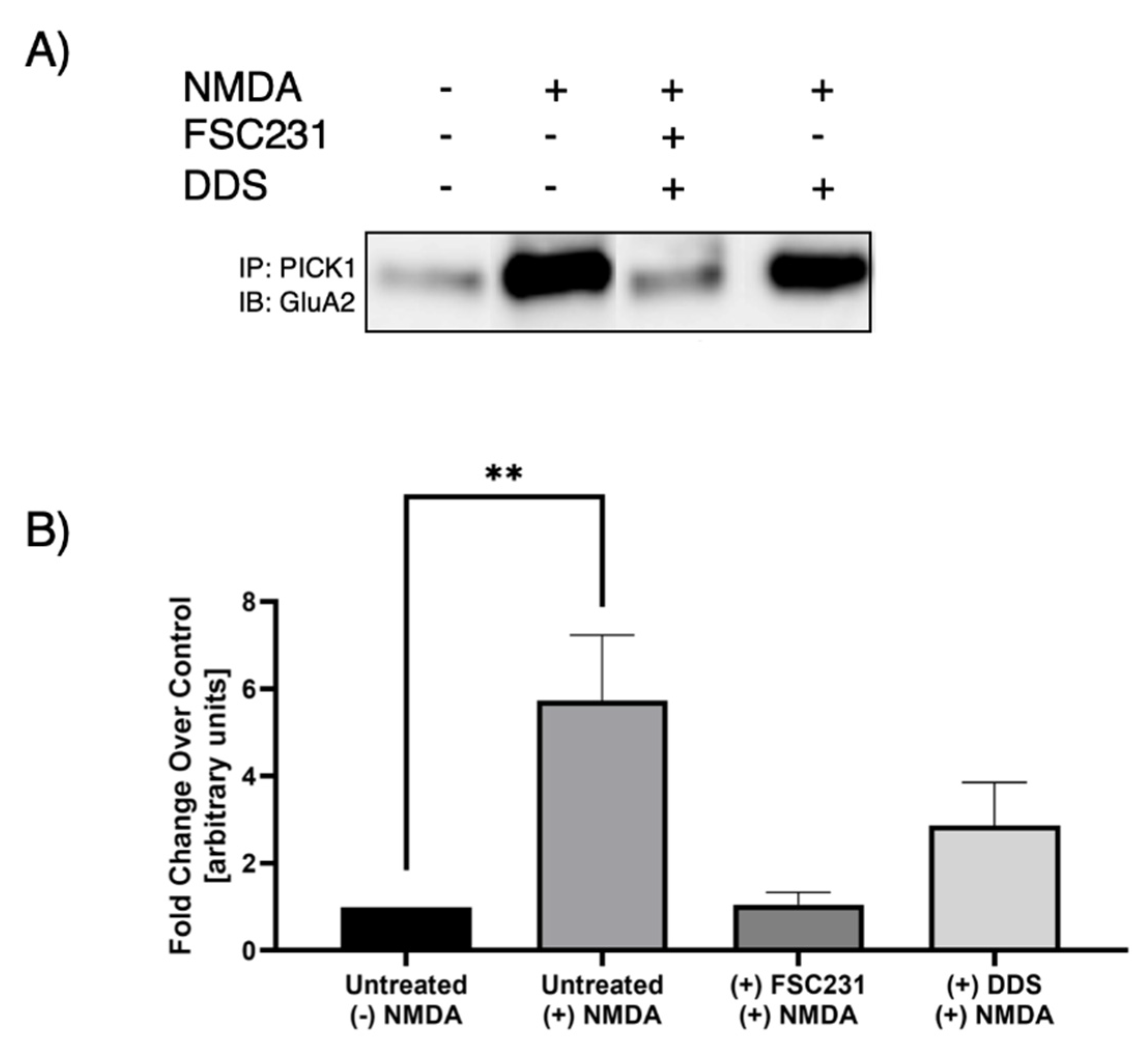
3.2. FSC231 Treatment Antagonizes NMDA-Induced PICK1–GluA2 Interaction
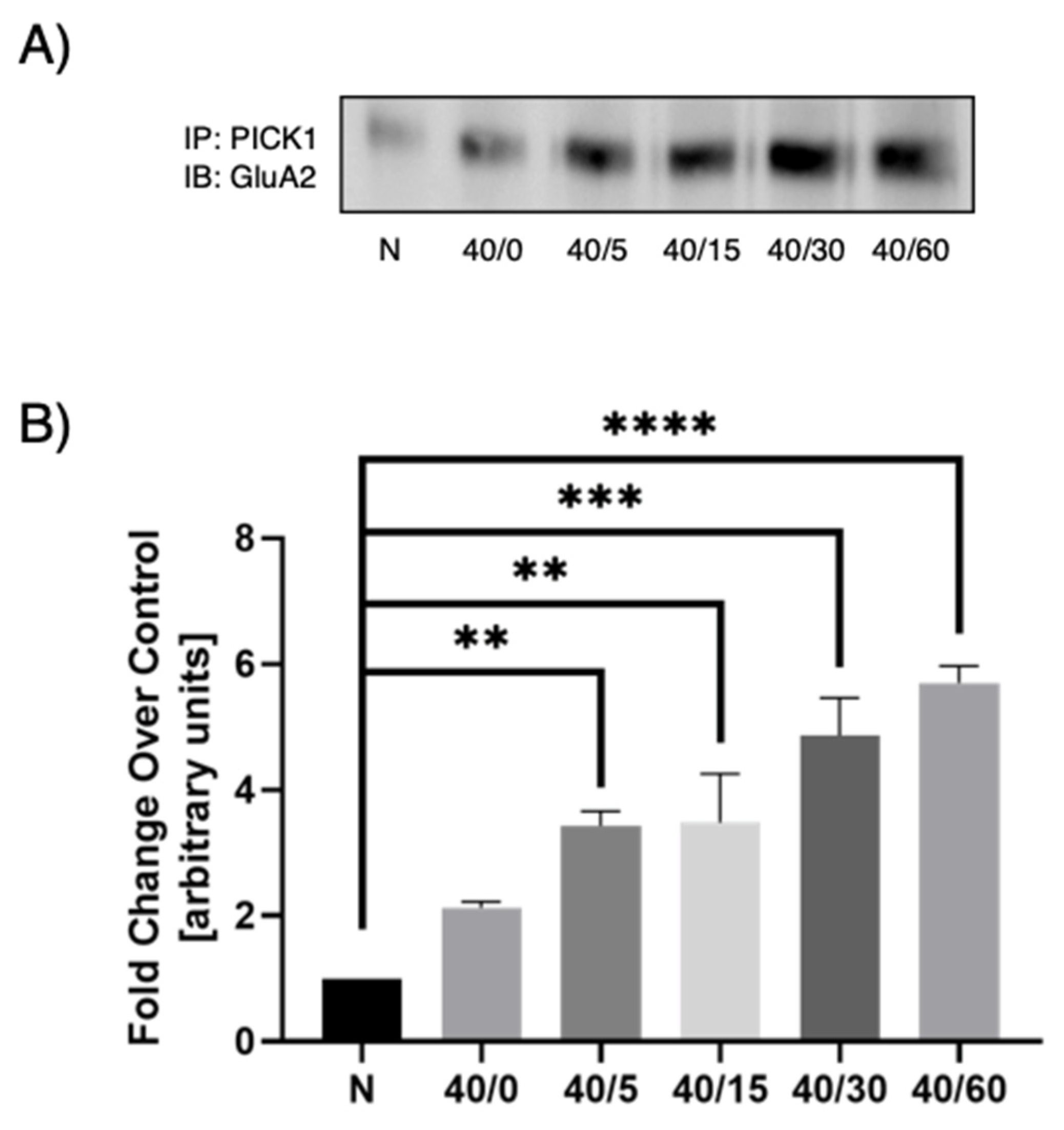
3.3. Optimal PICK1–GluA2 Association
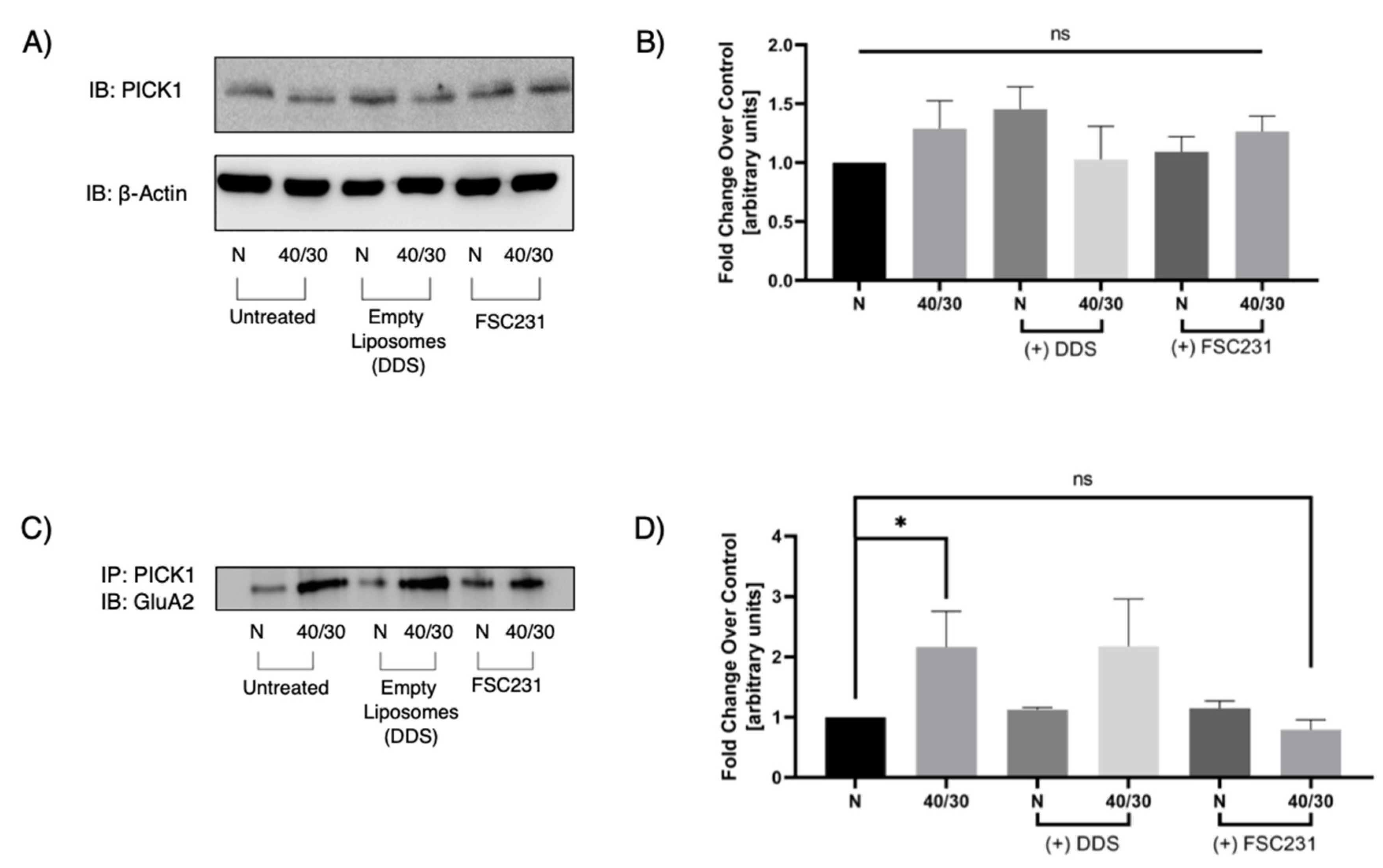
3.4. FSC231-Containing Liposomes Attenuate OGD/R-Induced PICK1–GluA2 Interaction
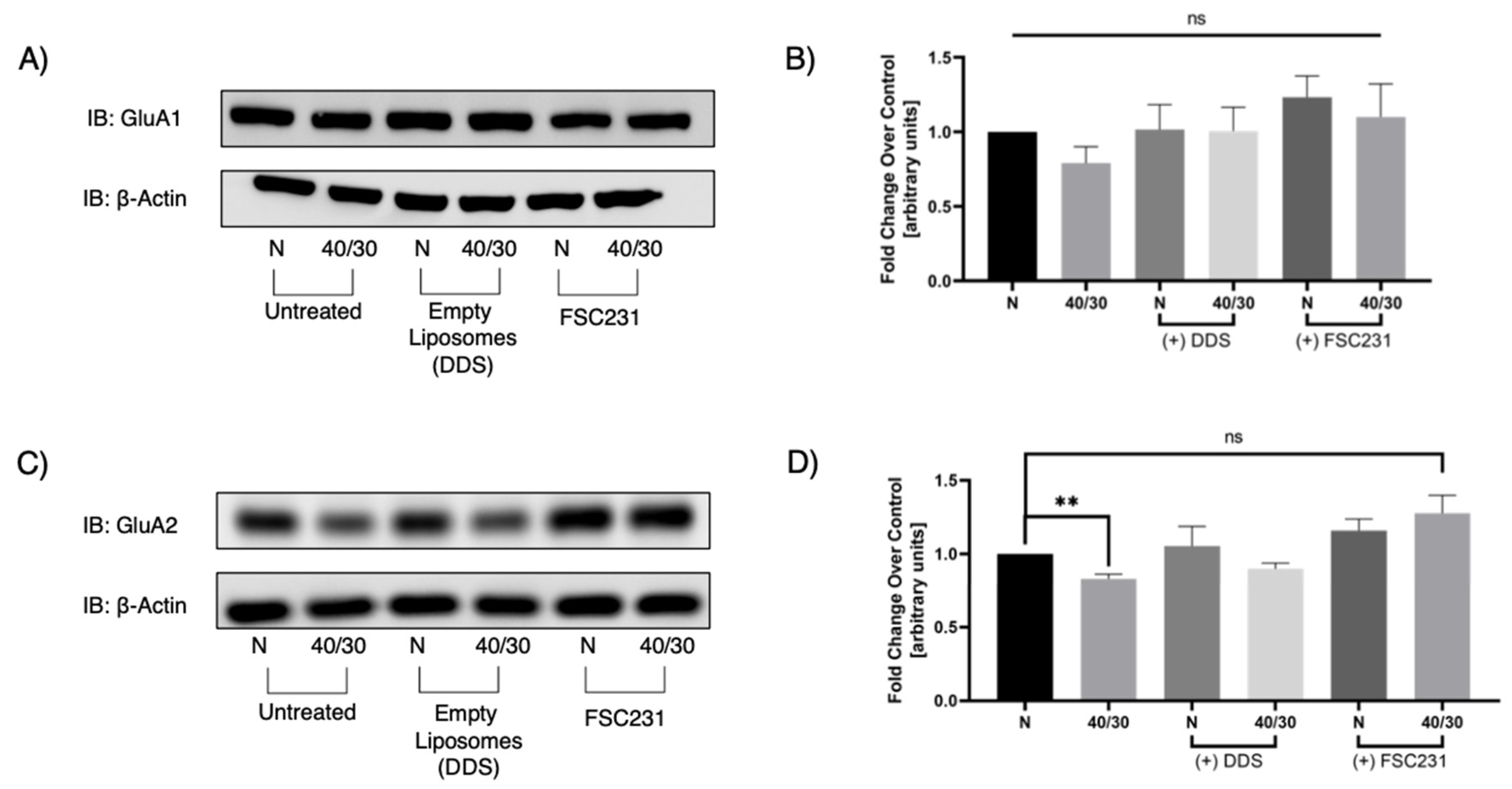
3.5. FSC231 Treatment Prevents OGD/R-Induced Degradation of GluA2 AMPAR Subunit
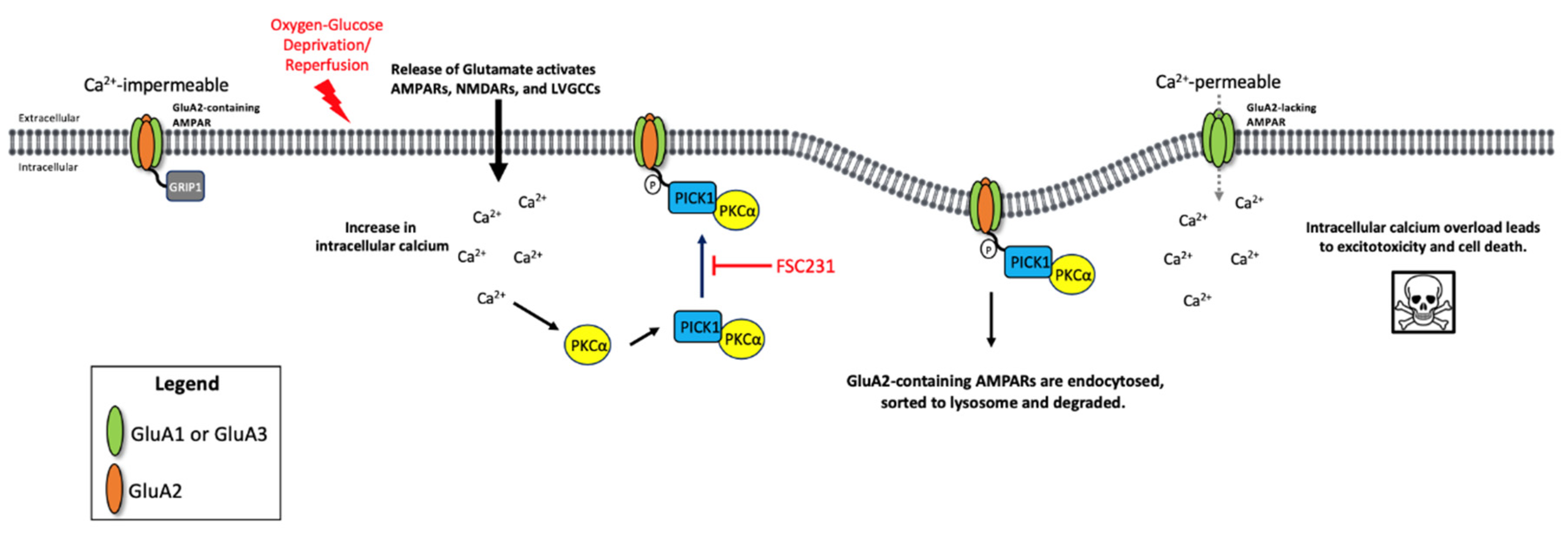
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Top Ten Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/index.html (accessed on 10 April 2020).
- Falluji, N.; Abou-Chebl, A.; Rodriguez-Castro, C.E.; Mukherjee, D. Reperfusion strategies for acute ischemic stroke. Angiology 2011, 1, 289–296. [Google Scholar] [CrossRef]
- Ford, L.M.; Sanberg, P.R.; Norman, A.B.; Fogelson, M.H. MK-801 prevents hippocampal neurodegeneration in neonatal hypoxic-ischemic rats. Arch. Neurol. 1989, 46, 1090–1096. [Google Scholar] [CrossRef]
- Mitani, A.; Namba, S.; Ikemune, K.; Yanase, H.; Arai, T.; Kataoka, K. Postischemic enhancements of N-Methyl-D-Aspartic Acid (NMDA) and non-NMDA receptor-mediated responses in hippocampal CA1 pyramidal neurons. J. Cereb. Blood Flow Metab. 1989, 18, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Massieu, L. Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch. Med Res. 2005, 37, 11–18. [Google Scholar] [CrossRef]
- Faden, A.; Demediuk, P.; Panter, S.S.; Vink, R. The role of excitatory amino acids in traumatic brain injury. Science 1989, 244, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Mcintosh, T.K.; Vink, R.; Soares, H.; Hayes, R.; Simon, R. Effects of the N-methyl-D-aspartate receptor blocker MK-801 on neurologic function after experimental brain injury. J. Neurotrama 1989, 6, 247–259. [Google Scholar] [CrossRef]
- Takag, N.; Shinno, K.; Teves, L.; Bissoon, N.; Wallace, M.C.; Gurd, J.W. Transient ischemia differentially increases tyrosine phosphorylation of NMDA receptor subunits 2A and 2B. J. Neurochem. 1997, 69, 1060–1065. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, T.P.; Aarts, M.; Rooyakkers, A.; Liu, L.; Lai, T.W.; Wu, D.C.; Lu, J.; Tymianski, M.; Craig, A.M.; et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007, 27, 2846–2857. [Google Scholar] [CrossRef]
- Liu, S.; Lau, L.; Wei, J.; Zhu, D.; Shengwei, Z.; Sun, H.; Fu, Y.; Liu, F.; Lu, Y. Expression of Ca2+-permeable AMPA receptor channels primes cell death in transient forebrain ischemia. Cell Press 2004, 43, 43–55. [Google Scholar] [CrossRef]
- Yin, H.; Sensi, S.; Ogoshi, F.; Weiss, J.H. Blockade of Ca2+-permeable AMPA/Kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J. Neurosci. 2002, 22, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Anzai, T.; Tsuzuki, K.; Yamada, N.; Hayashi, T.; Iwakuma, M.; Inada, K.; Kameyama, K.; Hoka, S.; Saji, M. Overexpression of Ca2+-permeable AMPA receptor promotes delayed cell death of hippocampal CA1 neurons following transient forebrain ischemia. Neurosci. Res. 2003, 46, 41–51. [Google Scholar] [CrossRef]
- Calderone, A.; Jover, T.; Mashiko, T.; Noh, K.M.; Tanaka, H.; Bennett, M.V.; Zukin, R.S. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J. Neurosci. 2004, 24, 9903–9913. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.M.; Yokota, H.; Mashiko, T.; Castillo, P.E.; Zukin, R.S.; Bennett, M.V. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc. Natl. Acad. Sci. USA 2005, 102, 12230–12235. [Google Scholar] [CrossRef]
- Liu, B.; Liao, M.; Mielke, J.G.; Ning, K.; Chen, Y.; Li, L.; El-Hayek, Y.H.; Gomez, E.; Zukin, R.S.; Fehlings, M.G.; et al. Ischemia insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J. Neurosci. 2006, 26, 5309–5319. [Google Scholar] [CrossRef] [PubMed]
- Kuner, T.; Beck, C.; Sakmann, B.; Seeburg, P.H. Channel-lining residues of the AMPA receptor M2 segment: Structural environment of the Q/R site and identification of the selectivity filter. J. Neurosci. 2001, 21, 4162–4172. [Google Scholar] [CrossRef]
- Kwak, S.; Weiss, J.H. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr. Opin. Neurobiol. 2006, 16, 281–287. [Google Scholar] [CrossRef]
- Lin, D.T.; Huganir, R.L. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J. Neurosci. 2007, 27, 13903–13908. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.M.; Mellor, J.R.; Hanley, J.G. PICK1-mediated glutamate receptor subunit 2 (GluR2) trafficking contributes to cell death in oxygen/glucose-deprived hippocampal neurons. J. Biol. Chem. 2009, 284, 14230–14235. [Google Scholar] [CrossRef]
- Thorsen, T.S.; Madsen, K.L.; Rebola, N.; Rathje, M.; Anggono, V.; Bach, A.; Moreira, I.S.; Stuhr-Hansen, N.; Dyhring, T.; Peters, D.; et al. Identification of a small-molecule inhibitor of the PICK1 PDZ domain that inhibits hippocampal LTP and LTD. Proc. Natl. Acad. Sci. USA 2010, 107, 413–418. [Google Scholar] [CrossRef]
- Fricker, G.; Kromp, T.; Wendel, A.; Blume, A.; Zirkel, J.; Rebmann, H.; Setzer, C.; Quinkert, R.O.; Martin, F.; Muller-Goymann, C. Phospholipids and lipid-based formulations in oral drug delivery. Pharm. Res. 2010, 27, 1469–1486. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Parnham, M.J.; Wetzig, H. Toxicity screening of liposomes. Chem. Phys. Lipids 1993, 64, 263–274. [Google Scholar] [CrossRef]
- Bach, A.; Stuhr-Hansen, N.; Thorsen, T.S.; Bork, N.; Moreira, I.S.; Frydenvang, K.; Padrah, S.; Christensen, S.B.; Madsen, K.L.; Weinstein, H.; et al. Structure-activity relationships of a small-molecule inhibitor of the PDZ domain of PICK1. Org. Biomol. Chem. 2010, 8, 4281–4288. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.J.; Kent, H.M.; Mills, I.G.; Vallis, Y.; Butler, P.J.G.; Evans, P.R.; McMahon, H.T. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science 2004, 303, 495–499. [Google Scholar] [CrossRef]
- Isaac, J.T.R.; Ashby, M.C.; McBain, C.J. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 2007, 54, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Scorziello, A.; Duchen, M.R. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 2007, 27, 1129–1138. [Google Scholar] [CrossRef]
- Gonzalez Bosc, L.V.; Plomaritas, D.R.; Herbert, L.M.; Giermakowska, W.; Browning, C.; Jernigan, N.L. ASIC-1 mediated calcium entry stimulates NFATc3 nuclear translocation via PICK1 coupling in pulmonary arterial mooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Herbert, L.M.; Nitta, C.H.; Yellowhair, T.R.; Browning, C.; Gonzalez Bosc, L.V.; Resta, T.C.; Jernigan, N.L. PICK1/calcineurin suppress ASIC1-mediated Ca2+ entry in rat pulmonary arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 2016, 310, 390–400. [Google Scholar] [CrossRef]
- Xie, J.; Wu, X.; Zhou, Q.; Yang, Y.; Tian, Y.; Huang, C.; Meng, X.; Li, J. PICK1 confers anti-inflammatory effects in acute liver injury via suppressing M1 macrophage polarization. Biochimie 2016, 127, 121–132. [Google Scholar] [CrossRef]
- Liu, T.; Qu, Z.; Ren, C.; Gan, X.; Qiu, C.; Hu, W. Prolactin potentiates the activity of acid-sensing ion channels in female rat primary sensory neurons. Neuropharmacology 2015, 103, 174–182. [Google Scholar] [CrossRef]
- Qiu, C.; Liu, Y.; Qiu, F.; Wu, J.; Zhou, Q.; Hu, W. Prokineticin 2 potentiates acid-sensing ion channel activity in rat dorsal root ganglion neurons. J. Neuroinflammation 2012, 9, 108–118. [Google Scholar] [CrossRef]
- Tang, Q.; Tan, L.; Yang, X.; Shen, Q.; Huang, X.; Wang, G.; Chen, H.; Nie, J.; Li, S.; Wu, L. Willed-movement training reduces motor deficits and induces a PICK1-dependent LTD in rats subjected to focal cerebral ischemia. Behav. Brain Res. 2013, 256, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.D.; Schassburger, R.L.; Guercio, L.A.; Pierce, R.C. Stimulation of mGluR5 in the Accumbens shell promotes cocaine seeking by activating PKC gamma. J. Neurosci. 2013, 33, 14160–14169. [Google Scholar] [CrossRef]
- Foerg, C.; Merkle, H.P. On the biomedical promise of cell penetrating peptides: Limits versus prospects. J. Pharm. Sci. 2008, 97, 144–162. [Google Scholar] [CrossRef]
- Madsen, K.L.; Thorsen, T.S.; Rahbek-Clemmensen, T.; Eriksen, J.; Gether, U. Protein interacting with C kinase 1 (PICK1) reduces reinsertion rates of interaction partners sorted to Rab11-dependent slow recycling pathway. J. Biol. Chem. 2012, 287, 12293–12308. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Xia, J.; Scannevin, R.H.; Zhang, X.; Huganir, R.L. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interactions with PDZ domain-containing proteins. J. Neurosci. 2000, 20, 7258–7267. [Google Scholar] [CrossRef] [PubMed]
- Seidenman, K.J.; Steinberg, J.P.; Huganir, R.; Malinow, R. Glutamate receptor subunit 2 serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J. Neurosci. 2003, 23, 872–882. [Google Scholar] [CrossRef]
- Lu, W.; Ziff, E.B. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron 2005, 47, 407–421. [Google Scholar] [CrossRef]
- Perez, J.L.; Khatri, L.; Chang, C.; Srivastava, S.; Osten, P.; Ziff, E.B. PICK1 targets activated protein kinase Cα to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J. Neurosci. 2001, 21, 5417–5428. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.A.; Beske, P.H.; Byrnes, N.M.; Astruc-Diaz, F. The post-ischemic increase in GluA2 Ser880 phosphorylation involves NADPH oxidase. J. Pharm. Sci. Ther. 2018, 4, 170–181. [Google Scholar] [CrossRef]
- Hirbec, H.I.N.; Francis, J.C.; Lauri, S.E.; Braithwaite, S.P.; Coussen, F.O.; Mulle, C.; Dev, K.K.; Couthino, V.; Meyer, G.; Isaac, J.T.R.; et al. Rapid and differential regulation of AMPA and Kainate receptors at hippocampal mossy fiber synapses by PICK1 and GRIP. Neuron 2003, 37, 625–638. [Google Scholar] [CrossRef]
- Jaulin-Bastard, F.; Saito, H.; Le Bivic, A.; Ollendorff, V.; Marchetto, S.; Birnbaum, D.; Borg, J.P. The ERBB2/HER2 receptor differentially interacts with ERBIN and PICK1 PSD-95/DLG/ZO-1 domain proteins. J. Biol. Chem. 2001, 276, 15256–15263. [Google Scholar] [CrossRef] [PubMed]
- Garry, E.M.; Moss, A.; Rosie, R.; Delaney, A.; Mitchell, R.; Fleetwood-Walker, S.M. Specific involvement in neuropathic pain of AMPA receptors and adaptor proteins for the GluR2 subunit. Mol. Cell. Neurosci. 2003, 24, 10–22. [Google Scholar] [CrossRef]
- Kim, C.H.; Chung, H.J.; Lee, H.K.; Huganir, R.L. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc. Natl. Acad. Sci. USA 2001, 98, 11725–11730. [Google Scholar] [CrossRef]
- Jin, W.; Ge, W.P.; Xu, J.; Cao, M.; Peng, L.; Yung, W.; Liao, D.; Duan, S.; Zhang, M.; Xia, J. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J. Neurosci. 2006, 26, 2380–2390. [Google Scholar] [CrossRef]
- Fukuta, T.; Ishii, T.; Asai, T.; Oku, N. Applications of liposomal drug delivery systems to develop neuroprotective agents for the treatment of ischemic stroke. Biol. Pharm. Bull. 2019, 42, 319–326. [Google Scholar] [CrossRef]
- Fukuta, T.; Asai, T.; Sato, A.; Namba, M.; Yanagida, Y.; Kikuchi, T.; Koide, H.; Shimizu, K.; Oku, N. Neuroprotection against cerebral ischemia/reperfusion injury by intravenous administration of liposomal fasudil. Int. J. Pharm. 2016, 506, 129–137. [Google Scholar] [CrossRef]

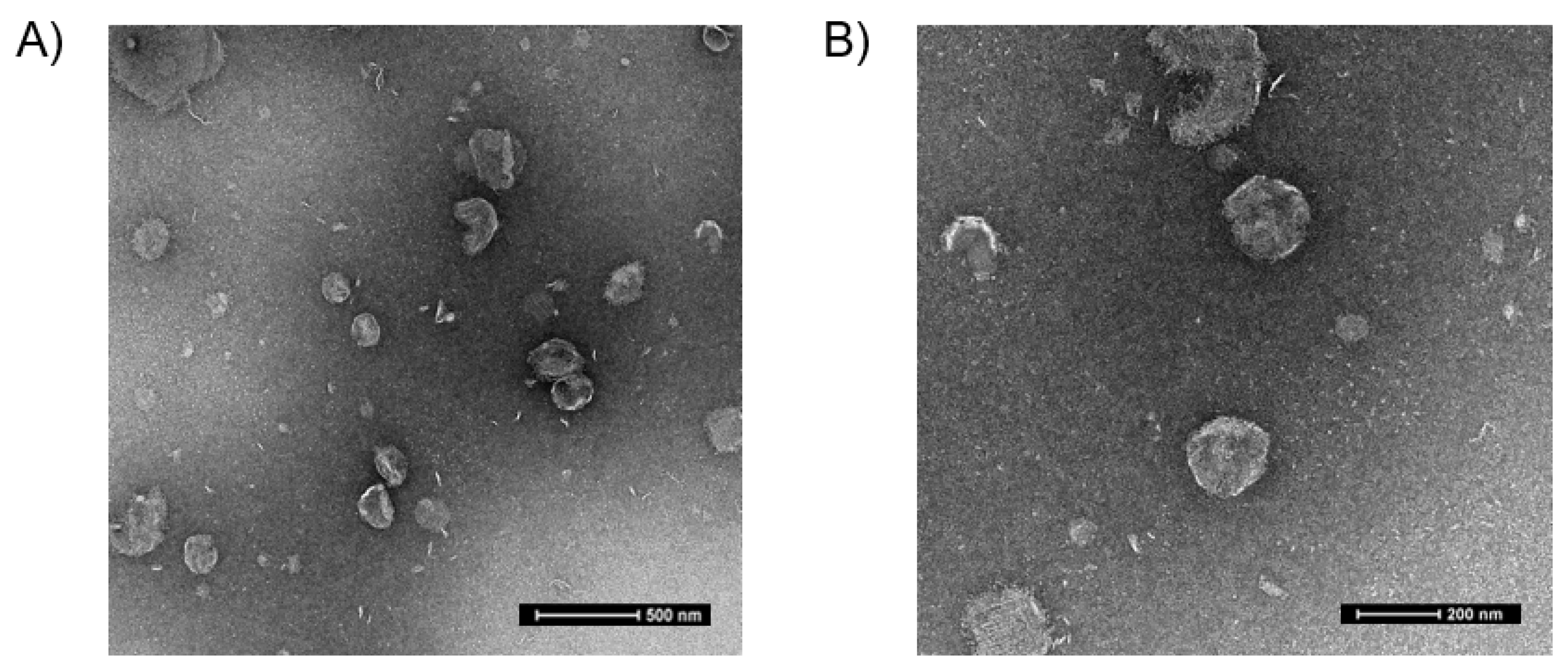
| Sample | Article Particle Size (nm) | Polydispersibility Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| Empty Multilamellar Vesicles | 173.3 | 0.369 | −2.89 |
| FSC231-loaded Multilamellar Vesicles | 256.1 | 0.312 | −7.01 |
| Wavelength (nm) | Encapsulation Efficiency (%) |
|---|---|
| 302 | 58 |
| 303 | 58 |
| 304 | 58 |
| 305 | 58 |
| 306 | 57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achzet, L.M.; Astruc-Diaz, F.; Beske, P.H.; Natale, N.R.; Denton, T.T.; Jackson, D.A. Liposomal Encapsulated FSC231, a PICK1 Inhibitor, Prevents the Ischemia/Reperfusion-Induced Degradation of GluA2-Containing AMPA Receptors. Pharmaceutics 2021, 13, 636. https://doi.org/10.3390/pharmaceutics13050636
Achzet LM, Astruc-Diaz F, Beske PH, Natale NR, Denton TT, Jackson DA. Liposomal Encapsulated FSC231, a PICK1 Inhibitor, Prevents the Ischemia/Reperfusion-Induced Degradation of GluA2-Containing AMPA Receptors. Pharmaceutics. 2021; 13(5):636. https://doi.org/10.3390/pharmaceutics13050636
Chicago/Turabian StyleAchzet, Lindsay M., Fanny Astruc-Diaz, Phillip H. Beske, Nicholas R. Natale, Travis T. Denton, and Darrell A. Jackson. 2021. "Liposomal Encapsulated FSC231, a PICK1 Inhibitor, Prevents the Ischemia/Reperfusion-Induced Degradation of GluA2-Containing AMPA Receptors" Pharmaceutics 13, no. 5: 636. https://doi.org/10.3390/pharmaceutics13050636
APA StyleAchzet, L. M., Astruc-Diaz, F., Beske, P. H., Natale, N. R., Denton, T. T., & Jackson, D. A. (2021). Liposomal Encapsulated FSC231, a PICK1 Inhibitor, Prevents the Ischemia/Reperfusion-Induced Degradation of GluA2-Containing AMPA Receptors. Pharmaceutics, 13(5), 636. https://doi.org/10.3390/pharmaceutics13050636






