Abstract
An increasing number of clinical studies worldwide are investigating the repurposing of antiviral, immune-modulatory, and anti-inflammatory agents to face the coronavirus disease-19 (COVID-19) pandemic. Nevertheless, few effective therapies exist to prevent or treat COVID-19, which demands increased drug discovery and repurposing efforts. In fact, many currently tested drugs show unknown efficacy and unpredictable drug interactions, such that interventions are needed to guarantee access to effective and safe medicines. Anti-inflammatory therapy has proven to be effective in preventing further injury in COVID-19 patients, but the benefit comes at a cost, as targeting inflammatory pathways can imply an increased risk of infection. Thus, optimization of the risk/benefit ratio is required in the anti-inflammatory strategy against COVID-19, which accounts for drug formulations and delivery towards regionalization and personalization of treatment approaches. In this perspective, we discuss how better knowledge of endogenous immunomodulatory pathways may optimize the clinical use of novel and repurposed drugs against COVID-19 in inpatient, outpatient, and home settings through innovative drug discovery, appropriate drug delivery systems and dedicated molecular pharmaceutics.
1. Introduction
Coronavirus disease-19 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a potentially fatal clinical syndrome that involves the lower airways and leads to interstitial pneumonia in humans with hyperinflammation and respiratory dysfunction [1]. The disease is characterized by three clinical stages: (i) an asymptomatic phase, accounting for 80 to 84% of cases, (ii) a non-severe symptomatic phase, potentially evolving to a hypoxemic pneumonia or (iii) to a severe, potentially lethal disease with hypoxia, lung infiltrates, and ultimate acute respiratory distress syndrome (ARDS) [1]. Drugs that inhibit key components of the coronavirus infection lifecycle have been repurposed in COVID-19 therapy [2], with the support of proper cheminformatic tools as well to expedite the identification of potential candidates and treatment modalities [3,4,5,6,7]. The mild to severe progression of COVID-19 depends on the extent and features of the individual immune response to the virus. Indeed, significant specific or non-specific organ damages can stem from the host’s own cellular and humoral immune responses to the infection. Therefore, COVID-19 pathogenesis is the result of a cascade of events starting from high levels of circulating proinflammatory cytokines that can evolve to a cytokine storm, responsible for non-specific inflammatory cell infiltration and contributing to downstream pulmonary and interstitial tissue damage [8]. Such conditions can quickly develop into ARDS with lethal consequences.
Thus, immunomodulatory agents capable of restraining or suppressing such progressions are logical candidates in COVID-19 therapy. Not surprisingly, interventions based on non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, intravenous immune globulins, immunosuppressants, chloroquine/hydroxychloroquine, IL-1 antagonists, IL-6R monoclonal antibodies, TNF inhibitors, and Janus kinase (JAK) inhibitors have been capable of relieving severe disease conditions in COVID-19 patients [9,10,11]. Nevertheless, a dark side in targeting inflammatory pathways exists, owing to a higher risk of opportunistic infections. In particular, the benefit of the use of glucocorticoids, IL-6 and Janus kinase inhibitors is likely outweighed by adverse effects, such as significantly increased risk of mortality and secondary infections [9]. However, an optimal risk/benefit ratio balance could be ideally granted by immunomodulatory agents capable of delivering anti-inflammatory input at the target organs, while preserving the immune system’s capacity to respond to pathogen invasions. This requires a better knowledge of immunoregulatory pathways underlying the homeostatic regulation of inflammation in the diseased organs to efficiently respond to infection while preventing damage.
Herein, we first describe current anti-inflammatory strategies, and then provide an example of how a better knowledge of inflammatory/anti-inflammatory endogenous pathways may optimize the clinical use of anti-inflammatory therapy in COVID-19 through innovative drug discovery, the selection of the appropriate administration route, drug delivery systems, and dedicated molecular pharmaceutics.
2. Current Anti-Inflammatory Approaches
The dramatic urgence of restraining the SARS-CoV-2 pandemic and preventing COVID-19 severity has granted several anti-inflammatory drugs an off-label use or a fast track to clinical trials (The Pharmaceutical Journal, February 2021; Online: doi: 10.1211/PJ.2021.20208126, last updated 24 February 2021, [12]). These include corticosteroids (i.e., dexamethasone), cytokines (i.e., interferons), drugs that interfere with cytokine activities (i.e., tocilizumab and sarilumab, which block IL-6 activity, canakinumab and anakinra, which block IL-1, or infliximab and adalimumab, which block TNFα) and signaling pathways (i.e., baricitinib and ruxolitinib, and JAK1/2 inhibitors).
Despite initial concerns [13], clinical evidence supports the efficacy of corticosteroids in the treatment of severe COVID-19 patients. Retrospective observational studies indicated that severe COVID-19 patients had a more favorable evolution if treated with corticosteroids [14], although other studies found either no effect [15] or a delayed healing [16] in hospitalized patients. A meta-analysis of seven randomized clinical trials, including 1703 hospitalized, critically ill patients, reported a lower 28-day all-cause mortality upon administration of systemic corticosteroids compared to usual care or placebo [17]. Currently, dexamethasone is strongly recommended for hospitalized patients requiring oxygen delivery through a high-flow device, non-invasive ventilation, invasive mechanical ventilation or extracorporeal membrane oxygenation (https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/, last updated 11 February 2021).
The use of NSAIDs also initially received concerns for COVID-19 treatment [18]. Several observational studies, however, have shown that NSAIDs are not associated with mortality or severity of disease [19,20,21,22,23,24] and their potential use in the treatment of COVID-19 has been proposed [25,26]. A recent retrospective analysis of data in Electronic Health Records (EHRs) to identify drugs with the potential to be repurposed to treat COVID-19 has identified, among others, ibuprofen as associated with a lower risk for COVID-19 outcomes [27]. This is in line with a previous study analyzing EHRs in six Eastern Massachusetts hospitals that identified a significant association between ibuprofen and diminished risk for hospitalization [28]. Currently ongoing clinical trials evaluating the efficacy and safety of ibuprofen will provide definite evidence for the potential clinical use of NSAIDs in COVID-19.
Alongside corticosteroids, monoclonal antibodies directed towards cytokine receptors look promising to decrease hyperinflammation. In fact, a recent preprint report on the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, showed that tocilizumab, a humanized antibody binding the IL-6 receptor, was effective in hospitalized patients with hypoxia and systemic inflammation and the benefits were present also in patients receiving systemic corticosteroids [29]. In addition, a retrospective analysis of data extracted from the RECOVERY study and seven previous randomized controlled trials confirmed a tocilizumab associated reduction of 28-day mortality [29]. Recently, the results from the REMAP-CAP trial have been published [30] demonstrating that not only tocilizumab, but also the other IL-6 receptor antagonist, sarilumab, improved outcomes, and this occurred also in patients treated with glucocorticoids.
The RECOVERY trial is also expected to provide results on colchicine, an alkaloid with anti-inflammatory effects, that may bear potential therapeutic efficacy in COVID-19 [31].
Another anti-inflammatory strategy includes inhibitors of signaling pathways mediating cytokine activity, such as the JAK/STAT pathway [32]. The results of the ACTT-2 trial in hospitalized adults with COVID-19 indicate that baricitinib plus remdesivir was superior to remdesivir alone in the primary outcome, i.e., the time to recovery, and the key secondary outcome, i.e., the clinical status at day 15 [33], thus showing promise for the use of JAK inhibitors, including not only baricitinib but also ruxolinitib and tofacitinib, in the treatment of COVID-19. However, evidence for the potential combination with corticosteroids remains to be provided.
Overall, targeting inflammation is a worthwhile strategy to combat COVID-19 and prevent disease severity. Nevertheless, even though promising results are emerging from clinical trials, systemic administration of anti-inflammatory drugs exposes the patients to additional risks and is associated with low compliance, especially in the case of biotechnological drugs. Therefore, specific approaches affording a localized action should be preferred to improve the efficacy/safety profile, as we will illustrate in the following sections for two endogenous pathways of immunomodulation.
3. The Inflammasome Pathway
The interferon and the NF-κB pathways have been recognized as being among the primary activated signaling cascades in SARS-CoV-2 infection [34], producing high IL-1β, TNF-α, and IL-6 serum and tissue levels [35,36]. Albeit being potentially protective by promoting CD8+ T cells and phagocytes responses against infected cells and the production of virus-specific antibodies, when highly expressed, these cytokines may contribute to COVID-19 pathogenesis for their role in the induction of the cytokine storm [37].
Notably, only the IL-1 pathway seems to affect the phases preceding the respiratory function, nadir [38], such that early blockade of the IL-1 receptor (IL-1R) was effective in treating acute hyperinflammatory respiratory failure in COVID-19 patients [39,40,41,42,43,44]. By causing the release of IL-1α and β, the activated IL-1 signaling pathway is considered a fundamental bridge between inflammasome disreactivity, mostly driven by dysfunctional NLRP3 activity and lung inflammation [45], including in acute lung injury after respiratory viral infections [46].
Upon different stimuli, inflammasomes lead to the synthesis of IL-1β by recruiting caspase-1 that cleaves the pro-IL-1β precursor to give the active form.
Being that inflammasome activity is dysregulated in COVID-19 [47,48,49], following the infection, alveolar macrophages secrete TNF-α and IL-1β, giving rise to cell death, damage, and NLRP3 activation that trigger the acute proinflammatory cascade. Furthermore, angiotensin-converting enzyme 2 signaling has also been implicated in NLRP3 activation [48].
In a subsequent phase, such initially localized inflammatory events spread to the vasculature, producing leakage, edema, and pneumonia, typical of COVID-19 [48].
The coronavirus tolerance observed in bats has been associated to a dampened transcriptional priming of NLRP3 [50], which confirms that targeting the NLRP3/IL-1β pathway is a successful strategy in COVID-19. Several clinical studies seem to confirm this, by showing the efficacy of IL-1R receptor antagonists (IL-1Ra), such as anakinra, against COVID-19, even in patients with co-morbidities and combined with antiviral drugs [39,40,41,42,43,44].
Anakinra is a recombinant non-glycosylated form of IL-1Ra showing higher affinity for IL-1R1 than that for IL-1 itself [51]. Anakinra (Kineret®) is a drug marketed in 2001 for the treatment of rheumatoid arthritis by subcutaneous administration of 100 mg daily and, more recently, of cryopyrin-associated periodic syndromes and systemic-onset juvenile idiopathic arthritis, and is widely used off-label [52]. Its therapeutic potential derives from the ability to prevent IL-1α and IL-1β driven inflammation. Anakinra clinical use is supported by a recognized safety and the evidence in murine lung and human bronchial epithelial cells of a potent inhibition of pathogenic NLRP3 activation and concurrent IL-1β, TNF-α and IL-6 suppression [53].
Together, these studies suggest that modulating NLRP3 or IL-1R1 related inflammatory responses could be a successful therapy in COVID-19 (Figure 1). It is worth mentioning that canakinumab, an antibody targeting IL-1β, has also been reported to improve outcomes [54,55,56]. However, the phase III CAN-COVID trial in hospitalized patients did not meet the primary endpoint, i.e., greater chance of survival without the need for invasive mechanical ventilation, and the key secondary endpoint of reduced COVID-19 mortality (https://www.novartis.com/news/media-releases/novartis-provides-update-can-covid-trial-hospitalized-patients-covid-19-pneumonia-and-cytokine-release-syndrome-crs; accessed 26 March 2021). Our recent observations that anakinra is capable of inhibiting NLRP3 and inducing autophagy by a mechanism independent of the known activity on IL-1R1 that involves a xenobiotic sensing pathway coupling mitochondrial redox balance to autophagy (manuscript submitted) suggest that the activity of anakinra is more complex than previously thought and may help to reconcile the results of the clinical trials.
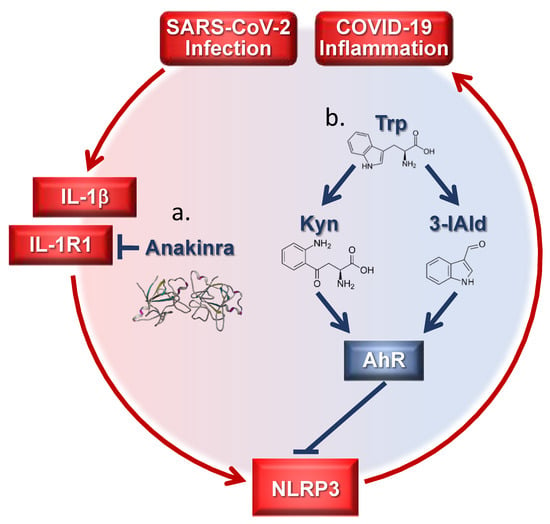
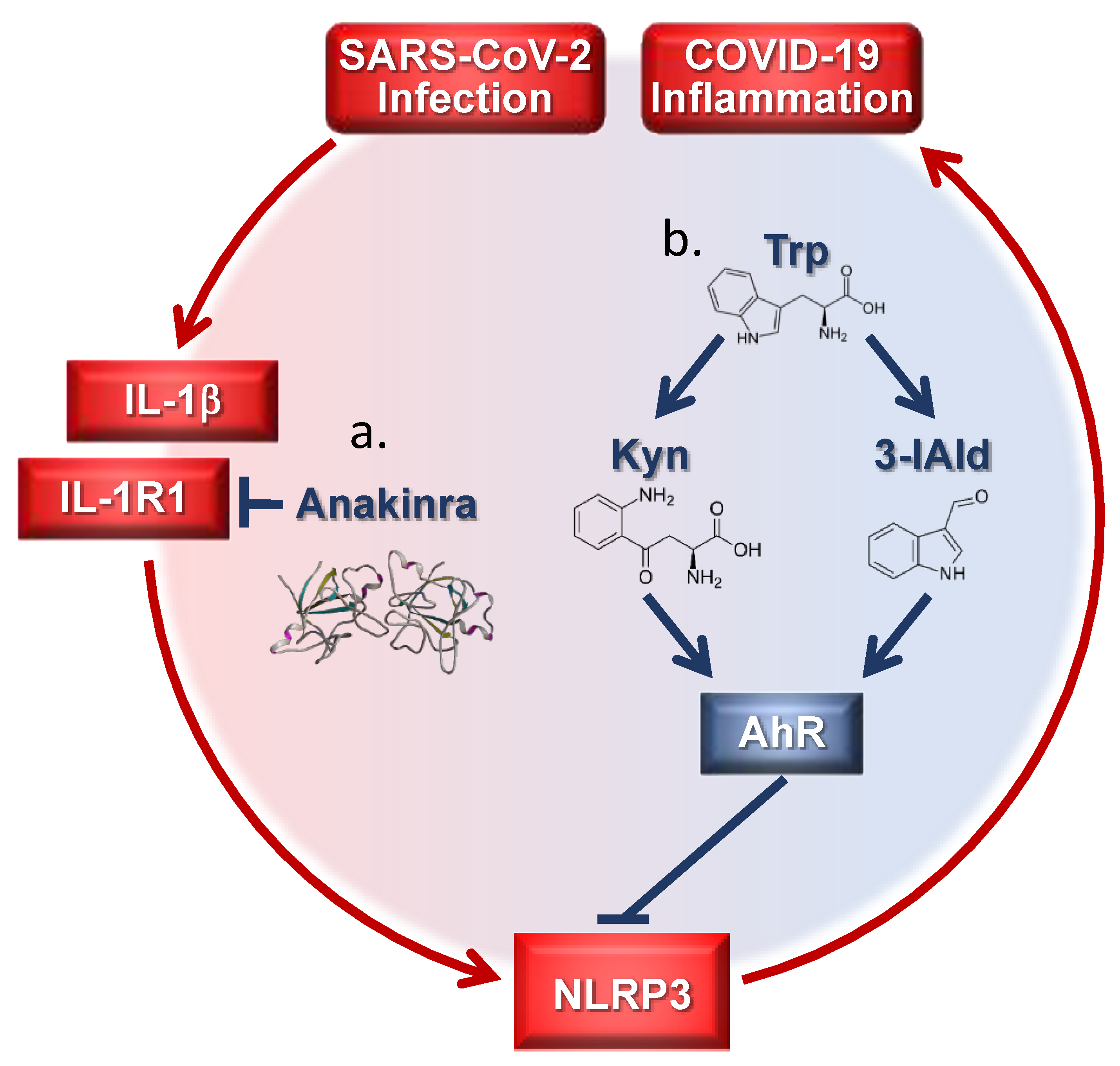
Figure 1.
Targeting inflammatory pathways in coronavirus disease-19 (COVID-19) by tryptophan (trp) metabolites and Anakinra. (a) Anakinra inhibits NLRP3 by blocking IL-1R1 activation; (b) endogenous trp metabolites target AhR downregulating the NLRP3 pathway. Kyn = kynurenine; 3-IAld = Indole-3-carboxaldehyde.
4. The Xenobiotic Pathway
Interferons (IFNs), either alone or combined with antiviral agents, are currently being explored for the treatment of COVID-19, owing to their role in innate immunity. Type I IFNs (alpha and beta) are secreted upon viral infection and are known to have antiviral activity against coronaviruses, which explains the considerable number of current clinical studies listed on ClinicalTrials.gov (US National Library of Medicine, 2020). IFNs are known to shift tryptophan (trp) catabolism away from serotonin toward kynurenines [57] via the enzyme indoleamine 2, 3-dioxygenase (IDO)1. IDO1 together with tryptophan-2, 3-dioxygenase have been related to inflammatory diseases, cancer, diabetes, and mental disorders in light of their regulatory role in kynurenine production in the trp metabolic pathway [58,59,60]. IDO1 has an important role in preserving immune tolerance and homeostasis in the lungs [61,62]. Therefore, it is not surprising that the IDO1/kynurenine pathway is upregulated in COVID-19 due to the rise in pro-inflammatory cytokines [63]. This implies that more than IDO1 mimetics, alternative pathways of trp utilization could be exploited for tolerance induction in the lung.
The Aryl Hydrocarbon Receptor (AhR) is a ubiquitous ligand-activated transcription factor mainly expressed in barrier organs, such as the lungs, skin, liver, and gut [64]. Particularly in these organs, AhR exerts a fundamental regulation of the immune response and the maintenance of mucosal homeostasis [65]. Albeit still debated, the increasing body of literature connects AhR signaling to the preservation of lung health [66]. Such an AhR role may help contrasting lung pathogens by sensing virulence factors and promoting the subsequent recruitment of inflammatory cells [67]. Activation of AhR by CoV may change disease phenotypic features based on time after infection but also on diet and environmental factors [68].
Evidence supporting the role of AhR in lung physiology, including negative NLRP3 regulation [69], could provide new COVID-19 therapeutic opportunities based on the AhR and/or other xenobiotic receptor biological functions (Figure 1). The AhR senses a wide variety of agonists, typically hydrophobic in nature, of either exogenous or endogenous origin [64], including metabolites produced by microbes [70]. However, it must be kept in mind that AhR biology relates to ligand nature, environment, and disease [64].
As a proof-of-concept that properly targeting AhR with an endogenous metabolite may result in beneficial effects, our preliminary observations indicate that local delivery of a microbial metabolite, administered either orally via microparticle encapsulation or via lung in a spray-dried formulation, could alleviate inflammation in mice with respiratory infection and inflammation (Puccetti et al. manuscript in preparation). Thus, the proper targeting of AhR in the lung alleviates the inflammatory response during infection.
5. Concluding Remarks
The requirements for drug formulations have increased significantly in recent decades, boosted by the current industry trends towards regionalization and personalization of treatment approaches. This trend is what demanded for the optimal delivery of anti-inflammatory agents in COVID-19, given the need for fine balancing benefits and risks. New formulations and techniques for the extended and precise dosing of medicines are now in place, such as spray-drying to produce enteric microparticles for local intestine release and inhalable dry powders for lung delivery [71]. Inhaled products can grant localization of therapeutic action, enabling dose reduction and lowering the risk of off-target effects [72], and are credited as an optimal delivery form for proteins and peptides [73]. Inhaled peptides have been already marketed or are under clinical development [71]. Likewise, despite the gut adverse environment, novel emerging formulations show promises for protein oral delivery [74]. Anakinra comes in prefilled syringes for subcutaneous injection at an individual dose of 100 mg/0.67 mL/day. Although highly bioavailable (95%) [75], reaching maximum plasma levels in 3–7 h with a terminal half-life of 6–8 h, the current once-a-day subcutaneous injection of anakinra is relatively low compliant and may show lower efficacy when delivered systemically in lung infections, such as in the case of COVID-19. Despite the safety profile and low toxicity, even in patients with asthma history, injection site reactions in addition to self-medication issues can result in patient discomfort that discourages this regimen. Thus, the high compliance of the lung and oral routes and the existence of enabling technologies for fast translation to the clinic make the oral and pulmonary delivery of anakinra a very attractive approach in COVID-19 therapy. Indeed, inhalation could be a pivotal approach against COVID-19, since the lungs represent the main infection site, and thus a therapeutic target, as even confirmed by in silico predictive tools [76,77]. The well-known and above-mentioned advantages of inhaled drugs, particularly in the form of dry powders, could be of great benefit for drugs like anakinra, justifying the likely higher cost of production compared to the injectable form. In this regard, considering the cost of the protein drug, dose reduction compared to Kineret® may partially counterbalance the above-mentioned higher expenses of the pulmonary products. Moreover, embedding the drug into a solid form extends the shelf life of the product, especially as far as biotechnological drugs are concerned, increasing its market value.
Similar to what was observed with the AhR-ligand formulations [78] (Puccetti et al., manuscript in preparation), our own ongoing project is in place with the expectation to optimize both the therapeutic efficacy of anakinra and the patient’s compliance. Thus, molecular pharmaceutics of repurposed and novel drugs may generate essential information useful for the development of anti-inflammatory-based drug discovery and delivery strategies in COVID-19.
In this regard, insightful investigation of immunological regulatory pathways has led to the identification of novel selective biologicals and small molecule drugs that have enabled tremendous advances in the treatment of chronic inflammatory diseases and tumor therapy [79].
The challenge ahead is to optimize the clinical use of biologicals to target inflammatory pathways in COVID-19 through novel drug delivery platforms and dedicated molecular pharmaceutics (Figure 2).
Figure 2.
Molecular pharmaceutics of tryptophan metabolites and anakinra in the treatment of COVID-19. The pulmonary route is preferred for enhanced local effect and dose reduction. The oral route is also considered as an alternative, highly compliant and cost-effective approach.
The recent development of inhaled forms of remdesivir for protecting and treating the respiratory mode of SARS-CoV-2 infection [80] and the relevant number of new or repurposed inhaled drugs under clinical development (Table 1) emphasize how molecular pharmaceutics, by allowing more widely available early-stage intervention methods to non-hospitalized patients, could significantly lessen symptoms before they become potentially life-threatening, lower costs, and reduce transmission.

Table 1.
Ongoing clinical trials of new and repurposed inhaled drugs in COVID-19 (ClinicalTrials.gov, accessed Oct. 2020, not intended to be exhaustive).
Author Contributions
Conceptualization, M.P. and S.G.; writing—original draft preparation, M.P. and S.G.; writing—review and editing, M.P., C.C., M.R. and S.G.; funding acquisition, S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Cystic Fibrosis Research Foundation, Research Project number FFC#17/2020 to S.G., M.P. gratefully acknowledge a fellowship from the Italian Cystic Fibrosis Research Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Sohag, A.A.M.; Hannan, M.A.; Rahman, S.; Hossain, M.; Hasan, M.; Khan, M.K.; Khatun, A.; Dash, R.; Uddin, M.J. Revisiting potential druggable targets against SARS-CoV-2 and repurposing therapeutics under preclinical study and clinical trials: A comprehensive review. Drug Dev. Res. 2020. [Google Scholar] [CrossRef]
- Ojha, P.K.; Kar, S.; Krishna, J.G.; Roy, K.; Leszczynski, J. Therapeutics for COVID-19: From computation to practices-where we are, where we are heading to. Mol. Divers 2021, 25, 625–659. [Google Scholar] [CrossRef] [PubMed]
- Tejera, E.; Munteanu, C.R.; Lopez-Cortes, A.; Cabrera-Andrade, A.; Perez-Castillo, Y. Drugs Repurposing Using QSAR, Docking and Molecular Dynamics for Possible Inhibitors of the SARS-CoV-2 M(pro) Protease. Molecules 2020, 25, 5172. [Google Scholar] [CrossRef]
- Egieyeh, S.; Egieyeh, E.; Malan, S.; Christofells, A.; Fielding, B. Computational drug repurposing strategy predicted peptide-based drugs that can potentially inhibit the interaction of SARS-CoV-2 spike protein with its target (humanACE2). PLoS ONE 2021, 16, e0245258. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Fast Identification of Possible Drug Treatment of Coronavirus Disease-19 (COVID-19) through Computational Drug Repurposing Study. J. Chem. Inf. Model 2020, 60, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.U.; Parida, S.; Lingaraju, M.C.; Kesavan, M.; Kumar, D.; Singh, R.K. Drug repurposing approach to fight COVID-19. Pharmacol. Rep. 2020, 72, 1479–1508. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020, 214, 108–393. [Google Scholar] [CrossRef]
- Felsenstein, S.; Herbert, J.A.; McNamara, P.S.; Hedrich, C.M. COVID-19: Immunology and treatment options. Clin. Immunol. 2020, 215, 108–448. [Google Scholar] [CrossRef]
- Ucciferri, C.; Vecchiet, J.; Falasca, K. Role of monoclonal antibody drugs in the treatment of COVID-19. World J. Clin. Cases 2020, 8, 4280–4285. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Tomino, C.; Puccetti, P.; Garaci, E. Off-label therapy targeting pathogenic inflammation in COVID-19. Cell Death Discov. 2020, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Rice, T.W. Corticosteroids in COVID-19 ARDS: Evidence and Hope During the Pandemic. JAMA 2020, 324, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Pascual Pareja, J.F.; Garcia-Caballero, R.; Soler Rangel, L.; Vazquez-Ronda, M.A.; Roa Franco, S.; Navarro Jimenez, G.; Moreno Palanco, M.A.; Gonzalez-Ruano, P.; Lopez-Menchaca, R.; Ruiz-Seco, P.; et al. Effectiveness of glucocorticoids in patients hospitalized for severe SARS-CoV-2 pneumonia. Med. Clin. 2021, 156, 221–228. [Google Scholar] [CrossRef]
- Andersen, K.M.; Mehta, H.B.; Palamuttam, N.; Ford, D.; Garibaldi, B.T.; Auwaerter, P.G.; Segal, J.; Alexander, G.C. Association Between Chronic Use of Immunosuppresive Drugs and Clinical Outcomes from Coronavirus Disease 2019 (COVID-19) Hospitalization: A Retrospective Cohort Study in a Large US Health System. Clin. Infect. Dis. 2021, ciaa1488, Online ahead of print. [Google Scholar] [CrossRef]
- D’Ardes, D.; Pontolillo, M.; Esposito, L.; Masciarelli, M.; Boccatonda, A.; Rossi, I.; Bucci, M.; Guagnano, M.T.; Ucciferri, C.; Santilli, F.; et al. Duration of COVID-19: Data from an Italian Cohort and Potential Role for Steroids. Microorganisms 2020, 8, 1327. [Google Scholar] [CrossRef]
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.H.; You, S.C.; Kim, J.; Yang, K. Non-steroidal anti-inflammatory agent use may not be associated with mortality of coronavirus disease 19. Sci. Rep. 2021, 11, 50–87. [Google Scholar] [CrossRef]
- Wong, A.Y.; MacKenna, B.; Morton, C.E.; Schultze, A.; Walker, A.J.; Bhaskaran, K.; Brown, J.P.; Rentsch, C.T.; Williamson, E.; Drysdale, H.; et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: An OpenSAFELY cohort analysis based on two cohorts. Ann. Rheum. Dis. 2021, 0, 1–9, Epub ahead of print: 21 January 2021. [Google Scholar] [CrossRef]
- Abu Esba, L.C.; Alqahtani, R.A.; Thomas, A.; Shamas, N.; Alswaidan, L.; Mardawi, G. Ibuprofen and NSAID Use in COVID-19 Infected Patients Is Not Associated with Worse Outcomes: A Prospective Cohort Study. Infect. Dis. Ther. 2021, 10, 253–268. [Google Scholar] [CrossRef]
- Kragholm, K.; Gerds, T.A.; Fosbol, E.; Andersen, M.P.; Phelps, M.; Butt, J.H.; Ostergaard, L.; Bang, C.N.; Pallisgaard, J.; Gislason, G.; et al. Association Between Prescribed Ibuprofen and Severe COVID-19 Infection: A Nationwide Register-Based Cohort Study. Clin. Transl. Sci. 2020, 13, 1103–1107. [Google Scholar] [CrossRef]
- Chandan, J.S.; Zemedikun, D.T.; Thayakaran, R.; Byne, N.; Dhalla, S.; Acosta-Mena, D.; Gokhale, K.M.; Thomas, T.; Sainsbury, C.; Subramanian, A.; et al. Non-steroidal anti-inflammatory drugs and susceptibility to COVID-19. Arthritis Rheumatol. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Kozer, E.; Shapira, Y.; Bar-Haim, A.; Youngster, I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1259.e5–1259.e7. [Google Scholar] [CrossRef] [PubMed]
- Kelleni, M.T. Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes. Biomed. Pharmacother. 2021, 133, 110–982. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Gunupuru, R. Targeting cyclooxygenase enzyme for the adjuvant COVID-19 therapy. Drug Dev. Res. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bejan, C.A.; Cahill, K.N.; Staso, P.J.; Choi, L.; Peterson, J.F.; Phillips, E.J. DrugWAS: Leveraging drug-wide association studies to facilitate drug repurposing for COVID-19. Med. Rxiv. 2021. 02.04.21251169. [Google Scholar] [CrossRef]
- Castro, V.M.; Ross, R.A.; McBride, S.M.; Perlis, R.H. Identifying common pharmacotherapies associated with reduced COVID-19 morbidity using electronic health records. Med. Rxiv. 2020. 04.11.20061994. [Google Scholar] [CrossRef]
- Horby, P.W.; Pessoa-Amorim, G.; Peto, L.; Brightling, C.E.; Sarkar, R.; Thomas, K.; Jeebun, V.; Ashish, A.; Tully, R.; Chadwick, D.; et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): Preliminary results of a randomised, controlled, open-label, platform trial. Med. Rxiv. 2021. 02.11.21249258. [Google Scholar] [CrossRef]
- Investigators, R.-C.; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Chiu, L.; Chow, R.; Chiu, N.; Lo, C.-H.; Aggarwal, R.; Lee, J.; Choi, Y.-G.; Lam, H.; Prsic, E.H.; Shin, H.J. Colchicine use in patients with COVID-19: A systematic review and meta-analysis. Med. Rxiv. 2021. 02.02.21250960. [Google Scholar] [CrossRef]
- Luo, W.; Li, Y.X.; Jiang, L.J.; Chen, Q.; Wang, T.; Ye, D.W. Targeting JAK-STAT Signaling to Control Cytokine Release Syndrome in COVID-19. Trends Pharmacol. Sci. 2020, 41, 531–543. [Google Scholar] [CrossRef]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Jamilloux, Y.; Henry, T.; Belot, A.; Viel, S.; Fauter, M.; El Jammal, T.; Walzer, T.; Francois, B.; Seve, P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020, 19, 102–567. [Google Scholar] [CrossRef]
- Cavalli, G.; de Luca, G.; Campochiaro, C.; Della-Torre, E.; Ripa, M.; Canetti, D.; Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Boffini, N.; et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020, 2, 325–331. [Google Scholar] [CrossRef]
- Cauchois, R.; Koubi, M.; Delarbre, D.; Manet, C.; Carvelli, J.; Blasco, V.B.; Jean, R.; Fouche, L.; Bornet, C.; Pauly, V.; et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 18951–18953. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Cron, R.Q.; Hartwell, J.; Manson, J.J.; Tattersall, R. Intravenous anakinra for cytokine storm syndromes—Authors’ reply. Lancet Rheumatol. 2020, 2, 522–523. [Google Scholar] [CrossRef]
- Iglesias-Julian, E.; Lopez-Veloso, M.; de-la-Torre-Ferrera, N.; Barraza-Vengoechea, J.C.; Delgado-Lopez, P.D.; Colazo-Burlato, M.; Ubeira-Iglesias, M.; Montero-Baladia, M.; Lorenzo-Martin, A.; Minguito-de-la-Iglesia, J.; et al. High dose subcutaneous Anakinra to treat acute respiratory distress syndrome secondary to cytokine storm syndrome among severely ill COVID-19 patients. J. Autoimmun. 2020, 115, 102–537. [Google Scholar] [CrossRef]
- Huet, T.; Beaussier, H.; Voisin, O.; Jouveshomme, S.; Dauriat, G.; Lazareth, I.; Sacco, E.; Naccache, J.M.; Bezie, Y.; Laplanche, S.; et al. Anakinra for severe forms of COVID-19: A cohort study. Lancet Rheumatol. 2020, 2, 393–400. [Google Scholar] [CrossRef]
- Dimopoulos, G.; de Mast, Q.; Markou, N.; Theodorakopoulou, M.; Komnos, A.; Mouktaroudi, M.; Netea, M.G.; Spyridopoulos, T.; Verheggen, R.J.; Hoogerwerf, J.; et al. Favorable Anakinra Responses in Severe Covid-19 Patients with Secondary Hemophagocytic Lymphohistiocytosis. Cell Host Microbe 2020, 28, 117–123. [Google Scholar] [CrossRef]
- Scambler, T.; Holbrook, J.; Savic, S.; McDermott, M.F.; Peckham, D. Autoinflammatory disease in the lung. Immunology 2018, 154, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Kurrer, M.; Bachmann, M.F.; Kopf, M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 2005, 79, 6441–6448. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, D.F.; Te Velde, A.A. Severe COVID-19: NLRP3 Inflammasome Dysregulated. Front Immunol. 2020, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- Yap, J.K.Y.; Moriyama, M.; Iwasaki, A. Inflammasomes and Pyroptosis as Therapeutic Targets for COVID-19. J. Immunol. 2020, 205, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Anderson, D.E.; Zhang, Q.; Tan, C.W.; Lim, B.L.; Luko, K.; Wen, M.; Chia, W.N.; Mani, S.; Wang, L.C.; et al. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat. Microbiol. 2019, 4, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Dinarello, C.A. Corrigendum: Anakinra Therapy for Non-cancer Inflammatory Diseases. Front Pharmacol. 2019, 10, 148. [Google Scholar] [CrossRef]
- Dinarello, C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 612–632. [Google Scholar] [CrossRef]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; de Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V.; et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 10791. [Google Scholar] [CrossRef] [PubMed]
- Ucciferri, C.; Auricchio, A.; di Nicola, M.; Potere, N.; Abbate, A.; Cipollone, F.; Vecchiet, J.; Falasca, K. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020, 2, 457–458. [Google Scholar] [CrossRef]
- Katia, F.; Myriam, D.P.; Ucciferri, C.; Auricchio, A.; di Nicola, M.; Marchioni, M.; Eleonora, C.; Emanuela, S.; Cipollone, F.; Vecchiet, J. Efficacy of canakinumab in mild or severe COVID-19 pneumonia. Immun. Inflamm. Dis. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Generali, D.; Bosio, G.; Malberti, F.; Cuzzoli, A.; Testa, S.; Romanini, L.; Fioravanti, A.; Morandini, A.; Pianta, L.; Giannotti, G.; et al. Canakinumab as treatment for COVID-19-related pneumonia: A prospective case-control study. Internation. J. Infect. Dis. IJID Off. Publ. Internation. Soc. Infect. Dis. 2020, 104, 433–440. [Google Scholar] [CrossRef]
- Wichers, M.C.; Koek, G.H.; Robaeys, G.; Verkerk, R.; Scharpe, S.; Maes, M. IDO and interferon-alpha-induced depressive symptoms: A shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry 2005, 10, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front Immunol. 2019, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Park, H.Y.; Suh, Y.S.; Yoon, E.H.; Kim, J.; Jang, W.H.; Lee, W.S.; Park, S.G.; Choi, I.W.; Choi, I.; et al. Inhibition of acute lethal pulmonary inflammation by the IDO-AhR pathway. Proc. Natl. Acad. Sci. USA 2017, 114, 5881–5890. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, P.; Grohmann, U. IDO and regulatory T cells: A role for reverse signalling and non-canonical NF-kappaB activation. Nat. Rev. Immunol. 2007, 7, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hansen, K.C.; Hod, E.A.; et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 2020, 5, e140327. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Puccetti, M.; Paolicelli, G.; Oikonomou, V.; de Luca, A.; Renga, G.; Borghi, M.; Pariano, M.; Stincardini, C.; Scaringi, L.; Giovagnoli, S.; et al. Towards Targeting the Aryl Hydrocarbon Receptor in Cystic Fibrosis. Mediators Inflamm. 2018, 2018, 1601486. [Google Scholar] [CrossRef]
- Moura-Alves, P.; Fae, K.; Houthuys, E.; Dorhoi, A.; Kreuchwig, A.; Furkert, J.; Barison, N.; Diehl, A.; Munder, A.; Constant, P.; et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 2014, 512, 387–392. [Google Scholar] [CrossRef]
- Grunewald, M.E.; Shaban, M.G.; Mackin, S.R.; Fehr, A.R.; Perlman, S. Murine Coronavirus Infection Activates the Aryl Hydrocarbon Receptor in an Indoleamine 2,3-Dioxygenase-Independent Manner, Contributing to Cytokine Modulation and Proviral TCDD-Inducible-PARP Expression. J. Virol. 2020, 94, e01743-19. [Google Scholar] [CrossRef]
- Huai, W.; Zhao, R.; Song, H.; Zhao, J.; Zhang, L.; Zhang, L.; Gao, C.; Han, L.; Zhao, W. Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription. Nat. Commun. 2014, 5, 4738. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Emami, F.; Vatanara, A.; Park, E.J.; Na, D.H. Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals. Pharmaceutics 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Rosiere, R.; Berghmans, T.; de Vuyst, P.; Amighi, K.; Wauthoz, N. The Position of Inhaled Chemotherapy in the Care of Patients with Lung Tumors: Clinical Feasibility and Indications According to Recent Pharmaceutical Progresses. Cancers 2019, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Fellner, R.C.; Terryah, S.T.; Tarran, R. Inhaled protein/peptide-based therapies for respiratory disease. Mol. Cell Pediatr. 2016, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Horava, S.D.; Moy, K.J.; Peppas, N.A. Biodegradable hydrophilic carriers for the oral delivery of hematological factor IX for hemophilia B treatment. Int. J. Pharm. 2016, 514, 220–228. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Assessment Report: Kineret; Procedure No. EMEA/H/C/000363/X/0042; European Medicines Agency: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kowalewski, J.; Ray, A. Predicting novel drugs for SARS-CoV-2 using machine learning from a >10 million chemical space. Heliyon 2020, 6, e04639. [Google Scholar] [CrossRef]
- Hathout, R.M.; Abdelhamid, S.G.; Metwally, A.A. Chloroquine and hydroxychloroquine for combating COVID-19: Investigating efficacy and hypothesizing new formulations using Bio/chemoinformatics tools. Inform. Med. Unlocked 2020, 21, 100–446. [Google Scholar] [CrossRef]
- Puccetti, M.; Giovagnoli, S.; Zelante, T.; Romani, L.; Ricci, M. Development of Novel Indole-3-Aldehyde-Loaded Gastro-Resistant Spray-Dried Microparticles for Postbiotic Small Intestine Local Delivery. J. Pharm. Sci. 2018, 107, 2341–2353. [Google Scholar] [CrossRef]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef]
- Sahakijpijarn, S.; Moon, C.; Koleng, J.J.; Christensen, D.J.; Williams Iii, R.O. Development of Remdesivir as a Dry Powder for Inhalation by Thin Film Freezing. Pharmaceutics 2020, 12, 1002. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).