Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Stability Study
2.3. Preparation of Stability Samples and Reference Standard Samples before LC-MS
2.4. LC-UV-MS Analysis of Stability Samples
2.5. Statistical Analysis
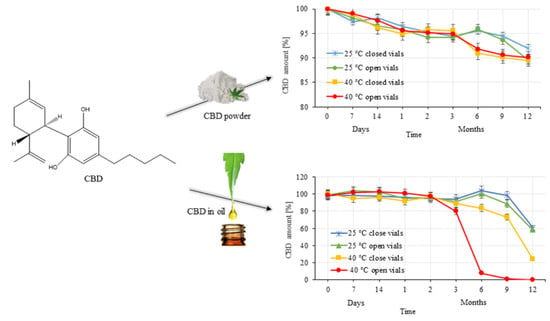
3. Results
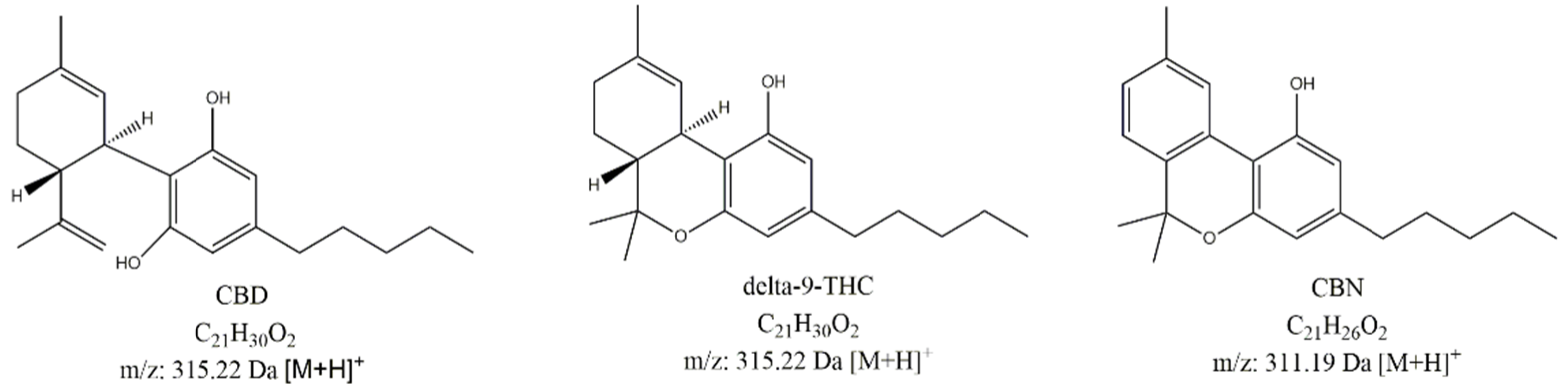
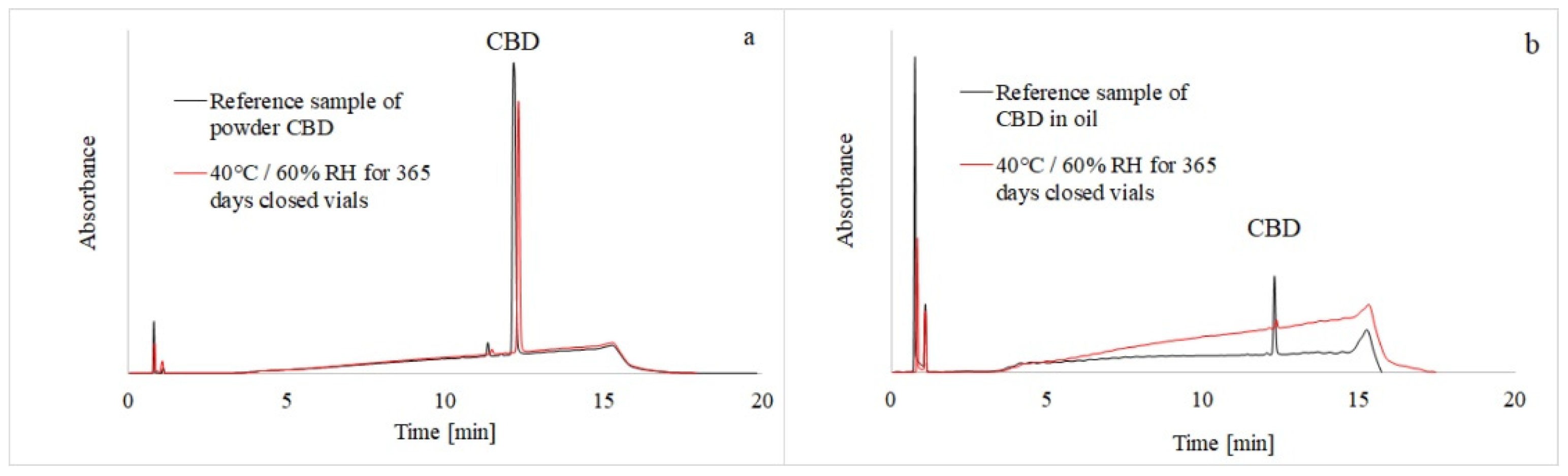
3.1. HPLC-MS Analysis
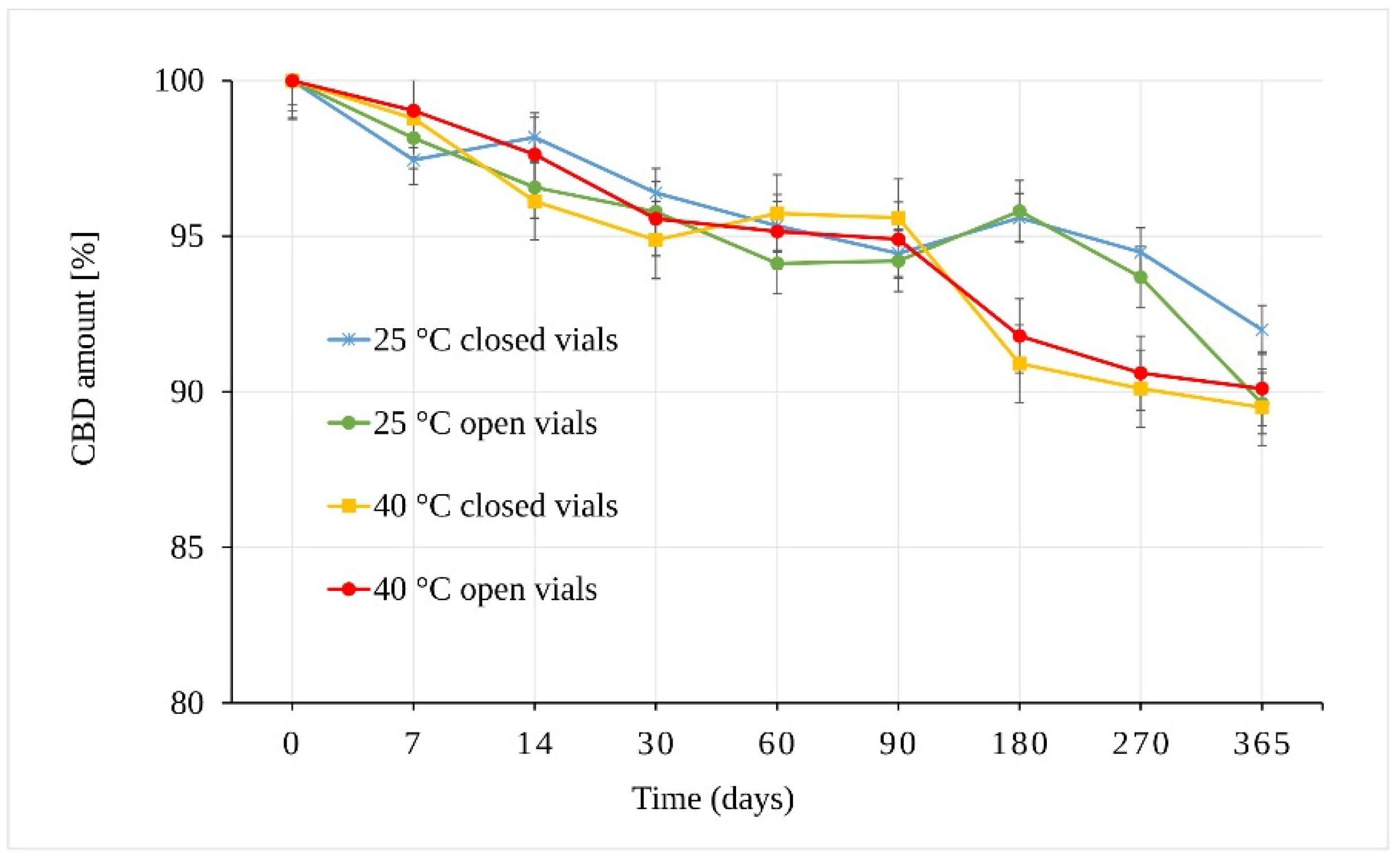
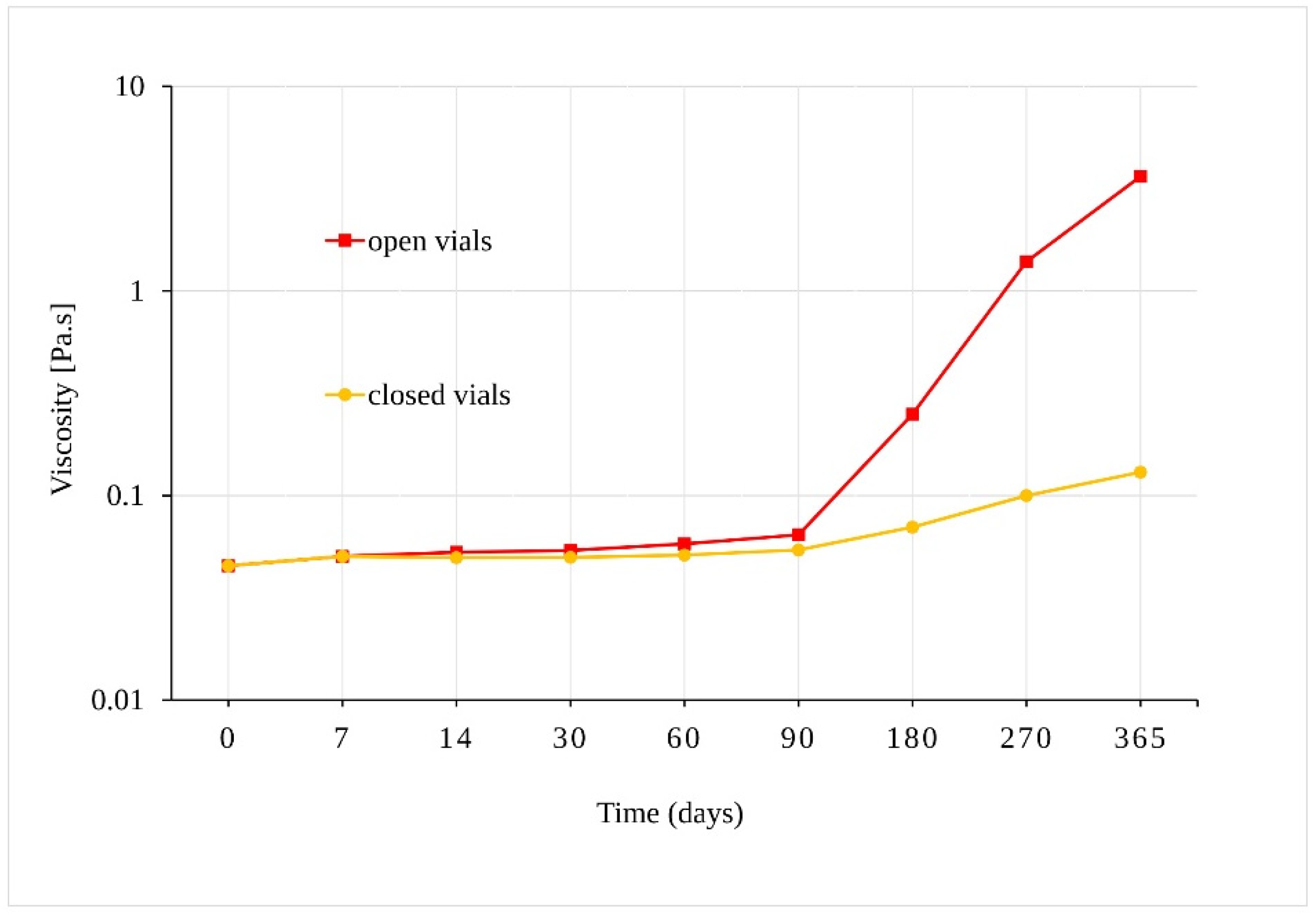
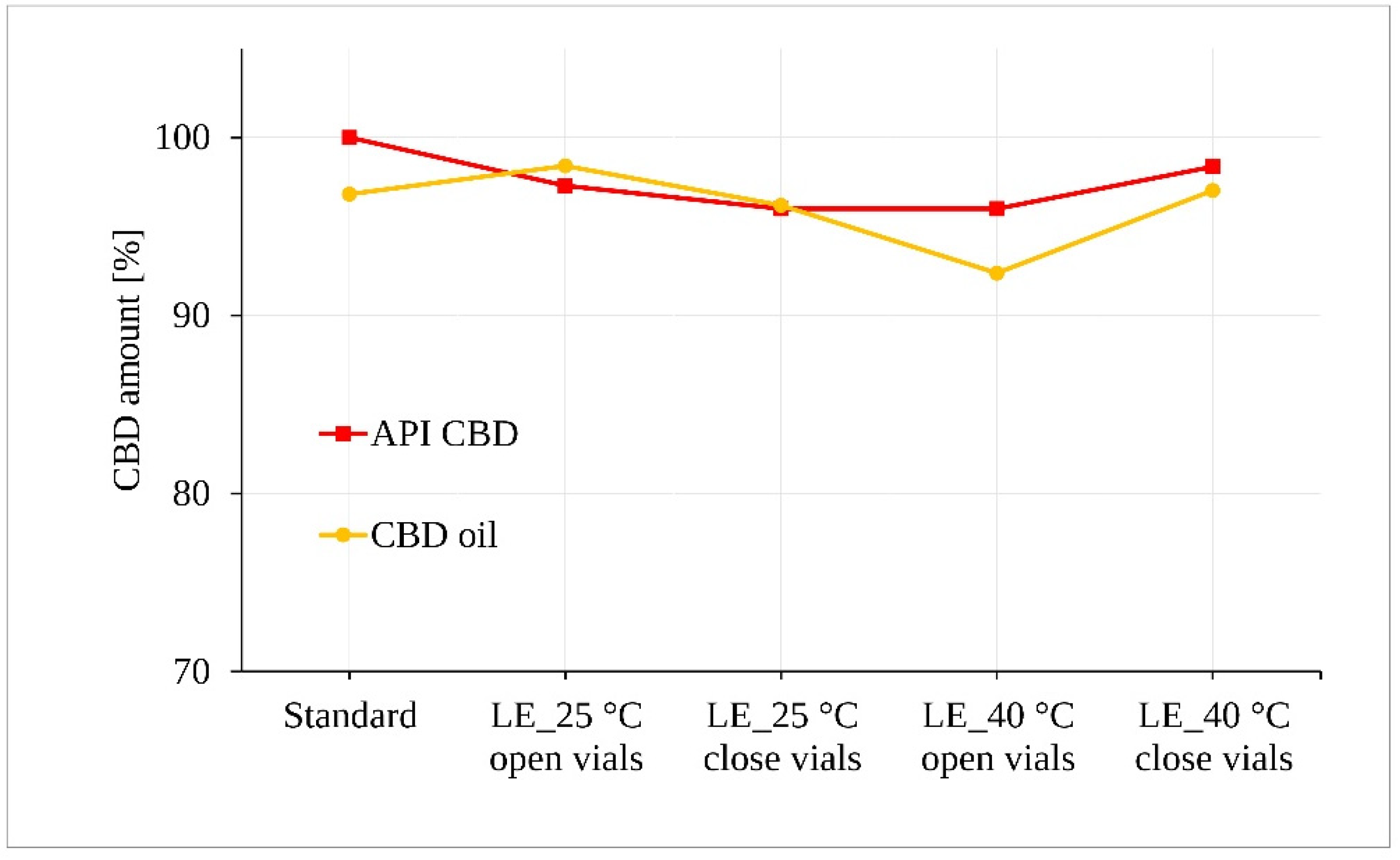
3.2. Evaluation of CBD Stability Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, G.; Grovey, B.; Furnish, T.; Wallace, M. Medical Cannabis for Neuropathic Pain. Curr. Pain Headache Rep. 2018, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Tomida, I.; Pertwee, R.G.; Azuara-Blanco, A. Cannabinoids and glaucoma. Br. J. Ophthalmol. 2004, 88, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.A.; Sansone, L.A. Marijuana and body weight. Innov. Clin. Neurosci. 2014, 11, 50–54. [Google Scholar] [PubMed]
- Parker, L.A.; Rock, E.M.; Limebeer, C.L. Regulation of nausea and vomiting by cannabinoids. Br. J. Pharmacol. 2011, 163, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardo, F.P.; Pichini, S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, P.M.; Brands, B.; Ekwere, I.T.; Elliott, S.; Gysling, K.; Jain, R.; Kaduri, P.; Kitanaka, J.; Rahimi-Movaghar, A.; Nudmamud-Thanoi, S.; et al. WHO Expert Committee on Drug Dependance: No. 1013, Cannabidiol; WHO Press: Geneva, Switzerland, 2018; pp. 1–27. [Google Scholar]
- Szaflarski, J.P.; Bebin, E.M.; Comi, A.M.; Patel, A.D.; Joshi, C.; Checketts, D.; Beal, J.C.; Laux, L.C.; De Boer, L.M.; Wong, M.H.; et al. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results. Epilepsia 2018, 59, 1540–1548. [Google Scholar] [CrossRef]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkotter, J.; Hellmich, M.R.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, 94. [Google Scholar] [CrossRef]
- Sulé-Suso, J.; Watson, N.A.; Van Pittius, D.G.; Jegannathen, A. Striking lung cancer response to self-administration of cannabidiol: A case report and literature review. SAGE Open Med. Case Rep. 2019, 7, 2050313X19832160. [Google Scholar] [CrossRef]
- Martinez-Martinez, E.; Martin-Ruiz, A.; Martin, P.; Calvo, V.; Provencio, M.; Garcia, J.M. CB2 cannabinoid receptor activation promotes colon cancer progression via AKT/GSK3beta signaling pathway. Oncotarget 2016, 7, 68781–68791. [Google Scholar] [CrossRef]
- Lindholst, C. Long term stability of cannabis resin and cannabis extracts. Aust. J. Forensic Sci. 2010, 42, 181–190. [Google Scholar] [CrossRef]
- Carbone, M.; Castelluccio, F.; Daniele, A.; Sutton, A.; Ligresti, A.; Di Marzo, V.; Gavagnin, M. Chemical characterisation of oxidative degradation products of Δ9-THC. Tetrahedron 2010, 66, 9497–9501. [Google Scholar] [CrossRef]
- Layton, C.; Runco, J.; Aubin, A. Forced Degradation of Cannabidiol; Waters Corporation: Milford, MA, USA, 2016; Volume 6, p. 720005766EN. [Google Scholar]
- International Conference on Harmonization Guideline: Impurities: Guideline for Residual Solvents Q3C (R5); ICH Steering Committee: London, UK, 2005; Volume 4, pp. 1–25. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-33.pdf (accessed on 25 July 2020).
- Guidance for Industry Q1A(R2): Stability Testing of New Drug Substances and Products; US. Food and Drug Administration: Rockville, MD, USA, 2003. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q1ar2-stability-testing-new-drug-substances-and-products (accessed on 25 July 2020).
- Palazzoli, F.; Citti, C.; Licata, M.; Vilella, A.; Manca, L.; Zoli, M.; Vandelli, M.A.; Forni, F.; Cannazza, G. Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC–MS/MS) method for the determination of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of a single high dose of CBD. J. Pharm. Biomed. Anal. 2018, 150, 25–32. [Google Scholar] [PubMed]
- Citti, C.; Ciccarella, G.; Braghiroli, D.; Parenti, C.; Vandelli, M.A.; Cannazza, G. Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J. Pharm. Biomed. Anal. 2016, 128, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Deidda, R.; Avohou, H.T.; Baronti, R.; Davolio, P.L.; Pasquini, B.; Del Bubba, M.; Hubert, C.; Hubert, P.; Orlandini, S.; Furlanetto, S. Analytical quality by design: Development and control strategy for a LC method to evaluate the cannabinoids content in cannabis olive oil extracts. J. Pharm. Biomed. Anal. 2019, 166, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Nemeškalová, A.; Hajkova, K.; Mikulu, L.; Sykora, D.; Kuchar, M. Combination of UV and MS/MS detection for the LC analysis of cannabidiol-rich products. Talanta 2020, 219, 121250. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.; Sabri, A.; Fischer, P.M.; Barrett, D.A.; Constantinescu, C.S.; Gershkovich, P. Development of a simple and sensitive HPLC-UV method for the simultaneous determination of cannabidiol and Delta(9)-tetrahydrocannabinol in rat plasma. J. Pharm. Biomed. Anal. 2015, 114, 145–151. [Google Scholar] [CrossRef]
- Turner, C.E.; Hadley, K.W.; Fetterman, P.S.; Doorenbos, N.J.; Quimby, M.W.; Waller, C. Constituents of Cannabis sativa L. IV: Stability of cannabinoids in stored plant material. J. Pharm. Sci. 1973, 62, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Trofin, I.; Dabija, G.; Vaireanu, D.I.; Laurentiu, F. The influence of long-term storage conditions on the stability of cannabinoids derived from cannabis resin. Rev. Chim. 2012, 63, 422–427. [Google Scholar]
- Maskan, M. Change in colour and rheological behaviour of sunflower seed oil during frying after adsorbent treatment of used oil. Eur. Food Res. Technol. 2003, 218, 20–25. [Google Scholar] [CrossRef]
- Tan, Y.A.; Ong, S.H.; Berger, K.G.; Oon, H.H.; Poh, L.B. A study of the cause of rapid color development of heated refined palm oil. J. Am. Oil Chem. Soc. 1985, 62, 999–1006. [Google Scholar] [CrossRef]
- Stevenson, S.G.; Vaisey-Genser, M.; Eskin, N.A.M. Quality control in the use of deep frying oils. J. Am. Oil Chem. Soc. 1984, 61, 1102–1108. [Google Scholar] [CrossRef]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality traits of “Cannabidiol oils”: Cannabinoids tontent, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, J.W.; Liebmann, J.A.; Rowan, M.G. The stability of cannabis and its preparations on storage. J. Pharm. Pharmacol. 1976, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Henry, J.T. Constituents of Cannabis sativa L. IX: Stability of synthetic and naturally occurring cannabinoids in chloroform. J. Pharm. Sci. 1975, 64, 357–359. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosović, E.; Sýkora, D.; Kuchař, M. Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics 2021, 13, 412. https://doi.org/10.3390/pharmaceutics13030412
Kosović E, Sýkora D, Kuchař M. Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics. 2021; 13(3):412. https://doi.org/10.3390/pharmaceutics13030412
Chicago/Turabian StyleKosović, Ema, David Sýkora, and Martin Kuchař. 2021. "Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution" Pharmaceutics 13, no. 3: 412. https://doi.org/10.3390/pharmaceutics13030412
APA StyleKosović, E., Sýkora, D., & Kuchař, M. (2021). Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics, 13(3), 412. https://doi.org/10.3390/pharmaceutics13030412