1. Introduction
In recent years, an increased contribution has been brought to the development of drugs capable of both imaging specific targets (tumours) and treating the malignant tissue via local irradiation with minimal detriment to the body. Research in the field of theranostic uses certain biological active molecules to which different radioisotopes can be bound for either diagnosis or treatment. This approach aims to find the molecule with high specificity for a tumour cell type, and then properly attach to it an imaging or a treatment radioisotope, according to its use. One molecule of this kind is the neurotensin (NT) for which it has been previously demonstrated to have the potential to target tumours such as: pancreatic cancer [
1,
2,
3], colorectal cancer [
4,
5,
6], lung cancer [
6], prostate cancer [
7,
8] or breast cancer [
9]. Neurotensin is a tridecapeptide (pGlu-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu) that acts as a neurotransmitter or neuromodulator in the central nervous system (CNS). It is a regulating factor in normal and cancerous tissue growth and also contributes to fat storage, obesity and metabolic disorders [
10]. Neurotensin exerts its effects primarily through neurotensin receptor subtype-1 (NTRS1) and with lower affinity through neurotensin receptor subtype-2 (NTRS2), both G-protein–coupled receptors (GPCR), and later-discovered neurotensin receptor subtype-3 (NTRS3), a single transmembrane domain sorting receptor. These findings suggest that the NT signalling pathway may provide a useful target for the development of diagnostic agents and antitumor drugs.
Although previous studies showed that neurotensin has a low stability in blood [
11], this work aimed to assess the reliability of neurotensin radiolabelling process, according to pharmaceutical requirements and the potential use for imaging and therapy, depending on the characteristics of the bound radioisotope. The foremost objective of this study was to evaluate the behaviour of the radiolabelled neurotensin to determine to which extent it can be used in natural conditions, and to draw a baseline for further biological studies, e.g., using inhibited proteolytic enzymes and/or pharmacokinetics modifiers.
The emerging interest in theranostic applications led to the development of peptides coupled with imaging/therapeutic radioisotopes pairs that quickly localize to cancer cells, delivering locally the diagnostic/therapeutic effect, respectively. A major and important choice is selecting the proper radionuclides for the intended use, as there are no “ideal” medical radioisotopes, and then choosing a pair of them for theranostic applications. Such an example of radioisotopes pair is
68Ga—used as imaging agent—and its therapeutic counterpart—
177Lu [
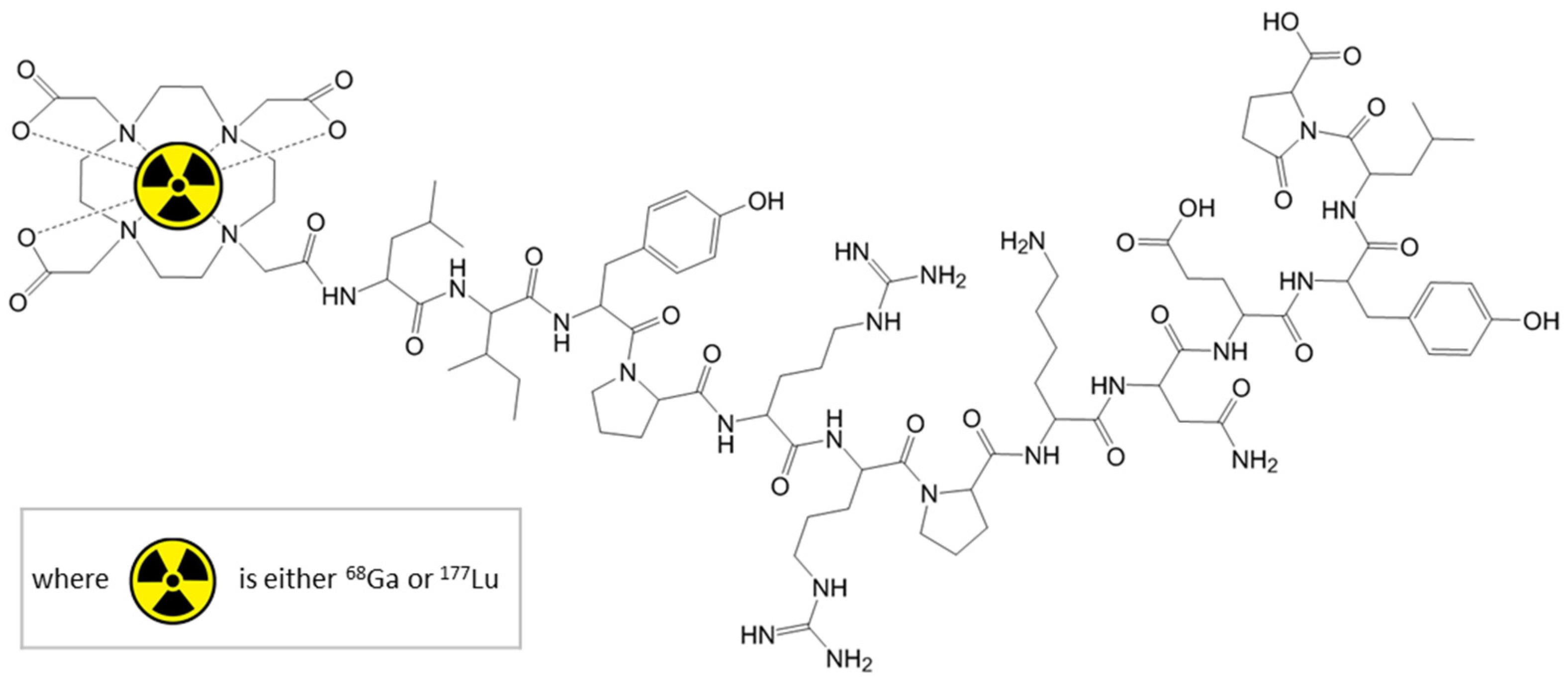
12]. Macrocyclic chelating agents, like DOTA can form complexes with radiometallic cations and can bind covalently to biomacromolecules. The ability to complex various cations offers the possibility to use the same biological active molecule both for diagnostic and therapy, depending on the complexed radioisotope (
Figure 1).
Gallium has no known natural role in biology, but
68Ga has a short half-life, compatible with the pharmacological distribution time of certain biomolecules (e.g., peptides, proteins and oligonucleotides). Furthermore,
68Ga and
177Lu are two radioisotopes which can be chelated with good results to a variety of biomolecules; this allowing to approach more diseases than e.g.,
99mTc. Therefore,
68Ga has gained increasing importance in clinical research in the last 25 years [
13] as a PET imaging agent, exhibiting more suitable characteristics than other PET radioisotopes in terms of higher detection resolution and sensitivity (T
1/2 = 67.83 min, E
β+ = 1.899 MeV) [
8,
14], a lower or comparative effective dose to that of
18F or
99mTc (2.3 mSv for [
68Ga]Ga-DOTA-TOC per PET scan, compared to 5.6 mSv for [
18F]FDG [
15]) and fast data acquisition. It decays by β
+ emission (88.88%) and electronic capture (11.11%) to stable
68Zn [
14].
Currently, the most common method for obtaining
68Ga is via
68Ge/
68Ga generator (
68Ge decays 100% by electronic capture to
68Ga), with the alternative of proton irradiation at medium energy cyclotrons via
68Zn(p,n)
68Ga nuclear reaction [
16]. Both methods have specific advantages (generator: no gallium impurities produced, virtually carrier-free compounds, minimal radioprotection equipment, ease of operation; cyclotron: much more activity available, no dead-time between batches), but also disadvantages (generator: maximum available activity is limited and decreasing with the age of the generator, limited elutions per day, limited number of patients per batch; cyclotron: radionuclidic impurities produced during irradiation, need of laborious purification methods, higher operation costs, dedicated vault, more expensive radiological protection). Considering these aspects and the needs for our studies, we chose the generator method of
68Ga production.
The most widely used
68Ga–based imaging/diagnostic agents are [
68Ga]Ga-DOTA-Somatostatin analogues like TOC, TATE or NOC for patients affected by neuroendocrine tumours (NET), and prostate specific membrane antigen [
68Ga]
68Ga-PSMA for prostate tumours [
17,
18].
177Lu is used for therapy due to low energy β
- emission (E
β- = 498 keV), but also provides imaging information due to its low energy gamma photons (E
γ = 208.36 keV (10%) and 113 keV (6.2%)) [
19,
20]. It is obtained in reactor-based production facilities, and its half-life (6.65 days) is sufficiently long for delivery to clinical sites. Major clinical applications of
177Lu include therapy with [
177Lu]Lu-DOTA-TATE for metastatic neuroendocrine tumours [
21], [
177Lu]Lu-PSMA for prostate cancer and [
177Lu]Lu-DOTA-Rituximab for non-Hodgkin lymphoma [
22].
Due to the short half-life of 68Ga, it is essential to establish standardized processes, with constant reproducibility, in order to shorten the radiolabelling time and to obtain radiolabelled peptide solution of pharmaceutical grade purity, suitable for injection. The automation of the process is also important, as long as this allows shortening of the overall process time, along with the reduction of radioactive dose to which the operators are exposed.
The present work describes our fast method for labelling the NT peptide with 68Ga and 177Lu respectively, and assesses the binding kinetics, as well as the biodistribution of 68Ga-DOTA-NT and 177Lu-DOTA-NT to organs for the applications of NT as a theranostic agent.
2. Materials and Methods
All reagents and solvents were purchased from commercial suppliers and used with no other purification. The peptide employed in all experiments (DOTA-NT) was provided already conjugated with DOTA chelator by piChem GmbH (Raaba-Grambach, Austria). Ultrapure water used in synthesis and for preparation of solutions was freshly prepared on-site with Millipore Milli-Q Direct 8 system (Millipore S.A.S., Molsheim, France).
Nomenclature statement:
68Ga and
177Lu employed in the studies presented in this work comply with the definitions of no-carrier-added and carrier-free compounds according to IUPAC nomenclature (Gold Book [
23,
24] and Orange Book [
25]), and Radiochemistry Society (Richland, WA, USA) [
26]. Therefore, the radiolabelled compounds produced and used in our studies are referred, and their chemical formulae are written according to the rules for mentioned definitions.
2.1. 68Ga-DOTA-NT Synthesis
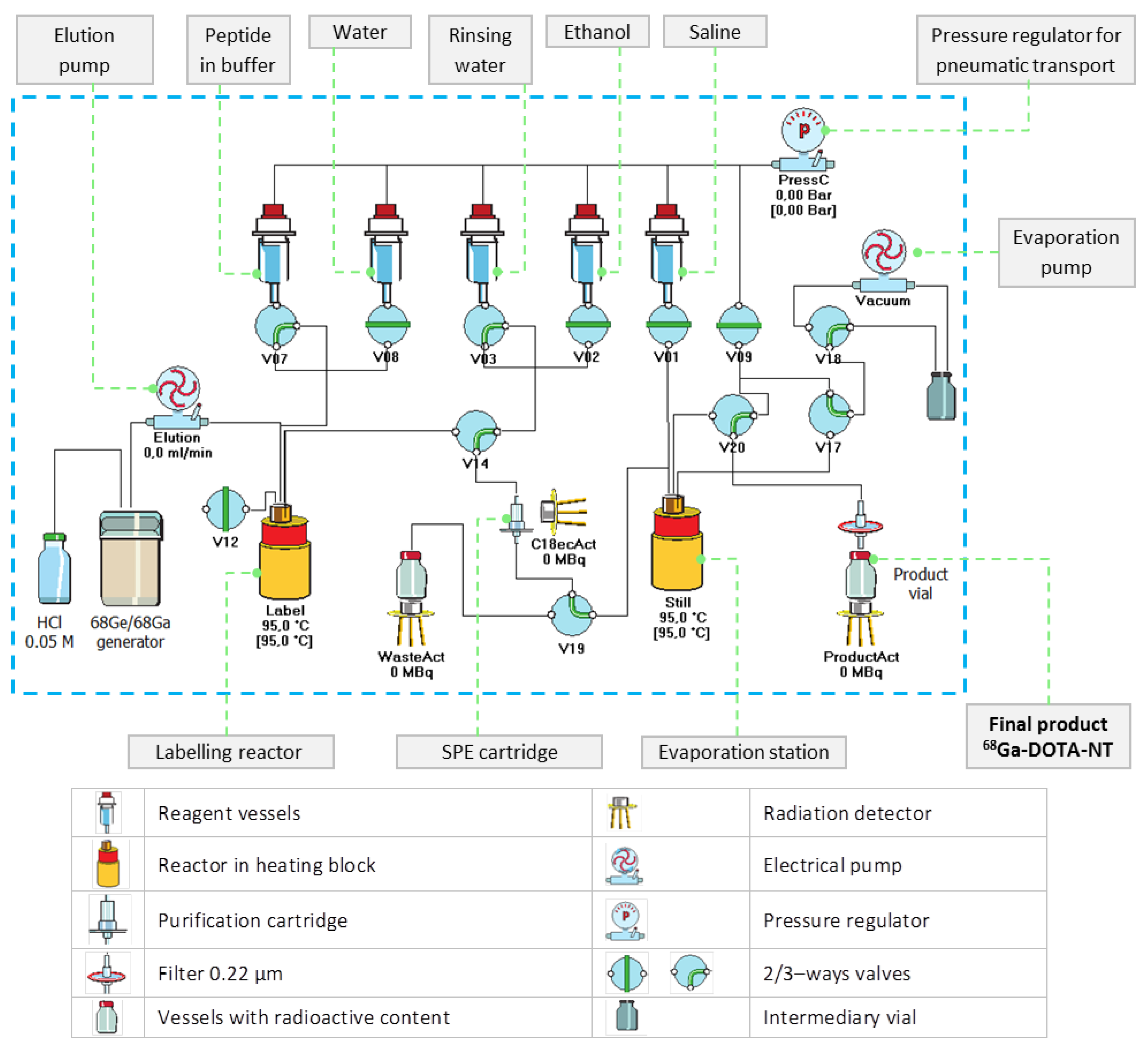
The preparation of 68Ga-DOTA-NT, consisting of radiolabelling with 68Ga, purification and formulation, was carried out on the automated synthesis module Galigand GAL-102 (Veenstra Instruments, Joure, The Netherlands).
In order to efficiently use the available activity, the syntheses were carried out just before the biological assays, and the elution was integrated as the first step of the synthesis sequence.
68Ga was freshly extracted for each batch from organic–column type 68Ge/68Ga generator (ITG Isotope Technologies Garching GmbH, Garching, Germany), which is coupled to synthesis module.
The buffer employed in all experiments is 1 M ammonium acetate solution prepared on-site from ammonium acetate salt (Carl Roth GmbH, Germany). For each batch, 68Ga was eluted according to manufacturer’s protocol with 4 mL 0.05 M HCl prepared on-site by diluting ultrapure-grade 30% HCl (Merck KGaA, Darmstadt, Germany).
The proper fraction of eluate, consisting of 2 mL of 68GaCl3 in 0.05 M HCl (pH = 1–1.3), which concentrates most of the activity eluted from the 68Ge/68Ga generator, was sent immediately in the reaction vessel, already heated to the labelling temperature (95 °C).
Aliquots of 50 µL containing 24 nmol of DOTA-NT dissolved in buffer solution were used for each batch. The amount of buffer required to adjust the pH of the reaction mixture in the range of 4.5–4.8 was previously mixed with the peptide aliquot, in order to protect the biomolecule and to avoid a possible degradation when added to the highly acidic eluate. The peptide-buffer mixture was sent to the labelling vial immediately after the transfer of the 68Ga eluate and left to react for 5 min at 95 °C.
After the labelling step, a small amount of ultrapure water (300 µL) was added in the labelling vial, and then the product was passed through a previously conditioned separation cartridge C18 Strata-X SPE (Phenomenex, Torrance, CA, USA). The SPE cartridge retains only the labelled peptide, while the free 68Ga impurities are evacuated as reaction waste. The complete removal of impurities was carried out by rinsing the cartridge with 1 mL mixture made of 950 µL ultrapure water and 50 µL ethanol (Chimreactiv S.R.L., Bucharest, Romania). After purification step, the peptide was recovered into the evaporation vial by passing 1 mL of ethanol through the SPE cartridge. Further, in the evaporation vial already heated at 95 °C, the ethanol is evaporated using dry heat and vacuum to hasten the process. The labelled peptide was reconstituted in 1 mL physiological saline solution (0.9% NaCl, B. Braun Melsungen AG, Melsungen, Germany). At the end of the synthesis, 68Ga-DOTA-NT was filtered through 0.22 µm membrane filter Millipore (Merck KGaA, Darmstadt, Germany) for sterilization. The pH was measured using pH strips (pH-Fix 2.0–9.0 and pH-Fix 3.6–6.1) from Macherey–Nagel GmbH & Co. (Düren, Germany).
2.2. 177Lu-DOTA-NT Synthesis
177Lu was acquired in form of 177LuCl3 from National Centre for Nuclear Research—Radioisotope Centre POLATOM (Otwock, Poland). The labelling of neurotensin with 177Lu was carried out manually in a preheated reaction vial at 100 °C. 370 MBq of 177LuCl3 solution was mixed with 1 M ammonium acetate solution in order to adjust the pH value to 5.5, and then 24 nmol of DOTA-NT was added. The mixture was left to react for 10 min under continuous stirring at 100 °C. Then, the heating was turned off and the mixture was left to cool down under stirring for 10 min. The pH was finally adjusted to 6.5–6.8 with buffer. The final formulation of 177Lu-DOTA-NT has been done with 1 mL of 0.9% NaCl solution, followed by filtration through a 0.22 μm membrane filter.
2.3. Radiochemical Purity and Stability
The radiochemical purity (RCP) of the radiolabelled DOTA-Neurotensin conjugates was assessed by radio-HPLC, using a Shimadzu Prominence 20 A series system (Shimadzu Corporation, Kyoto, Japan) equipped with LB513 BGO γ–detector (Berthold Technologies GmbH & Co.KG, Bad Wildbad, Germany). The stationary phase used was a C18 column—Nucleosil® 5µm, 250 mm × 4.6 mm, (Supelco Inc., Bellefonte, PA, USA). The mobile phase was composed of water and acetonitrile, both acidified with 0.1% TFA, at 1 mL/min flow rate. The following gradient was used: 0–2 min 5% B, 2–32 min 5–65% B, 32–35 min 65–5% B, 35–40 min 5% B (where A = water with 0.1% TFA, and B = acetonitrile with 0.1% TFA).
The stability of 68Ga-DOTA-NT was assessed by radio-HPLC for 4 h after the end of the labelling and purification process (EOS). 5 samples taken from the same vial were injected at different moments after the end of the synthesis, as follows: immediately, after 1, 2, 3 and 4 h respectively, and evaluated in terms of radiochemical purity. The radiochemical stability of 177Lu-DOTA-NT was checked up to 48 h from the end of preparation. At the end of the evaluation period, the radiochemical purity should be higher than 95%.
2.4. Cell Culture
The human colon cancer cell line HT-29 (ATCC, Manassas, VA, USA) was incubated in Dulbecco’s Modified Eagle’s Medium (DMEM) (Biological Industries, Beit-Haemek, Israel), at 37 °C in controlled atmosphere containing 5% CO2.
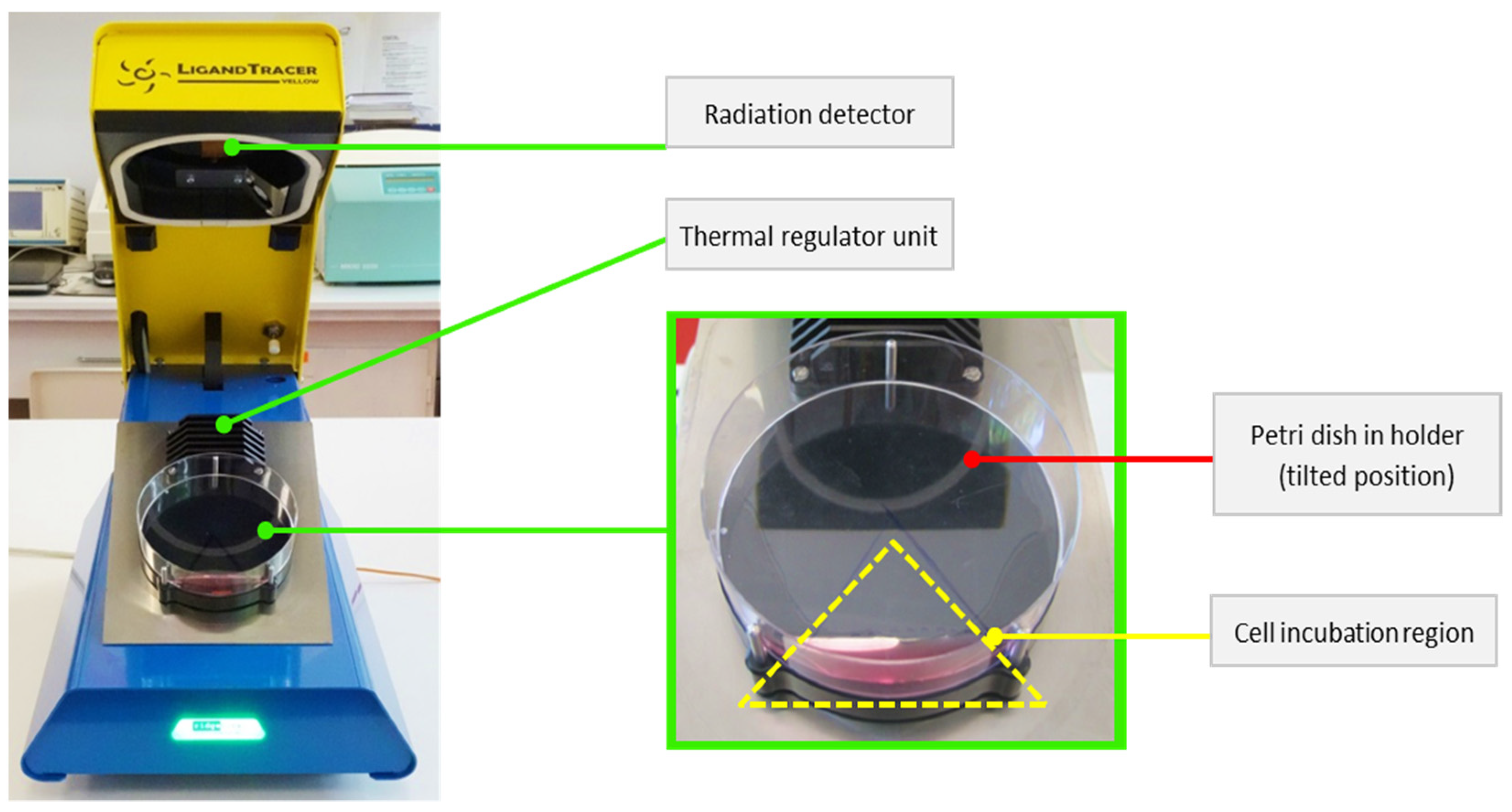
For binding assay, Petri dishes (NunclonTM Delta Surface 150350, ThermoFisher Scientific, Roskilde, Denmark) were prepared with cell culture 24 h before experiments, in order to allow the cells to firmly attach to the dish surface. 4 × 105 cells were seeded in a well-defined area of the dish, while keeping the Petri dish in a tilted position. Once the cells have attached, the remaining cell solution was removed, and 2 mL of DMEM was added. Each dish was inspected under a microscope to check that all cells have attached in the designated area.
2.5. In Vitro Uptake and Retention Assessment of 68Ga-DOTA-NT
The LigandTracer Yellow system (Ridgeview Instruments AB, Uppsala, Sweden) was used to evaluate the amount of tracer that binds to specific receptors expressed on the surface of the tumour cell membrane. This instrument uses the rotating radioimmunoassay (RIA) method to evaluate real-time dynamics of tracer binding to tumour cells during analysis. Every 5.6 min, the system records the activity in 12 points (6 points corresponding to the upper position of the tilted dish and 6 points for the base position—see
Figure 2 [
27]). The recorded result represents the difference in detected signal between each corresponding point from the two areas [
28,
29].
To perform this analysis, the background was measured (eliminating any radioactive source around the device for baseline acquisition). 0.6 nmol of 68Ga-DOTA-NT with 10 MBq was added over cells in Petri dish and recording was started to obtain the tracer uptake profile. After reaching the steady state, the radioactive medium was removed and the cells were washed with 2 mL of fresh DMEM (1 mL at a time) to remove additional activity (labelled peptide left unbound) from the Petri dish. After this washing step, 2 mL of medium was added over the cells to ensure an optimal survival environment during the next step of the analysis. Cell retention is obtained by restarting the measurement, the retention curve is recorded until a plateau is reached due to the equilibrium state. The same procedure was used in the case of 177Lu, but using 0.6 nmol of 177Lu-DOTA-NT with 25 MBq activity.
2.6. In Vitro Receptor Binding of DOTA-NT and Competition Assay
Neurotensin exhibits three specific receptors with increased affinity against NTRS1 compared to NTRS2 and NTRS3. In order to evaluate the specific binding due to the exclusive interaction of the peptide with its receptors, a competition assay was performed. This assay in which all NT receptors are blocked before incubating the labelled peptide is intended to highlight non-specific binding of NT to the cells. The difference between the results obtained by incubating the non-blocked peptide (total binding) and those obtained with peptide after blocking all receptors (non-specific binding) represents specific binding. For this assessment, three different NT receptors antagonists were used, each having a preferential increased affinity for one of the receptors, allowing the blocking of all NTRs at the same time:
NTRC 824,
N-[2-[5-[[(4-Methylphenyl) sulfonyl] amino]-3-(trifluoro acetyl)-1H-indol-1-yl] acetyl]-L-leucine is a non-peptide NTSR2 antagonist that exhibits 150-fold selectivity for NTRS2 over NTRS1 [
30].
SR 48692, 2-[[1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxyphenyl)-1H-pyrazol-3-carbonyl]amino] adamantane-2-carboxylic acid is a neurotensin antagonist selective for NTRS1 over NTRS2 20.
SR 142948, 2-[[5-(2,6-dimethoxyphenyl)-1-(4-(
N-(3-dimethylaminopropyl)-
N-methylcabamoyl)-2-isopropylphenyl)-1H-pyrazole-3-carbonyl]amino]adamantane-2-carboxylic acid is a synthetic NTR antagonist that completely inhibits [
125I]ITyr
3-NT specific binding to HT-29 cells [
31].
To evaluate specific binding, 5 × 105 HT-29 cells previously prepared according to the cell culture protocol were incubated with 70 nM of NTRC 824 (Bio-Techne Ltd., Abingdon, UK, dissolved in 0.1 M DMSO), 30 nM of SR 48692 (Bio-Techne Ltd., Abingdon, UK, dissolved in 0.1 M DMSO), and 10 nM of SR 142948 (Bio-Techne Ltd., Abingdon, UK, dissolved in 0.1 M DMSO) for 1.5 h. Afterwards, the medium containing unbound antagonists was removed and the cells were washed with 2 mL of fresh DMEM medium. After the baseline acquisition, 0.6 nmol of 68Ga-DOTA-NT was incubated and the uptake and cell retention were assessed according to the method described above.
2.7. Biodistribution Studies
All experiments were conducted according to the guidelines of EU Directive 2010/63 approved by The Romanian National Sanitary Veterinary and Food Safety Authority (NSVFSA, approval no. 442/25.02.2019).
Biodistribution studies of 68Ga-DOTA-NT and 177Lu-DOTA-NT were carried out on 8–10 weeks old, athymic nude male NU/J mice (Jackson Laboratory, Bar Harbor, ME, USA), weighting 26 ± 2.5 g.
The mice were inoculated 7 days prior to peptide administration with 5 × 106 HT-29 colon cancer cells. All animals were anesthetized before testing by administration of a mixture of 7.5 mg/mL ketamine (Narkamon Bio, Bioveta Romania S.R.L., Cluj-Napoca, Romania), 1.5 mg/mL xylazine (Narcoxyl 2, MSD Animal Health, Kenilworth, NJ, USA) and 0.25 mg/mL acepromazine (Sedam, Pasteur Filiala Filipești S.A., Filipeștii de Pădure, Romania)
The radiopharmaceuticals were injected intravenously in the tail vein with 2.8 ± 0.4 Bq of 68Ga-DOTA-NT per animal or 9.7 ± 1.6 MBq of 177Lu-DOTA-NT, respectively. The actual injected activity took over in the bloodstream was corrected by subtraction of residual activity left in the syringe, and the activity remaining at the injection point (animal tail). Mice injected with 68Ga-DOTA-NT were sacrificed at 30- or 60-min post-injection (p.i.). Mice injected with 177Lu-DOTA-NT were divided into three groups, corresponding to different dissection time (60 min, 7 days, 14 days p.i.). The organs of interest were weighted on an analytical scale ALT 220-4M (Kern & Sohn GmbH, Balingen, Germany) and their activities were measured with calibrated multi-channel-analyser for γ-spectroscopy equipped with NaI (Tl) detector Mucha (Raytest GmbH, Straubenhardt, Germany). The biodistribution was calculated as percent of injected dose per gram of organ (% ID/g) ± standard deviation.
2.8. Statistical Analysis
The data were expressed as means ± standard deviation (n = 4). Comparison of results among the groups (%ID/g vs. tracers, %ID/g vs. tracers and organs, %ID/g vs. tracers and tumour cells), was carried out by one-way analysis of variance (ANOVA) and Tukey post-hoc analysis using SPSS v26 (IBM, New York, NY, USA). The results were considered significant for a p-value lower than 0.05.
3. Results
3.1. Labelling of DOTA-NT
There are two limitations in 68Ga use, namely its short half-life and the limited activity available from a generator (which is dependent on the initial activity of 68Ge loaded on the generator). Also, the maximum generated activity is continuously decreasing in time due to the decay of the loaded parent nuclide—68Ge. Therefore, each batch of 68Ga–labelled peptide was freshly prepared just before its use. These limitations compel to shorten the time required by synthesis and experiments, and also to maximize the overall yield of the preparation process.
The sole Ga isotope produced in the generator is 68Ga, produced from 68Ge by electronic capture, as carrier-free 68Ga. This is a huge advantage over the cyclotron route, which requires additional, time-consuming purifying steps and provides radiolabelled compounds with lower purity; the drawback consists of limited activity and the dead time between two batches (approx. 5–6 h), required by re-generation of sufficient quantity of 68Ga for the next synthesis.
According to the manufacturer of the generator, a total volume of 4 mL HCl solution must be used for each synthesis. Only the most useful 2 mL of the eluate (the main fraction), concentrating the highest amount of
68Ga (extracted from the generator in form of
68GaCl
3) should be used for labelling. The first and the last fractions of the eluate (a total of 2 mL—containing higher amount of zinc, produced during
68Ga decay inside the generator and HCl) are transferred to the waste. Compared with the tin oxide based generator, whose eluate usually contains small amounts of other impurities (cations of Ge, Fe, Sn, Zn) and should be purified prior to entering in contact with the peptides [
32], this organic–column type generator does not require that step of purification. Moreover, this generator requires a much lower concentration of hydrochloric acid (0.05 M) compared to a tin oxide-based generator which typically requires a concentrations of 5 M and 10 M. To achieve high purity, short duration and high yield for
68Ga-labelling, preliminary experiments were carried out to investigate the process parameters (e.g., reaction temperature, pH, reaction time, evaporation time) that lead to optimal results. The optimal values of the automated process were implemented in a customized production script of proprietary Absynth controlling software. The synthesis flowchart and the scheme in the graphical interface of Absynth are presented in
Figure 3 and
Figure 4, respectively.
Preliminary tests revealed that for the labelling of neurotensin with
68Ga a more acidic pH is necessary (optimal 4.5) compared to the slightly higher pH in the case of
177Lu (pH = 5.5–6.0). In the case of
68Ga, the reaction time for total binding is shorter, 5 min at a temperature of 95 °C being sufficient to completely bind the radioisotope to the peptide. The labelling temperature was increased from 37 °C up to 95 °C to reduce the reaction time, without any detectable degradation of the radiolabelled peptide. The values of process parameters are presented in
Table 1.
3.2. Radiochemical Purity and Stability
For avoiding background noise in imaging and harmful radioactive dose to the body after administration of radiopharmaceuticals, it is very important that the product does not undergo structural degradation over the shelf-life, as this may affect its pharmacokinetics and, therefore, the correctness of the effects it provides. The stability of a radiopharmaceutical depends on several factors such as: temperature, exposure to light, physical and chemical properties of the active substances, excipients, pharmaceutical form and properties of packaging materials. Radiochemical impurities may occur as a result of the degradation of the peptide due to radiolysis, temperature variations and by the action of oxidizing or reducing agents. Another possible cause can be the unstable trapping of the metallic ions by the chelator. In our studies, DOTA chelator exhibited good affinity both for Ga3+ and Lu3+, providing fast and stable binding (up to 4 h from EOS for 68Ga-DOTA-NT and 48 h from EOS for 177Lu-DOTA-NT).
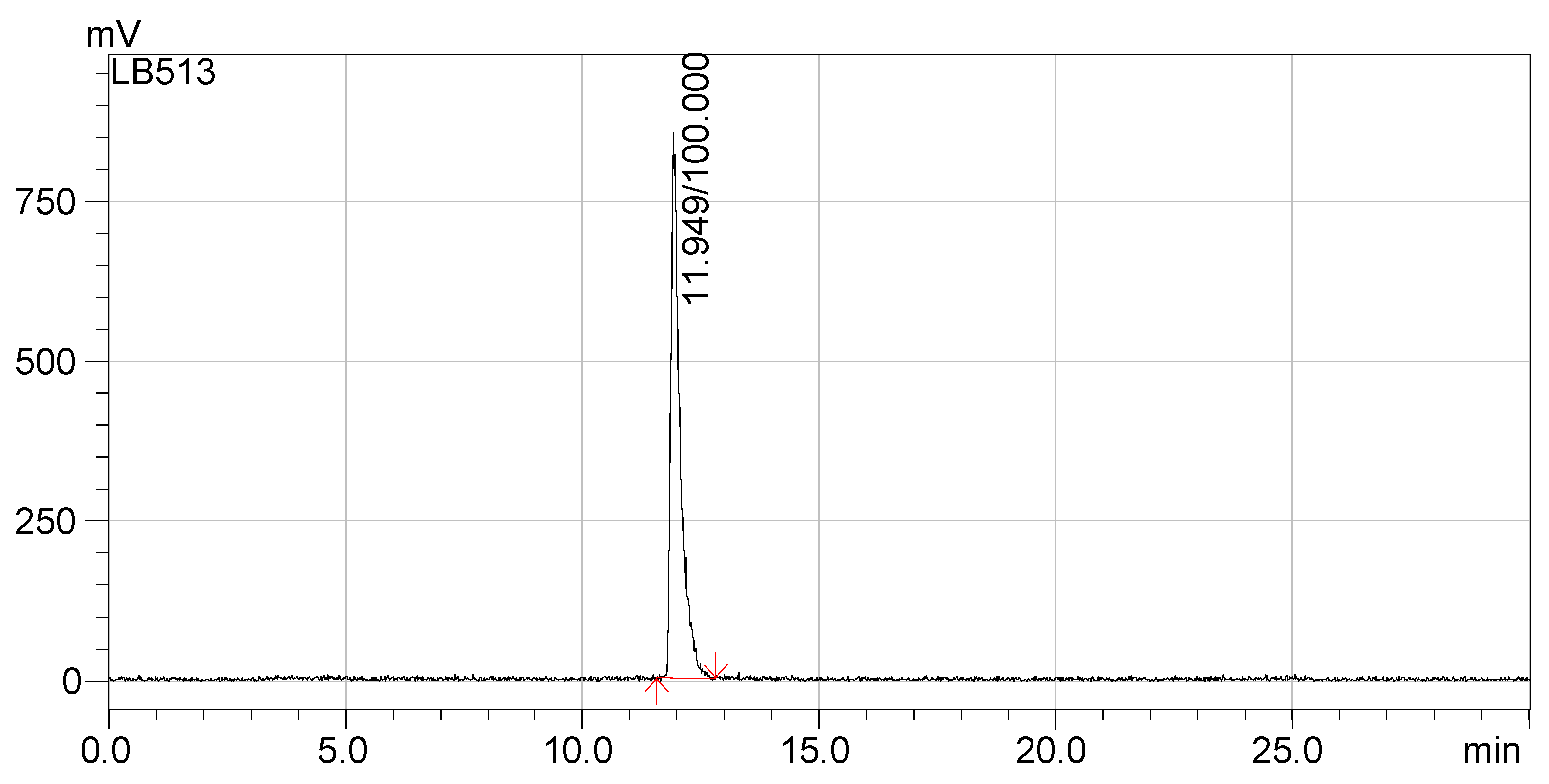
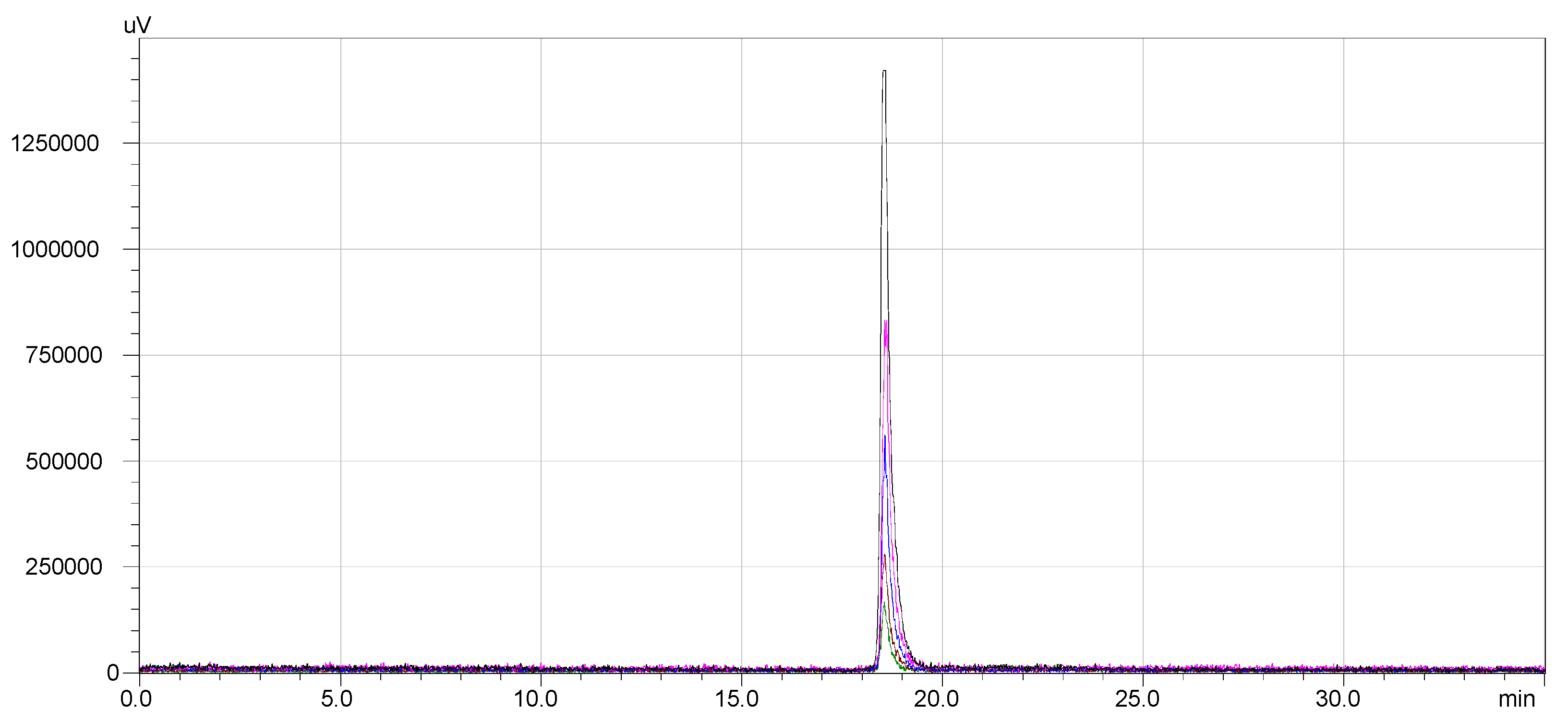
The radio-HPLC chromatograms presented in
Figure 5 and
Figure 6 indicate that the synthesized compounds meet the radiochemical purity condition, with RCP > 99% for
68Ga-DOTA-NT (retention time 18.5 min) and >99% for
177Lu-DOTA-NT (retention time 11.9 min).
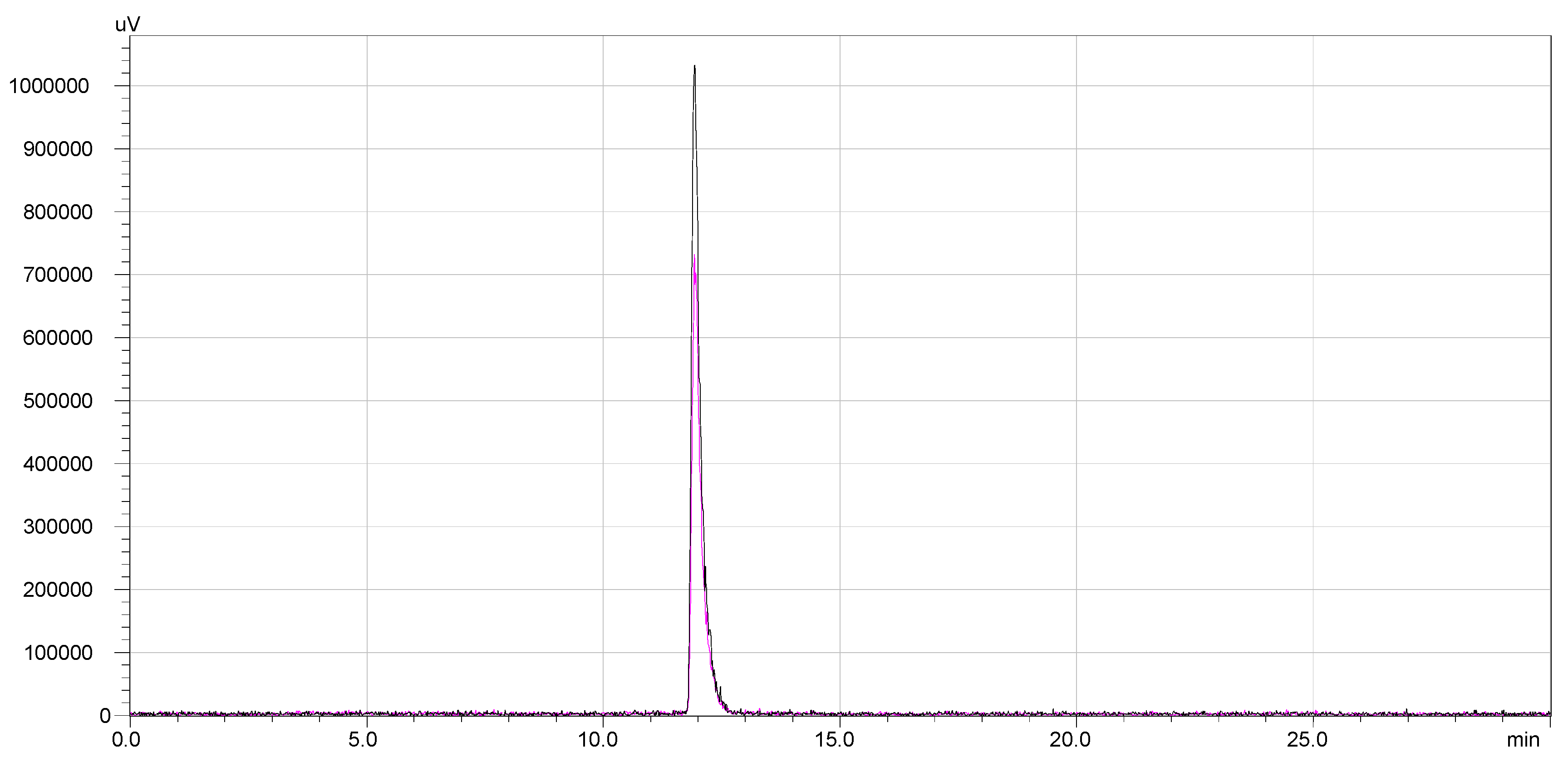
The stability of
68Ga-DOTA-NT was assessed by radio-HPLC for 4 h after the end of labelling and purification process (EOS). Following the RCP analysis at the end of synthesis, other 4 samples were taken from the same vial at equal time intervals (1 h). The overlapped chromatograms are presented in
Figure 7.The radiochemical stability of
177Lu-DOTA-NT was checked up to 48 h from the end of preparation. Both labelled compounds maintained their radiochemical purity throughout the investigation periods. No other peaks were identified in the subsequent chromatograms. Therefore, DOTA-NT did not undergo any structural modification and the radioisotopes were stably trapped by DOTA chelator. The overlapped chromatograms are presented in
Figure 8.
3.3. In Vitro Uptake and Retention Measurement of 68Ga-DOTA-NT
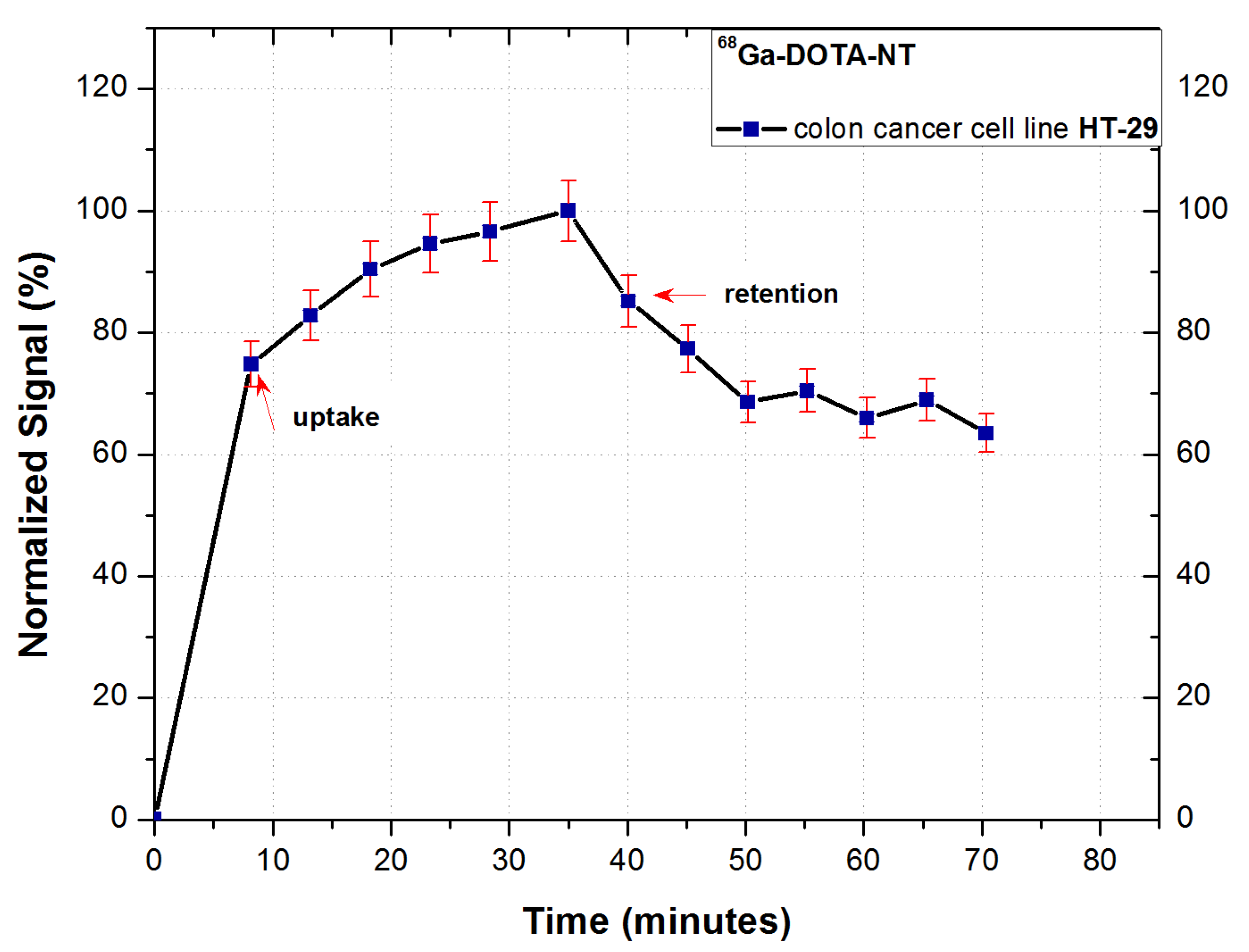
The binding kinetics of
68Ga-DOTA-NT to NTR-expressing HT-29 cancer cells during incubation is presented in
Figure 9.
Following the analysis of the cellular uptake of 68Ga-DOTA-NT, an exponential increase of the cell-associated activity can be observed, the trend of the uptake profile indicating a good dynamic of the tracer.
The highest cell retention was obtained for 0.6 nmol of NT, the maximum retention reaching 70% of the maximum measured activity during the uptake stage of analysis. This result confirms the expression of NT receptors on tumour cells membrane, as well as the ability of the peptide to form stable bonds with receptors. To verify the stability of binding cell, retention was studied for 35 min. A slight decrease in activity can be observed in the first 10 min of retention analysis (from 83% immediately after starting) followed by its stabilization around 70%.
3.4. In Vitro Specific Binding Assay
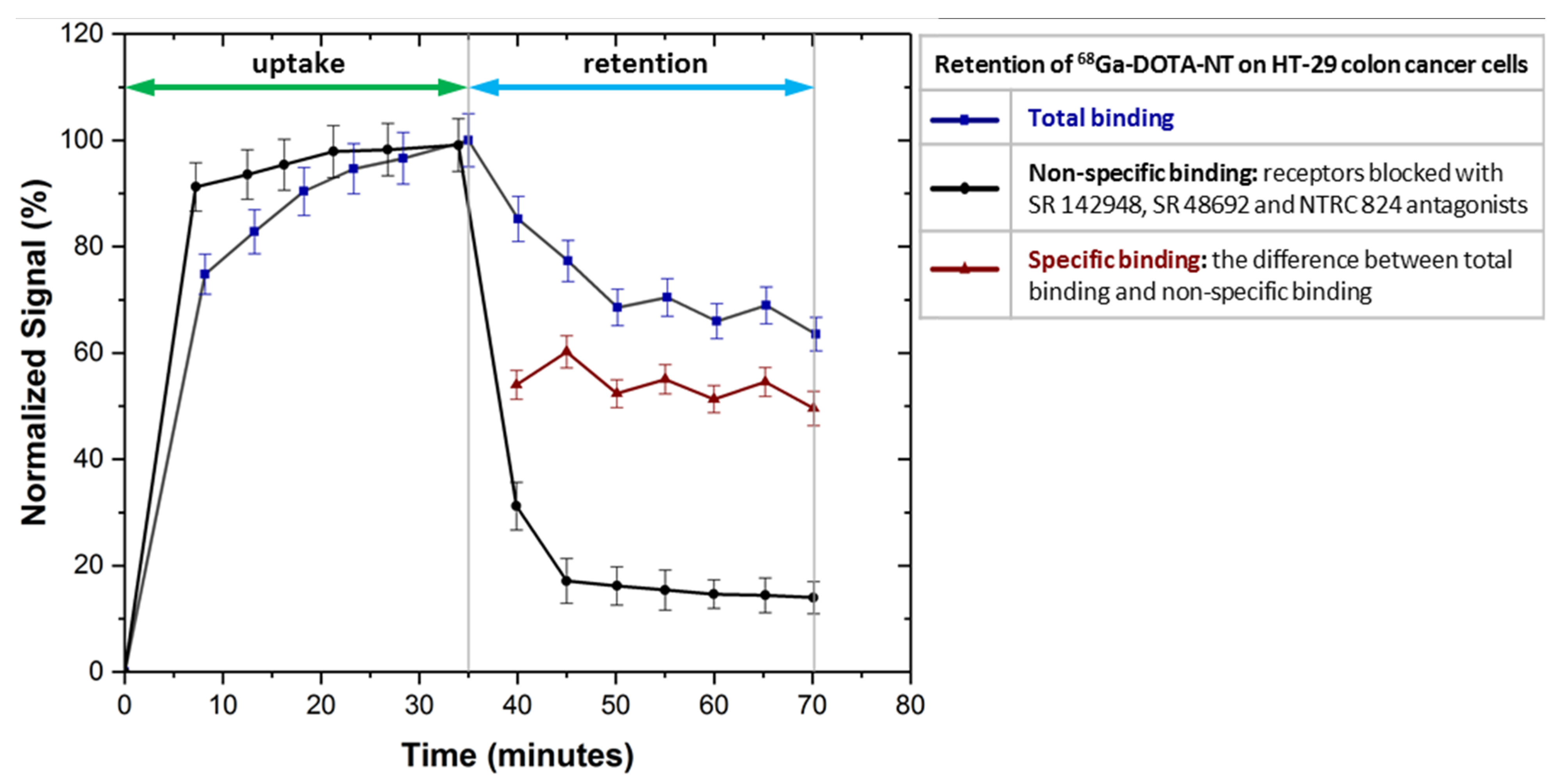
As can be seen in
Figure 10, NTRC 824, SR 48692 and SR 142948 antagonists used to block NTRS1, NTRS2 and NTRS3 receptors inhibit the
68Ga-DOTA-NT binding to colon HT-29 tumour cells as the uptake profile trend is almost linear.
After removal of the radioactive medium the amount of non-specific binding of
68Ga-DOTA-NT to cells is about 18% compared to the value of total binding stabilized at 70%, normalized for the maximum detected value. Because all receptors are blocked, the amount of peptide retained by the cells is presumably due to other non-specific binding mechanisms such as: channel-forming mechanism, inverted micelle, barrel-stave pores, endocytosis mechanism etc. [
33].
Subtracting the non-specific binding from the total binding we obtain a specific binding of about 52%. This result allows us to assume that applied in vivo 68Ga-DOTA-NT will be distributed mainly in tissues that express neurotensin receptors, the non-specific binding being too low to generate unwanted accumulations in other tissues.
3.5. In Vitro Binding Kinetics of 177Lu-DOTA-NT
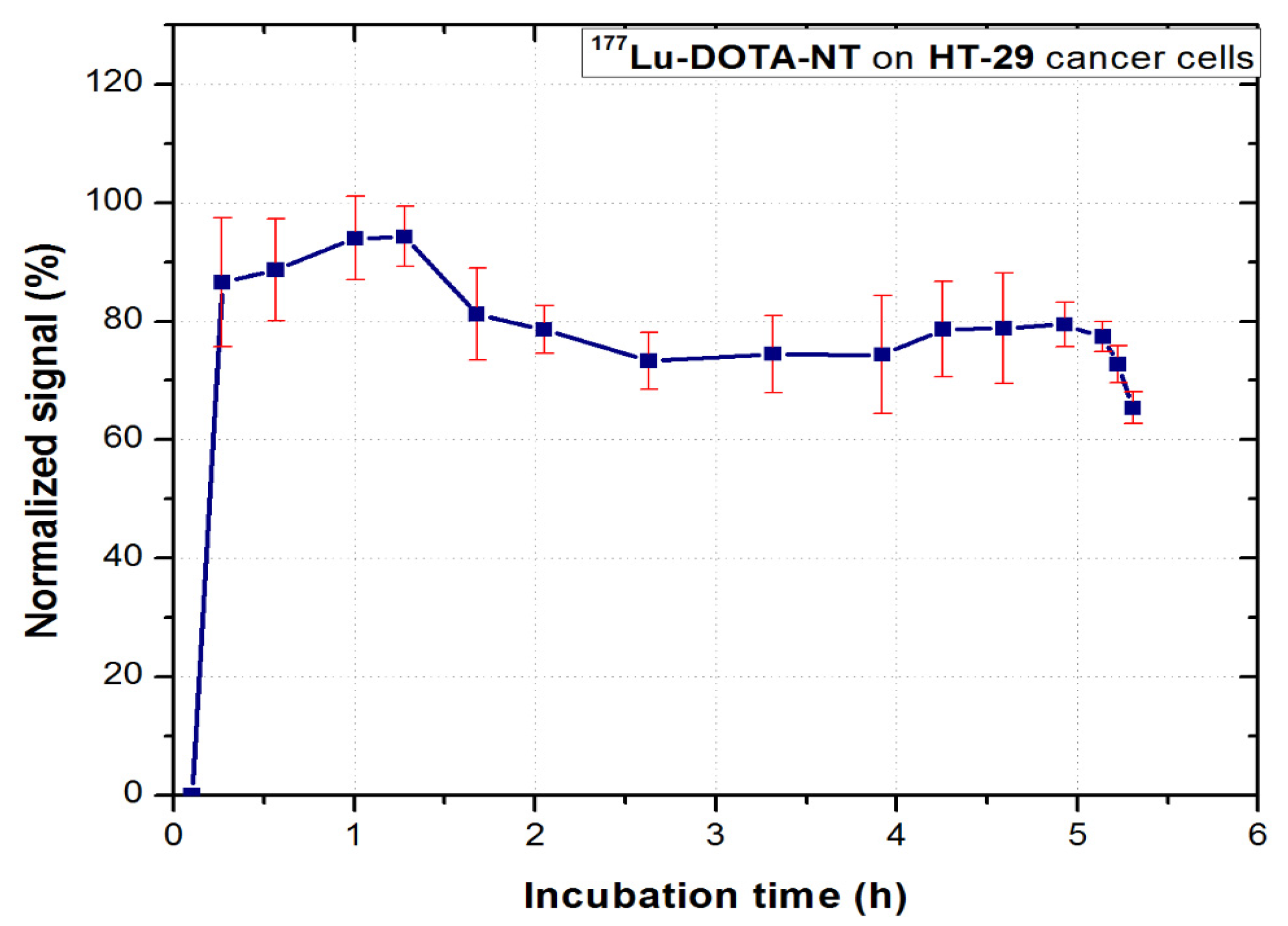
177Lu-DOTA-NT was evaluated in vitro for 6–8 h to observe the binding kinetic profile of the compound to HT-29 tumour cells. The real-time dynamic measurement is shown in
Figure 11.
In the first 90 min of incubation, the compound follows a trend of association with NT receptors. After removing the excess free compound in the medium, the cell dynamics was continuously measured. Stable retention on the cells (approx. 70% of the maximum association activity for 3.5 h) can be observed. This value is consistent with that obtained for 68Ga-DOTA-NT, which indicates that the 68Ga and 177Lu radioisotopes do not interfere in the process of peptide binding to its receptors.
After approx. 5 h from the incubation of the tracer, a sudden decrease of activity can be observed, which coincides with the second phase, critical for the therapy tracers, respectively the dissociation phase. At this stage, the tumour cells detach from the Petri dish due to the processes of apoptosis. They float in the culture medium and at subsequent rotations of the support they are no longer detected in the region they were initially attached. This method of analysis allows real-time visualization of these processes which confirms the possibility of using DOTA-NT as a theranostic agent. Since the stability of 177Lu-DOTA-NT has been assessed for up to 48 h post-synthesis with no degradation observed, the possibility of peptide degradation or release of radionuclide in the medium can be excluded.
3.6. Biodistribution
3.6.1. 68Ga-DOTA-NT
The results of the biodistribution studies of
68Ga-DOTA-NT are summarized in
Figure 12.
Significant uptake of
68Ga-DOTA-NT was observed in kidneys at 30 min p.i (34.26 ± 0.14%ID/g). It can be observed that 60 min p.i., the amount of tracer in the kidneys halves down to 14.82 ± 1.51% ID/g, most likely due to excretion through the renal system. No significant retention was observed in the liver (1.95 ± 0.28% ID/g at 30 min p.i. and 1.42 ± 0.073% ID/g at 60 min p.i.), suggesting that
68Ga-DOTA-NT was fairly water-soluble and does not associate into larger polypeptides chains, according to other studies [
34,
35].
The second most significant measured activity was detected in the blood, 14.87 ± 0.55% ID/g at 30 min p.i. respectively 8.17 ± 2.79% ID/g at 60 min p.i. This result, along with the observed decrease of the kidneys’ activity after 60 min, indicates a fast clearance of neurotensin.
The lungs and the tumour are the only organs in which a higher activity (accumulation) was registered at 60 min p.i. compared to the activity measured at 30 min p.i. Thus, in the lungs the activity increases from 3.71 ± 0.34% ID/g at 30 min p.i., up to 6.44 ± 3.36% ID/g at 60 min p.i.
Tumour vasculature is a critical micro-environmental factor associated with tumour response to the therapy and heterogeneous in both time- and location- dependent manner. Thus, for an accurate assessment, the tumour was sectioned into two parts: the centre of the tumour and the periphery. At 30 min p.i. the distribution of the tracer (activity) throughout the tumour mass is homogeneous, as the centre and the periphery of the tumour have close values: 4 ± 0.38% ID/g and 4.78 ± 1.91% ID/g respectively. At 60 min p.i. the activity at the tumour edge increases to 11.56 ± 2.14% ID/g compared to 4.5 ± 0.8% ID/g in the centre. This result highlights better vessel function and higher microvascular density (MVD) of the tumour periphery, than the tumour core.
3.6.2. 177Lu-DOTA-NT
The biodistribution of
177Lu-DOTA-NT was studied at 3 different times in order to observe the evolution of the tumour under the action of β
- radiation, but also how this compound can affect other organs by accumulation on them. The results are presented in
Figure 13 and
Figure 14.
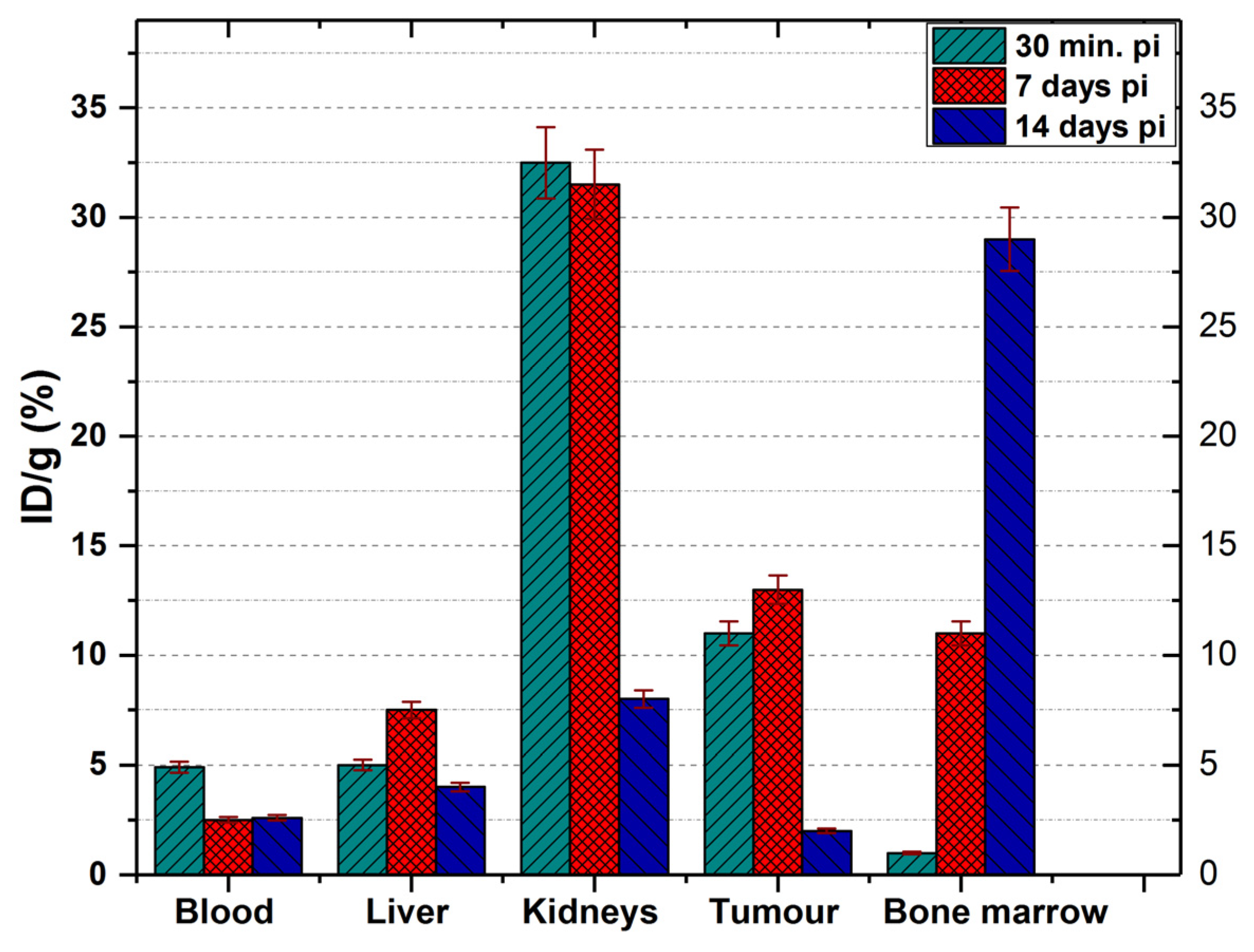
At 30 min p.i. the tracer was found mainly in the kidneys (32.5 ± 1.6% ID/g), tumour (11 ± 0.6% ID/g), blood (4.9 ± 0.2% ID/g) and liver (5.1 ± 0.21% ID/g). At 7 days p.i., the amount of tracer in the blood decreases significantly (reaching 2.5 ± 0.15% ID/g) but increased the accumulation in the liver (7.5 ± 0.4% ID/g). At this time, the amount of 177Lu-DOTA-NT in the kidneys showed a slight decrease to 31.5 ± 1.2% ID/g.
Recent studies showed radiologic signs of liver steatosis during the treatment with
177Lu-DOTA-TATE, but an improvement by the end of the treatment period [
36]. These findings, correlated with increased liver radioactivity at 7 days p.i., indicate a possibility that
177Lu-DOTA-NT may have a hepatotoxicity effect, but further evaluation is needed.
At 14 days p.i., the activity in the blood is kept at 2.6 ± 0.23% ID/g (similar to that reported at 7 days p.i.), and in the liver decreases significantly compared to that at 7 days p.i., reaching 4.3 ± 0.98% ID/g.
In the kidneys, the activity at 14 days decreases down to 8.1 ± 1.2% ID/g, a proportional decrease to that of the tumour (1.99 ± 0.35% ID/g).
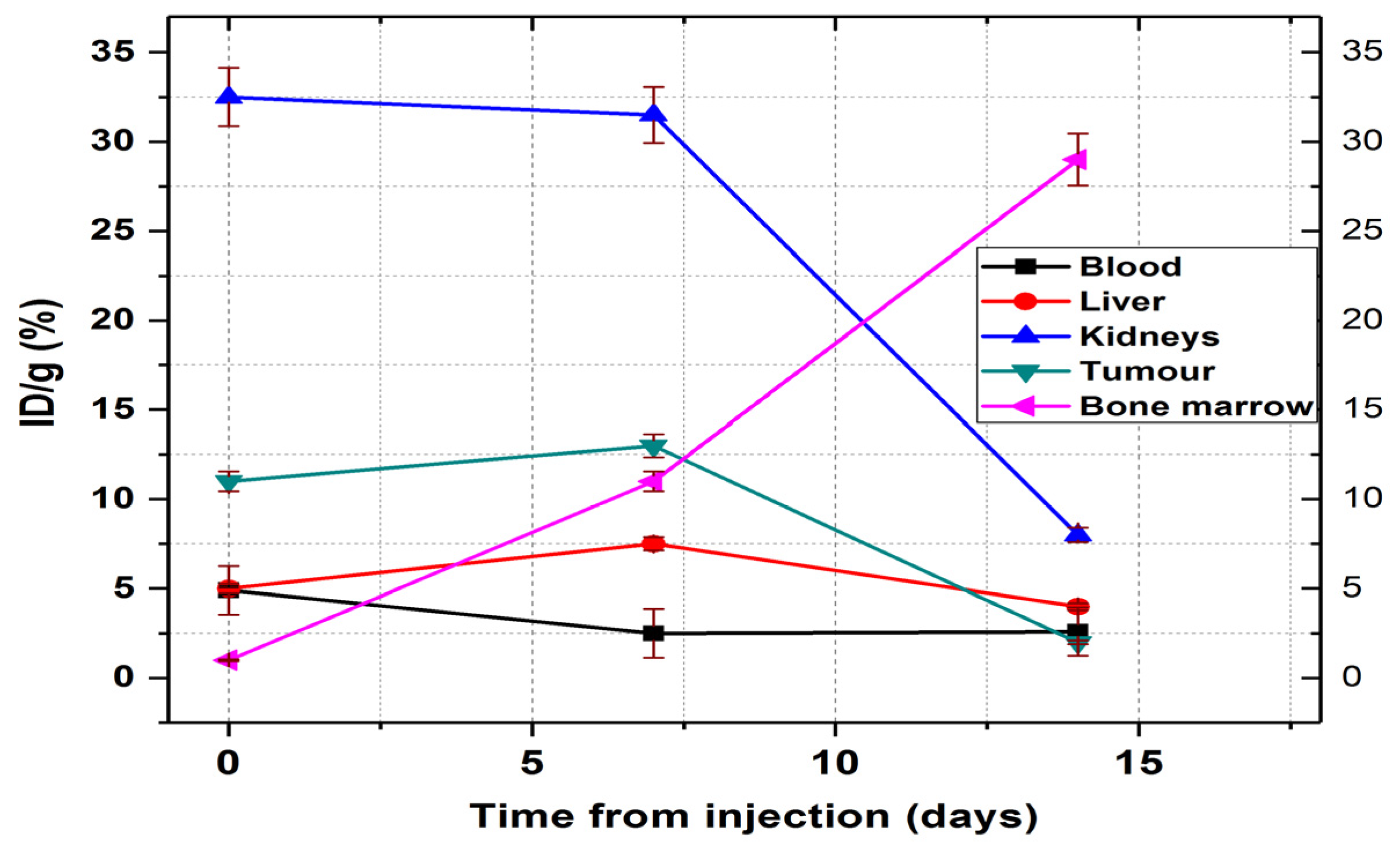
The only organ in which activity growth is maintained 14 days after injection is the bone marrow, where it reaches 29.45 ± 2.1% ID/g. Comparing the accumulated activity in the bone marrow with the accumulated radioactivity of the radiopharmaceutical in the blood, we could easily observe in
Figure 14 that the activity measured in the femur marrow increases much more than the decrease in the blood. This dynamic process indicates a very rapid absorption of the
177Lu–radiolabelled peptide in the bone marrow.
4. Discussion
Regulatory peptides, such as neurotensin, are suitable agents for specific delivery of radioisotopes to tumour cells, for both diagnostic and therapeutic applications [
37,
38]. The aim of this study was the investigation of neurotensin as a potential agent used in diagnosis and therapy of colon cancer when coupled with
68Ga and
177Lu, respectively.
In vitro evaluation of the binding kinetics (uptake and retention) on HT-29 colon cancer cells revealed a maximum of 70% retained activity from the maximum recorded during uptake. This result confirms the expression of NT receptors on tumour cells membrane, as well as the ability of the peptide to form stable bonds with receptors. Based on the results obtained for the specific binding assay, we were able to further determine that the non-specific binding represents 18% of the total binding, and the remaining 52% is attributed to the specific binding of neurotensin to its receptors (NTRS1, NTRS2 and NTRS3), In the case of 177Lu-DOTA-NT, 5 h after cell incubation, the cell retention profile follows a sudden decreasing trend of radioactivity, which might signify the colon cancer cells death and detachment from the Petri dish. These results demonstrate the need for additional, more precise investigation of 177Lu-DOTA-NT cytotoxic effects.
In vivo biodistribution studies in athymic nude mice bearing colon tumours confirmed the increased potential of 68Ga-DOTA-NT to target this type of cells, with 4.78 ± 1.91% ID/g at the tumour periphery.
Although previous studies showed that neurotensin has a low stability in blood, our results indicate that the use of 68Ga-radiolabelled peptide may be feasible for imaging of tumour from 30 min up to 1 h p.i. This time interval seems to sufficiently allow the distribution and accumulation of radiolabelled peptide into the body. A longer time p.i. is not feasible, as long as it leads to losing too much activity due to the short half-life of 68Ga.
A high uptake (6.44 ± 3.36% ID/g at 60 min p.i) was measured in the lungs. This result can be explained by the previous discoveries that pulmonary parenchyma is an important site of neurotensin metabolism [
39] and a high density of NTRS1 is expressed by lung pleura [
40]. No uptake of
68Ga-DOTA-NT was detected in the brain, most certainly due to low blood brain barrier (BBB) penetration of neurotensin, otherwise a non–BBB–penetrant neuropeptide.
In the case of
177Lu-DOTA-NT, at 30 min p.i, the highest amount of injected dose is found in the kidneys. This level is maintained at 7 days p.i., but at 14 days p.i. it decreases to 8.1 ± 1.2% ID/g. From previously reported data, treatment with
177Lu administered in the form of
177Lu-DOTA-TATE has been shown to cause radiation-induced renal damage [
41]. To reduce or avoid this side effect, different molecules (like Human Antioxidation Protein α
1-Microglobulin) have been developed that can effectively limit this effect. To assess whether the residual activity of
177Lu-DOTA-NT after 14 days p.i. causes nephrotoxicity, future studies are needed.
The challenge associated with the use of small peptides is the rapid in vivo degradation by peptidases that might require their activity inhibition [
42], before peptide–based drug administration. An interesting finding was that, even without inhibiting the proteolytic enzymes, the activity continued to accumulate in the tumours for up to 7 days and then decreased. The possible causes of decreasing may be: the peptide instability (although in this case the peptide was stable for more than a few hours), or the tumour cells apoptosis, which is a desirable effect of the
177Lu. However, further investigations and an additional study in conditions of inhibited degrading enzymes are needed.
Despite these promising results, a cause for concern is the constant accumulation in the bone marrow, increasing from 0.98 ± 0.32% ID/g at 30 min p.i. to 29.45 ± 2.1% ID/g at 7 days p.i. Future toxicity studies will be performed to evaluate long-term bone marrow toxicity, whether can cause myelosuppression, and also to optimize the dose at which the treatment with 177Lu-DOTA-NT is safe for administration.