RETRACTED: Pyrvinium Treatment Confers Hepatic Metabolic Benefits via β-Catenin Downregulation and AMPK Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Dual-luciferase Assa
2.3. Western Blot Analysis
2.4. Quantitative Real-Time PCR Analysis
2.5. Gene Knockdown and Overexpression
2.6. Glucose Production Assay
2.7. Nile Red Staining
2.8. Animal Studies
2.9. Statistical Analysis
3. Results
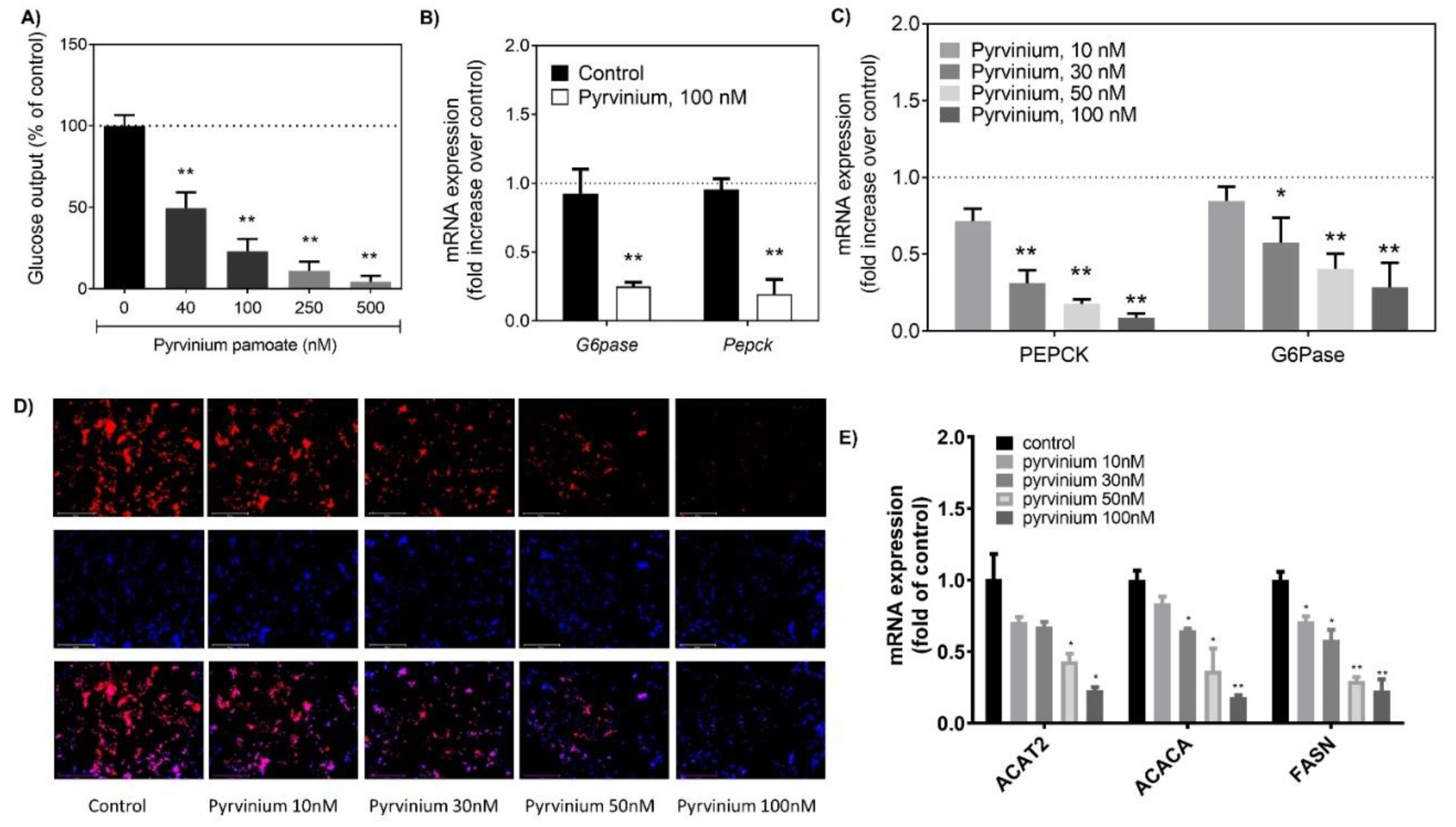
3.1. Pyrvinium Decreased Gluconeogenesis and Lipogenesis in Hepatocytes
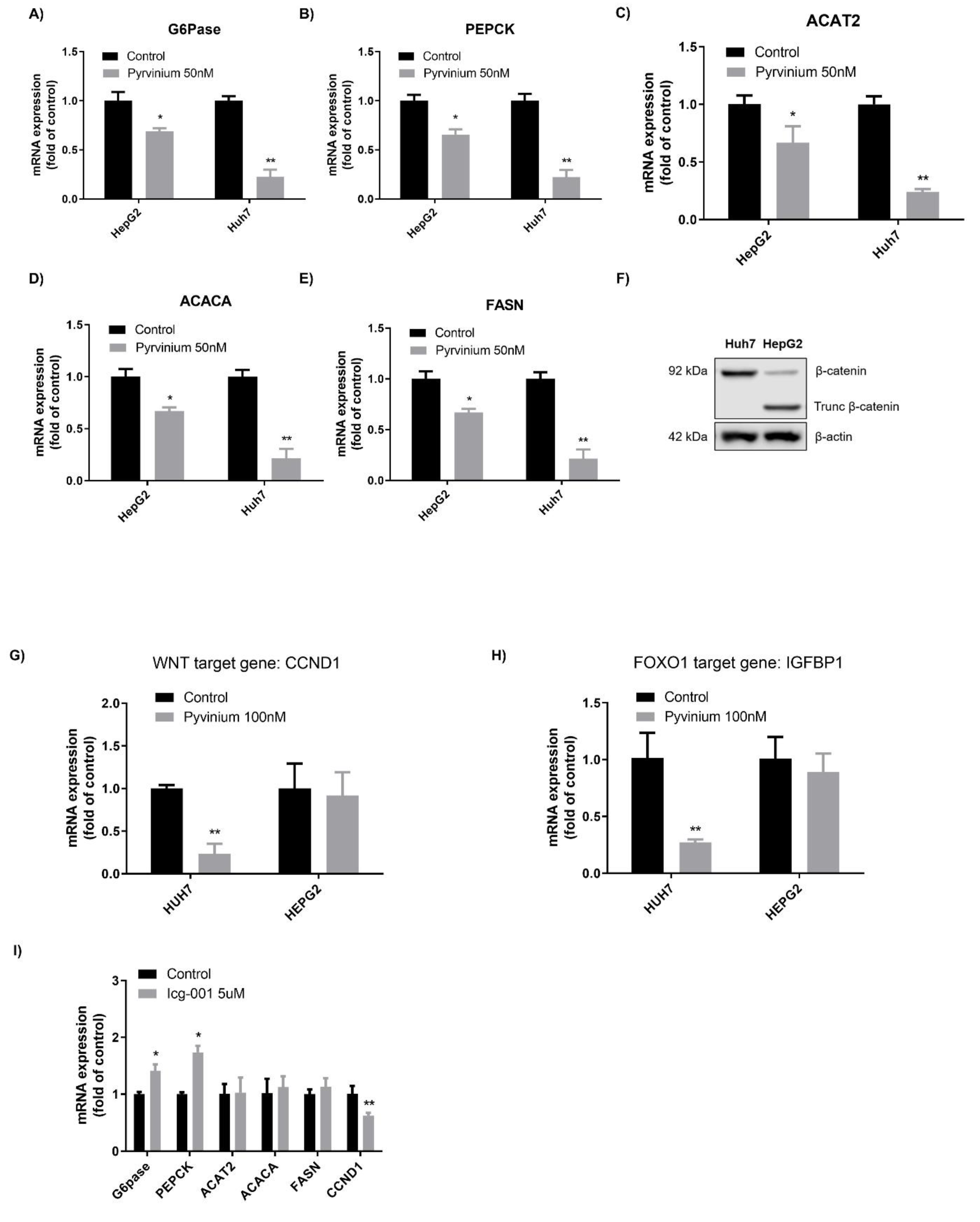
3.2. Decrease of Gluconeogenesis and Lipogenesis by Pyrvinium Involved β-catenin Downregulation
3.3. Effects of Pyrvinium on Gluconeogenesis and Lipogenesis Were Affected by β-catenin Mutation
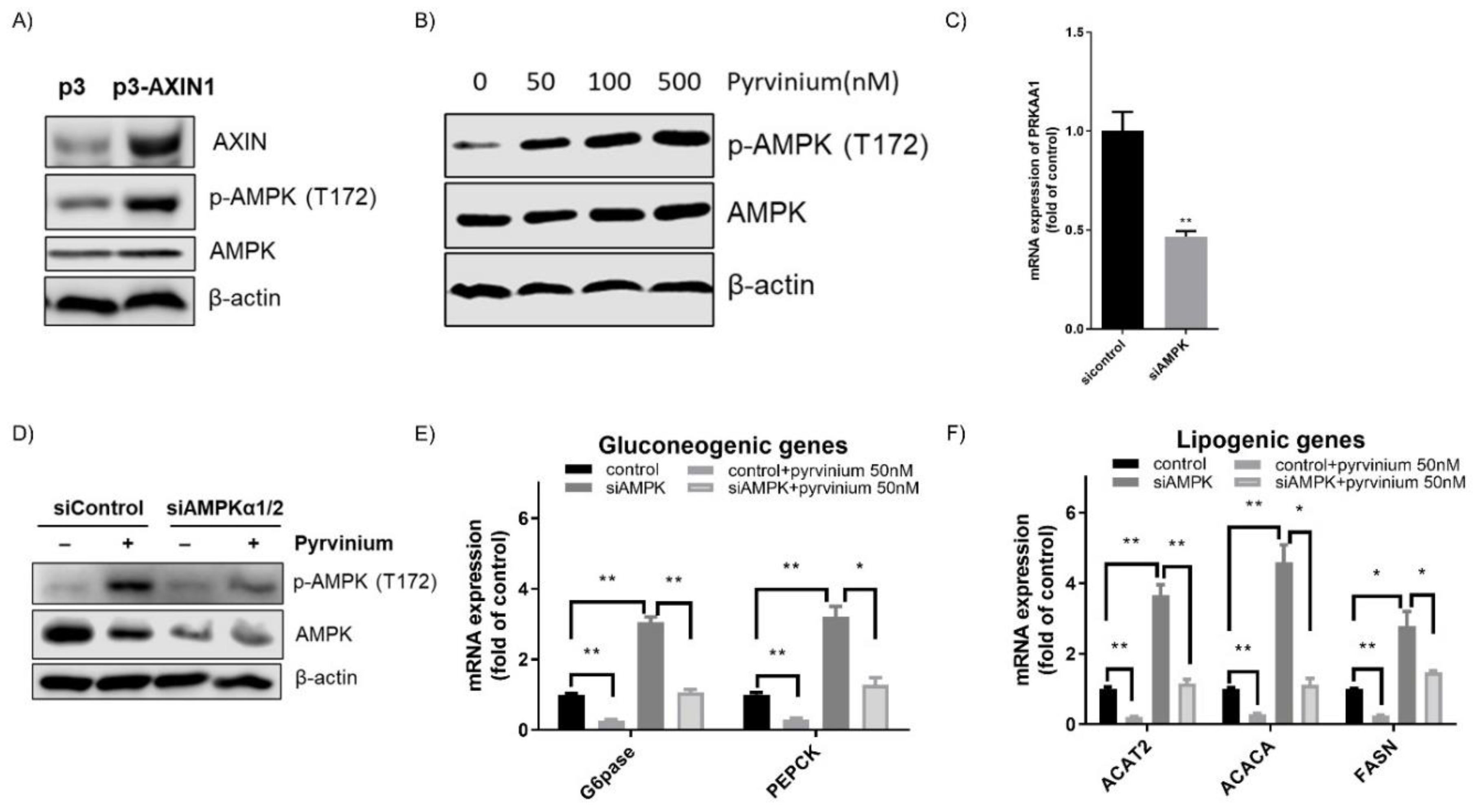
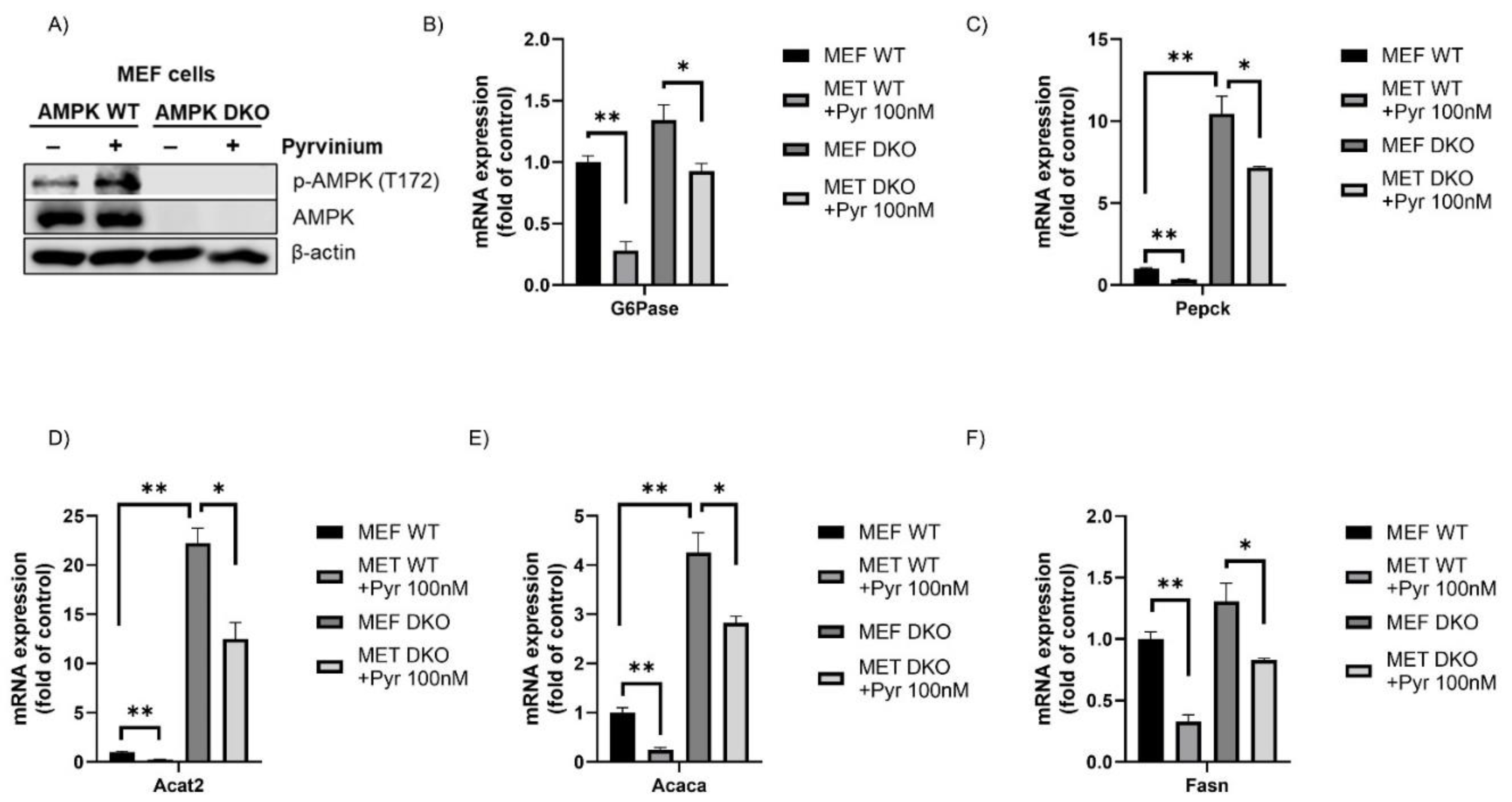
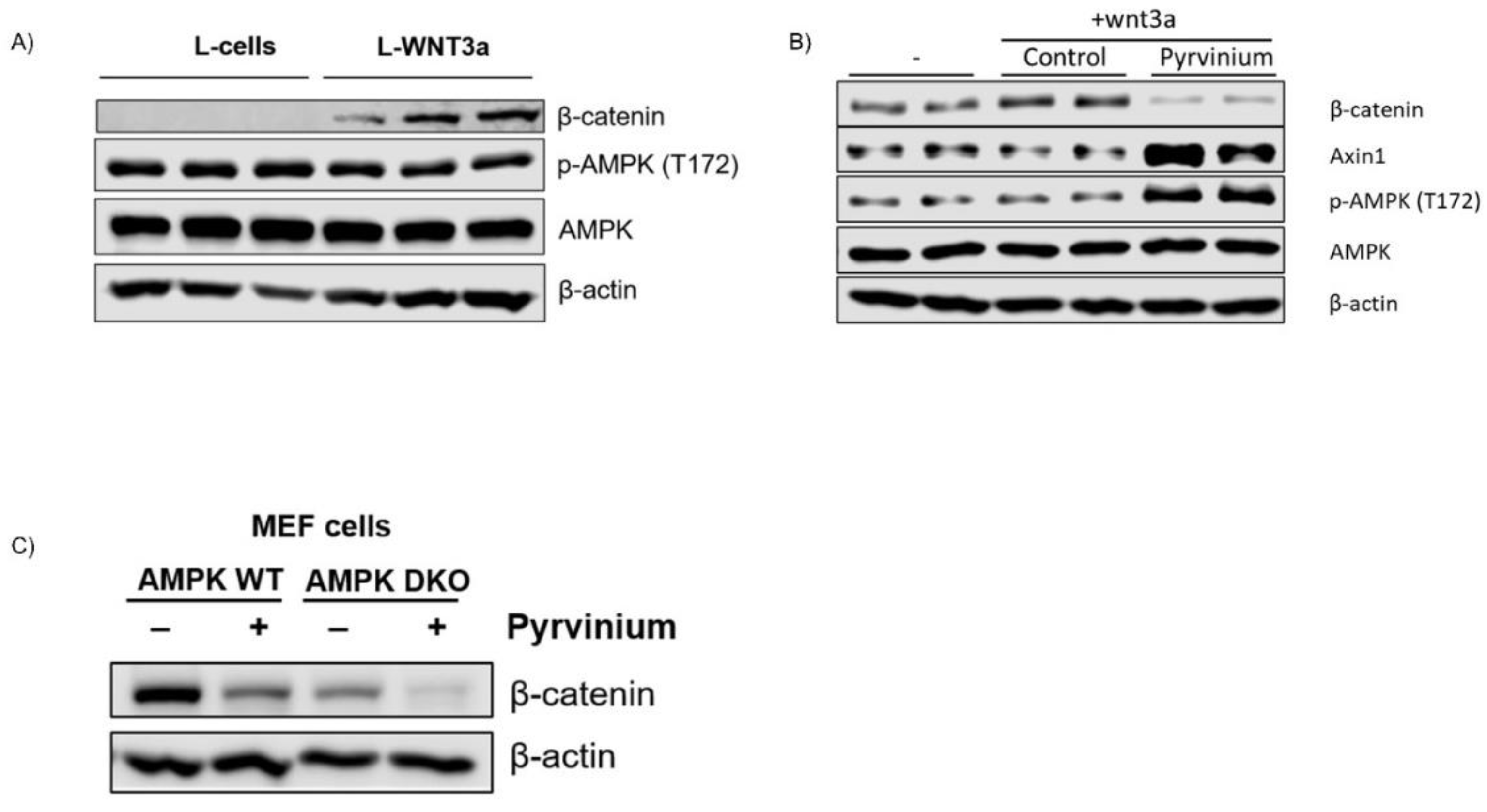
3.4. Pyrvinium Treatment Led to the Activation of AMPK, a Master Regulator of Cellular Metabolism
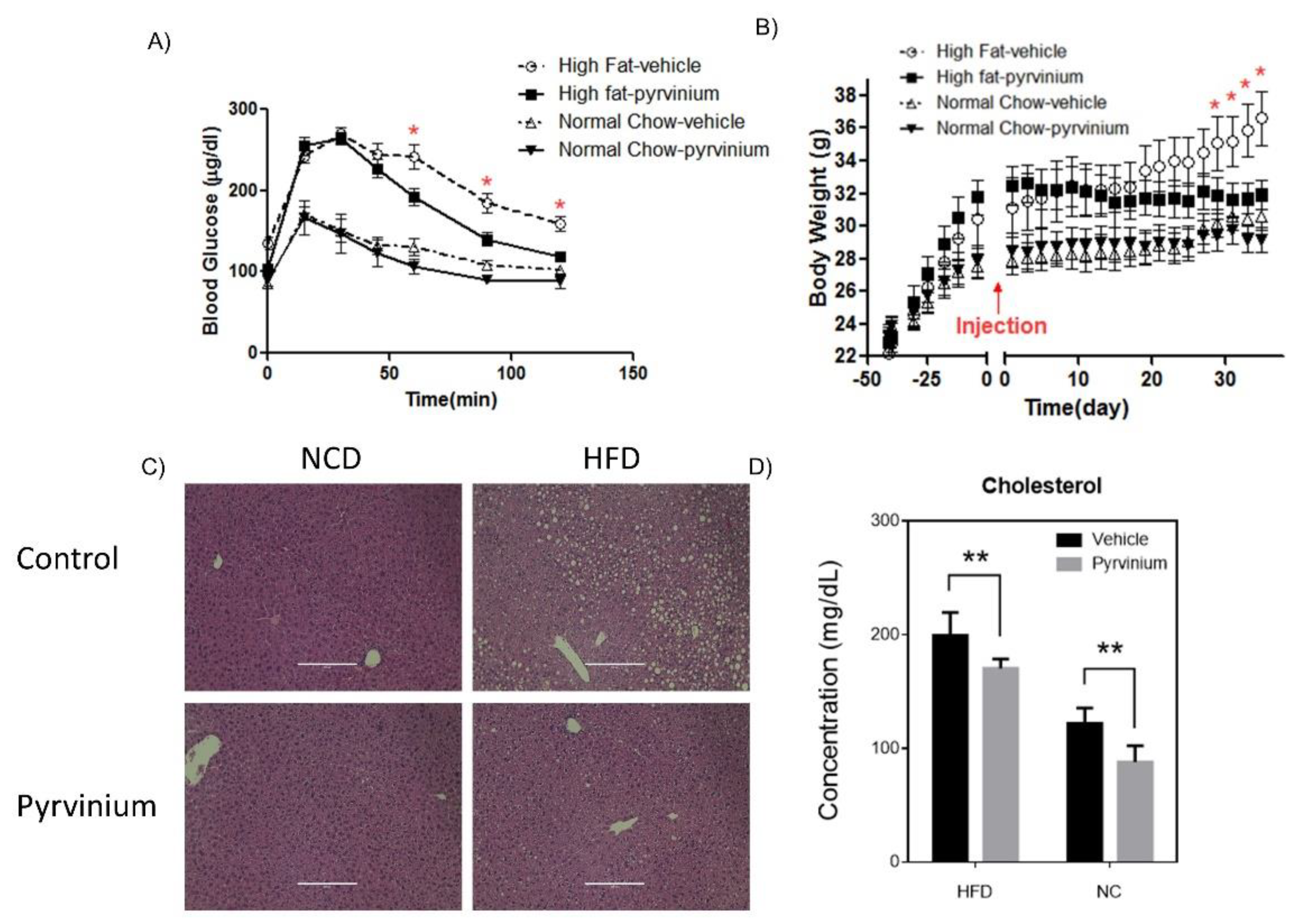
3.5. Efficacy of Pyrvinium in Treatment of High Fat Diet-Induced Metabolic Disorders in Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owen, O.E.; Reichard, G.A., Jr.; Patel, M.S.; Boden, G. Energy metabolism in feasting and fasting. Adv. Exp. Med. Biol. 1979, 111, 169–188. [Google Scholar] [CrossRef]
- Behari, J.; Li, H.; Liu, S.; Stefanovic-Racic, M.; Alonso, L.; O’Donnell, C.P.; Shiva, S.; Singamsetty, S.; Watanabe, Y.; Singh, V.P.; et al. beta-catenin links hepatic metabolic zonation with lipid metabolism and diet-induced obesity in mice. Am. J. Pathol. 2014, 184, 3284–3298. [Google Scholar] [CrossRef]
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009, 13, 9–19. [Google Scholar]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Essers, M.A.; de Vries-Smits, L.M.; Barker, N.; Polderman, P.E.; Burgering, B.M.; Korswagen, H.C. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 2005, 308, 1181–1184. [Google Scholar] [CrossRef]
- Morris, S.L.; Huang, S. Crosstalk of the Wnt/beta-catenin pathway with other pathways in cancer cells. Genes Dis. 2016, 3, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Guo, Y.F.; Xiong, D.H.; Shen, H.; Zhao, L.J.; Xiao, P.; Guo, Y.; Wang, W.; Yang, T.L.; Recker, R.R.; Deng, H.W. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J. Med. Genet. 2006, 43, 798–803. [Google Scholar] [CrossRef]
- Yi, F.; Sun, J.; Lim, G.E.; Fantus, I.G.; Brubaker, P.L.; Jin, T. Cross talk between the insulin and Wnt signaling pathways: Evidence from intestinal endocrine L cells. Endocrinology 2008, 149, 2341–2351. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, L.; Hunter, D.J.; Meigs, J.B.; Manson, J.E.; van Dam, R.M.; Hu, F.B. Variant of transcription factor 7-like 2 (TCF7L2) gene and the risk of type 2 diabetes in large cohorts of U.S. women and men. Diabetes 2006, 55, 2645–2648. [Google Scholar] [CrossRef]
- Cauchi, S.; Meyre, D.; Dina, C.; Choquet, H.; Samson, C.; Gallina, S.; Balkau, B.; Charpentier, G.; Pattou, F.; Stetsyuk, V.; et al. Transcription factor TCF7L2 genetic study in the French population: Expression in human beta-cells and adipose tissue and strong association with type 2 diabetes. Diabetes 2006, 55, 2903–2908. [Google Scholar] [CrossRef]
- Grant, S.F.; Thorleifsson, G.; Reynisdottir, I.; Benediktsson, R.; Manolescu, A.; Sainz, J.; Helgason, A.; Stefansson, H.; Emilsson, V.; Helgadottir, A.; et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006, 38, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef]
- Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997, 16, 3797–3804. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Miao, Z.; Xu, X.; Liu, C.F. XAV939, a small molecular inhibitor, provides neuroprotective effects on oligodentrocytes. J. Neurosci. Res. 2014, 92, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Obianom, O.N.; Ai, Y.; Li, Y.; Yang, W.; Guo, D.; Yang, H.; Sakamuru, S.; Xia, M.; Xue, F.; Shu, Y. Triazole-Based Inhibitors of the Wnt/beta-Catenin Signaling Pathway Improve Glucose and Lipid Metabolisms in Diet-Induced Obese Mice. J. Med. Chem. 2019, 62, 727–741. [Google Scholar] [CrossRef]
- Rossi, M.; Ruiz de Azua, I.; Barella, L.F.; Sakamoto, W.; Zhu, L.; Cui, Y.; Lu, H.; Rebholz, H.; Matschinsky, F.M.; Doliba, N.M.; et al. CK2 acts as a potent negative regulator of receptor-mediated insulin release in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, E6818–E6824. [Google Scholar] [CrossRef] [PubMed]
- Thorne, C.A.; Hanson, A.J.; Schneider, J.; Tahinci, E.; Orton, D.; Cselenyi, C.S.; Jernigan, K.K.; Meyers, K.C.; Hang, B.I.; Waterson, A.G.; et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat. Chem. Biol. 2010, 6, 829–836. [Google Scholar] [CrossRef]
- Saraswati, S.; Alfaro, M.P.; Thorne, C.A.; Atkinson, J.; Lee, E.; Young, P.P. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS ONE 2010, 5, e15521. [Google Scholar] [CrossRef] [PubMed]
- Sudigbia, I.; Notokarsono, S.; Darmawan, S.; Tjoen, L.W. Single-dose treatment of oxyuriasis with pyrvinium pamoate (Vanquin). Paediatr. Indones 1970, 10, 125–130. [Google Scholar]
- Li, W.C.; Ralphs, K.L.; Tosh, D. Isolation and culture of adult mouse hepatocytes. Methods Mol. Biol. 2010, 633, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P.; Consortium, N.I.H.M.M.P.C. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef]
- De La Coste, A.; Romagnolo, B.; Billuart, P.; Renard, C.A.; Buendia, M.A.; Soubrane, O.; Fabre, M.; Chelly, J.; Beldjord, C.; Kahn, A.; et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc. Natl. Acad. Sci. USA 1998, 95, 8847–8851. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Liu, H.; Fergusson, M.M.; Wu, J.J.; Rovira, I.I.; Liu, J.; Gavrilova, O.; Lu, T.; Bao, J.; Han, D.; Sack, M.N. Wnt signaling regulates hepatic metabolism. Sci. Signal. 2011, 4, ra6. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Guo, H.; Zhang, C.S.; Lin, S.Y.; Yin, Z.; Peng, Y.; Luo, H.; Shi, Y.; Lian, G.; Zhang, C.; et al. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell Metab. 2013, 18, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Schinzari, V.; Timperi, E.; Pecora, G.; Palmucci, F.; Gallerano, D.; Grimaldi, A.; Covino, D.A.; Guglielmo, N.; Melandro, F.; Manzi, E.; et al. Wnt3a/beta-Catenin Signaling Conditions Differentiation of Partially Exhausted T-effector Cells in Human Cancers. Cancer Immunol Res. 2018, 6, 941–952. [Google Scholar] [CrossRef]
- Zhao, J.; Yue, W.; Zhu, M.J.; Sreejayan, N.; Du, M. AMP-activated protein kinase (AMPK) cross-talks with canonical Wnt signaling via phosphorylation of beta-catenin at Ser 552. Biochem. Biophys. Res. Commun. 2010, 395, 146–151. [Google Scholar] [CrossRef]
- Smith, T.C.; Kinkel, A.W.; Gryczko, C.M.; Goulet, J.R. Absorption of pyrvinium pamoate. Clin. Pharmacol. Ther. 1976, 19, 802–806. [Google Scholar] [CrossRef]
- Elghazi, L.; Gould, A.P.; Weiss, A.J.; Barker, D.J.; Callaghan, J.; Opland, D.; Myers, M.; Cras-Meneur, C.; Bernal-Mizrachi, E. Importance of beta-Catenin in glucose and energy homeostasis. Sci. Rep. 2012, 2, 693. [Google Scholar] [CrossRef]
- Gu, X.; Sun, R.; Chen, L.; Chu, S.; Doll, M.A.; Li, X.; Feng, W.; Siskind, L.; McClain, C.J.; Deng, Z. Neutral ceramidase mediates nonalcoholic steatohepatitis by regulating monounsaturated fatty acids and gut IgA(+) B cells. Hepatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Boj, S.F.; van Es, J.H.; Huch, M.; Li, V.S.; Jose, A.; Hatzis, P.; Mokry, M.; Haegebarth, A.; van den Born, M.; Chambon, P.; et al. Diabetes risk gene and Wnt effector Tcf7l2/TCF4 controls hepatic response to perinatal and adult metabolic demand. Cell 2012, 151, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Lezmi, S.; Wang, H.; Davis, J.; Banz, W.; Chen, H. Fat accumulation in the liver of obese rats is alleviated by soy protein isolate through beta-catenin signaling. Obesity (Silver Spring) 2014, 22, 151–158. [Google Scholar] [CrossRef]
- Venerando, A.; Girardi, C.; Ruzzene, M.; Pinna, L.A. Pyrvinium pamoate does not activate protein kinase CK1, but promotes Akt/PKB down-regulation and GSK3 activation. Biochem. J. 2013, 452, 131–137. [Google Scholar] [CrossRef]
- Shen, C.; Li, B.; Astudillo, L.; Deutscher, M.P.; Cobb, M.H.; Capobianco, A.J.; Lee, E.; Robbins, D.J. The CK1alpha Activator Pyrvinium Enhances the Catalytic Efficiency (kcat/Km) of CK1alpha. Biochemistry 2019, 58, 5102–5106. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef]
- Cha, P.H.; Cho, Y.H.; Lee, S.K.; Lee, J.; Jeong, W.J.; Moon, B.S.; Yun, J.H.; Yang, J.S.; Choi, S.; Yoon, J.; et al. Small-molecule binding of the axin RGS domain promotes beta-catenin and Ras degradation. Nat. Chem. Biol. 2016, 12, 593–600. [Google Scholar] [CrossRef]
- Monga, S.P. Beta-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology 2015, 148, 1294–1310. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, M.H.; Hwang, C.J.; Kim, Y.; Hwang, D.Y.; Han, S.B.; Hong, J.T. Parkin deficiency prevents chronic ethanol-induced hepatic lipid accumulation through beta-catenin accumulation. Cell Commun. Signal. 2019, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, S.; Wang, J.; Fei, F.; Dong, Z.; Ke, A.W.; He, R.; Wang, L.; Zhang, L.; Ji, M.B.; et al. Canonical Wnt Signaling Remodels Lipid Metabolism in Zebrafish Hepatocytes following Ras Oncogenic Insult. Cancer Res. 2018, 78, 5548–5560. [Google Scholar] [CrossRef]
- Tian, L.; Shao, W.; Ip, W.; Song, Z.; Badakhshi, Y.; Jin, T. The developmental Wnt signaling pathway effector beta-catenin/TCF mediates hepatic functions of the sex hormone estradiol in regulating lipid metabolism. PLoS Biol. 2019, 17, e3000444. [Google Scholar] [CrossRef]
- Seo, M.H.; Lee, J.; Hong, S.W.; Rhee, E.J.; Park, S.E.; Park, C.Y.; Oh, K.W.; Park, S.W.; Lee, W.Y. Exendin-4 Inhibits Hepatic Lipogenesis by Increasing beta-Catenin Signaling. PLoS ONE 2016, 11, e0166913. [Google Scholar] [CrossRef]
- Ip, W.; Shao, W.; Chiang, Y.T.; Jin, T. The Wnt signaling pathway effector TCF7L2 is upregulated by insulin and represses hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1166–E1176. [Google Scholar] [CrossRef]
- Matsumoto, M.; Han, S.; Kitamura, T.; Accili, D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J. Clin. Investig. 2006, 116, 2464–2472. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Z.; Jiang, S.; Hu, W.; Li, T.; Di, S.; Wang, D.; Yang, Y. A global perspective on FOXO1 in lipid metabolism and lipid-related diseases. Prog. Lipid Res. 2017, 66, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liong, E.C.; Ching, Y.P.; Chang, R.C.; Fung, M.L.; Xu, A.M.; So, K.F.; Tipoe, G.L. Lycium barbarum polysaccharides protect rat liver from non-alcoholic steatohepatitis-induced injury. Nutr. Diabetes 2013, 3, e81. [Google Scholar] [CrossRef]
- Xiao, J.; Ho, C.T.; Liong, E.C.; Nanji, A.A.; Leung, T.M.; Lau, T.Y.; Fung, M.L.; Tipoe, G.L. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur. J. Nutr. 2014, 53, 187–199. [Google Scholar] [CrossRef]
- Nakae, J.; Kitamura, T.; Silver, D.L.; Accili, D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Investig. 2001, 108, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Cheruvu, V.K.; Ismail-Beigi, F. Stimulation of glucose transport in response to activation of distinct AMPK signaling pathways. Am. J. Physiol. Cell Physiol. 2008, 295, C1071–C1082. [Google Scholar] [CrossRef]
- Pyun, D.H.; Kim, T.J.; Kim, M.J.; Hong, S.A.; Abd El-Aty, A.M.; Jeong, J.H.; Jung, T.W. Endogenous metabolite, kynurenic acid, attenuates nonalcoholic fatty liver disease via AMPK/autophagy- and AMPK/ORP150-mediated signaling. J. Cell Physiol. 2020. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, H.W.; Rhyu, I.J.; Kee, S.H. Axin is expressed in mitochondria and suppresses mitochondrial ATP synthesis in HeLa cells. Exp. Cell Res. 2016, 340, 12–21. [Google Scholar] [CrossRef]
- Teguh, S.C.; Klonis, N.; Duffy, S.; Lucantoni, L.; Avery, V.M.; Hutton, C.A.; Baell, J.B.; Tilley, L. Novel conjugated quinoline-indoles compromise Plasmodium falciparum mitochondrial function and show promising antimalarial activity. J. Med. Chem. 2013, 56, 6200–6215. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Ouyang, H.; Zhu, T.; Lindvall, C.; Wang, Y.; Zhang, X.; Yang, Q.; Bennett, C.; Harada, Y.; Stankunas, K.; et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006, 126, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Y.; Ai, Y.; Obianom, O.N.; Guo, D.; Yang, H.; Sakamuru, S.; Xia, M.; Shu, Y.; Xue, F. Pyrazole-4-Carboxamide (YW2065): A Therapeutic Candidate for Colorectal Cancer via Dual Activities of Wnt/beta-Catenin Signaling Inhibition and AMP-Activated Protein Kinase (AMPK) Activation. J. Med. Chem. 2019, 62, 11151–11164. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lacerda, L.; Debeb, B.G.; Atkinson, R.L.; Solley, T.N.; Li, L.; Orton, D.; McMurray, J.S.; Hang, B.I.; Lee, E.; et al. The antihelmintic drug pyrvinium pamoate targets aggressive breast cancer. PLoS ONE 2013, 8, e71508. [Google Scholar] [CrossRef]

| Genes | Human | |
|---|---|---|
| Forward | Reverse | |
| AXIN1 | GACCTGGGGTATGAGCCTGA | GGCTTATCCCATCTTGGTCATC |
| CSNK1A1 | AGTGGCAGTGAAGCTAGAATCT | CGCCCAATACCCATTAGGAAGTT |
| CTNNB1 | CATCTACACAGTTTGATGCTGCT | GCAGTTTTGTCAGTTCAGGGA |
| PEPCK | GTCAGCCTGATCACATCCACA | CCGTCTTGCTTTCGATCCTG |
| G6Pase | CTACTACAGCAACACTTCCGTG | GGTCGGCTTTATCTTTCCCTGA |
| CYCD1 | CAATGACCCCGCACGATTTC | CATGGAGGGCGGATTGGAA |
| IGFBP1 | ACCTATGATGGCTCGAAGGC | ATGGATGTCTCACACTGTCTGC |
| ACAT2 | GCGGACCATCATAGGTTCCTT | ACTGGCTTGTCTAACAGGATTCT |
| ACACA | CTCTTGGCCTTTTCCCGGTC | GCCAGACATGCTGGACCTTA |
| FASN | GCAAGCTGAAGGACCTGTCT | AATCTGGGTTGATGCCTCCG |
| PRKAA1 | ACAGCCGAGAAGCAGAAACA | GGGCCTGCATACAATCTTCCT |
| Mouse | ||
| G6Pase | TGTTGCTGTAGTAGTCGGTGTCC | CATCAATCTCCTCTGGGTGGC |
| Pepck | GCAGTGAGGAAGTTCGTGGA | TACATGGTGCGGCCTTTCAT |
| Acat2 | AAACCCCGAGAAGGTCAACAT | GCTGCCCACCTTTCAACAAT |
| Acaca | GTGGTACCTGGCTGCTAGTC | GCCAGACATGCTGGATCTCAT |
| Fasn | AACCTCTCCCAGGTGTGTG | AGAGACTGAACCGAAGGCAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Obianom, O.N.; Huang, J.; Guo, D.; Yang, H.; Li, Q.; Shu, Y. RETRACTED: Pyrvinium Treatment Confers Hepatic Metabolic Benefits via β-Catenin Downregulation and AMPK Activation. Pharmaceutics 2021, 13, 330. https://doi.org/10.3390/pharmaceutics13030330
Zhou S, Obianom ON, Huang J, Guo D, Yang H, Li Q, Shu Y. RETRACTED: Pyrvinium Treatment Confers Hepatic Metabolic Benefits via β-Catenin Downregulation and AMPK Activation. Pharmaceutics. 2021; 13(3):330. https://doi.org/10.3390/pharmaceutics13030330
Chicago/Turabian StyleZhou, Shiwei, Obinna N. Obianom, Jiangsheng Huang, Dong Guo, Hong Yang, Qing Li, and Yan Shu. 2021. "RETRACTED: Pyrvinium Treatment Confers Hepatic Metabolic Benefits via β-Catenin Downregulation and AMPK Activation" Pharmaceutics 13, no. 3: 330. https://doi.org/10.3390/pharmaceutics13030330
APA StyleZhou, S., Obianom, O. N., Huang, J., Guo, D., Yang, H., Li, Q., & Shu, Y. (2021). RETRACTED: Pyrvinium Treatment Confers Hepatic Metabolic Benefits via β-Catenin Downregulation and AMPK Activation. Pharmaceutics, 13(3), 330. https://doi.org/10.3390/pharmaceutics13030330








