Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multicomponent Systems
Abstract
1. Introduction
2. SAS Process: From Batch to Continuous
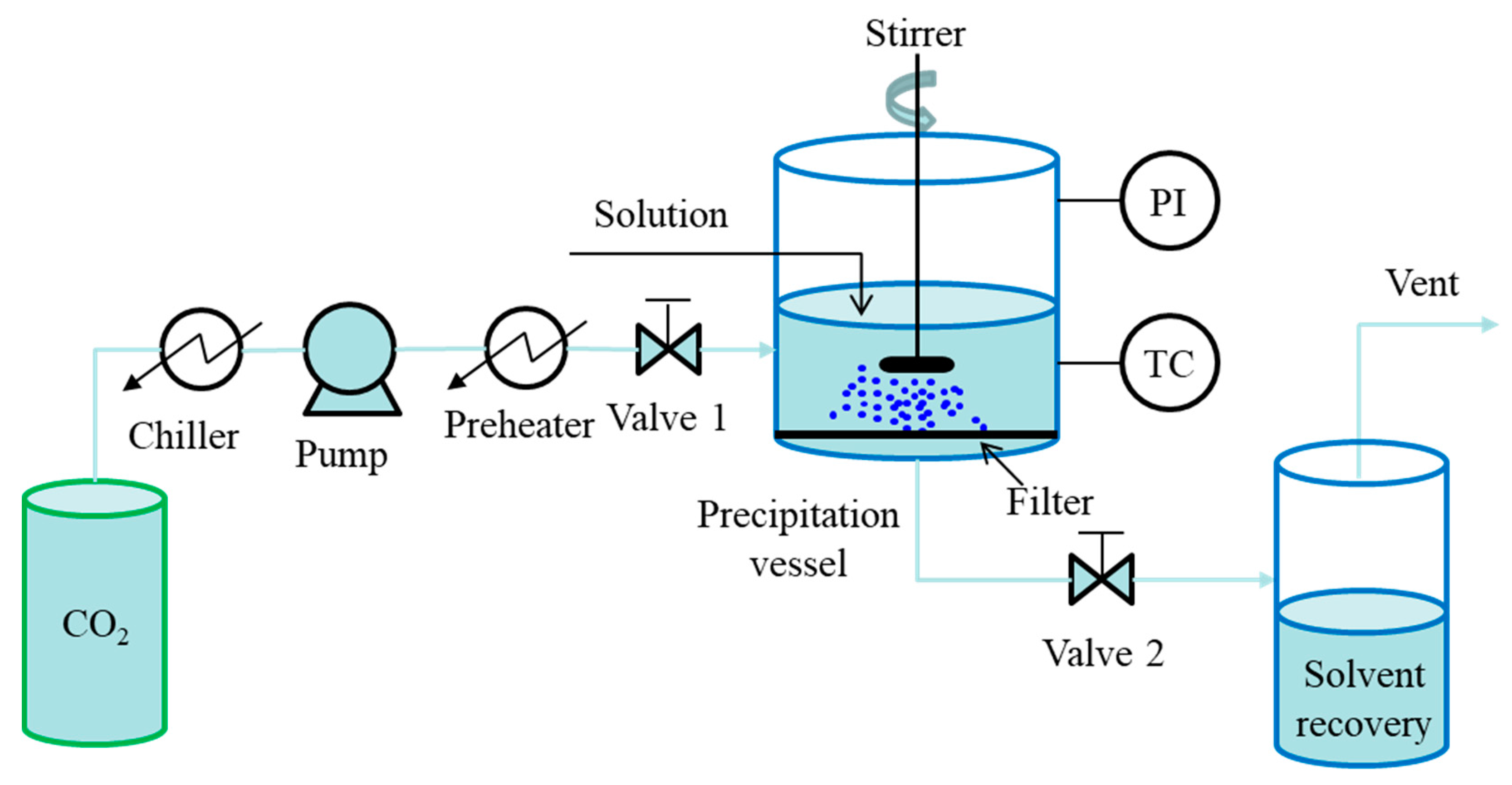
2.1. Batch Mode
2.2. Semi-Continuous Mode
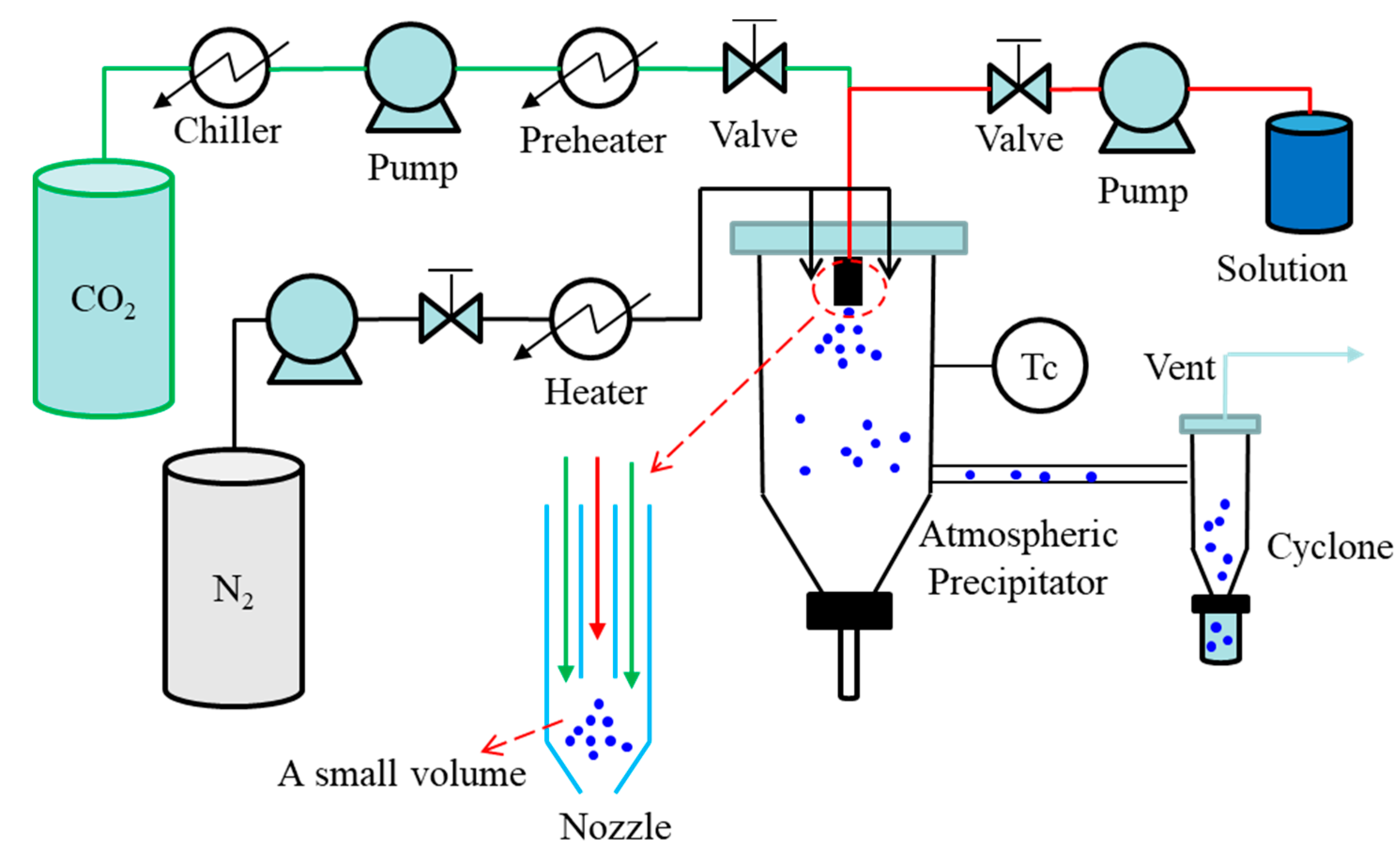
2.3. Continuous Mode
3. Applications of SAS Process for Solid Multicomponent Systems
3.1. Pharmaceutical Co-Crystals
3.2. Solid Dispersions
4. Discussion of the Manipulation of the Solid-State Properties of APIs
4.1. Influence Mechanisms of Operating Parameters
4.2. Applications of On-Line Monitoring and Computational Techniques
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tran, P.H.L.; Tran, T.T.D. Dosage form designs for the controlled drug release of solid dispersions. Int. J. Pharm. 2020, 581, 119274. [Google Scholar] [CrossRef]
- Byrn, S.R.; Zografi, G.; Chen, X.S. Solid State Properties of Pharmaceutical Materials, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Zhong, L.; Gao, L.; Li, L.; Zang, H. Trends-process analytical technology in solid oral dosage manufacturing. Eur. J. Pharm. Biopharm. 2020, 153, 187–199. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Engineering cocrystals of poorly water-soluble drugs to enhance dissolution in aqueous medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Morissette, S.L.; Almarsson, Ö.; Peterson, M.L.; Remenar, J.F.; Read, M.J.; Lemmo, A.V.; Ellis, S.; Cima, M.J.; Gardner, C.R. High-throughput crystallization: Polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv. Drug Deliver. Rev. 2004, 56, 275–300. [Google Scholar] [CrossRef]
- Rasenack, N.; Müller, B.W. Micron-size drug particles: Common and novel micronization techniques. Pharm. Dev. Technol. 2004, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Van den Mooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Girotra, P.; Singh, S.K.; Nagpal, K. Supercritical fluid technology: A promising approach in pharmaceutical research. Pharm. Dev. Technol. 2013, 18, 22–38. [Google Scholar] [CrossRef]
- Campardelli, R.; Baldino, L.; Reverchon, E. Supercritical fluids applications in nanomedicine. J. Supercrit. Fluids 2015, 101, 193–214. [Google Scholar] [CrossRef]
- Long, B.; Ryan, K.M.; Padrela, L. From batch to continuous—New opportunities for supercritical CO2 technology in pharmaceutical manufacturing. Eur. J. Pharm. Sci. 2019, 137, 104971. [Google Scholar] [CrossRef] [PubMed]
- Soh, S.H.; Lee, L.Y. Microencapsulation and nanoencapsulation using supercritical fluid (SCF) techniques. Pharmaceutics 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, T.K.R.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications—A review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of food components and emerging technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Marcus, Y. Some advances in supercritical fluid extraction for fuels, bio-materials and purification. Processes 2019, 7, 156. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, Y.; Wang, X. Tailoring particle microstructures via supercritical CO2 processes for particular drug delivery. Curr. Pharm. Des. 2015, 21, 2543–2562. [Google Scholar] [CrossRef]
- Badens, E.; Masmoudi, Y.; Mouahid, A.; Crampon, C. Current situation and perspectives in drug formulation by using supercritical fluid technology. J. Supercrit. Fluids 2018, 134, 274–283. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals—A comprehensive review. Adv. Drug Deliver. Rev. 2018, 131, 22–78. [Google Scholar] [CrossRef]
- De Marco, I.; Rossmann, M.; Prosapio, V.; Reverchon, E.; Braeuer, A. Control of particle size, at micrometric and nanometric range, using supercritical antisolvent precipitation from solvent mixtures: Application to pvp. Chem. Eng. J. 2015, 273, 344–352. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, I.; Reverchon, E. Supercritical antisolvent coprecipitation mechanisms. J. Supercrit. Fluids 2018, 138, 247–258. [Google Scholar] [CrossRef]
- Kalani, M.; Yunus, R. Application of supercritical antisolvent method in drug encapsulation: A review. Int. J. Nanomed. 2011, 6, 1429–1442. [Google Scholar] [CrossRef]
- MacEachern, L.; Kermanshahi-pour, A.; Mirmehrabi, M. Supercritical carbon dioxide for pharmaceutical co-crystal production. Cryst. Growth Des. 2020, 20, 6226–6244. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Caputo, G.; De Marco, I. Spherical microparticles production by supercritical antisolvent precipitation: Interpretation of results. J. Supercrit. Fluids 2008, 47, 70–84. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I.; Torino, E. Nanoparticles production by supercritical antisolvent precipitation: A general interpretation. J. Supercrit. Fluids 2007, 43, 126–138. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I.; Adami, R.; Caputo, G. Expanded micro-particles by supercritical antisolvent precipitation: Interpretation of results. J. Supercrit. Fluids 2008, 44, 98–108. [Google Scholar] [CrossRef]
- Rossmann, M.; Braeuer, A.; Dowy, S.; Gallinger, T.G.; Leipertz, A.; Schluecker, E. Solute solubility as criterion for the appearance of amorphous particle precipitation or crystallization in the supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2012, 66, 350–358. [Google Scholar] [CrossRef]
- Liu, G.; Wang, W.; Wang, H.; Jiang, Y. Preparation of 10-hydroxycamptothecin proliposomes by the supercritical CO2 anti-solvent process. Chem. Eng. J. 2014, 243, 289–296. [Google Scholar] [CrossRef]
- Wang, W.; Liu, G.; Wu, J.; Jiang, Y. Co-precipitation of 10-hydroxycamptothecin and poly (l-lactic acid) by supercritical CO2 anti-solvent process using dichloromethane/ethanol co-solvent. J. Supercrit. Fluids 2013, 74, 137–144. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Jiang, Y. Recrystallization and micronization of camptothecin by the supercritical antisolvent process: Influence of solvents. Ind. Eng. Chem. Res. 2013, 52, 15049–15056. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Q.; Huang, Y.; Guan, G.; Jiang, Y. Tailoring the particle microstructures of gefitinib by supercritical CO2 anti-solvent process. J. CO2 Util. 2017, 20, 43–51. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, G.; Lin, Q.; Jiang, Y. Co-crystal of paracetamol and trimethylglycine prepared by a supercritical CO2 anti-solvent process. Chem. Eng. Technol. 2018, 41, 1122–1131. [Google Scholar] [CrossRef]
- Liu, G.; Gong, L.; Zhang, J.; Wu, Z.; Deng, H.; Deng, S. Development of nimesulide amorphous solid dispersions via supercritical anti-solvent process for dissolution enhancement. Eur. J. Pharm. Sci. 2020, 152, 105457. [Google Scholar] [CrossRef]
- Liu, G.; Wu, Z.; Zhang, J.; Gong, L.; Zhang, Y.; Deng, S. Particle design of itraconazole by supercritical anti-solvent technology: Processing-microstructure-solubility relationship. Chem. Eng. Process. 2020, 154, 108013. [Google Scholar] [CrossRef]
- Gallagher, P.M.; Coffey, M.P.; Krukonis, V.J.; Klasutis, N. Gas antisolvent recrystallization: New process to recrystallize compounds insoluble in supercritical fluids. In Supercritical Fluid Science and Technology; American Chemical Society: Washington, DC, USA, 1989; Volume 406, pp. 334–354. [Google Scholar]
- Ober, C.A.; Montgomery, S.E.; Gupta, R.B. Formation of itraconazole/l-malic acid cocrystals by gas antisolvent cocrystallization. Powder Technol. 2013, 236, 122–131. [Google Scholar] [CrossRef]
- Bleich, J.; Müller, B.W.; Waßmus, W. Aerosol solvent extraction system—A new microparticle production technique. Int. J. Pharm. 1993, 97, 111–117. [Google Scholar] [CrossRef]
- Dixon, D.J.; Johnston, K.P.; Bodmeier, R.A. Polymeric materials formed by precipitation with a compressed fluid antisolvent. AIChE J. 1993, 39, 127–139. [Google Scholar] [CrossRef]
- Palakodaty, S.; York, P.; Pritchard, J. Supercritical fluid processing of materials from aqueous solutions: The application of seds to lactose as a model substance. Pharm. Res. 1998, 15, 1835–1843. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Gupta, R.B. Production of griseofulvin nanoparticles using supercritical CO2 antisolvent with enhanced mass transfer. Int. J. Pharm. 2001, 228, 19–31. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Velaga, S.P.; Matos, H.A.; de Azevedo, E.G. Formation of indomethacin–saccharin cocrystals using supercritical fluid technology. Eur. J. Pharm. Sci. 2009, 38, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.A.; Padrela, L.; Geraldes, V.; Santos, J.; Matos, H.A.; Azevedo, E.G. Theophylline polymorphs by atomization of supercritical antisolvent induced suspensions. J. Supercrit. Fluids 2011, 58, 303–312. [Google Scholar] [CrossRef]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef]
- Boel, E.; Koekoekx, R.; Dedroog, S.; Babkin, I.; Vetrano, M.R.; Clasen, C.; Van den Mooter, G. Unraveling particle formation: From single droplet drying to spray drying and electrospraying. Pharmaceutics 2020, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- U.S. FDA. Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry; U.S. FDA: Silver Spring, MD, USA, 2018. [Google Scholar]
- Pando, C.; Cabañas, A.; Cuadra, I.A. Preparation of pharmaceutical co-crystals through sustainable processes using supercritical carbon dioxide: A review. RSC Adv. 2016, 6, 71134–71150. [Google Scholar] [CrossRef]
- Neurohr, C.; Revelli, A.L.; Billot, P.; Marchivie, M.; Lecomte, S.; Laugier, S.; Massip, S.; Subra-Paternault, P. Naproxen–nicotinamide cocrystals produced by CO2 antisolvent. J. Supercrit. Fluids 2013, 83, 78–85. [Google Scholar] [CrossRef]
- Erriguible, A.; Neurohr, C.; Revelli, A.L.; Laugier, S.; Fevotte, G.; Subra-Paternault, P. Cocrystallization induced by compressed CO2 as antisolvent: Simulation of a batch process for the estimation of nucleation and growth parameters. J. Supercrit. Fluids 2015, 98, 194–203. [Google Scholar] [CrossRef]
- Cuadra, I.A.; Cabañas, A.; Cheda, J.A.R.; Pando, C. Polymorphism in the co-crystallization of the anticonvulsant drug carbamazepine and saccharin using supercritical CO2 as an anti-solvent. J. Supercrit. Fluids 2018, 136, 60–69. [Google Scholar] [CrossRef]
- Neurohr, C.; Erriguible, A.; Laugier, S.; Subra-Paternault, P. Challenge of the supercritical antisolvent technique sas to prepare cocrystal-pure powders of naproxen-nicotinamide. Chem. Eng. J. 2016, 303, 238–251. [Google Scholar] [CrossRef]
- Shikhar, A.; Bommana, M.M.; Gupta, S.S.; Squillante, E. Formulation development of carbamazepine–nicotinamide co-crystals complexed with γ-cyclodextrin using supercritical fluid process. J. Supercrit. Fluids 2011, 55, 1070–1078. [Google Scholar] [CrossRef]
- Wichianphong, N.; Charoenchaitrakool, M. Application of box–behnken design for processing of mefenamic acid–paracetamol cocrystals using gas anti-solvent (GAS) process. J. CO2 Util. 2018, 26, 212–220. [Google Scholar] [CrossRef]
- Pessoa, A.S.; Aguiar, G.P.S.; Vladimir Oliveira, J.; Bortoluzzi, A.J.; Paulino, A.; Lanza, M. Precipitation of resveratrol-isoniazid and resveratrol-nicotinamide cocrystals by gas antisolvent. J. Supercrit. Fluids 2019, 145, 93–102. [Google Scholar] [CrossRef]
- Cuadra, I.A.; Cabañas, A.; Cheda, J.A.R.; Türk, M.; Pando, C. Cocrystallization of the anticancer drug 5-fluorouracil and coformers urea, thiourea or pyrazinamide using supercritical CO2 as an antisolvent (SAS) and as a solvent (CSS). J. Supercrit. Fluids 2020, 160, 104813. [Google Scholar] [CrossRef]
- Cuadra, I.A.; Cabañas, A.; Cheda, J.A.R.; Martínez-Casado, F.J.; Pando, C. Pharmaceutical co-crystals of the anti-inflammatory drug diflunisal and nicotinamide obtained using supercritical CO2 as an antisolvent. J. CO2 Util. 2016, 13, 29–37. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; Sun, W.-J.; Lu, T.; Tong, H.H.Y.; Sun, C.C.; Zheng, Y. Resveratrol cocrystals with enhanced solubility and tabletability. Int. J. Pharm. 2016, 509, 391–399. [Google Scholar] [CrossRef]
- He, H.; Zhang, Q.; Li, M.; Wang, J.-R.; Mei, X. Modulating the dissolution and mechanical properties of resveratrol by cocrystallization. Cryst. Growth Des. 2017, 17, 3989–3996. [Google Scholar] [CrossRef]
- Park, H.; Jin Seo, H.; Hong, S.-H.; Ha, E.-S.; Lee, S.; Kim, J.-S.; Baek, I.-H.; Kim, M.-S.; Hwang, S.-J. Characterization and therapeutic efficacy evaluation of glimepiride and l-arginine co-amorphous formulation prepared by supercritical antisolvent process: Influence of molar ratio and preparation methods. Int. J. Pharm. 2020, 581, 119232. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef] [PubMed]
- Vo, C.L.; Park, C.; Lee, B.J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical antisolvent process for pharmaceutical applications: A review. Processes 2020, 8, 938. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Liu, T.; Zhao, L.; Zhao, J.; Feng, N. Development and in-vivo assessment of the bioavailability of oridonin solid dispersions by the gas anti-solvent technique. Int. J. Pharm. 2011, 411, 172–177. [Google Scholar] [CrossRef]
- Adeli, E. The use of supercritical anti-solvent (SAS) technique for preparation of irbesartan-pluronic® F-127 nanoparticles to improve the drug dissolution. Powder Technol. 2016, 298, 65–72. [Google Scholar] [CrossRef]
- Franco, P.; Reverchon, E.; De Marco, I. Zein/diclofenac sodium coprecipitation at micrometric and nanometric range by supercritical antisolvent processing. J. CO2 Util. 2018, 27, 366–373. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Eudragit: A novel carrier for controlled drug delivery in supercritical antisolvent coprecipitation. Polymers 2020, 12, 234. [Google Scholar] [CrossRef]
- Dittanet, P.; Phothipanyakun, S.; Charoenchaitrakool, M. Co-precipitation of mefenamic acid−polyvinylpyrrolidone k30 composites using gas anti-solvent. J. Taiwan Inst. Chem. Eng. 2016, 63, 17–24. [Google Scholar] [CrossRef]
- Charoenchaitrakool, M.; Polchiangdee, C.; Srinophakun, P. Production of theophylline and polyethylene glycol 4000 composites using gas anti-solvent (GAS) process. Mater. Lett. 2009, 63, 136–138. [Google Scholar] [CrossRef]
- Park, J.; Cho, W.; Kang, H.; Lee, B.B.J.; Kim, T.S.; Hwang, S.-J. Effect of operating parameters on pvp/tadalafil solid dispersions prepared using supercritical anti-solvent process. J. Supercrit. Fluids 2014, 90, 126–133. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Xu, P.-Y.; Chen, B.-Q.; Fu, C.-P.; Kankala, R.K.; Chen, A.-Z.; Wang, S.-B. Supercritical antisolvent process-assisted fabrication of chrysin-polyvinylpyrrolidone sub-microparticles for improved anticancer efficiency. J. Supercrit. Fluids 2020, 162, 104847. [Google Scholar] [CrossRef]
- Machmudah, S.; Winardi, S.; Wahyudiono; Kanda, H.; Goto, M. Formation of fine particles from curcumin/pvp by the supercritical antisolvent process with a coaxial nozzle. ACS Omega 2020, 5, 6705–6714. [Google Scholar] [CrossRef]
- Wu, K.; Li, J.; Wang, W.; Winstead, D.A. Formation and characterization of solid dispersions of piroxicam and polyvinylpyrrolidone using spray drying and precipitation with compressed antisolvent. J. Pharm. Sci. 2009, 98, 2422–2431. [Google Scholar] [CrossRef]
- Lee, C.-W.; Kim, S.-J.; Youn, Y.-S.; Widjojokusumo, E.; Lee, Y.-H.; Kim, J.; Lee, Y.-W.; Tjandrawinata, R.R. Preparation of bitter taste masked cetirizine dihydrochloride/β-cyclodextrin inclusion complex by supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2010, 55, 348–357. [Google Scholar] [CrossRef]
- Won, D.-H.; Kim, M.-S.; Lee, S.; Park, J.-S.; Hwang, S.-J. Improved physicochemical characteristics of felodipine solid dispersion particles by supercritical anti-solvent precipitation process. Int. J. Pharm. 2005, 301, 199–208. [Google Scholar] [CrossRef]
- Moseson, D.E.; Parker, A.S.; Beaudoin, S.P.; Taylor, L.S. Amorphous solid dispersions containing residual crystallinity: Influence of seed properties and polymer adsorption on dissolution performance. Eur. J. Pharm. Sci. 2020, 146, 105276. [Google Scholar] [CrossRef]
- Brough, C.; Williams, R.O., 3rd. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef]
- Long, B.; Walker, G.M.; Ryan, K.M.; Padrela, L. Controlling polymorphism of carbamazepine nanoparticles in a continuous supercritical-CO2-assisted spray drying process. Cryst. Growth Des. 2019, 19, 3755–3767. [Google Scholar] [CrossRef]
- de la Fuente Badilla, J.C.; Peters, C.J.; de Swaan Arons, J. Volume expansion in relation to the gas–antisolvent process. J. Supercrit. Fluids 2000, 17, 13–23. [Google Scholar] [CrossRef]
- Mukhopadhyay, M. Partial molar volume reduction of solvent for solute crystallization using carbon dioxide as antisolvent. J. Supercrit. Fluids 2003, 25, 213–223. [Google Scholar] [CrossRef]
- Reverchon, E.; Torino, E.; Dowy, S.; Braeuer, A.; Leipertz, A. Interactions of phase equilibria, jet fluid dynamics and mass transfer during supercritical antisolvent micronization. Chem. Eng. J. 2010, 156, 446–458. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, E. Influence of pressure, temperature and concentration on the mechanisms of particle precipitation in supercritical antisolvent micronization. J. Supercrit. Fluids 2011, 58, 295–302. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Mechanisms controlling supercritical antisolvent precipitate morphology. Chem. Eng. J. 2011, 169, 358–370. [Google Scholar] [CrossRef]
- Dukhin, S.S.; Shen, Y.; Dave, R.; Pfeffer, R. Droplet mass transfer, intradroplet nucleation and submicron particle production in two-phase flow of solvent-supercritical antisolvent emulsion. Colloid. Surf. A 2005, 261, 163–176. [Google Scholar] [CrossRef]
- Marra, F.; De Marco, I.; Reverchon, E. Numerical analysis of the characteristic times controlling supercritical antisolvent micronization. Chem. Eng. Sci. 2012, 71, 39–45. [Google Scholar] [CrossRef]
- Werling, J.O.; Debenedetti, P.G. Numerical modeling of mass transfer in the supercritical antisolvent process. J. Supercrit. Fluids 1999, 16, 167–181. [Google Scholar] [CrossRef]
- Werling, J.O.; Debenedetti, P.G. Numerical modeling of mass transfer in the supercritical antisolvent process: Miscible conditions. J. Supercrit. Fluids 2000, 18, 11–24. [Google Scholar] [CrossRef]
- Clegg, I. Process analytical technology. In Specification of Drug Substances and Products, 2nd ed.; Riley, C.M., Rosanske, T.W., Reid, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 7; pp. 149–173. [Google Scholar]
- Braeuer, A.; Dowy, S.; Leipertz, A. Simultaneous raman and elastic light scattering imaging for particle formation investigation. Opt. Lett. 2010, 35, 2553–2555. [Google Scholar] [CrossRef]
- Dowy, S.; Braeuer, A.; Reinhold-López, K.; Leipertz, A. In situ optical monitoring of the solution concentration influence on supercritical particle precipitation. J. Supercrit. Fluids 2010, 55, 282–291. [Google Scholar] [CrossRef]
- Braeuer, A.; Adami, R.; Dowy, S.; Rossmann, M.; Leipertz, A. Observation of liquid solution volume expansion during particle precipitation in the supercritical CO2 antisolvent process. J. Supercrit. Fluids 2011, 56, 121–124. [Google Scholar] [CrossRef]
- Vorobei, A.M.; Pokrovskiy, O.I.; Ustinovich, K.B.; Parenago, O.O.; Lunin, V.V. A method for measuring solubility in multi-component sub- and supercritical fluids using an online hyphenation of supercritical antisolvent precipitation and supercritical fluid chromatography. J. Mol. Liq. 2019, 280, 212–217. [Google Scholar] [CrossRef]
- Pokrovskiy, O.; Vorobei, A.; Zuev, Y.; Kostenko, M.; Lunin, V. Investigation of precipitation selectivity and particle size concentration dependences in supercritical antisolvent method via online supercritical fluid chromatography. Adv. Powder Technol. 2020, 31, 2257–2266. [Google Scholar] [CrossRef]
- Muthancheri, I.; Long, B.; Ryan, K.M.; Padrela, L.; Ramachandran, R. Development and validation of a two-dimensional population balance model for a supercritical CO2 antisolvent batch crystallization process. Adv. Powder Technol. 2020, 31, 3191–3204. [Google Scholar] [CrossRef]
- Cardoso, F.A.R.; Vogel, E.M.; Souza, M.F.; Cardozo-Filho, L. Mathematical modeling to predict the size and nucleation rate of micro and nanoparticles using the scale-up process with supercritical CO2. J. Supercrit. Fluids 2019, 154, 104608. [Google Scholar] [CrossRef]
- Clercq, S.; Mouahid, A.; Pèpe, G.; Badens, E. Prediction of crystal–solvent interactions in a supercritical medium: A possible way to control crystal habit at high supersaturations with molecular modeling. Cryst. Growth Des. 2020, 20, 6863–6876. [Google Scholar] [CrossRef]
- Sierra-Pallares, J.; Marchisio, D.L.; Parra-Santos, M.T.; García-Serna, J.; Castro, F.; Cocero, M.J. A computational fluid dynamics study of supercritical antisolvent precipitation: Mixing effects on particle size. AIChE J. 2012, 58, 385–398. [Google Scholar] [CrossRef]
- Cardoso, F.A.R.; Rezende, R.V.P.; Almeida, R.A.; Meier, H.F.; Cardozo-Filho, L. Effect of precipitation chamber geometry on the production of microparticles by antisolvent process. J. Supercrit. Fluids 2018, 133, 357–366. [Google Scholar] [CrossRef]
- Yongda, S. Supercritical fluid particle design for poorly water-soluble drugs (review). Curr. Pharm. Des. 2014, 20, 349–368. [Google Scholar]
- Supercritical Fluids for Particle Engineering. Available online: http://www.crystecpharma.com/science/supercritical-fluids.html (accessed on 23 March 2021).
- Superfluids Crystallization Process. Available online: https://aphios.com/technology/crystallization/superfluids-crystallization-process/ (accessed on 23 March 2021).
- Large Pilot and Production Scale System and Equipment Supply. Available online: https://supercriticalfluid.net/supercritical-fluid-production-systems/ (accessed on 23 March 2021).
- What Kind of Research does Lavipharm Specialize in? Available online: https://www.lavipharm.com/what-kind-of-research-does-lavipharm-specialize-in/ (accessed on 23 March 2021).



| API/Co-former Method | Operating Parameters a | Solid-State Properties | Ref. |
|---|---|---|---|
| Itraconazole/L-malic acid GAS | R = 1:1 mass ratio, FCO2 = 1 g/min C = 50 mg/mL, V = 10 mL THF p = 10.3 MPa, T = 40 °C | (a) Contain a certain amorphous material; (b) Slightly larger than those produced from n-heptane. | [35] |
| Naproxen/nicotinamide GAS | R = 1:2–3:1 molar ratio C = 12–74 mg/mL, V = 40 mL acetone p = 10 MPa, T = 35 °C FCO2 = 2–20 g/min Stirring speed = 60–500 rpm | (a) The same hydrogen-bond network and stoichiometry than co-crystals produced by cooling crystallization, grinding or evaporation techniques; (b) The co-crystal purity was affected by R; (c) Pure co-crystals with sizes < 180 μm was obtained. | [46] |
| Carbamazepine/nicotinamide GAS | R = 1:2 or 1:1 molar ratio V = 6–8 mL ethanol, p = 11 MPa T = 40 °C, FCO2 = 90–95 mL/min | (a) The co-crystals were needle shaped; (b) 2.5-fold increase in dissolution rate. | [50] |
| Mefenamic acid (MEF)/paracetamol GAS process | R = 3:1–5:1 mass ratio C = 70–90% MEF saturation V = 5 mL acetone, T = 25 °C−45 °C p = 9 MPa, FCO2 = 10 mL/min | Optimized co-crystals improved the MEF dissolution rate by 6.0, 5.3 and 2.3-fold when compared to pure MEF, sieved co-crystals prepared by an evaporation technique and sieved marketed combination drugs, respectively. | [51] |
| Resveratrol/isoniazid or nicotinamide GAS | R = 1:1 molar ratio V = 15–25 mL ethanol p = 9 MPa, T = 45 °C FCO2 = 10 g/min | (a) Their crystal structures similar to those reported; (b) Had non-cocrystallized components; (c) Showed a higher dissolution rate than those obtained by liquid anti-solvent technique. | [52] |
| Paracetamol/trimethylglycine Semi-continuous | R = 1:1 molar ratio, FCO2 = 30 g/min DCM/methanol (1:4–1:1, v/v) C = 10–40 mg/mL, p = 9–12 MPa T = 35–50 °C, Fs = 0.5–1.6 mL/min | (a) Mean particle size < 10 μm, much smaller than those obtained using the ball milling process; (b) Enhanced dissolution rate and tableting performance; (c) Residual solvent contents were less than ICH limits. | [31] |
| Carbamazepine (CBZ)/saccharin Semi-continuous | R = 1:1 molar ratio methanol, ethanol, DCM, DMSO Fs = 1 mL/min, D = 100 μm CCBZ = 15–30 mg/mL, FCO2 = 20 g/min T = 40.0–60.0 °C, p = 10.0–15.0 MPa | (a) Pure co-crystal polymorph I was obtained, but pure co-crystal polymorph II could not be obtained; (b) Showed the same crystal structure and morphology as those previously obtained by other methodologies; (c) Without the presence of homo-crystals and solvent free. | [48] |
| Naproxen/ nicotinamide Semi-continuous | R = 2:1 molar ratio, Acetone C = 40 mg/mL, Fs = 2–13 mL/min T = 37 °C, p = 10 MPa FCO2 = 7–59 g/min | (a) The same hydrogen bond interactions and crystal structure than co-crystals obtained by other techniques; (b) A thin plate-like morphology and a size 20 μm−1 mm; (c) At high FCO2/Fs, had homo-crystals of naproxen. | [49] |
| 5-Fluorouracil/urea, thiourea or pyrazinamide Semi-continuous | Methanol, C5-Fu = 2.5–5 mg/mL Fs = 1 mL/min, FCO2 = 20 g/min p = 7–15 MPa, T = 40 °C, D = 100 μm | (a) Pure 5-Fu/urea co-crystals were obtained; (b) 5-Fu/pyrazinamide co-crystal did not be obtained; (c) There were 5-Fu homo-crystal impurities in the precipitate of 5-Fu/thiourea co-crystals. | [53] |
| Diflunisal/nicotinamide Semi-continuous | R = 2:1 molar ratio, ethanol, acetone C = 18.64–37.28 mg/mL, D = 100 μm T = 35- 40 °C, p = 10.0–12.0 MPa Fs = 1 mL/min, FCO2 = 20 g/min | (a) Same crystal structure to those obtained by liquid assisted ball mill grinding and solution crystallization; (b) Needles with uniform width and variable length; (c) Improved diflunisal release slightly. | [54] |
| Indomethacin/saccharin AAS and ASES | Coaxial nozzle (a small mixing chamber~30 μL, D = 200 μm) p = 6–12 MPa, T = 50–70 °C C = 4.35 mg/g, Fs/FCO2 = 0.03–0.19 g/g | (a) Pure co-crystals with different morphologies and sizes (0.2–5 μm) were obtained; (b) AAS process: nearly spherical particles; (c) ASES process: larger and elongated particles. | [40] |
| API/Carrier a Method | Operating Parameters b | Solid-State Properties | Ref. |
|---|---|---|---|
| Oridonin/ PVP K17 GAS | ethanol p = 14 MPa, T = 55 °C | (a) Large and adhesive particles, amorphous form; (b) Improved drug dissolution rate greatly; (c) 26.4-fold improvement in the absorption of oridonin. | [61] |
| Mefenamic acid (MEF)/PVP K30 GAS | acetone/ethanol, FCO2 = 10 mL/min R = 1:2–1:0.5 (w/w) C = 50–75% MEF saturation p = 9 MPa, T = 25–35 °C | (a) Porous and irregularly shaped; (b) R, C and T affected morphology and size greatly; (c) Possessed the crystalline structure of MEF; (d) Improved the drug dissolution rate greatly. | [65] |
| Theophylline (THEO)/ PEG 4000 GAS | V = 5 mL ethanol/DCM R = 2:3–2:7 (w/w), CTHEO = 1–2 wt.% T = 25–45 °C, p = 9 MPa FCO2 = 10 mL/min | (a) Contained some rectangular shape of THEO particles; (b) The excess drugs on the composites can be removed effectively by washing with ethyl acetate; (d) Improved the drug dissolution rate greatly. | [66] |
| Tadalafil/ PVP K30 Semi-continuous | R = 1:4 molar ratio, ethanol/DCM Fs = 1 mL/min, FCO2 = 50 g/min C = 5–15 mg/mL, T = 40.0–50.0 °C p = 9.0–15.0 MPa | (a) Mean particle size 200–900 nm, affected by T,C, and p; (b) C = 15 mg/mL, irregular, micron-sized crystalline particles were obtained, resulting in low dissolution rate; (c) C = 5–10 mg/mL, spherical, nano-sized amorphous particles were obtained, resulting in fast dissolution rate. | [67] |
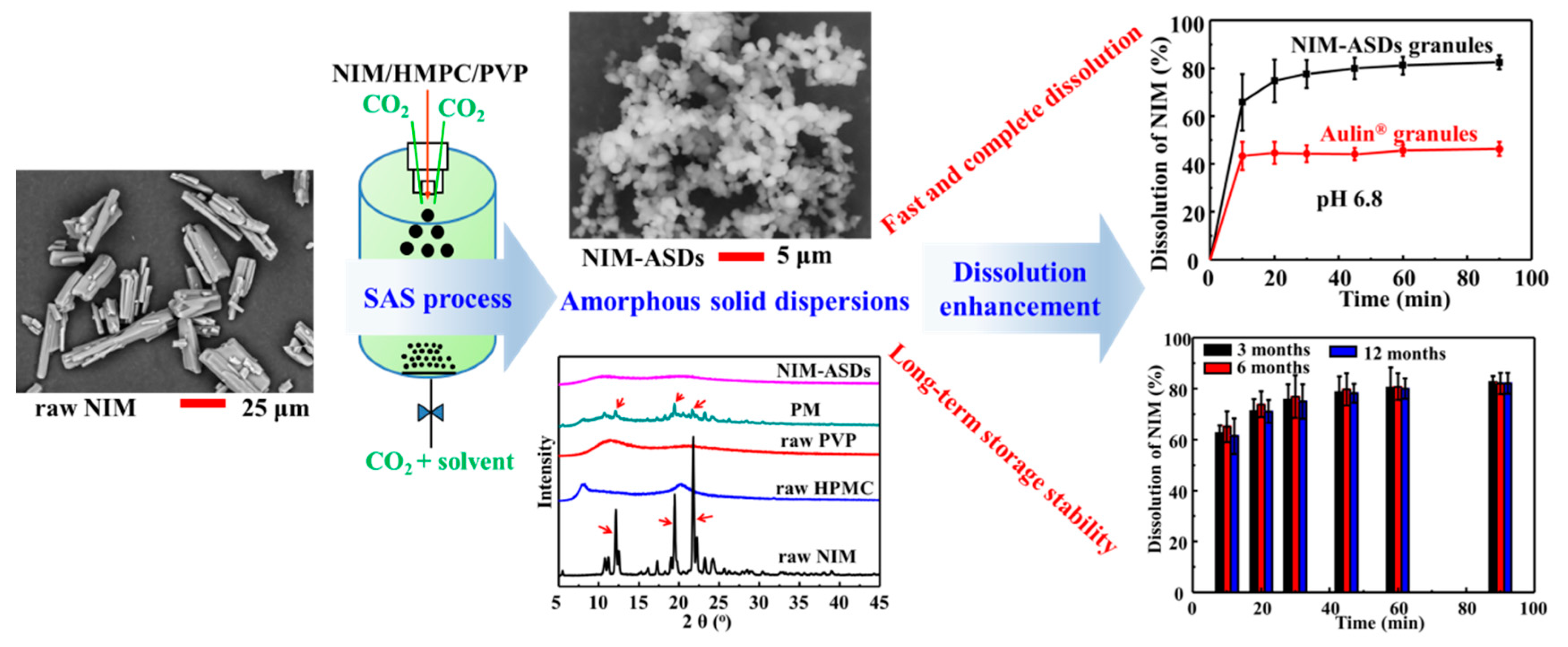
| Nimesulide (NIM)/HPMC and PVP K30 Semi-continuous | DCM/methanol (1:1–3:1, v/v) R(NIM/HPMC/PVP, mass ratio) = 1:1–4:0–2 C = 25–55 mg/mL, Fs = 2 mL/min D = 100 μm, FCO2 = 100 mL/min T = 40 °C, p = 8 MPa | (a) Existed in crystal forms or amorphous, R and solvent ratio affected the particle solid-state properties greatly; (d) Spherical, micro-sized amorphous particles were obtained, resulting in fast and high dissolution rate; (e) The residual solvents were far below the ICH limits; (f) Amorphous NIM was stable during 12-month storage. | [32] |
| Itraconazole (ITZ)/HPMC Semi-continuous | methanol/DCM, D = 70–650 μm R = 3:1–1:4 (w/w), C = 1–5 mg/mL T = 35–55 °C, p = 9–17 MPa Fs = 0.5–5 mL/min, FCO2 = 50 g/min | (a) Enhanced ITZ solubility from 4.4 μg/mL to 108.5 μg/mL; (b) Spherical, micro-sized amorphous particles were obtained, resulting in fast and high dissolution rate; (c) Hydrogen bond interaction was formed between HPMC and ITZ, hindering the recrystallization of dissolved ITZ. | [33] |
| Irbesartan/ Pluronic® F-127 Semi-continuous | Ethanol, R = 1:1 (w/w) p = 10–20 MPa, T = 40–70 °C Fs = 0.2–2 mL/min | (a) Spherical, amorphous particles with size 97 ± 19 nm; (b) The dissolution was 13 times more than the pure drug. | [62] |
| Diclofenac/zein Semi-continuous | DMSO, R = 1:5–1:30 (w/w) C = 30–50 mg/mL, p = 9 MPa T = 40 °C, D = 100 μm Fs = 1 mL/min, FCO2 = 30 g/min | (a) Spherical, amorphous microparticles were obtained with mean diameters 0.416–1.308 μm; (b) The drug release is slower as R decreases; (d) When R = 1:30, prolonged the drug release successfully. | [63] |
| Diclofenac or theophylline/Eudragit L100-55 Semi-continuous | DMSO, R = 1:10–1:20 (w/w) C = 20–50 mg/mL, D = 100 μm p = 9–15 MPa, T = 40 °C Fs = 1 mL/min, FCO2 = 30 g/min | (a) R, p and C affected the morphology and size greatly; (b) Spherical, amorphous microparticles were obtained; (c) The drug release delayed up to 28 and 57 times for diclofenac and theophylline, respectively. | [64] |
| Chrysin (CHS)/PVP Semi-continuous | acetone/ethanol, R = 1:4 (w/w) CCHS = 1–3 mg/mL, Fs = 1 mL/min T = 40–60 °C, p = 12 MPa | (a) Spherical, crystalline particles with an average size of 273.7 nm ± 38.9 nm to 958.8 nm ± 83.2 nm were obtained; (b) The dissolution rate was 2.8 times higher than pure CHS; | [68] |
| Curcumin (CUR)/PVP Semi-continuous | acetone/ethanol, R = 2–4 wt% PVP T = 40–60 °C, p = 8–12 MPa Fs = 0.5 mL/min, FCO2 = 15 mL/min | (a) Spherical particles with size < 1 μm were abtained; (b) The PVP addition enhanced the CUR dissolution in distilled water significantly. | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Li, J.; Deng, S. Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multicomponent Systems. Pharmaceutics 2021, 13, 475. https://doi.org/10.3390/pharmaceutics13040475
Liu G, Li J, Deng S. Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multicomponent Systems. Pharmaceutics. 2021; 13(4):475. https://doi.org/10.3390/pharmaceutics13040475
Chicago/Turabian StyleLiu, Guijin, Junjian Li, and Shiming Deng. 2021. "Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multicomponent Systems" Pharmaceutics 13, no. 4: 475. https://doi.org/10.3390/pharmaceutics13040475
APA StyleLiu, G., Li, J., & Deng, S. (2021). Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multicomponent Systems. Pharmaceutics, 13(4), 475. https://doi.org/10.3390/pharmaceutics13040475






