Efficacy and Safety of Azelaic Acid Nanocrystal-Loaded In Situ Hydrogel in the Treatment of Acne Vulgaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the AZA Nanosuspensions (AZA-NS)
2.3. Freeze Drying of the AZA Nanosuspensions
2.4. Particle Size Analyses
2.5. AZA-NC Hydrogel Formulation
2.6. Spreadability Test
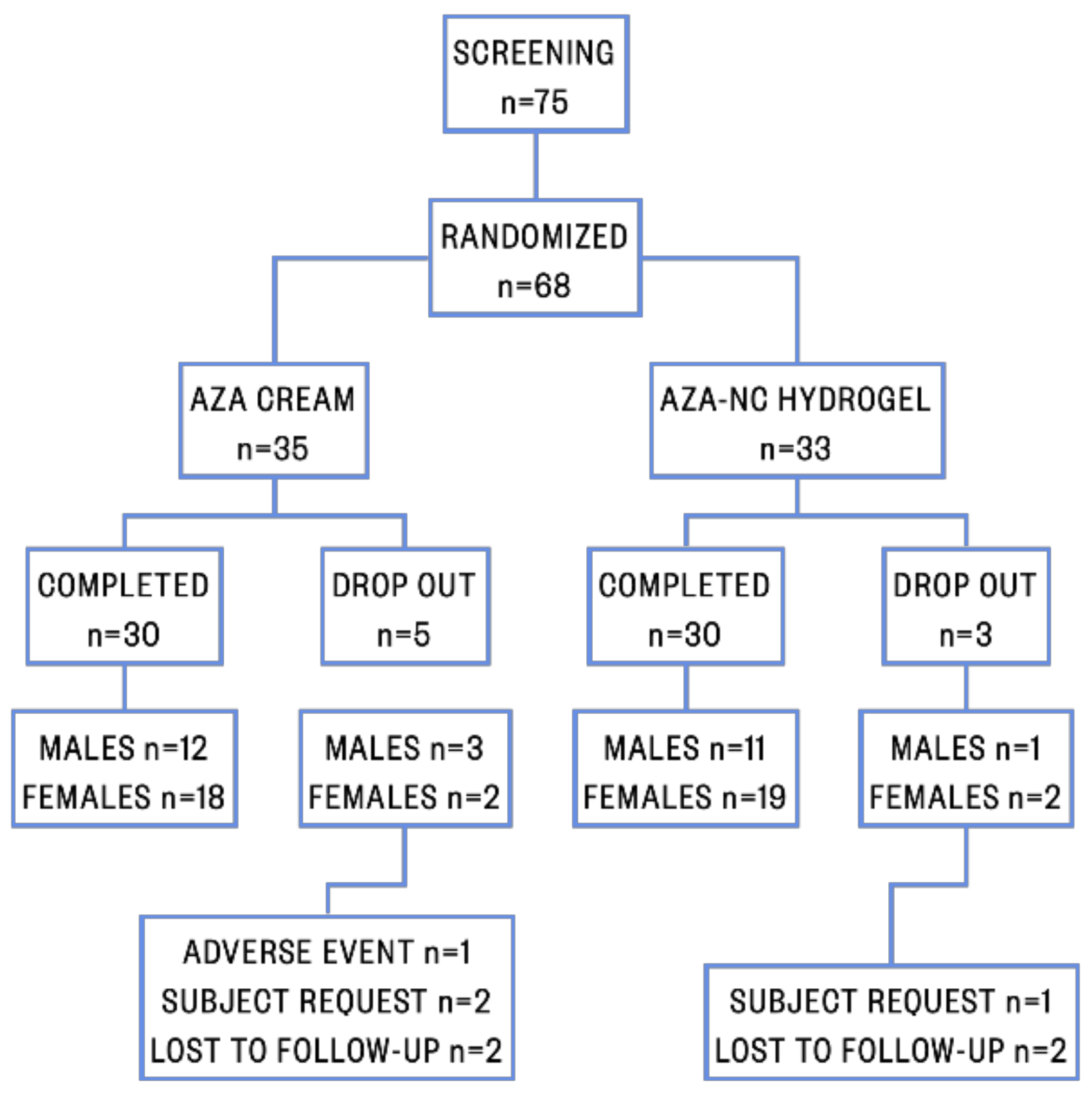
2.7. Patients and Clinical Study Design
2.8. Statistical Analysis
3. Results and Discussion
3.1. AZA-NS Particle Size and PDI
3.2. Spreadability of AZA-NC Hydrogel
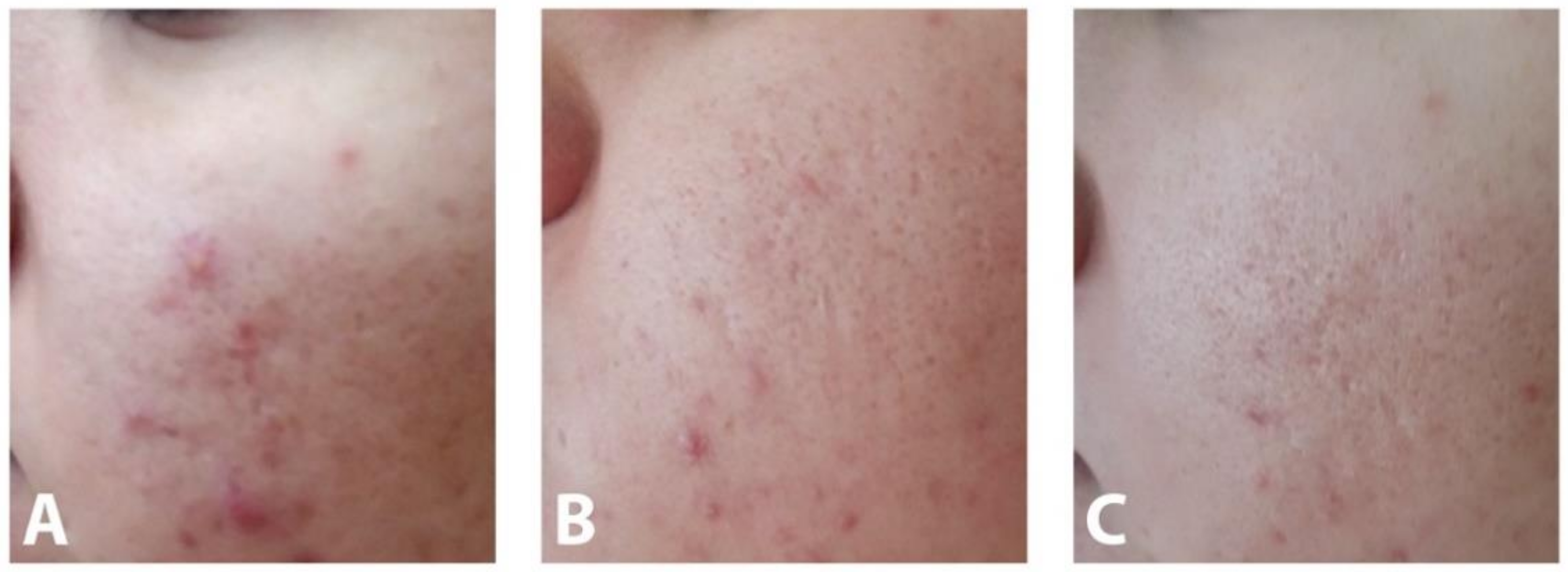
3.3. Efficacy of AZA-NC Hydrogel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohiuddin, A. A Comprehensive Review of Acne Vulgaris. J. Clin. Res. Dermatol. 2019, 6, 1–34. [Google Scholar] [CrossRef]
- Dréno, B. Recent data on epidemiology of acne. Ann. Dermatol. Vénéréologie 2010, 137, 3–5. [Google Scholar] [CrossRef]
- Taylor, M.; Gonzalez, M.; Porter, R. Pathways to inflammation: Acne pathophysiology. Eur. J. Dermatol. 2011, 21, 323–333. [Google Scholar] [CrossRef]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Otlewska, A.; Baran, W.; Batycka-Baran, A. Adverse events related to topical drug treatments for acne vulgaris. Expert Opin. Drug Saf. 2020, 19, 513–521. [Google Scholar] [CrossRef]
- Fox, L.; Csongradi, C.; Aucamp, M.; Du Plessis, J.; Gerber, M. Treatment Modalities for Acne. Molecules 2016, 21, 1063. [Google Scholar] [CrossRef]
- Dikicier, B.S. Topical treatment of acne vulgaris: Efficiency, side effects, and adherence rate. J. Int. Med. Res. 2019, 47, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Katsambas, A.; Dessinioti, C. New and emerging treatments in dermatology: Acne. Dermatol. Ther. 2008, 21, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.; Hegel, J. Azelaic Acid: Properties and Mode of Action. Ski. Pharmacol. Physiol. 2014, 27, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Young, M.C.; Zito, P.M. Azelaic Acid in Acne Vulgaris. J. Dermatol. Nurses Assoc. 2018, 10, 152–153. [Google Scholar] [CrossRef]
- Patel, V.; Sharma, O.P.; Mehta, T. Nanocrystal: A novel approach to overcome skin barriers for improved topical drug delivery. Expert Opin. Drug Deliv. 2018, 15, 351–368. [Google Scholar] [CrossRef]
- Lohan, S.B.; Saeidpour, S.; Colombo, M.; Staufenbiel, S.; Unbehauen, M.; Wolde-Kidan, A.; Netz, R.R.; Bodmeier, R.; Haag, R.; Teutloff, C.; et al. Nanocrystals for Improved Drug Delivery of Dexamethasone in Skin Investigated by EPR Spectroscopy. Pharmaceutics 2020, 12, 400. [Google Scholar] [CrossRef]
- Colombo, M.; Staufenbiel, S.; Rühl, E.; Bodmeier, R. In situ determination of the saturation solubility of nanocrystals of poorly soluble drugs for dermal application. Int. J. Pharm. 2017, 521, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kumar, S.; Gokhale, R.; Burgess, D.J. Physical stability of nanosuspensions: Investigation of the role of stabilizers on Ostwald ripening. Int. J. Pharm. 2011, 406, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef]
- Zhai, X.; Lademann, J.; Keck, C.M.; Müller, R.H. Dermal nanocrystals from medium soluble actives—Physical stability and stability affecting parameters. Eur. J. Pharm. Biopharm. 2014, 88, 85–91. [Google Scholar] [CrossRef]
- Tomić, I.; Juretić, M.; Jug, M.; Pepić, I.; Čižmek, B.C.; Filipović-Grčić, J. Preparation of in situ hydrogels loaded with azelaic acid nanocrystals and their dermal application performance study. Int. J. Pharm. 2019, 563, 249–258. [Google Scholar] [CrossRef]
- Hayashi, N.; Akamatsu, H.; Kawashima, M. Acne Study Group Establishment of grading criteria for acne severity. J. Dermatol. 2008, 35, 255–260. [Google Scholar] [CrossRef]
- Möschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Mohammady, M.; Mohammadi, Y.; Yousefi, G. Freeze-Drying of Pharmaceutical and Nutraceutical Nanoparticles: The Effects of Formulation and Technique Parameters on Nanoparticles Characteristics. J. Pharm. Sci. 2020, 109, 3235–3247. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiss, B.; Schaefer, U.F.; Lehr, C.-M.; Wepf, R. Nanoparticles—An efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, Y. Critical freezing rate in freeze drying nanocrystal dispersions. J. Control. Release 2006, 111, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kulawik-Pióro, A.; Ptaszek, A.; Kruk, J. Effective tool for assessment of the quality of barrier creams—Relationships between rheological, textural and sensory properties. Regul. Toxicol. Pharmacol. 2019, 103, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Engesland, A.; Kermany, B.P.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2011, 125, 180–188. [Google Scholar] [CrossRef]
- Schaller, M.; Sebastian, M.; Ress, C.; Seidel, D.; Hennig, M. A multicentre, randomized, single-blind, parallel-group study comparing the efficacy and tolerability of benzoyl peroxide 3%/clindamycin 1% with azelaic acid 20% in the topical treatment of mild-to-moderate acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Mastrofrancesco, A.; Ottaviani, M.; Aspite, N.; Cardinali, G.; Izzo, E.; Graupe, K.; Zouboulis, C.C.; Camera, E.; Picardo, M. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARγ activation. Exp. Dermatol. 2010, 19, 813–820. [Google Scholar] [CrossRef]
- Mazzarello, V.; Gavini, E.; Rassu, G.; Donadu, M.G.; Usai, D.; Piu, G.; Pomponi, V.; Sucato, F.; Zanetti, S.; Montesu, M.A. Clinical Assessment of New Topical Cream Containing Two Essential Oils Combined with Tretinoin in the Treatment of Acne. Clin. Cosmet. Investig. Dermatol. 2020, 13, 233–239. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]

| AZA-NS | Grinding Time (h) | z-Average (nm ± SD) | PDI |
|---|---|---|---|
| AZA-NS-1 | 2 | 188.7 ± 34.1 | 0.49 |
| AZA-NS-2 | 3 | 148.1 ± 6.5 | 0.40 |
| AZA-NS-3 | 4 | 144.8 ± 4.7 | 0.36 |
| AZA-NS-FD * | 3 | 269.9 ± 14.8 | 0.43 |
| Patient Characteristic | AZA Cream | AZA-NC Hydrogel | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 18.83 ± 2.321 | 18.57 ± 3.093 | 0.707 |
| Gender (n; %) | <1 | ||
| M | 12; 40.0% | 11; 36.7% | |
| F | 18; 60.0% | 19; 63.3% | |
| Smoking (n; %) | 12; 40.0% | 11; 36.7% | <1 |
| Alcohol consumption (n; %) | 9; 30.0% | 9; 30.0% | <1 |
| Work status (n; %) | |||
| Employed | 2; 6.7% | 4; 13.3% | |
| Student | 20; 66.7% | 13; 43.3% | |
| Pupil | 8; 26.7% | 13; 43.3% |
| Lesions type_week | AZA Cream Mean ± SD | AZA-NC Hydrogel Mean ± SD | p-Value |
|---|---|---|---|
| Inflammatory lesions_0 | 5.43 ± 3.421 | 6.13 ± 3.501 | 0.437 |
| Non-inflammatory lesions_0 | 7.63 ± 4.189 | 8.00 ± 3.877 | 0.726 |
| Total lesions_0 | 13.07 ± 7.080 | 14.13 ± 6.872 | 0.556 |
| Lesions type_week(s) | AZA Cream Mean ± SD | AZA-NC Hydrogel Mean ± SD | p-Value |
|---|---|---|---|
| Inflammatory lesions_0 | 5.43 ± 3.421 | 6.13 ± 3.501 | 0.437 |
| Inflammatory lesions_4 | 4.80 ± 3.253 | 5.30 ± 3.153 | 0.548 |
| Inflammatory lesions_8 | 3.60 ± 2.430 | 3.73 ± 2.612 | 0.839 |
| Diff. inflammatory lesion 0–4 | 0.63 ± 0.556 | 0.83 ± 0.791 | 0.262 |
| Reduction_4 (%) | 11.66 | 13.59 | |
| Diff. inflammatory lesion 0–8 | 1.83 ± 1.206 | 2.40 ± 1.248 | 0.079 |
| Reduction_8 (%) | 33.76 | 39.15 | |
| Non-inflammatory lesions_0 | 7.63 ± 4.189 | 8.00 ± 3.877 | 0.726 |
| Non-inflammatory lesions_4 | 6.73 ± 3.823 | 6.90 ± 3.614 | 0.863 |
| Non-inflammatory lesions_8 | 5.50 ± 3.330 | 5.23 ± 3.137 | 0.751 |
| Diff. non-inflammatory lesion 0–4 | 0.93 ± 0.740 | 1.10 ± 0.845 | 0.420 |
| Reduction_4 (%) | 12.23 | 13.75 | |
| Diff. non-inflammatory lesion 0–8 | 2.13 ± 1.279 | 2.77 ± 1.194 | 0.052 |
| Reduction_8 (%) | 27.96 | 34.58 |
| Lesions type_week(s) | AZA Cream Mean ± SD | p-Value | η2 | AZA-NC Hydrogel Mean ± SD | p-Value | η2 |
|---|---|---|---|---|---|---|
| Inflammatory lesions_0 | 5.43 ± 3.421 | <0.001 | 0.710 | 6.13 ± 3.501 | <0.001 | 0.794 |
| Inflammatory lesions_8 | 3.60 ± 2.430 | 3.73 ± 2.612 | ||||
| Reduction in lesion count 0–8 (%) | 33.76 | 39.15 | ||||
| Non-inflammatory lesions_0 | 7.63 ± 4.189 | <0.001 | 0.745 | 8.00 ± 3.877 | <0.001 | 0.848 |
| Non-inflammatory lesions_8 | 5.50 ± 3.330 | 5.23 ± 3.137 | ||||
| Reduction in lesion count 0–8 (%) | 27.96 | 34.58 | ||||
| Total lesions_0 | 13.07 ± 7.080 | <0.001 | 0.797 | 14.13 ± 6.872 | <0.001 | 0.846 |
| Total lesions_8 | 9.10 ± 5.422 | 8.97 ± 5.189 | ||||
| Reduction in lesion count 0–8 (%) | 30.37 | 36.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomić, I.; Miočić, S.; Pepić, I.; Šimić, D.; Filipović-Grčić, J. Efficacy and Safety of Azelaic Acid Nanocrystal-Loaded In Situ Hydrogel in the Treatment of Acne Vulgaris. Pharmaceutics 2021, 13, 567. https://doi.org/10.3390/pharmaceutics13040567
Tomić I, Miočić S, Pepić I, Šimić D, Filipović-Grčić J. Efficacy and Safety of Azelaic Acid Nanocrystal-Loaded In Situ Hydrogel in the Treatment of Acne Vulgaris. Pharmaceutics. 2021; 13(4):567. https://doi.org/10.3390/pharmaceutics13040567
Chicago/Turabian StyleTomić, Ivona, Sandra Miočić, Ivan Pepić, Dubravka Šimić, and Jelena Filipović-Grčić. 2021. "Efficacy and Safety of Azelaic Acid Nanocrystal-Loaded In Situ Hydrogel in the Treatment of Acne Vulgaris" Pharmaceutics 13, no. 4: 567. https://doi.org/10.3390/pharmaceutics13040567
APA StyleTomić, I., Miočić, S., Pepić, I., Šimić, D., & Filipović-Grčić, J. (2021). Efficacy and Safety of Azelaic Acid Nanocrystal-Loaded In Situ Hydrogel in the Treatment of Acne Vulgaris. Pharmaceutics, 13(4), 567. https://doi.org/10.3390/pharmaceutics13040567







