Endophytic Bacteria Enterobacter hormaechei Fabricated Silver Nanoparticles and Their Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of the Endophyte and Preparation of Cell-Free Extract
2.2. Synthesis of Eh-AgNPs
2.3. Characterization of Eh-AgNPs
2.4. Antimicrobial Activity
2.5. Statistical Analysis
3. Results
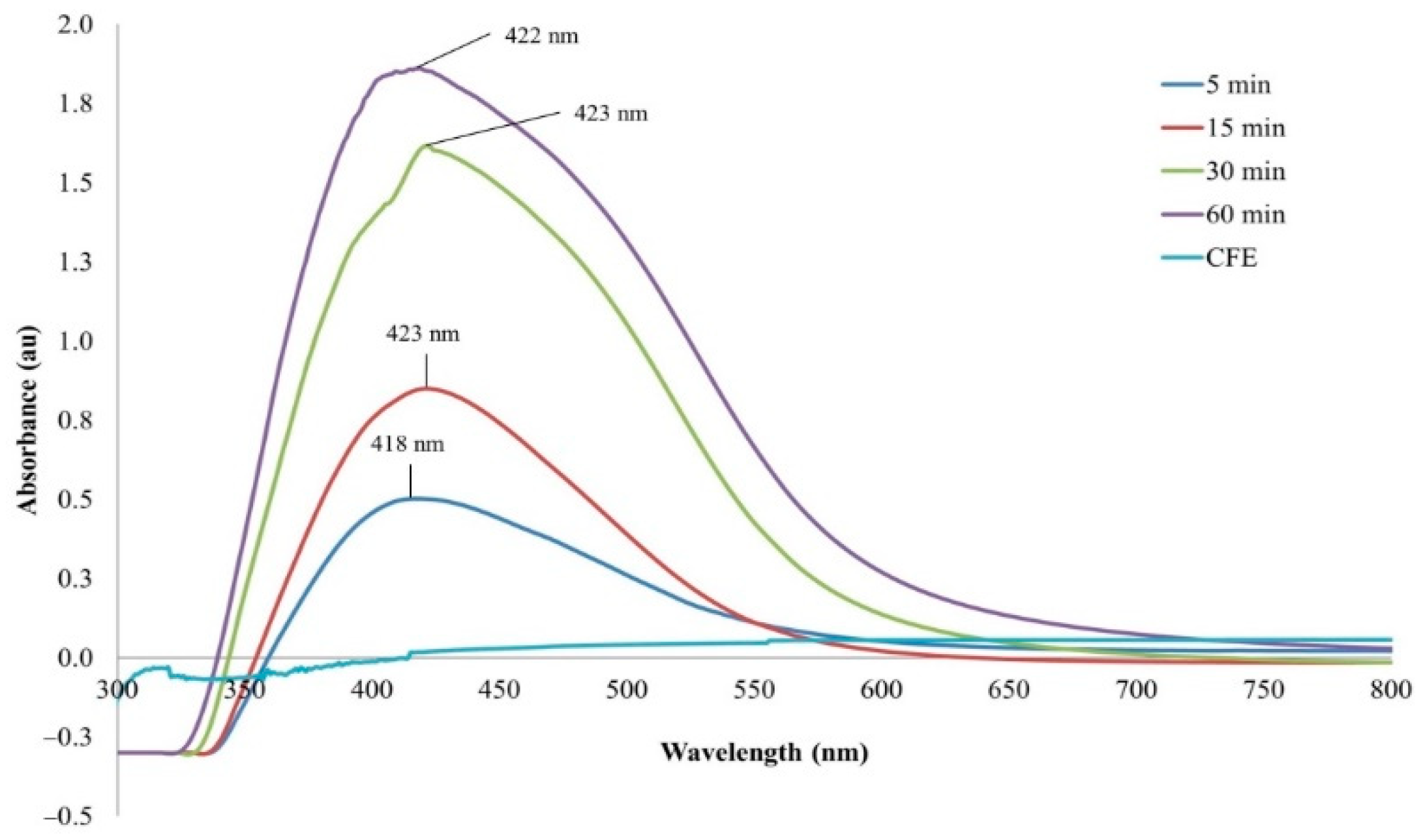
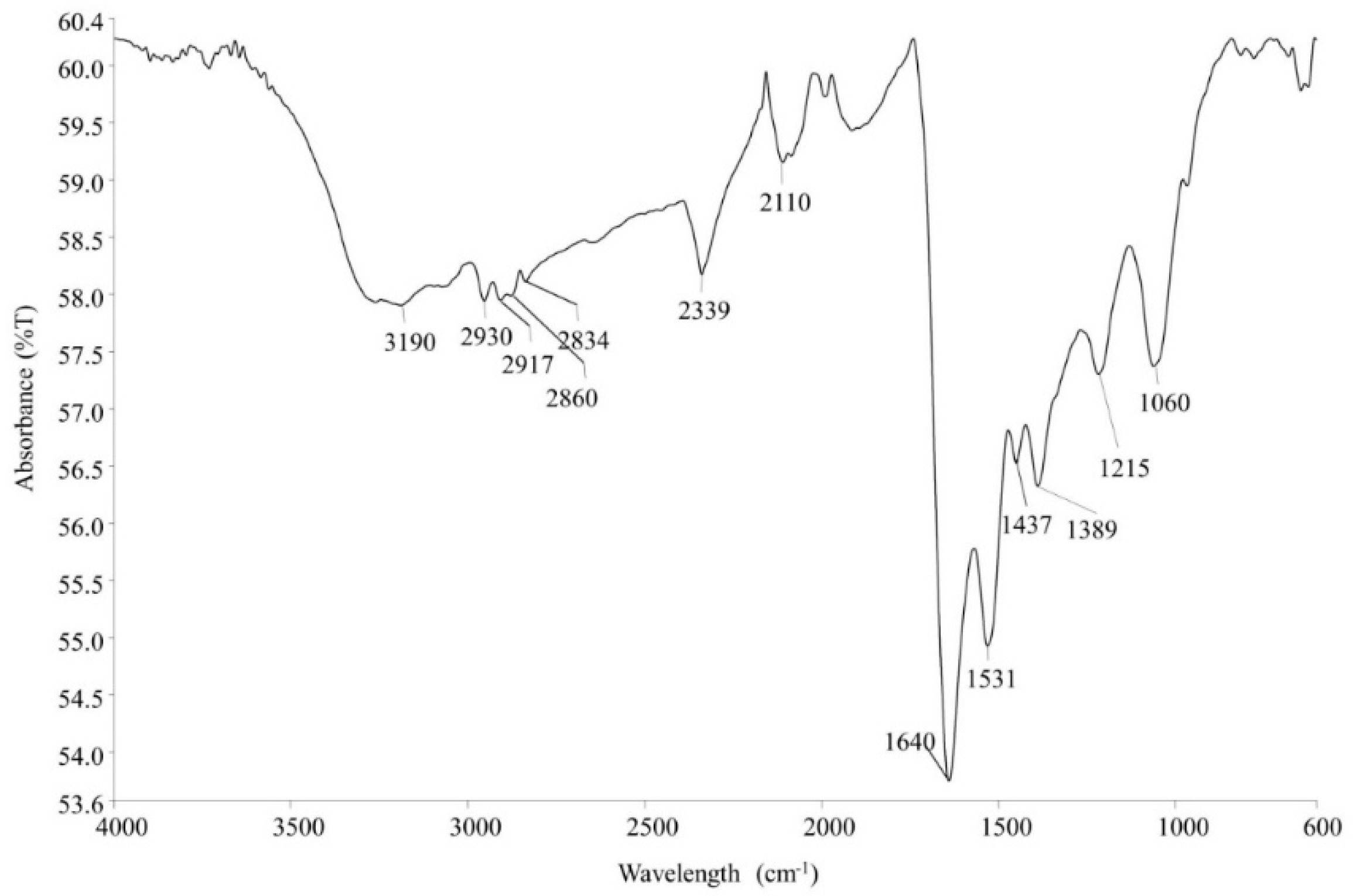
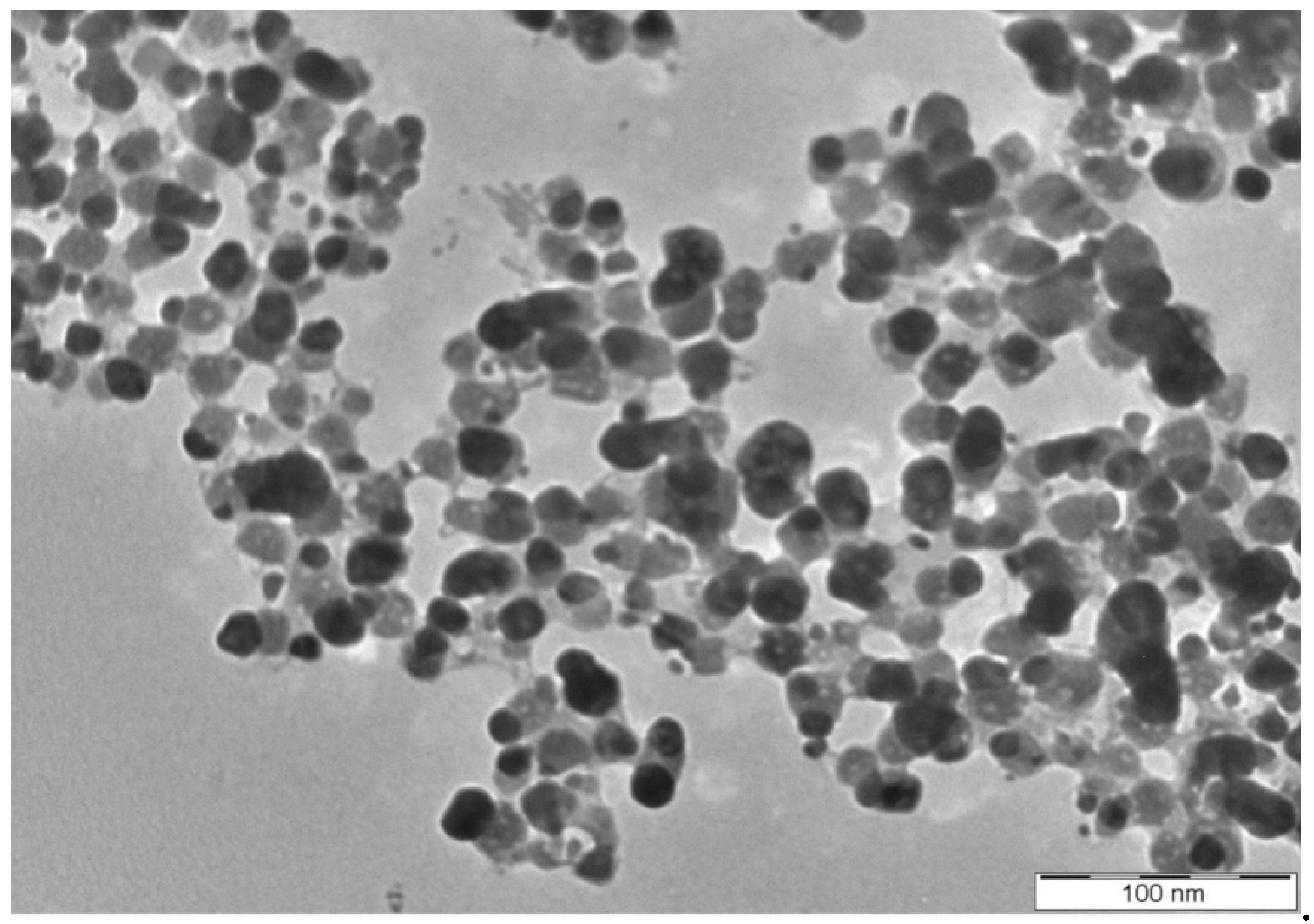
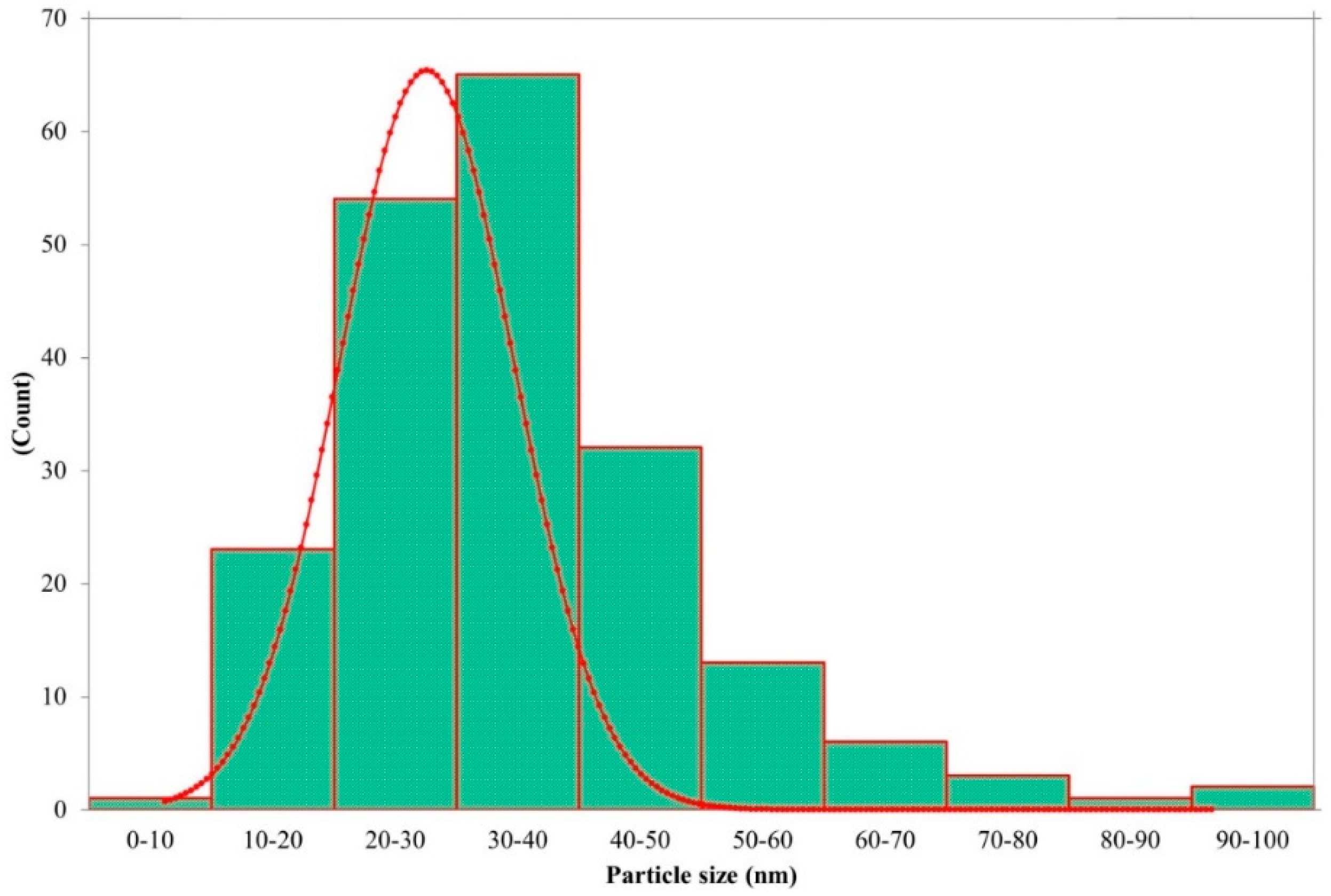
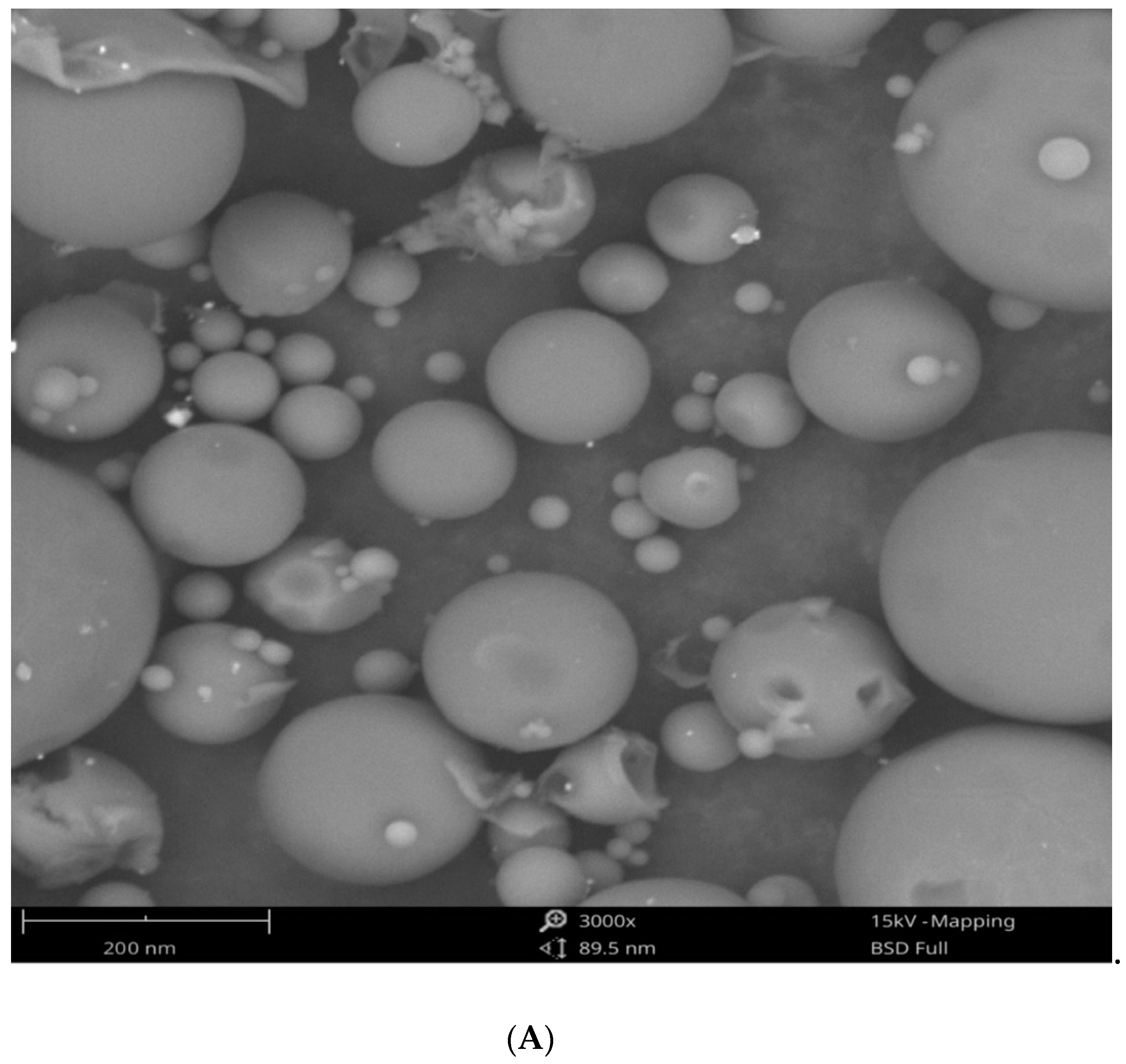
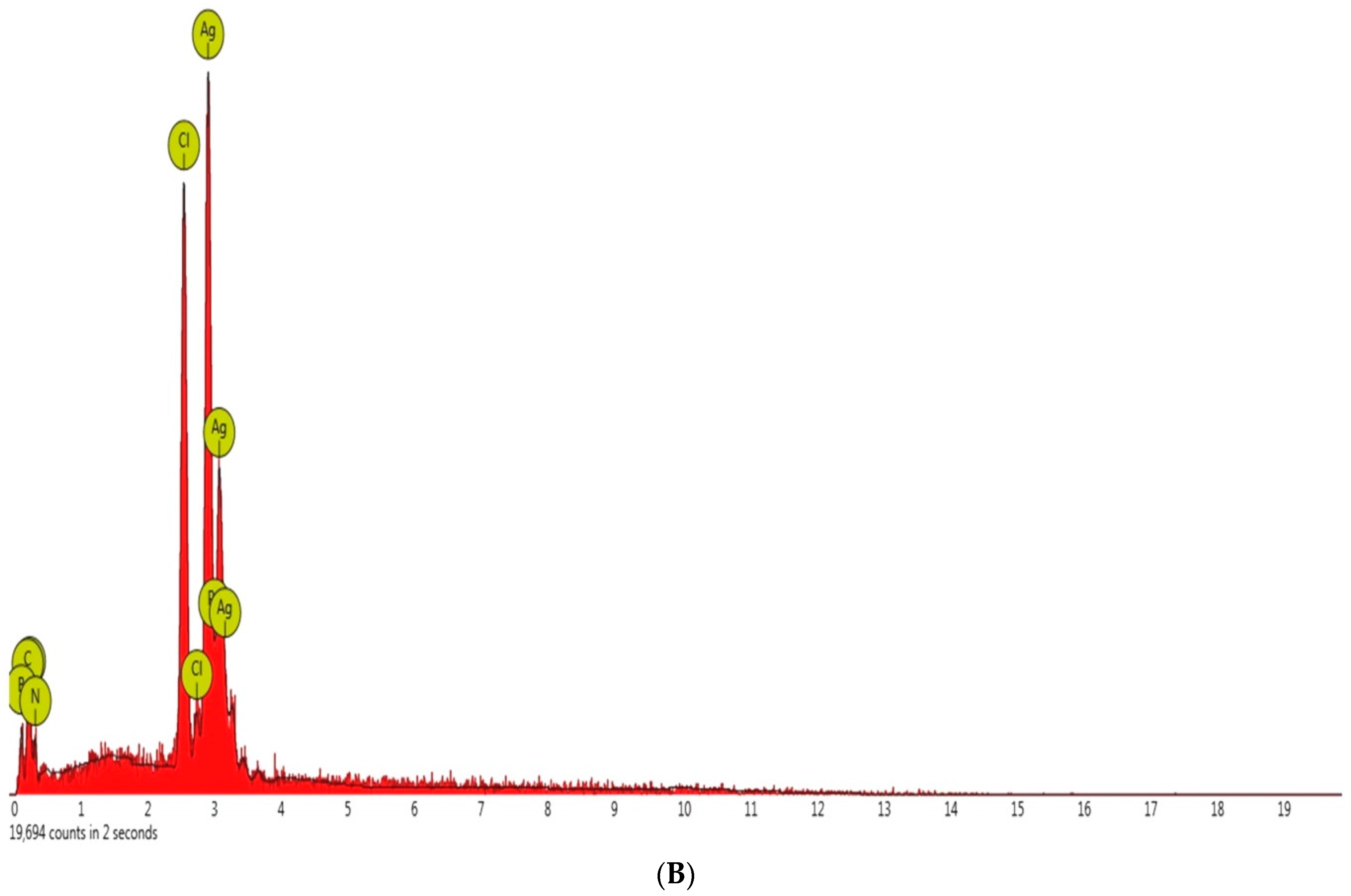
3.1. Synthesis and Characterization of Eh-AgNPs
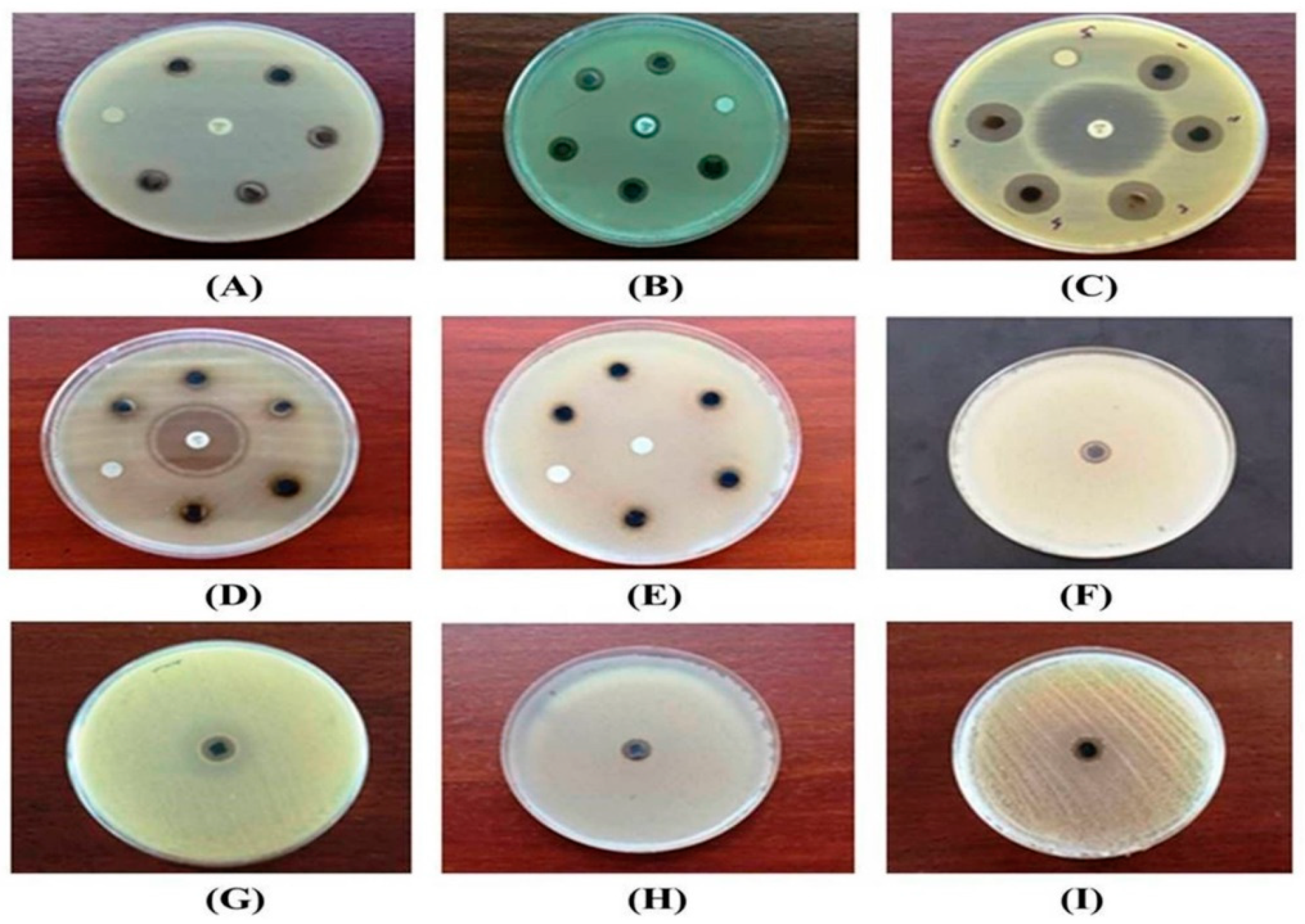
3.2. Antimicrobial Activity
4. Discussion
4.1. Synthesis and Characterization of Eh-AgNPs
4.2. Antimicrobial Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019; p. 139.
- IACG. No Time to Wait: Securing the Future from Drug-Resistant Infections; Report to the Secretary-General of the United Nations; United Nations Interagency Coordination Group on Antimicrobial Resistance: Geneva, Switzerland, 2019; p. 25. [Google Scholar]
- CDC. Infographic: Antibiotic Resistance The Global Threat; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019; p. 1.
- WHO. World Health Statistics 2018: Monitoring Health for the SDGs; World Health Organization: Geneva, Switzerland, 2018; p. 86. [Google Scholar]
- WHO. Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline; World Health Organization: Geneva, Switzerland, 2019; p. 35. [Google Scholar]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and Their Antimicrobial Properties against Pathogens Including Bacteria, Fungi, Parasites and Viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.; Fulaz, S.; Stanisic, D.; Tasic, L. Biogenic Nanosilver against Multidrug-Resistant Bacteria (MDRB). Antibiotics 2018, 7, 69. [Google Scholar] [CrossRef]
- Das, C.G.A.; Kumar, V.G.; Dhas, T.S.; Karthick, V.; Govindaraju, K.; Joselin, J.M.; Baalamurugan, J. Antibacterial Activity of Silver Nanoparticles (Biosynthesis): A Short Review on Recent Advances. Biocatal. Agric. Biotechnol. 2020, 27, 101593. [Google Scholar] [CrossRef]
- Javaid, A.; Oloketuyi, S.F.; Khan, M.M.; Khan, F. Diversity of Bacterial Synthesis of Silver Nanoparticles. BioNanoScience 2018, 8, 43–59. [Google Scholar] [CrossRef]
- Kawish, M.; Ullah, F.; Ali, H.S.; Saifullah, S.; Ali, I.; Rehman, J.U.; Imran, M. Bactericidal potentials of silver nanoparticles: Novel Aspects against Multidrug Resistance Bacteria. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Shah, M.R., Imran, M., Ullah, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 175–188. [Google Scholar]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A Comprehensive Review on Green Synthesis of Nature-Inspired Metal Nanoparticles: Mechanism, Application and Toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green Synthesis of Silver Nanoparticles: Biomolecule-Nanoparticle Organizations Targeting Antimicrobial Activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green Synthesis of Metallic Nanoparticles as Effective Alternatives to Treat Antibiotics Resistant Bacterial Infections: A Review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green Nanotechnology: A Review on Green Synthesis of Silver Nanoparticles—An Ecofriendly Approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A Review on Biosynthesis of Silver Nanoparticles and Their Biocidal Properties. J. Nanobiotechnol. 2018, 16. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver Nanoparticles: Various Methods of Synthesis, Size Affecting Factors and Their Potential Applications–a Review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Sharma, A.; Malhotra, B.; Kharkwal, H.; Kulkarni, G.T.; Kaushik, N. Therapeutic Agents from Endophytes Harbored in Asian Medicinal Plants. Phytochem. Rev. 2020, 19, 691–720. [Google Scholar] [CrossRef]
- Bagur, H.; Poojari, C.C.; Melappa, G.; Rangappa, R.; Chandrasekhar, N.; Somu, P. Biogenically Synthesized Silver Nanoparticles Using Endophyte Fungal Extract of Ocimum Tenuiflorum and Evaluation of Biomedical Properties. J. Clust. Sci. 2019. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Rao, M.P.N.; Xiao, M.; Wang, H.-F.; Hozzein, W.N.; Chen, W.; Li, W.-J. Antibacterial Activity of Silver Nanoparticles against Staphylococcus Warneri Synthesized Using Endophytic Bacteria by Photo-Irradiation. Front. Microbiol. 2017, 8, 1090. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-Synthesization of Silver Nanoparticles Using Endophytic Bacteria Isolated from Garlic and Its Antifungal Activity against Wheat Fusarium Head Blight Pathogen Fusarium Graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of Silver Nanoparticles Using Endophytic Bacteria and Their Role in Inhibition of Rice Pathogenic Bacteria and Plant Growth Promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef]
- Monowar, T.; Rahman, M.; Bhore, S.; Raju, G.; Sathasivam, K. Silver Nanoparticles Synthesized by Using the Endophytic Bacterium Pantoea Ananatis Are Promising Antimicrobial Agents against Multidrug Resistant Bacteria. Molecules 2018, 23, 3220. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, L.; Liu, F.; Hu, Y.; Leng, C.; Kan, Y.; Yao, L.; Shi, H. First Report of Enterobacter Hormaechei with Respiratory Disease in Calves. BMC Vet. Res. 2020, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Dyabi, F.Z.; Bennaoui, F.; Slitine, N.E.I.; Soraa, N.; Maoulainine, F.M.R. Enterobacter Hormaechei: New Neonatal Infection in Morocco. Open Infect. Dis. J. 2018, 10, 147–150. [Google Scholar] [CrossRef]
- Monahan, L.G.; DeMaere, M.Z.; Cummins, M.L.; Djordjevic, S.P.; Roy Chowdhury, P.; Darling, A.E. High Contiguity Genome Sequence of a Multidrug-Resistant Hospital Isolate of Enterobacter Hormaechei. Gut Pathog. 2019, 11, 3. [Google Scholar] [CrossRef]
- Gou, J.; Liu, N.; Guo, L.; Xu, H.; Lv, T.; Yu, X.; Chen, Y.; Guo, X.; Rao, Y.; Zheng, B. Carbapenem-Resistant Enterobacter Hormaechei ST1103 with IMP-26 Carbapenemase and ESBL Gene BlaSHV-178. Infect. Drug Resist. 2020, 13, 597–605. [Google Scholar] [CrossRef]
- John, M.S.; Nagoth, J.A.; Ramasamy, K.P.; Mancini, A.; Giuli, G.; Natalello, A.; Ballarini, P.; Miceli, C.; Pucciarelli, S. Synthesis of Bioactive Silver Nanoparticles by a Pseudomonas Strain Associated with the Antarctic Psychrophilic Protozoon Euplotes focardii. Mar. Drugs 2020, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Syed, B.; Prasad, M.N.N.; Satish, S. Synthesis and Characterization of Silver Nanobactericides Produced by Aneurinibacillus Migulanus 141, a Novel Endophyte Inhabiting Mimosa Pudica L. Arab. J. Chem. 2019, 12, 3743–3752. [Google Scholar] [CrossRef]
- Syed, B.; Prasad, M.N.N.; Dhananjaya, B.L.; Yallappa, S.; Satish, S. Synthesis of Silver Nanoparticles by Endosymbiont Pseudomonas Fluorescens CA 417 and Their Bactericidal Activity. Enzyme Microb. Technol. 2016, 95, 128–136. [Google Scholar] [CrossRef]
- Syed, B.; Yashavantha Rao, H.C.; Nagendra-Prasad, M.N.; Prasad, A.; Harini, B.P.; Azmath, P.; Rakshith, D.; Satish, S. Biomimetic Synthesis of Silver Nanoparticles Using Endosymbiotic Bacterium Inhabiting Euphorbia Hirta L. and Their Bactericidal Potential. Scientifica 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Loh, C.Y.; Tan, Y.Y.; Rohani, R.; Weber, J.-F.F.; Bhore, S.J. Diversity of Endophytic Bacteria in Malaysian Plants as Revealed by 16S rRNA Encoding Gene Sequence Based Method of Bacterial Identification. J. Young Pharm. 2013, 5, 95–97. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; p. 296. [Google Scholar]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline, 2nd ed.; CLSI supplement M44-A2; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2009; p. 25. [Google Scholar]
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A10; Approved Standard, 10th ed.; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2015; p. 92. [Google Scholar]
- Finch, R. Antimicrobial Therapy: Principles of Use. Medicine (Baltim.) 2009, 37, 545–550. [Google Scholar] [CrossRef]
- Ur Rehman, I.; Movasaghi, Z.; Rehman, S. Vibrational Spectroscopy for Tissue Analysis; CRC Press: Boca Raton, FA, USA, 2012; pp. 213–294. [Google Scholar]
- Anon. Infrared Spectroscopy Absorption Table. Available online: https://chem.libretexts.org/@go/page/22645 (accessed on 27 March 2021).
- Khan, T.; Yasmin, A.; Townley, H.E. An Evaluation of the Activity of Biologically Synthesized Silver Nanoparticles against Bacteria, Fungi and Mammalian Cell Lines. Colloids Surf. B Biointerfaces 2020, 194, 111156. [Google Scholar] [CrossRef]
- Alsharif, S.M.; Salem, S.S.; Abdel-Rahman, M.A.; Fouda, A.; Eid, A.M.; El-Din Hassan, S.; Awad, M.A.; Mohamed, A.A. Multifunctional Properties of Spherical Silver Nanoparticles Fabricated by Different Microbial Taxa. Heliyon 2020, 6, e03943. [Google Scholar] [CrossRef]
- Wypij, M.; Czarnecka, J.; Świecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, Characterization and Evaluation of Antimicrobial and Cytotoxic Activities of Biogenic Silver Nanoparticles Synthesized from Streptomyces Xinghaiensis OF1 Strain. World J. Microbiol. Biotechnol. 2018, 34. [Google Scholar] [CrossRef]
- Wang, M.; Fu, H.; Ruan, R. A Small Horizontally Transferred Gene Cluster Contributes to the Sporulation of Alternaria Alternata. Genome Biol. Evol. 2019, 11, 3436–3444. [Google Scholar] [CrossRef]
- Chahar, V.; Sharma, B.; Shukla, G.; Srivastava, A.; Bhatnagar, A. Study of Antimicrobial Activity of Silver Nanoparticles Synthesized Using Green and Chemical Approach. Colloids Surf. Physicochem. Eng. Asp. 2018, 554, 149–155. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; CLSI document M100-S23; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013; p. 199. [Google Scholar]
- Khaleghi, M.; Khorrami, S.; Ravan, H. Identification of Bacillus Thuringiensis Bacterial Strain Isolated from the Mine Soil as a Robust Agent in the Biosynthesis of Silver Nanoparticles with Strong Antibacterial and Anti-Biofilm Activities. Biocatal. Agric. Biotechnol. 2019, 18, 101047. [Google Scholar] [CrossRef]
- Toda, M.; Williams, S.R.; Berkow, E.L.; Farley, M.M.; Harrison, L.H.; Bonner, L.; Marceaux, K.M.; Hollick, R.; Zhang, A.Y.; Schaffner, W.; et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia—Four Sites, United States, 2012–2016. MMWR Surveill. Summ. 2019, 68, 1–15. [Google Scholar] [CrossRef]
- Lateef, A.; Ojo, S.A.; Oladejo, S.M. Anti-Candida, Anti-Coagulant and Thrombolytic Activities of Biosynthesized Silver Nanoparticles Using Cell-Free Extract of Bacillus Safensis LAU 13. Process Biochem. 2016, 51, 1406–1412. [Google Scholar] [CrossRef]
- Wang, C.; Kim, Y.J.; Singh, P.; Mathiyalagan, R.; Jin, Y.; Yang, D.C. Green Synthesis of Silver Nanoparticles by Bacillus Methylotrophicus and Their Antimicrobial Activity. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1127–1132. [Google Scholar] [CrossRef]
- Mouton, J.W.; Meletiadis, J.; Voss, A.; Turnidge, J. Variation of MIC Measurements: The Contribution of Strain and Laboratory Variability to Measurement Precision. J. Antimicrob. Chemother. 2018, 73, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.T.E.; El-Sayed, A.S. Biocidal Activity of Metal Nanoparticles Synthesized by Fusarium Solani against Multidrug-Resistant Bacteria and Mycotoxigenic Fungi. J. Microbiol. Biotechnol. 2020, 30, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.; Ansari, M.; Alzohairy, M.; Ali, S.; Khan, H.; Almatroudi, A.; Raees, K. Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida Glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi. Nanomaterials 2018, 8, 586. [Google Scholar] [CrossRef]
- Khatoon, N.; Sharma, Y.; Sardar, M.; Manzoor, N. Mode of Action and Anti-Candida Activity of Artemisia Annua Mediated-Synthesized Silver Nanoparticles. J. Mycol. Médicale 2019, 29, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, V.S.; Reddy Mudiam, M.K.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver Nanoparticles Induced Alterations in Multiple Cellular Targets, Which Are Critical for Drug Susceptibilities and Pathogenicity in Fungal Pathogen (Candida Albicans). Int. J. Nanomed. 2018, 13, 2647–2663. [Google Scholar] [CrossRef]
- Robles-Martínez, M.; Patiño-Herrera, R.; Pérez-Vázquez, F.J.; Montejano-Carrizales, J.M.; González, J.F.C.; Pérez, E. Mentha Piperita as a Natural Support for Silver Nanoparticles: A New Anti- Candida Albicans Treatment. Colloid Interface Sci. Commun. 2020, 35, 100253. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ghilan, A.-K.M.; Arasu, M.V.; Duraipandiyan, V. Green Biosynthesis of Silver Nanoparticles Produced from Marine Streptomyces Sp. Al-Dhabi-89 and Their Potential Applications against Wound Infection and Drug Resistant Clinical Pathogens. J. Photochem. Photobiol. B 2018, 189, 176–184. [Google Scholar] [CrossRef]
- Khan, M.H.; Unnikrishnan, S.; Ramalingam, K. Bactericidal Potential of Silver-Tolerant Bacteria Derived Silver Nanoparticles against Multi Drug Resistant ESKAPE Pathogens. Biocatal. Agric. Biotechnol. 2019, 18, 100939. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; Ali, D.M.; Prakash, P.; Pugazhendhi, A. Synthesis of Silver Nanoparticles from Bacillus Brevis (NCIM 2533) and Their Antibacterial Activity against Pathogenic Bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.A.; Shaaban, M.I. Synthesis of Silver Nanoparticles with Antimicrobial and Anti-Adherence Activities against Multidrug-Resistant Isolates from Acinetobacter Baumannii. J. Taibah Univ. Med. Sci. 2017, 12, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; AlYahya, S.; Govarthanan, M.; ALjahdali, N.; Al-Enazi, N.; Alsamhary, K.; Alshehri, W.A.; Alwakeel, S.S.; Alharbi, S.A. Soil Bacteria Cupriavidus Sp. Mediates the Extracellular Synthesis of Antibacterial Silver Nanoparticles. J. Mol. Struct. 2020, 1202, 127233. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Yazgan, I.; Akgul, A.; Eshun, G.B.; Sakhaee, L.; Sadik, O.A. Size and Shape-Dependent Antimicrobial Activities of Silver and Gold Nanoparticles: A Model Study as Potential Fungicides. Molecules 2020, 25, 2682. [Google Scholar] [CrossRef]
- Ghosh, P.; Sowdhamini, R. Bioinformatics Comparisons of RNA-Binding Proteins of Pathogenic and Non-Pathogenic Escherichia Coli Strains Reveal Novel Virulence Factors. BMC Genom. 2017, 18, 658. [Google Scholar] [CrossRef] [PubMed]
- Sela, U.; Euler, C.W.; Correa da Rosa, J.; Fischetti, V.A. Strains of Bacterial Species Induce a Greatly Varied Acute Adaptive Immune Response: The Contribution of the Accessory Genome. PLOS Pathog. 2018, 14, e1006726. [Google Scholar] [CrossRef]

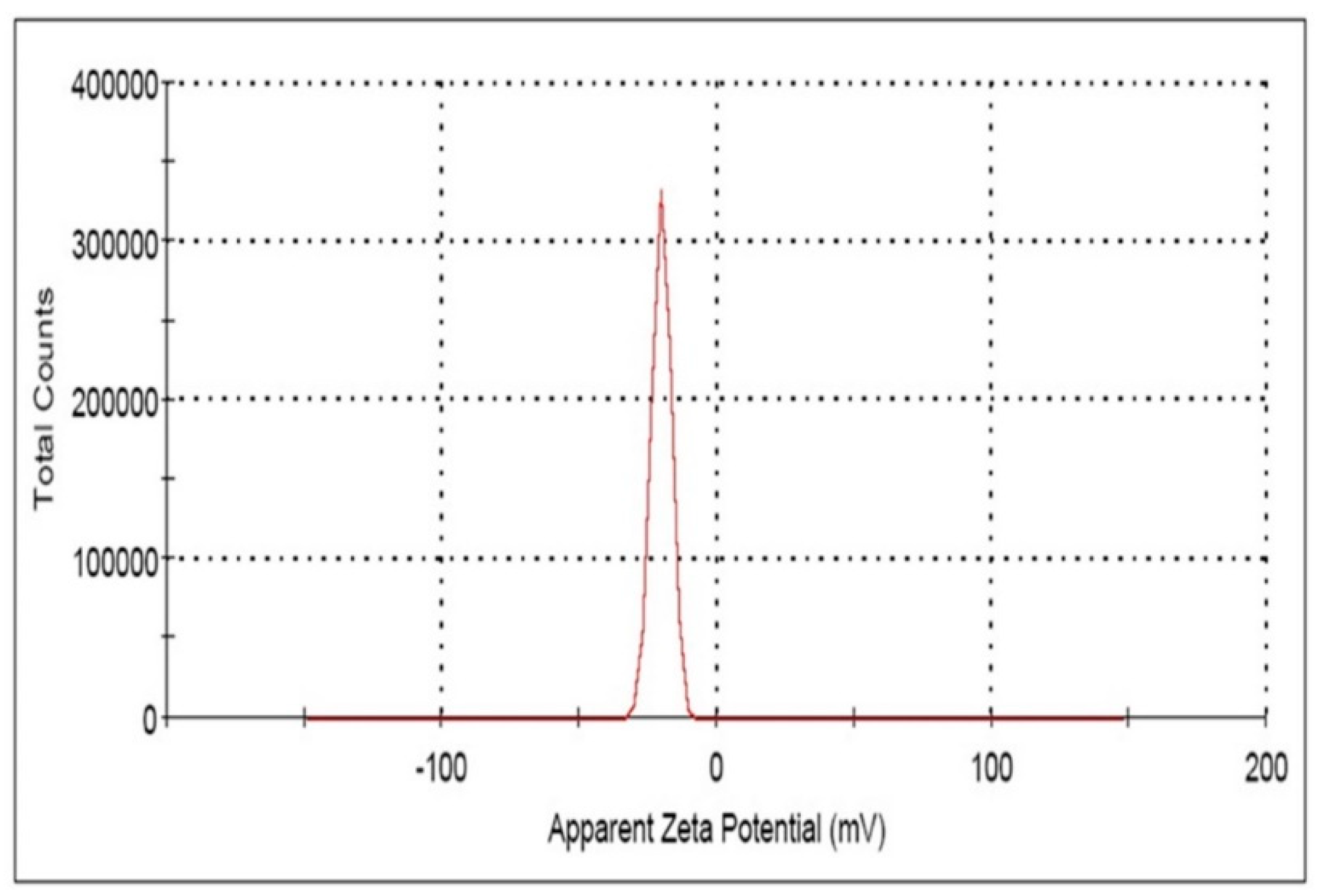
| Triplicate Study | Zeta Potential (ζ) (mV) | Area (%) | Conductivity (mS/cm) |
|---|---|---|---|
| Mean ± SD (mV) | |||
| 1. | −19.3 ± 3.97 | 100 | 0.0362 |
| 2. | −19.6 ± 4.32 | 100 | 0.0101 |
| 3. | −20.3 ± 3.53 | 100 | 0.00980 |
| Microbes | ZOI (mm) | MIC (μg/mL) | Control |
|---|---|---|---|
| Pathogenic Microbes 1 | |||
| B. cereus (ATCC 10876) | 9.14 ± 0.05 c | 1.75 | Ampicillin (10 μg): resistant |
| S. aureus subsp. aureus (ATCC 11632) | 11.20 ± 0.07 b | 1.50 | Ampicillin (10 µg): 10.14 ± 0.05 |
| E. coli (ATCC 10536) | 15.16 ± 0.05 a | 1.25 | Ciprofloxacin (5 µg): 30.48 ± 0.08 |
| P. aeruginosa (ATCC 10145) | 7.18 ± 0.04 e | 2.25 | Ciprofloxacin (5 µg): 30.10 ± 0.07 |
| C. albicans (ATCC 10231) | 8.24 ± 0.05 d | 2.00 | Itraconazole (10 µg): resistant |
| MDR bacteria 2 | |||
| S. pneumoniae (ATCC 700677) | 11.24 ± 0.05 C | 5.25 | - |
| E. faecium (ATCC 700221) | 13.20 ± 0.07 A | 2.00 | - |
| S. aureus subsp. aureus (ATCC 33592) | 10.98 ± 0.08 D | 6.00 | - |
| E. coli (NCTC 13351) | 12.24 ± 0.05 B | 3.75 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monowar, T.; Rahman, M.S.; Bhore, S.J.; Sathasivam, K.V. Endophytic Bacteria Enterobacter hormaechei Fabricated Silver Nanoparticles and Their Antimicrobial Activity. Pharmaceutics 2021, 13, 511. https://doi.org/10.3390/pharmaceutics13040511
Monowar T, Rahman MS, Bhore SJ, Sathasivam KV. Endophytic Bacteria Enterobacter hormaechei Fabricated Silver Nanoparticles and Their Antimicrobial Activity. Pharmaceutics. 2021; 13(4):511. https://doi.org/10.3390/pharmaceutics13040511
Chicago/Turabian StyleMonowar, Tahmina, Md. Sayedur Rahman, Subhash J. Bhore, and Kathiresan V. Sathasivam. 2021. "Endophytic Bacteria Enterobacter hormaechei Fabricated Silver Nanoparticles and Their Antimicrobial Activity" Pharmaceutics 13, no. 4: 511. https://doi.org/10.3390/pharmaceutics13040511
APA StyleMonowar, T., Rahman, M. S., Bhore, S. J., & Sathasivam, K. V. (2021). Endophytic Bacteria Enterobacter hormaechei Fabricated Silver Nanoparticles and Their Antimicrobial Activity. Pharmaceutics, 13(4), 511. https://doi.org/10.3390/pharmaceutics13040511







