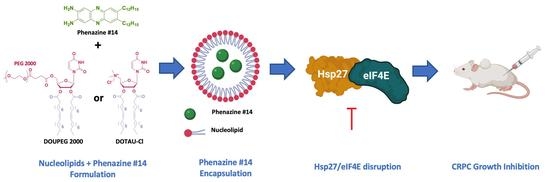
Nucleoside-Lipid-Based Nanoparticles for Phenazine Delivery: A New Therapeutic Strategy to Disrupt Hsp27-eIF4E Interaction in Castration Resistant Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
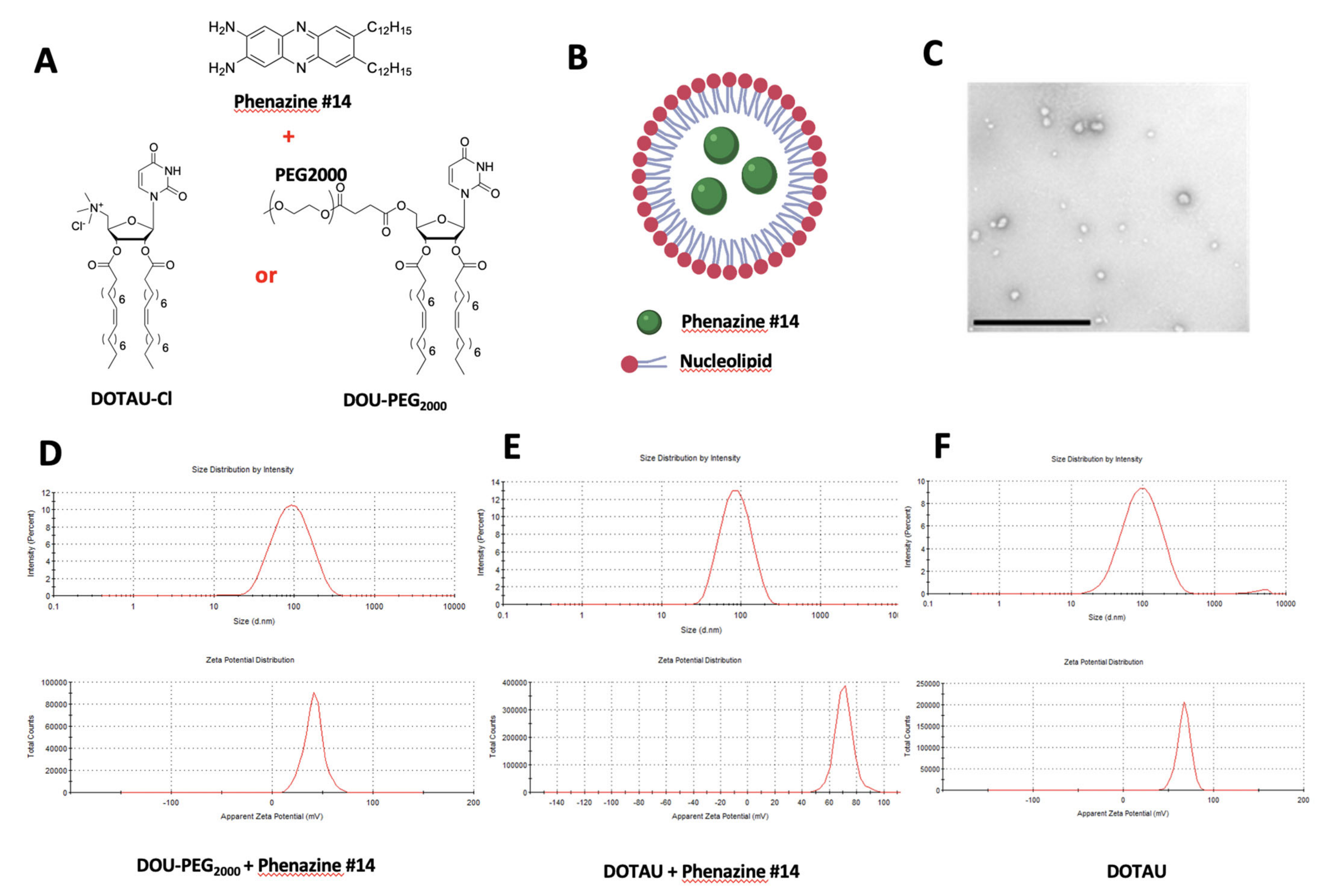
2.2. Phenazine Derivative Compound#14
2.3. Nanoparticles DOTAU (NPDOTAU) Loading with Phenazine #14
2.4. Nanoparticles DOU-PEG2000 (NPDOU-PEG) Loading with Phenazine #14
2.5. Transmission Electron Microscopy (TEM)
2.6. Treatment of PC-3 Cells with the Phenazine #14 and the NPs
2.7. In Vivo Tumor Growth Evaluation
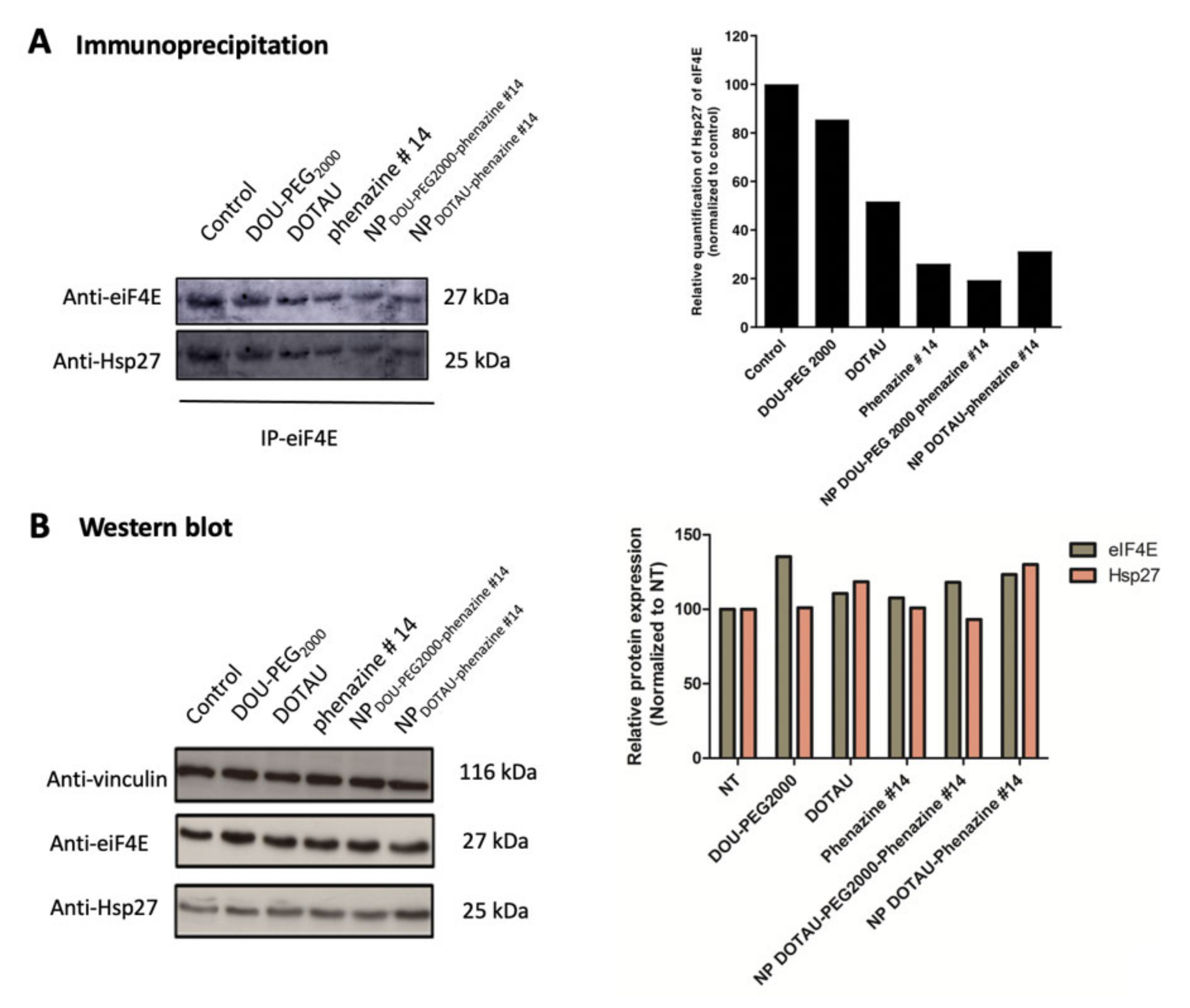
2.8. Immunoprecipitation (IP)
2.9. Western Blot
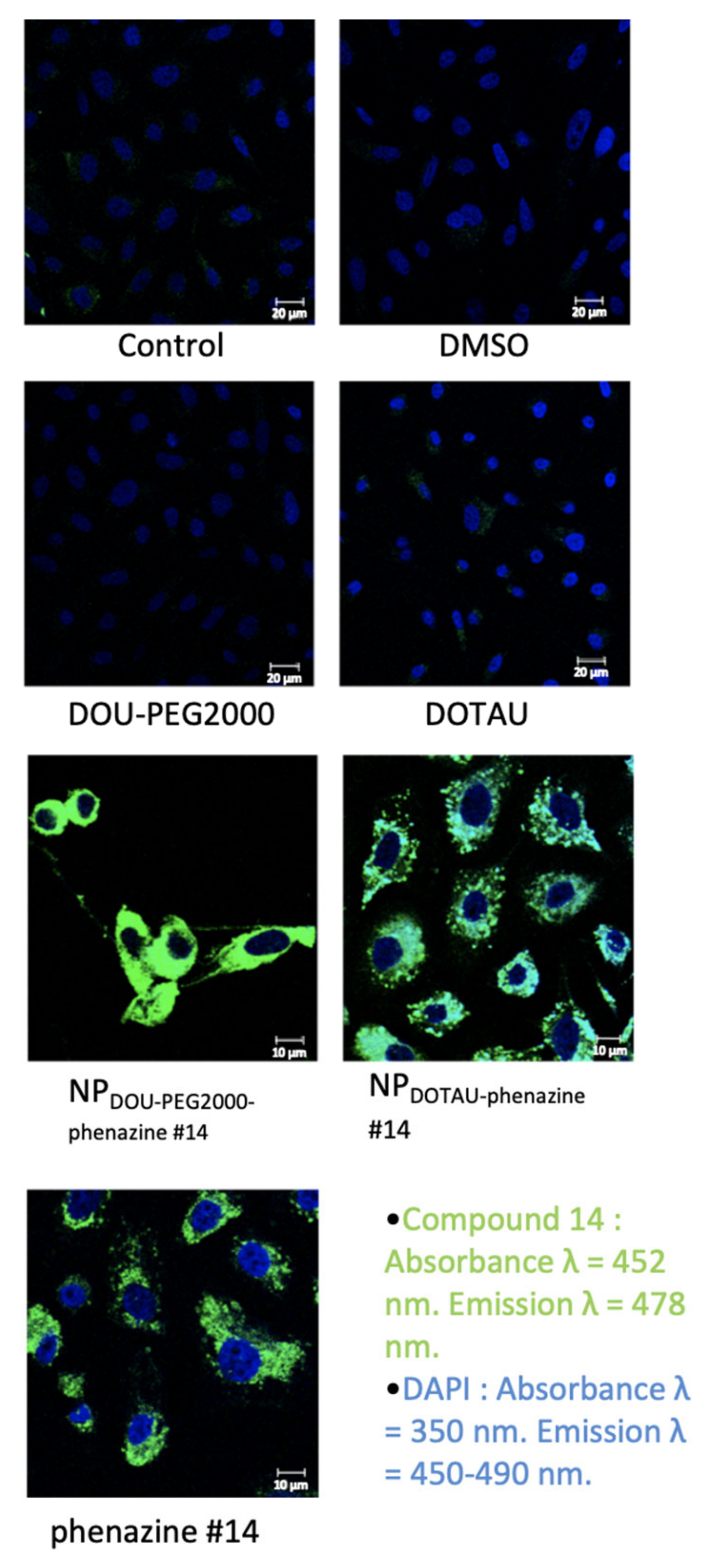
2.10. Confocal Microscopy
2.11. Cell Viability Assay
2.12. Flow Cytometry
2.13. Immunohistochemistry
2.14. Statistical Analysis
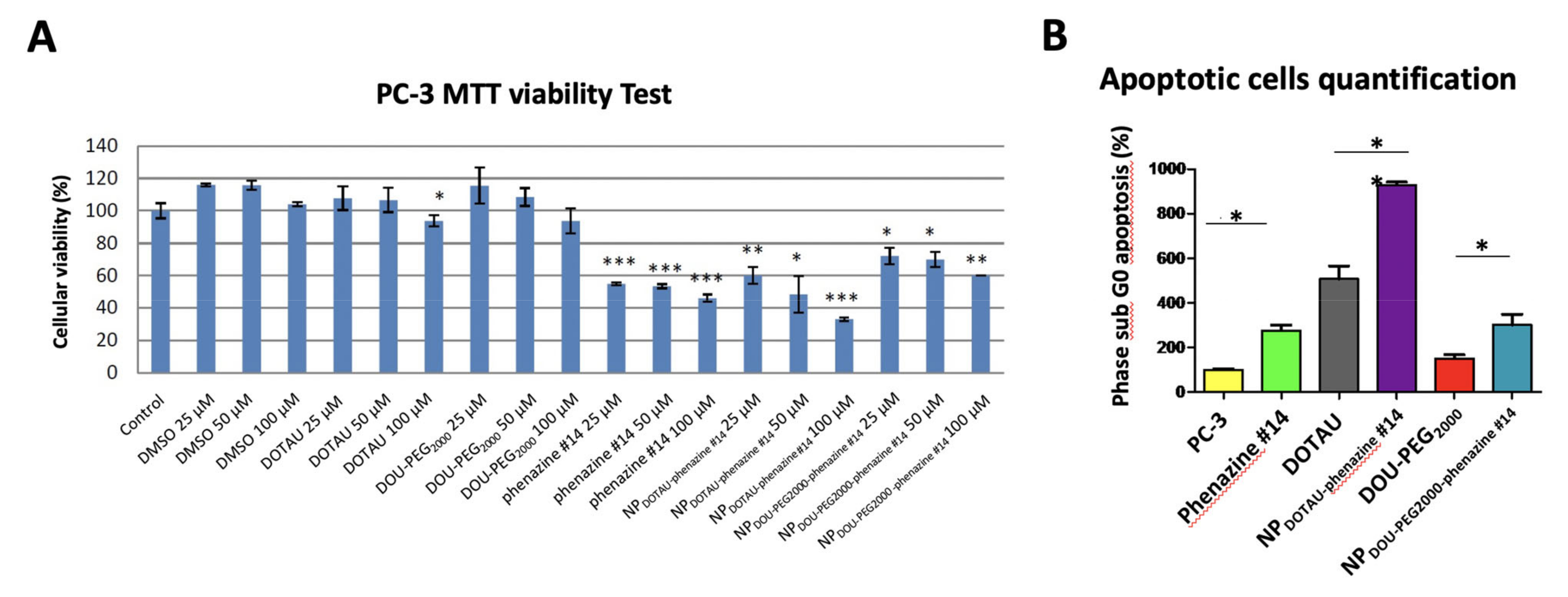
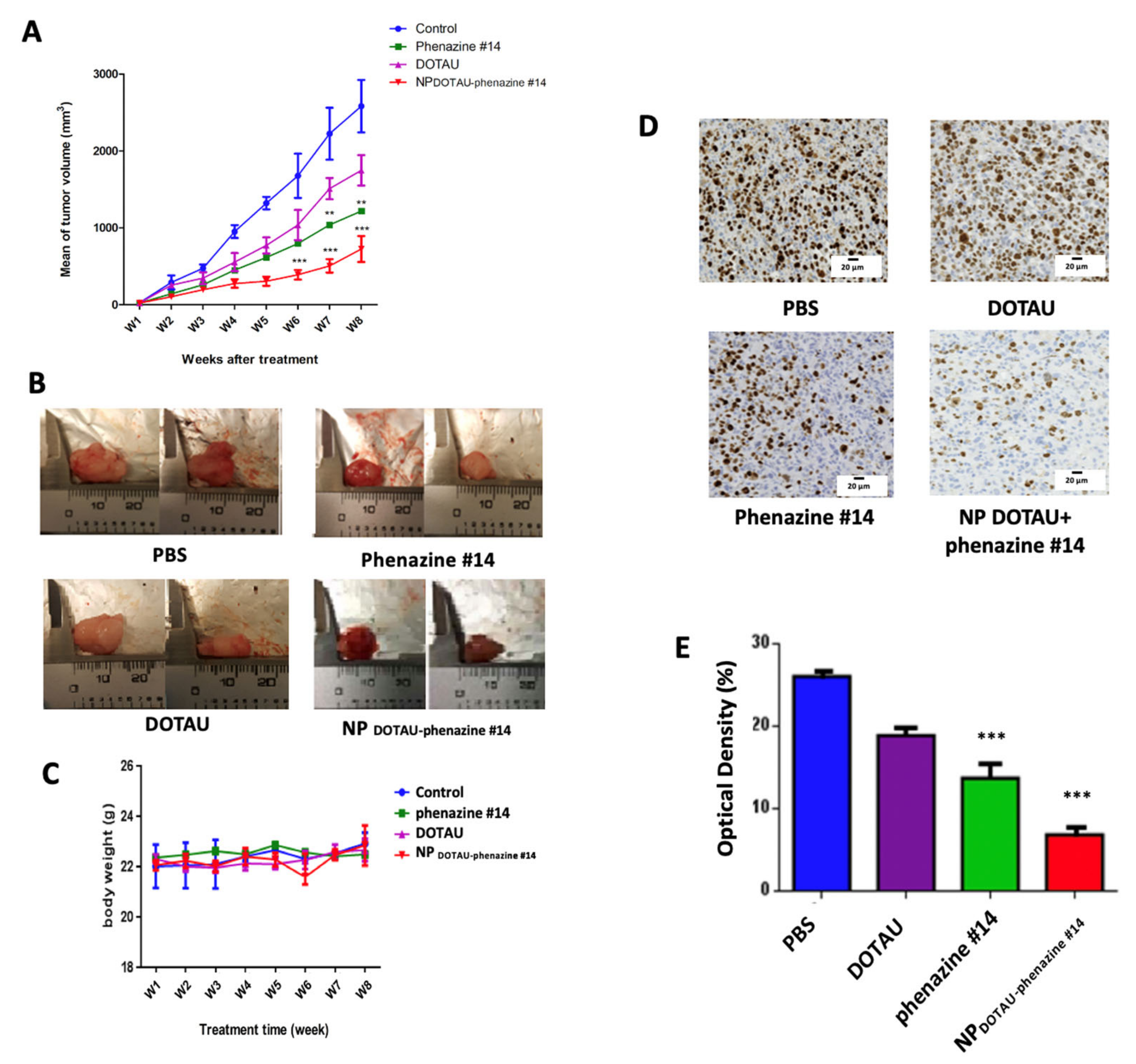
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.A.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of Docetaxel, Zoledronic Acid, or Both to First-Line Long-Term Hormone Therapy in Prostate Cancer (STAMPEDE): Survival Results from an Adaptive, Multiarm, Multistage, Platform Randomised Controlled Trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Fizazi, K.; Kramer, G.; Eymard, J.-C.; Sternberg, C.N.; de Bono, J.; Castellano, D.; Tombal, B.; Wülfing, C.; Liontos, M.; Carles, J.; et al. Quality of Life in Patients with Metastatic Prostate Cancer Following Treatment with Cabazitaxel versus Abiraterone or Enzalutamide (CARD): An Analysis of a Randomised, Multicentre, Open-Label, Phase 4 Study. Lancet Oncol. 2020, 21, 1513–1525. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer (TheraP): A Randomised, Open-Label, Phase 2 Trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Autio, K.A.; Dreicer, R.; Anderson, J.; Garcia, J.A.; Alva, A.; Hart, L.L.; Milowsky, M.I.; Posadas, E.M.; Ryan, C.J.; Graf, R.P.; et al. Safety and Efficacy of BIND-014, a Docetaxel Nanoparticle Targeting Prostate-Specific Membrane Antigen for Patients with Metastatic Castration-Resistant Prostate Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, P.; So, A.; Kojima, S.; Signaevsky, M.; Beraldi, E.; Fazli, L.; Hurtado-Coll, A.; Yamanaka, K.; Gleave, M. Heat Shock Protein 27 Increases after Androgen Ablation and Plays a Cytoprotective Role in Hormone-Refractory Prostate Cancer. Cancer Res. 2004, 64, 6595–6602. [Google Scholar] [CrossRef]
- Rocchi, P.; Beraldi, E.; Ettinger, S.; Fazli, L.; Vessella, R.L.; Nelson, C.; Gleave, M. Increased Hsp27 after Androgen Ablation Facilitates Androgen-Independent Progression in Prostate Cancer via Signal Transducers and Activators of Transcription 3-Mediated Suppression of Apoptosis. Cancer Res. 2005, 65, 11083–11093. [Google Scholar] [CrossRef]
- Yu, E.Y.; Ellard, S.L.; Hotte, S.J.; Gingerich, J.R.; Joshua, A.M.; Gleave, M.E.; Chi, K.N. A Randomized Phase 2 Study of a HSP27 Targeting Antisense, Apatorsen with Prednisone versus Prednisone Alone, in Patients with Metastatic Castration Resistant Prostate Cancer. Investig. New Drugs 2018, 36, 278–287. [Google Scholar] [CrossRef]
- Andrieu, C.; Taieb, D.; Baylot, V.; Ettinger, S.; Soubeyran, P.; De-Thonel, A.; Nelson, C.; Garrido, C.; So, A.; Fazli, L.; et al. Heat Shock Protein 27 Confers Resistance to Androgen Ablation and Chemotherapy in Prostate Cancer Cells through EIF4E. Oncogene 2010, 29, 1883–1896. [Google Scholar] [CrossRef]
- Baylot, V.; Katsogiannou, M.; Andrieu, C.; Taieb, D.; Acunzo, J.; Giusiano, S.; Fazli, L.; Gleave, M.; Garrido, C.; Rocchi, P. Targeting TCTP as a New Therapeutic Strategy in Castration-Resistant Prostate Cancer. Mol. Ther. 2012, 20, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.D.; Sonenberg, N. Regulation of Cap-Dependent Translation by EIF4E Inhibitory Proteins. Nature 2005, 433, 477–480. [Google Scholar] [CrossRef]
- Ziouziou, H.; Andrieu, C.; Laurini, E.; Karaki, S.; Fermeglia, M.; Oueslati, R.; Taieb, D.; Camplo, M.; Siri, O.; Pricl, S.; et al. Targeting Hsp27/EIF4E Interaction with Phenazine Compound: A Promising Alternative for Castration-Resistant Prostate Cancer Treatment. Oncotarget 2017, 8, 77317–77329. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Prévot, G.; Mousli, Y.; Barthélémy, P.; Crauste-Manciet, S.; Dehay, B.; Desvergnes, V. Synthesis and Intracellular Uptake of Rhodamine–Nucleolipid Conjugates into a Nanoemulsion Vehicle. ACS Omega 2020, 5, 5815–5823. [Google Scholar] [CrossRef]
- Benizri, S.; Ferey, L.; Alies, B.; Mebarek, N.; Vacher, G.; Appavoo, A.; Staedel, C.; Gaudin, K.; Barthélémy, P. Nucleoside-Lipid-Based Nanocarriers for Sorafenib Delivery. Nanoscale Res. Lett. 2018, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Ramin, M.A.; Sindhu, K.R.; Appavoo, A.; Oumzil, K.; Grinstaff, M.W.; Chassande, O.; Barthélémy, P. Cation Tuning of Supramolecular Gel Properties: A New Paradigm for Sustained Drug Delivery. Adv. Mater. 2017, 29, 1605227. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Pasut, G. PEGylation, Successful Approach to Drug Delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Baillet, J.; Desvergnes, V.; Hamoud, A.; Latxague, L.; Barthélémy, P. Lipid and Nucleic Acid Chemistries: Combining the Best of Both Worlds to Construct Advanced Biomaterials. Adv. Mater. Weinh. 2018, 30. [Google Scholar] [CrossRef]
- Chabaud, P.; Camplo, M.; Payet, D.; Serin, G.; Moreau, L.; Barthélémy, P.; Grinstaff, M.W. Cationic Nucleoside Lipids for Gene Delivery. Bioconjug. Chem. 2006, 17, 466–472. [Google Scholar] [CrossRef]
- Oumzil, K.; Khiati, S.; Grinstaff, M.W.; Barthélémy, P. Reduction-Triggered Delivery Using Nucleoside-Lipid Based Carriers Possessing a Cleavable PEG Coating. J. Control. Release 2011, 151, 123–130. [Google Scholar] [CrossRef]
- Torchilin, V.P. Micellar Nanocarriers: Pharmaceutical Perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef]
- Gregoriadis, G. Liposome Research in Drug Delivery: The Early Days. J. Drug Target. 2008, 16, 520–524. [Google Scholar] [CrossRef]
- Oh, J.K.; Siegwart, D.J.; Lee, H.; Sherwood, G.; Peteanu, L.; Hollinger, J.O.; Kataoka, K.; Matyjaszewski, K. Biodegradable Nanogels Prepared by Atom Transfer Radical Polymerization as Potential Drug Delivery Carriers: Synthesis, Biodegradation, in Vitro Release, and Bioconjugation. J. Am. Chem. Soc. 2007, 129, 5939–5945. [Google Scholar] [CrossRef] [PubMed]
- Milani, S.; Berti, D.; Dante, S.; Hauss, T.; Baglioni, P. Intercalation of Single-Strand Oligonucleotides between Nucleolipid Anionic Membranes: A Neutron Diffraction Study. Langmuir 2009, 25, 4084–4092. [Google Scholar] [CrossRef] [PubMed]
- Bildstein, L.; Dubernet, C.; Marsaud, V.; Chacun, H.; Nicolas, V.; Gueutin, C.; Sarasin, A.; Bénech, H.; Lepêtre-Mouelhi, S.; Desmaële, D.; et al. Transmembrane Diffusion of Gemcitabine by a Nanoparticulate Squalenoyl Prodrug: An Original Drug Delivery Pathway. J. Control. Release 2010, 147, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Moreau, L.; Barthélémy, P.; Li, Y.; Luo, D.; Prata, C.A.H.; Grinstaff, M.W. Nucleoside Phosphocholine Amphiphile for in Vitro DNA Transfection. Mol. Biosyst. 2005, 1, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, C.; Prata, C.A.H.; Giorgio, S.; Garzino, F.; Payet, D.; Barthélémy, P.; Grinstaff, M.W.; Camplo, M. Cationic Nucleoside Lipids Based on a 3-Nitropyrrole Universal Base for SiRNA Delivery. Bioconjug. Chem. 2009, 20, 193–196. [Google Scholar] [CrossRef]
- Ceballos, C.; Khiati, S.; Prata, C.A.H.; Zhang, X.-X.; Giorgio, S.; Marsal, P.; Grinstaff, M.W.; Barthélémy, P.; Camplo, M. Cationic Nucleoside Lipids Derived from Universal Bases: A Rational Approach for SiRNA Transfection. Bioconjug. Chem. 2010, 21, 1062–1069. [Google Scholar] [CrossRef]
- Khiati, S.; Pierre, N.; Andriamanarivo, S.; Grinstaff, M.W.; Arazam, N.; Nallet, F.; Navailles, L.; Barthélémy, P. Anionic Nucleotide--Lipids for in Vitro DNA Transfection. Bioconjug. Chem. 2009, 20, 1765–1772. [Google Scholar] [CrossRef]
- Mavrodi, D.V.; Blankenfeldt, W.; Thomashow, L.S. Phenazine Compounds in Fluorescent Pseudomonas Spp. Biosynthesis and Regulation. Annu. Rev. Phytopathol. 2006, 44, 417–445. [Google Scholar] [CrossRef] [PubMed]
- Huigens, R.W.; Abouelhassan, Y.; Yang, H. Phenazine Antibiotic-Inspired Discovery of Bacterial Biofilm-Eradicating Agents. Chembiochem 2019, 20, 2885–2902. [Google Scholar] [CrossRef]
- Hussain, H.; Specht, S.; Sarite, S.R.; Saeftel, M.; Hoerauf, A.; Schulz, B.; Krohn, K. A New Class of Phenazines with Activity against a Chloroquine Resistant Plasmodium Falciparum Strain and Antimicrobial Activity. J. Med. Chem. 2011, 54, 4913–4917. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; Xing, Y.; Ma, Y.; Lu, J.; Bao, W.; Wang, Y.; Xi, T. A Novel Anticancer and Antifungus Phenazine Derivative from a Marine Actinomycete BM-17. Microbiol. Res. 2012, 167, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Evidente, A. Phenazine as an Anticancer Agent. In Microbial Phenazines: Biosynthesis, Agriculture and Health; Chincholkar, S., Thomashow, L., Eds.; Springer: Berlin, Germany, 2013; pp. 217–243. ISBN 978-3-642-40573-0. [Google Scholar]
- Moris, M.-A.; Andrieu, C.; Rocchi, P.; Seillan, C.; Acunzo, J.; Brunel, F.; Garzino, F.; Siri, O.; Camplo, M. 2,3-Dialkoxyphenazines as Anticancer Agents. Tetrahedron Lett. 2015, 56, 2695–2698. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Vasey, P.A.; Kaye, S.B.; Morrison, R.; Twelves, C.; Wilson, P.; Duncan, R.; Thomson, A.H.; Murray, L.S.; Hilditch, T.E.; Murray, T.; et al. Phase I Clinical and Pharmacokinetic Study of PK1 [N-(2-Hydroxypropyl)Methacrylamide Copolymer Doxorubicin]: First Member of a New Class of Chemotherapeutic Agents-Drug-Polymer Conjugates. Cancer Research Campaign Phase I/II Committee. Clin. Cancer Res. 1999, 5, 83–94. [Google Scholar]
- Dunkan, R. Tumor Targeting by Enhanced Permeability and Retention (EPR) Effect. Ann. Oncol. 2001, 9, 39. [Google Scholar]
- Chauhan, V.P.; Stylianopoulos, T.; Martin, J.D.; Popović, Z.; Chen, O.; Kamoun, W.S.; Bawendi, M.G.; Fukumura, D.; Jain, R.K. Normalization of Tumour Blood Vessels Improves the Delivery of Nanomedicines in a Size-Dependent Manner. Nat. Nanotechnol. 2012, 7, 383–388. [Google Scholar] [CrossRef]
- Khiati, S.; Luvino, D.; Oumzil, K.; Chauffert, B.; Camplo, M.; Barthélémy, P. Nucleoside-Lipid-Based Nanoparticles for Cisplatin Delivery. ACS Nano 2011, 5, 8649–8655. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer Drug Nanomicelles Formed by Self-Assembling Amphiphilic Dendrimer to Combat Cancer Drug Resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziouziou, H.; Paris, C.; Benizri, S.; Le, T.K.; Andrieu, C.; Nguyen, D.T.; Appavoo, A.; Taïeb, D.; Brunel, F.; Oueslati, R.; et al. Nucleoside-Lipid-Based Nanoparticles for Phenazine Delivery: A New Therapeutic Strategy to Disrupt Hsp27-eIF4E Interaction in Castration Resistant Prostate Cancer. Pharmaceutics 2021, 13, 623. https://doi.org/10.3390/pharmaceutics13050623
Ziouziou H, Paris C, Benizri S, Le TK, Andrieu C, Nguyen DT, Appavoo A, Taïeb D, Brunel F, Oueslati R, et al. Nucleoside-Lipid-Based Nanoparticles for Phenazine Delivery: A New Therapeutic Strategy to Disrupt Hsp27-eIF4E Interaction in Castration Resistant Prostate Cancer. Pharmaceutics. 2021; 13(5):623. https://doi.org/10.3390/pharmaceutics13050623
Chicago/Turabian StyleZiouziou, Hajer, Clément Paris, Sébastien Benizri, Thi Khanh Le, Claudia Andrieu, Dang Tan Nguyen, Ananda Appavoo, David Taïeb, Frédéric Brunel, Ridha Oueslati, and et al. 2021. "Nucleoside-Lipid-Based Nanoparticles for Phenazine Delivery: A New Therapeutic Strategy to Disrupt Hsp27-eIF4E Interaction in Castration Resistant Prostate Cancer" Pharmaceutics 13, no. 5: 623. https://doi.org/10.3390/pharmaceutics13050623
APA StyleZiouziou, H., Paris, C., Benizri, S., Le, T. K., Andrieu, C., Nguyen, D. T., Appavoo, A., Taïeb, D., Brunel, F., Oueslati, R., Siri, O., Camplo, M., Barthélémy, P., & Rocchi, P. (2021). Nucleoside-Lipid-Based Nanoparticles for Phenazine Delivery: A New Therapeutic Strategy to Disrupt Hsp27-eIF4E Interaction in Castration Resistant Prostate Cancer. Pharmaceutics, 13(5), 623. https://doi.org/10.3390/pharmaceutics13050623