Oral Proteasomal Inhibitors Ixazomib, Oprozomib, and Delanzomib Upregulate the Function of Organic Anion Transporter 3 (OAT3): Implications in OAT3-Mediated Drug-Drug Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Transport Measurement
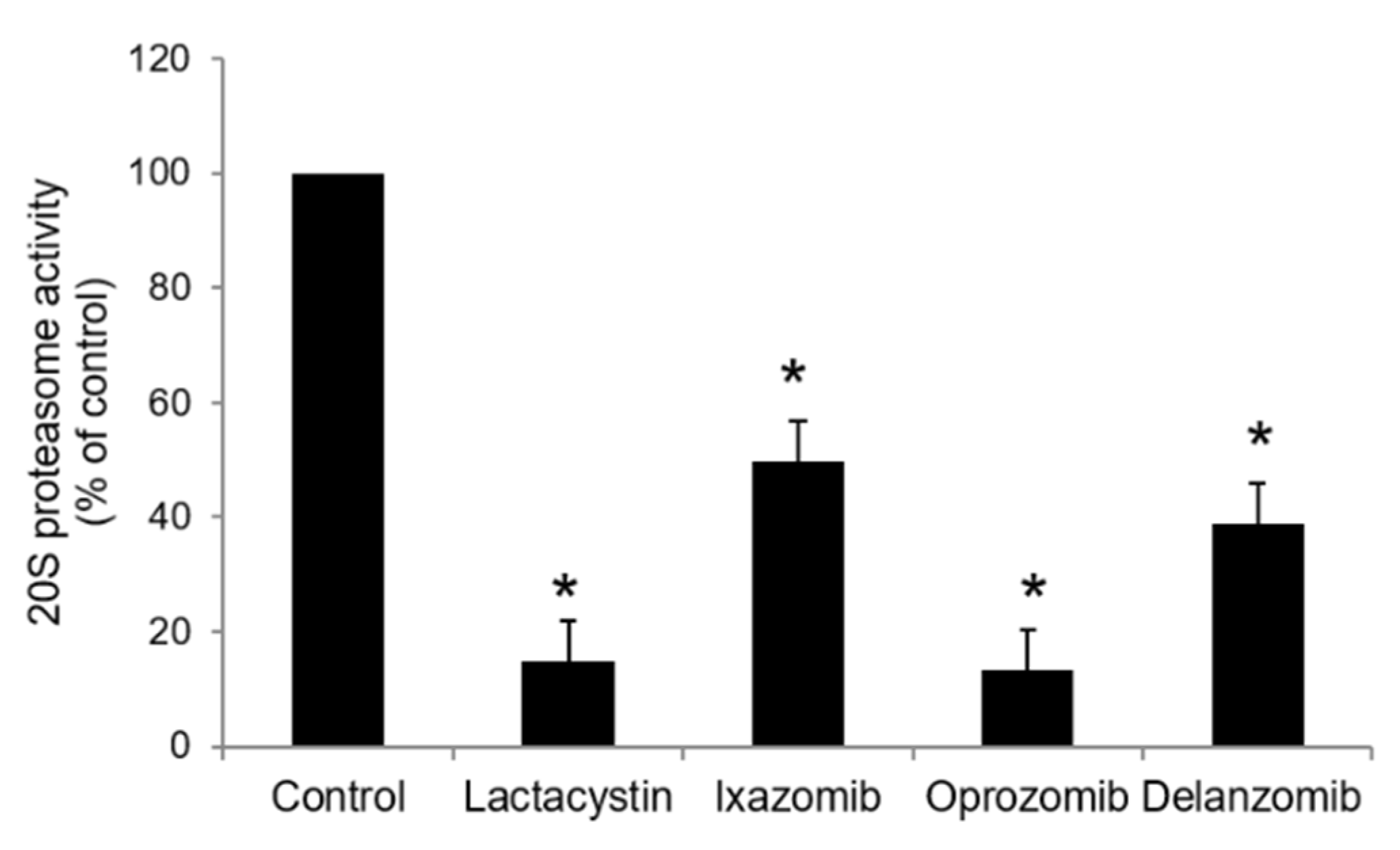
2.4. 20S Proteasome Activity Assay
2.5. Cell-Surface Biotinylation
2.6. Immunoprecipitation
2.7. Degradation Assay of OAT3
2.8. Electrophoresis and Immunoblotting
2.9. Data Analysis
3. Results
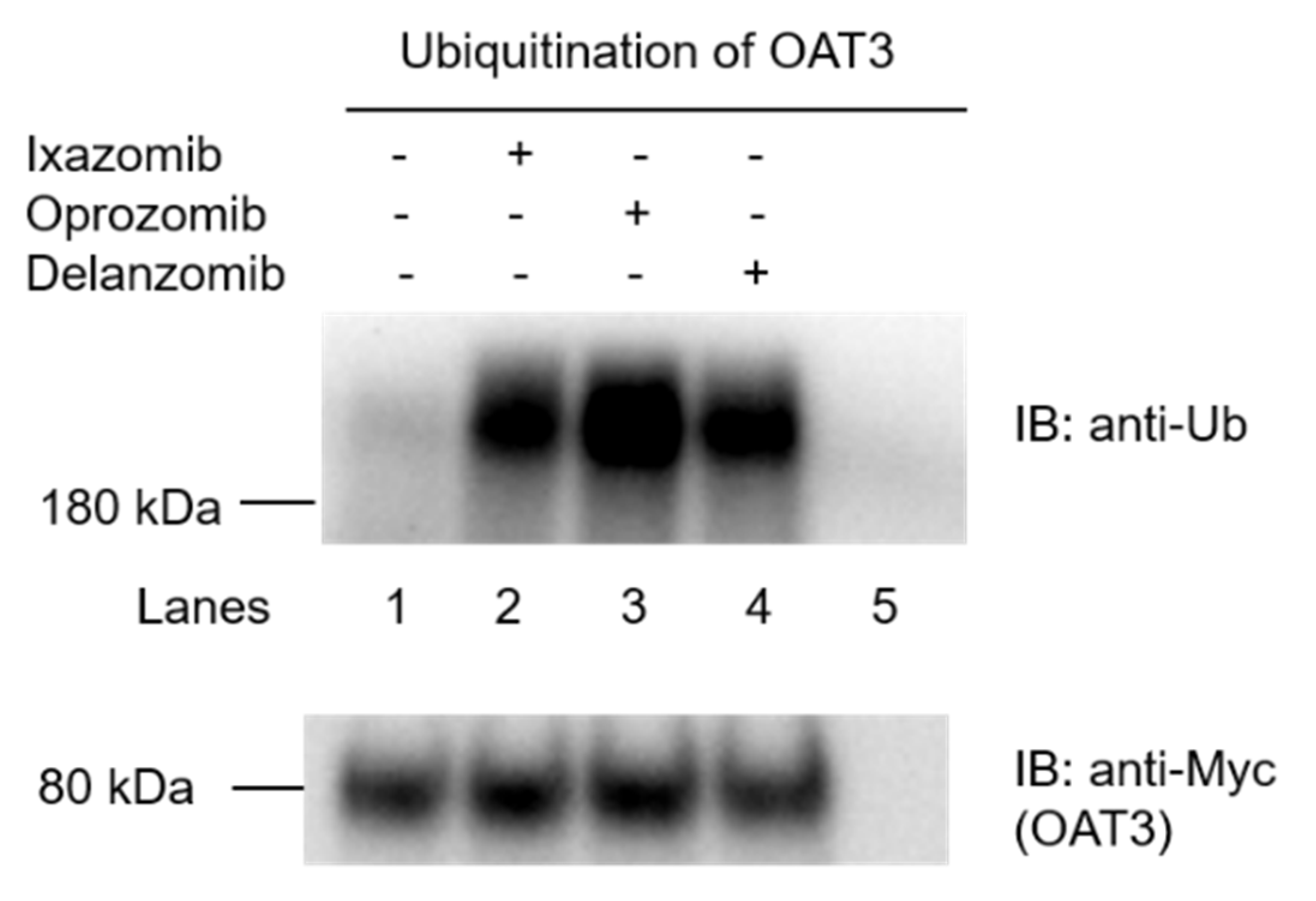
3.1. Effects of Ixazomib, Oprozomib, and Delanzomib on the Ubiquitination of OAT3
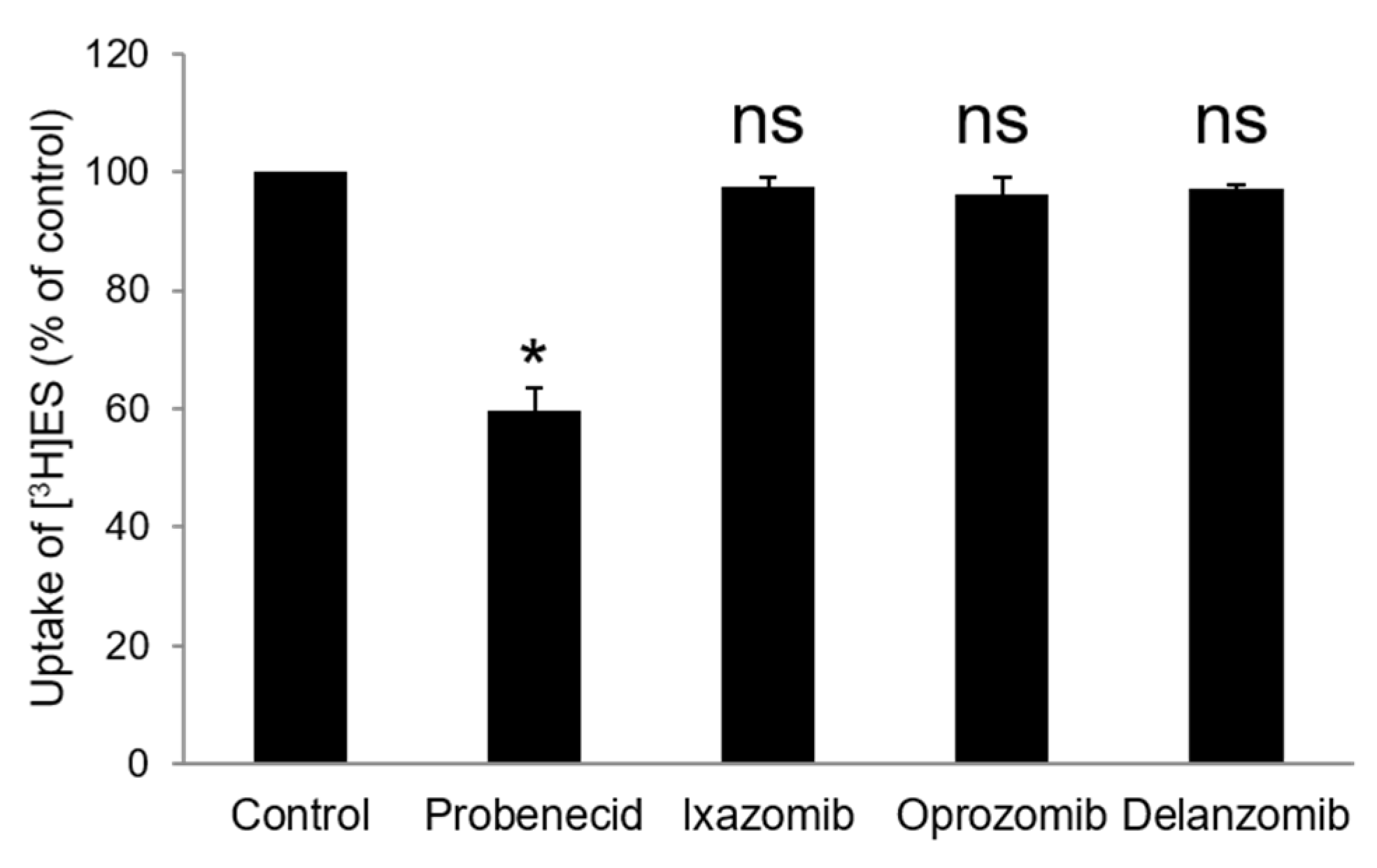
3.2. Cis-Effect of Ixazomib, Oprozomib, or Delanzomib on OAT3-Mediated Uptake of Estrone Sulfate
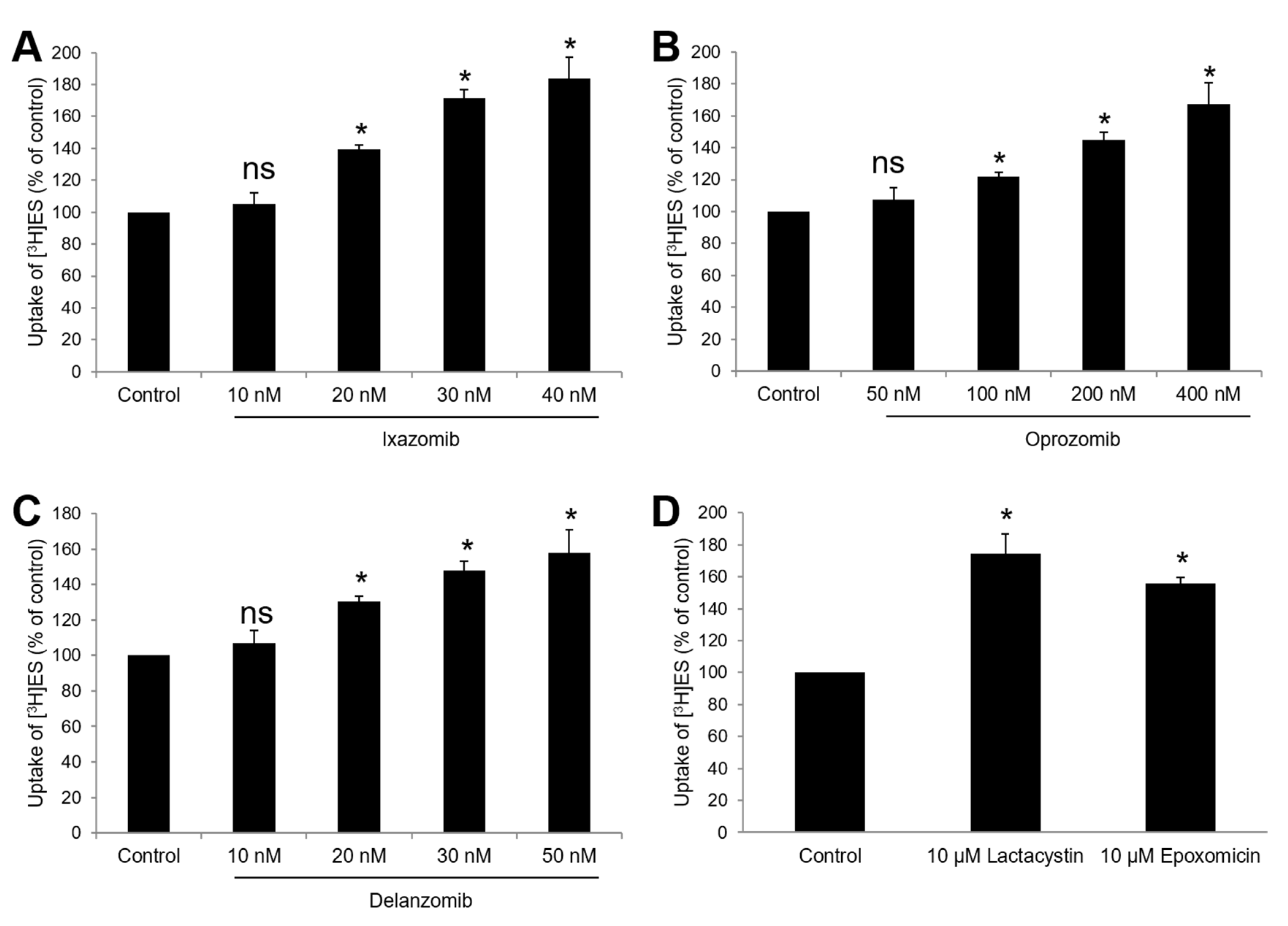
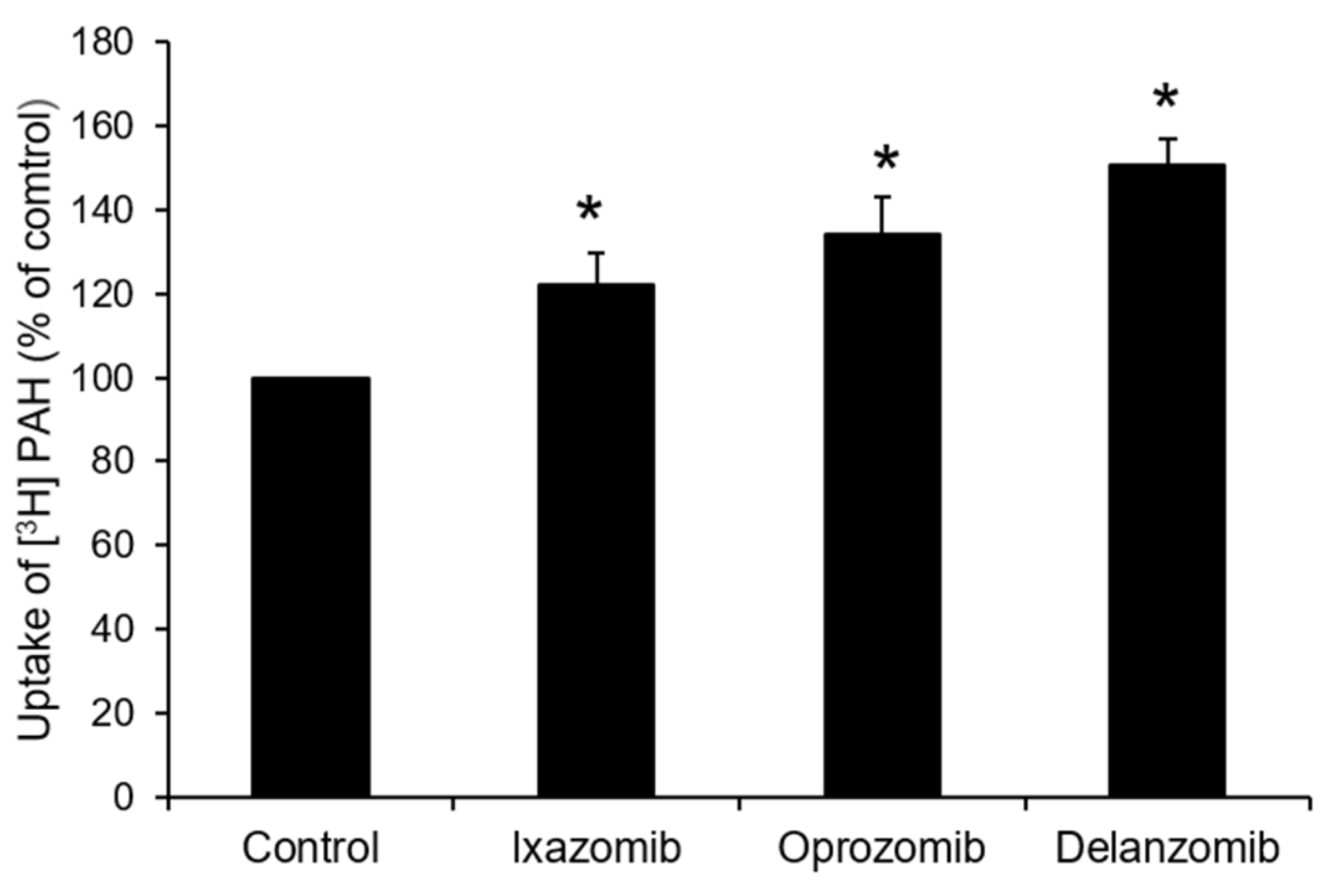
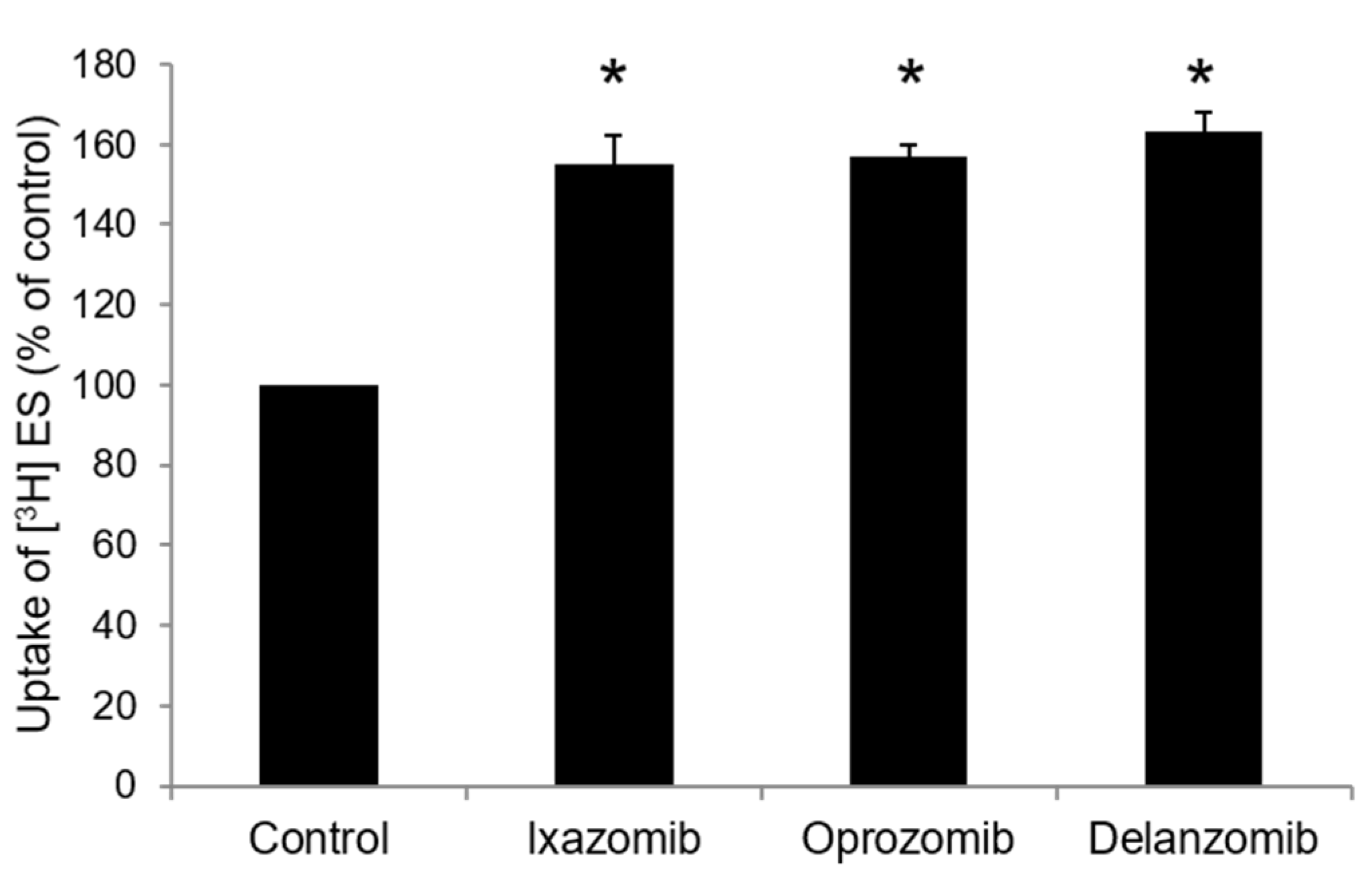
3.3. Effects of Ixazomib, Oprozomib, or Delanzomib on OAT3-Mediated Uptake of Estrone Sulfate or P-Aminohippuric Acid
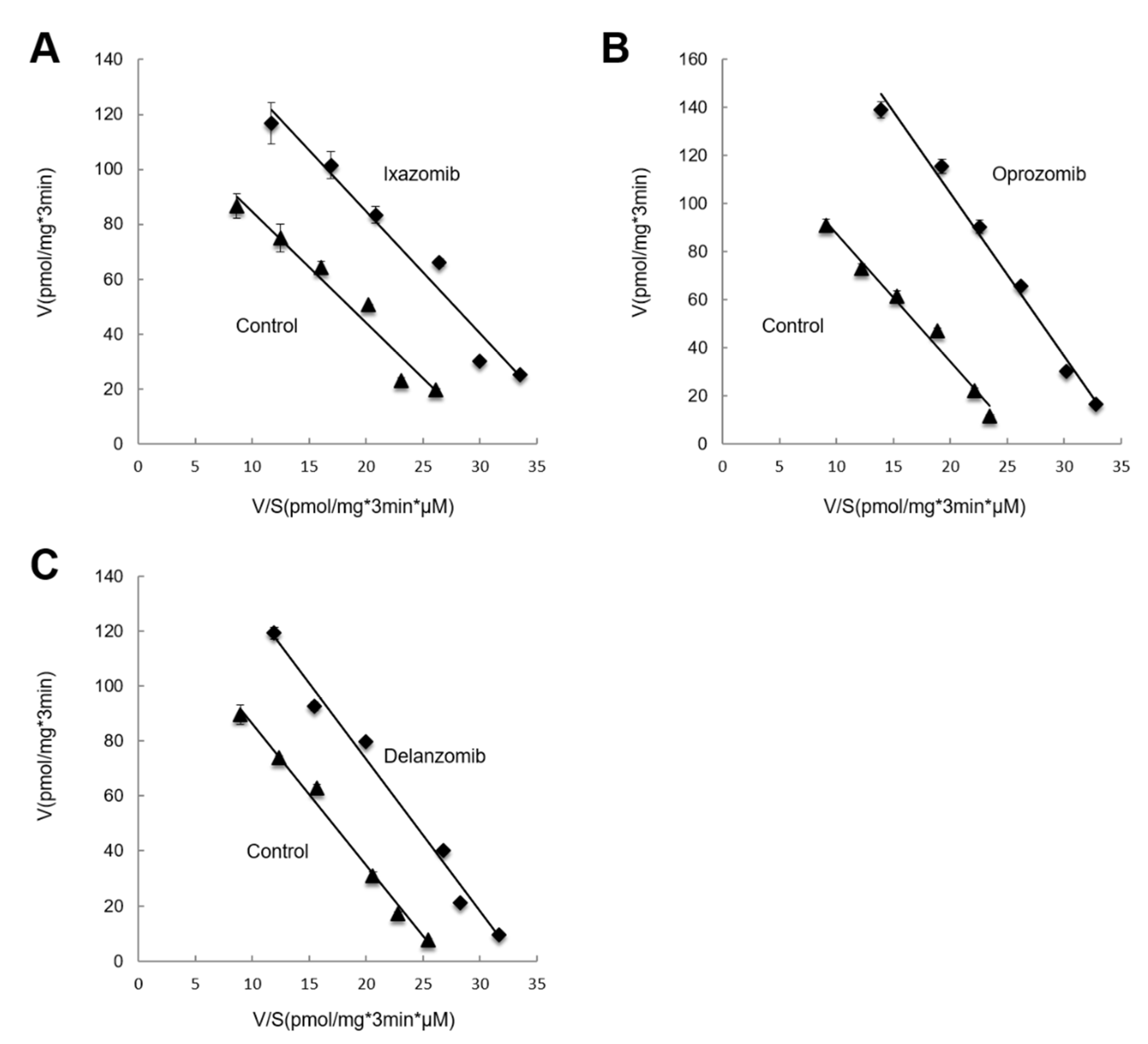
3.4. Kinetic Analysis of the Effects of Ixazomib, Oprozomib, or Delanzomib on OAT3-Mediated Uptake of Estrone Sulfate
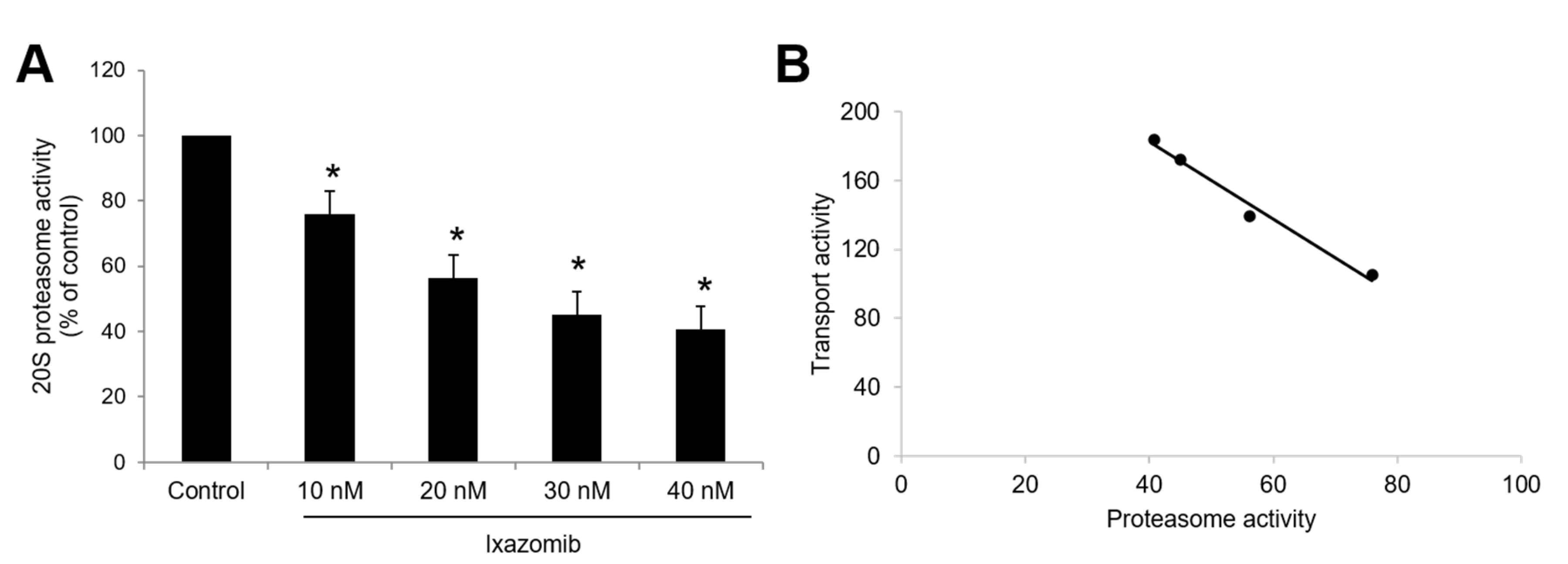
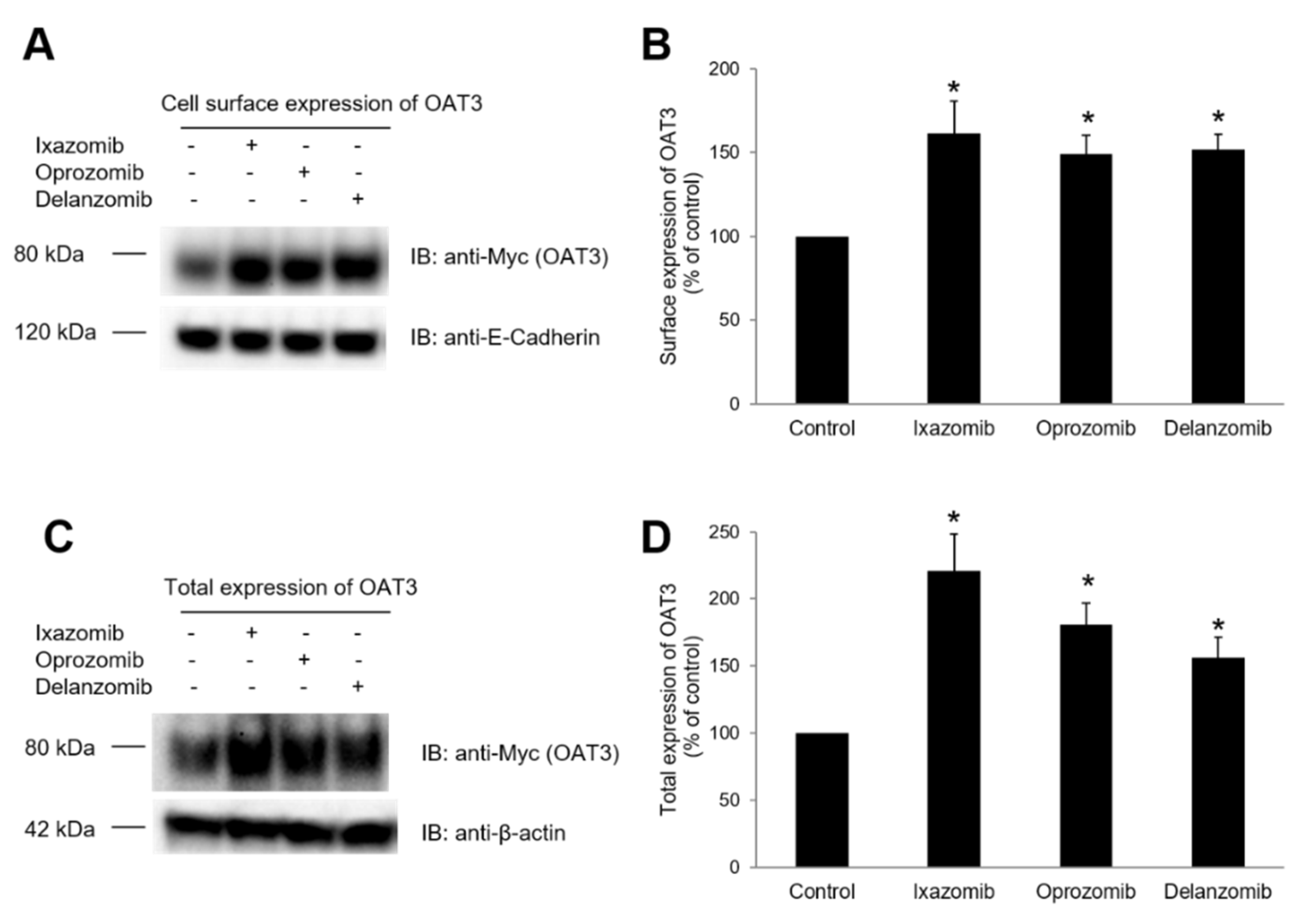
3.5. Effect of Ixazomib, Oprozomib, or Delanzomib on OAT3 Expression
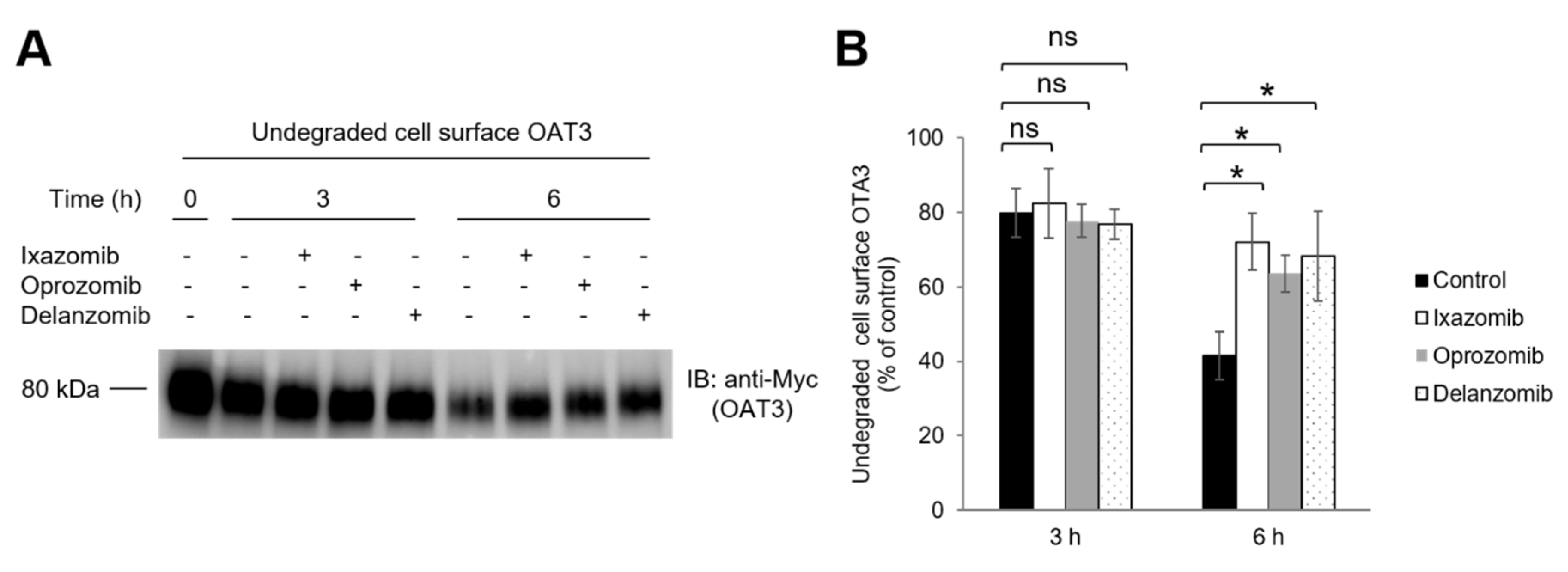
3.6. Effect of Ixazomib, Oprozomib, and Delanzomib on OAT3 Degradation
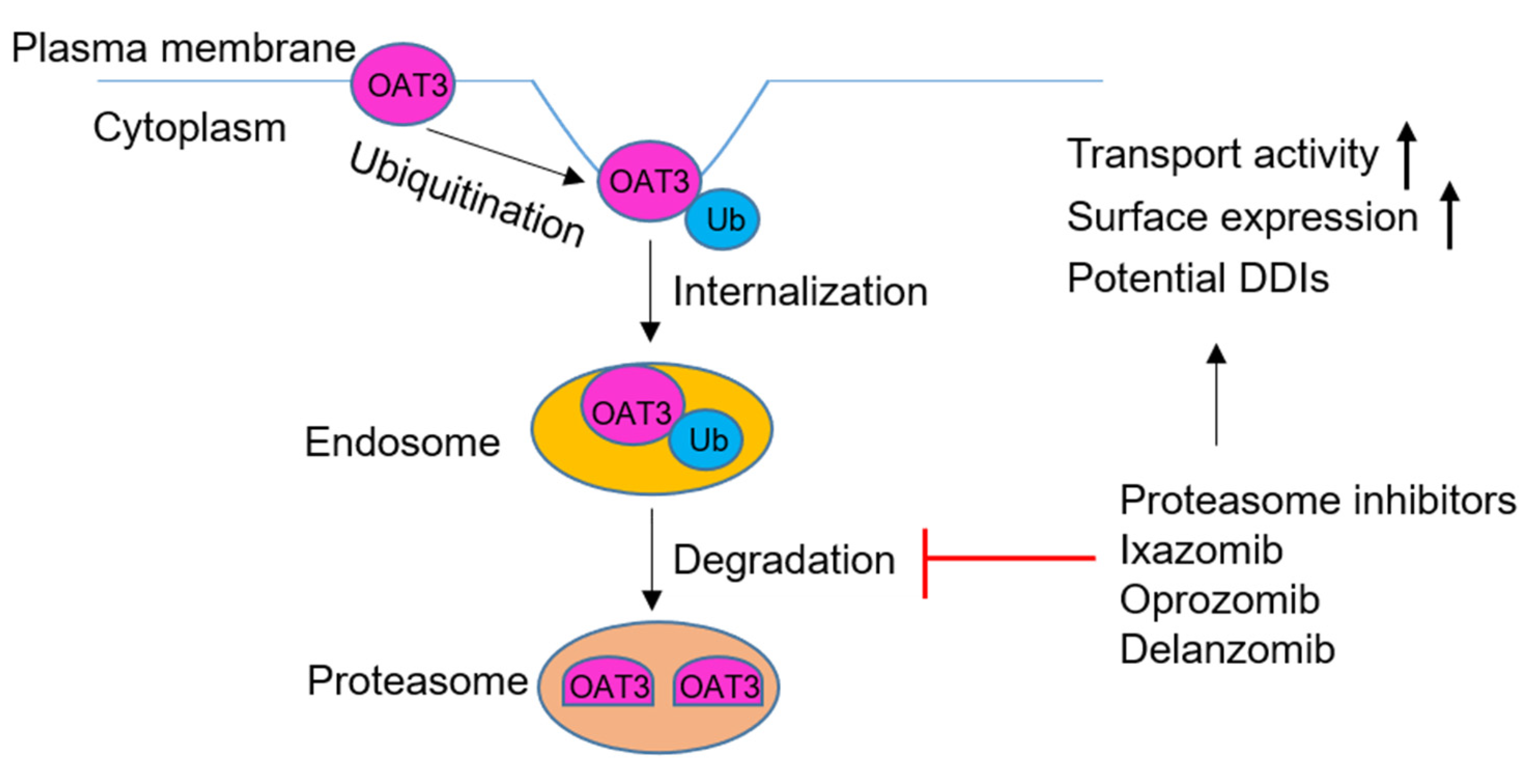
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- You, G. Structure, function, and regulation of renal organic anion transporters. Med. Res. Rev. 2002, 22, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef]
- Wang, L.; Sweet, D.H. Renal organic anion transporters (SLC22 family): Expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 2013, 15, 53–69. [Google Scholar] [CrossRef]
- Liang, Y.; Li, S.; Chen, L. The physiological role of drug transporters. Protein Cell 2015, 6, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, G. Drug transport by Organic Anion Transporters (OATs). Pharmacol. Ther. 2012, 136, 106–130. [Google Scholar] [CrossRef]
- Nigam, S.K. What do drug transporters really do? Nat. Rev. Drug Discov. 2015, 14, 29–44. [Google Scholar] [CrossRef]
- The International Transporter Consortium; Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef]
- Zhu, Y.; Huo, X.; Wang, C.; Meng, Q.; Liu, Z.; Sun, H.; Tan, A.; Ma, X.; Peng, J.; Liu, K. Organic anion transporters also mediate the drug-drug interaction between imipenem and cilastatin. Asian J. Pharm. Sci. 2020, 15, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Wang, C.; Duan, Y.; Huo, X.; Meng, Q.; Liu, Z.; Yang, S.; Zhu, Y.; Sun, H.; Ma, X.; et al. OAT1 and OAT3 also mediate the drug-drug interaction between piperacillin and tazobactam. Int. J. Pharm. 2018, 537, 172–182. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, C.; Liu, Q.; Meng, Q.; Huo, X.; Liu, Z.; Sun, P.; Yang, X.; Sun, H.; Qin, J.; et al. Bezafibrate-mizoribine interaction: Involvement of organic anion transporters OAT1 and OAT3 in rats. Eur. J. Pharm. Sci. 2016, 81, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, C.; Meng, Q.; Huo, X.; Sun, H.; Peng, J.; Ma, X.; Sun, P.; Liu, K. MDR1 and OAT1/OAT3 mediate the drug-drug interaction between puerarin and methotrexate. Pharm. Res. 2014, 31, 1120–1132. [Google Scholar] [CrossRef]
- Ye, J.; Liu, Q.; Wang, C.; Meng, Q.; Sun, H.; Peng, J.; Ma, X.; Liu, K. Benzylpenicillin inhibits the renal excretion of acyclovir by OAT1 and OAT3. Pharmacol. Rep. 2013, 65, 505–512. [Google Scholar] [CrossRef]
- Xue, X.; Gong, L.K.; Maeda, K.; Luan, Y.; Qi, X.M.; Sugiyama, Y.; Ren, J. Critical role of organic anion transporters 1 and 3 in kidney accumulation and toxicity of aristolochic acid I. Mol. Pharm. 2011, 8, 2183–2192. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Bi, Y.; Yu, H.; Wei, J.; Zhang, Y.; Han, L.; Zhang, Y. Potent Inhibitors of Organic Anion Transporters 1 and 3 from natural compounds and their protective effect on aristolochic acid nephropathy. Toxicol. Sci. 2020, 175, 279–291. [Google Scholar] [CrossRef]
- Huo, X.; Meng, Q.; Wang, C.; Zhu, Y.; Liu, Z.; Ma, X.; Ma, X.; Peng, J.; Sun, H.; Liu, K. Cilastatin protects against imipenem-induced nephrotoxicity via inhibition of renal organic anion transporters (OATs). Acta Pharm. Sin. B 2019, 9, 986–996. [Google Scholar] [CrossRef]
- Huo, X.; Meng, Q.; Wang, C.; Wu, J.; Wang, C.; Zhu, Y.; Ma, X.; Sun, H.; Liu, K. Protective effect of cilastatin against diclofenac-induced nephrotoxicity through interaction with diclofenac acyl glucuronide via organic anion transporters. Br. J. Pharmacol. 2020, 177, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Meng, Q.; Wang, C.; Wu, J.; Zhu, Y.; Sun, P.; Ma, X.; Sun, H.; Liu, K. Targeting renal OATs to develop renal protective agent from traditional Chinese medicines: Protective effect of Apigenin against Imipenem-induced nephrotoxicity. Phytother. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Liu, Q.; Wang, C.; Meng, Q.; Guo, X.; Sun, H.; Peng, J.; Ma, X.; Kaku, T.; Liu, K. Inhibitory effect of 1alpha,25-dihydroxyvitamin D(3) on excretion of JBP485 via organic anion transporters in rats. Eur. J. Pharm. Sci. 2013, 48, 351–359. [Google Scholar] [CrossRef]
- Di Giusto, G.; Anzai, N.; Ruiz, M.L.; Endou, H.; Torres, A.M. Expression and function of Oat1 and Oat3 in rat kidney exposed to mercuric chloride. Arch. Toxicol. 2009, 83, 887–897. [Google Scholar] [CrossRef]
- Shibayama, Y.; Ushinohama, K.; Ikeda, R.; Yoshikawa, Y.; Motoya, T.; Takeda, Y.; Yamada, K. Effect of methotrexate treatment on expression levels of multidrug resistance protein 2, breast cancer resistance protein and organic anion transporters Oat1, Oat2 and Oat3 in rats. Cancer Sci. 2006, 97, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Zlender, V.; Breljak, D.; Ljubojevic, M.; Flajs, D.; Balen, D.; Brzica, H.; Domijan, A.M.; Peraica, M.; Fuchs, R.; Anzai, N.; et al. Low doses of ochratoxin A upregulate the protein expression of organic anion transporters Oat1, Oat2, Oat3 and Oat5 in rat kidney cortex. Toxicol. Appl. Pharmacol. 2009, 239, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Phatchawan, A.; Chutima, S.; Varanuj, C.; Anusorn, L. Decreased renal organic anion transporter 3 expression in type 1 diabetic rats. Am. J. Med. Sci. 2014, 347, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Thongnak, L.; Pongchaidecha, A.; Jaikumkao, K.; Chatsudthipong, V.; Chattipakorn, N.; Lungkaphin, A. The additive effects of atorvastatin and insulin on renal function and renal organic anion transporter 3 function in diabetic rats. Sci. Rep. 2017, 7, 13532. [Google Scholar] [CrossRef]
- Wanchai, K.; Yasom, S.; Tunapong, W.; Chunchai, T.; Eaimworawuthikul, S.; Thiennimitr, P.; Chaiyasut, C.; Pongchaidecha, A.; Chatsudthipong, V.; Chattipakorn, S.; et al. Probiotic Lactobacillus paracasei HII01 protects rats against obese-insulin resistance-induced kidney injury and impaired renal organic anion transporter 3 function. Clin. Sci. (Lond.) 2018, 132, 1545–1563. [Google Scholar] [CrossRef] [PubMed]
- Wanchai, K.; Yasom, S.; Tunapong, W.; Chunchai, T.; Thiennimitr, P.; Chaiyasut, C.; Pongchaidecha, A.; Chatsudthipong, V.; Chattipakorn, S.; Chattipakorn, N.; et al. Prebiotic prevents impaired kidney and renal Oat3 functions in obese rats. J. Endocrinol. 2018, 237, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Z.; You, G. Insulin-like growth factor 1 modulates the phosphorylation, expression, and activity of organic anion transporter 3 through protein kinase A signaling pathway. Acta Pharm. Sin. B 2020, 10, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, H.; You, G. An Essential Role of Nedd4-2 in the Ubiquitination, expression, and function of organic anion Transporter-3. Mol. Pharm. 2016, 13, 621–630. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; You, G. Activation of protein kinase a stimulates SUMOylation, expression, and transport activity of organic anion transporter 3. AAPS J. 2019, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Suh, W.; Pan, Z.; You, G. Short-term and long-term effects of protein kinase C on the trafficking and stability of human organic anion transporter 3. Int. J. Biochem. Mol. Biol. 2012, 3, 242–249. [Google Scholar]
- Zhang, Q.; Hong, M.; Duan, P.; Pan, Z.; Ma, J.; You, G. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J. Biol. Chem. 2008, 283, 32570–32579. [Google Scholar] [CrossRef]
- Jandial, D.D.; Farshchi-Heydari, S.; Larson, C.A.; Elliott, G.I.; Wrasidlo, W.J.; Howell, S.B. Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin. Cancer Res. 2009, 15, 553–560. [Google Scholar] [CrossRef]
- Hu, M.C.; Di Sole, F.; Zhang, J.; McLeroy, P.; Moe, O.W. Chronic regulation of the renal Na(+)/H(+) exchanger NHE3 by dopamine: Translational and posttranslational mechanisms. Am. J. Physiol. Renal. Physiol. 2013, 304, F1169–F1180. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Ayaori, M.; Terao, Y.; Hisada, T.; Iizuka, M.; Takiguchi, S.; Uto-Kondo, H.; Yakushiji, E.; Nakaya, K.; Sasaki, M.; et al. Proteasomal inhibition promotes ATP-binding cassette transporter A1 (ABCA1) and ABCG1 expression and cholesterol efflux from macrophages in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Farasyn, T.; Crowe, A.; Ding, K.; Yue, W. Treatment with proteasome inhibitor bortezomib decreases organic anion transporting polypeptide (OATP) 1B3-mediated transport in a substrate-dependent manner. PLoS ONE 2017, 12, e0186924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, A.S.; Worthen, C.; Knutson, M.D.; Enns, C.A. An iron-regulated and glycosylation-dependent proteasomal degradation pathway for the plasma membrane metal transporter ZIP14. Proc. Natl. Acad. Sci. USA 2014, 111, 9175–9180. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; You, G. Proteasome Inhibitors Bortezomib and carfilzomib stimulate the transport activity of human organic anion transporter 1. Mol. Pharmacol. 2020, 97, 384–391. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; You, G. The activity of organic anion transporter-3: Role of dexamethasone. J. Pharmacol. Sci. 2018, 136, 79–85. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; You, G. AG490, a JAK2-specific inhibitor, downregulates the expression and activity of organic anion transporter-3. J. Pharmacol. Sci. 2018, 136, 142–148. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Patterson, C.; You, G. Lysine 48-linked polyubiquitination of organic anion transporter-1 is essential for its protein kinase C-regulated endocytosis. Mol. Pharmacol. 2013, 83, 217–224. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Liu, Q.; Meng, Q.; Cang, J.; Sun, H.; Peng, J.; Ma, X.; Huo, X.; Liu, K. Aspirin and probenecid inhibit organic anion transporter 3-mediated renal uptake of cilostazol and probenecid induces metabolism of cilostazol in the rat. Drug Metab. Dispos. 2014, 42, 996–1007. [Google Scholar] [CrossRef]
- Antonescu, I.E.; Karlgren, M.; Pedersen, M.L.; Simoff, I.; Bergstrom, C.A.S.; Neuhoff, S.; Artursson, P.; Steffansen, B.; Nielsen, C.U. Acamprosate is a substrate of the human organic anion transporter (OAT) 1 without OAT3 Inhibitory properties: Implications for renal acamprosate secretion and drug-drug interactions. Pharmaceutics 2020, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.H.; Miller, D.S.; Pritchard, J.B.; Fujiwara, Y.; Beier, D.R.; Nigam, S.K. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J. Biol. Chem. 2002, 277, 26934–26943. [Google Scholar] [CrossRef]
- Barros, S.A.; Srimaroeng, C.; Perry, J.L.; Walden, R.; Dembla-Rajpal, N.; Sweet, D.H.; Pritchard, J.B. Activation of protein kinase Czeta increases OAT1 (SLC22A6)- and OAT3 (SLC22A8)-mediated transport. J. Biol. Chem. 2009, 284, 2672–2679. [Google Scholar] [CrossRef]
- Soodvilai, S.; Chatsudthipong, V.; Evans, K.K.; Wright, S.H.; Dantzler, W.H. Acute regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am. J. Physiol. Renal. Physiol. 2004, 287, F1021–F1029. [Google Scholar] [CrossRef]
- Soodvilai, S.; Wright, S.H.; Dantzler, W.H.; Chatsudthipong, V. Involvement of tyrosine kinase and PI3K in the regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am. J. Physiol. Renal. Physiol. 2005, 289, F1057–F1064. [Google Scholar] [CrossRef]
- Wang, H.; You, G. SGK1/Nedd4-2 signaling pathway regulates the activity of human organic anion transporters 3. Biopharm. Drug Dispos. 2017, 38, 449–457. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.; Greupink, R.; Wortelboer, H.M.; van den Heuvel, J.J.; Schreurs, M.; Koenderink, J.B.; Masereeuw, R.; Russel, F.G. Interaction of immunosuppressive drugs with human organic anion transporter (OAT) 1 and OAT3, and multidrug resistance-associated protein (MRP) 2 and MRP4. Transl. Res. 2013, 162, 398–409. [Google Scholar] [CrossRef]
- Bhardwaj, R.K.; Herrera-Ruiz, D.; Eltoukhy, N.; Saad, M.; Knipp, G.T. The functional evaluation of human peptide/histidine transporter 1 (hPHT1) in transiently transfected COS-7 cells. Eur. J. Pharm. Sci. 2006, 27, 533–542. [Google Scholar] [CrossRef]
- Goyal, S.; Vanden Heuvel, G.; Aronson, P.S. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am. J. Physiol. Renal. Physiol. 2003, 284, F467–F473. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Hanley, M.J.; Xia, C.; Labotka, R.; Harvey, R.D.; Venkatakrishnan, K. Clinical Pharmacology of Ixazomib: The first oral proteasome inhibitor. Clin. Pharm. 2019, 58, 431–449. [Google Scholar] [CrossRef] [PubMed]
- FDA. Pharmacology Review(s) for NINLARO® (Ixazomib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208462Orig1s000PharmR.pdf (accessed on 29 July 2020).
- Infante, J.R.; Mendelson, D.S.; Burris, H.A., 3rd; Bendell, J.C.; Tolcher, A.W.; Gordon, M.S.; Gillenwater, H.H.; Arastu-Kapur, S.; Wong, H.L.; Papadopoulos, K.P. A first-in-human dose-escalation study of the oral proteasome inhibitor oprozomib in patients with advanced solid tumors. Investig. New Drugs 2016, 34, 216–224. [Google Scholar] [CrossRef]
- Muchamuel, T.; Basler, M.; Aujay, M.A.; Suzuki, E.; Kalim, K.W.; Lauer, C.; Sylvain, C.; Ring, E.R.; Shields, J.; Jiang, J.; et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat. Med. 2009, 15, 781–787. [Google Scholar] [CrossRef]
- Gallerani, E.; Zucchetti, M.; Brunelli, D.; Marangon, E.; Noberasco, C.; Hess, D.; Delmonte, A.; Martinelli, G.; Bohm, S.; Driessen, C.; et al. A first in human phase I study of the proteasome inhibitor CEP-18770 in patients with advanced solid tumours and multiple myeloma. Eur. J. Cancer 2013, 49, 290–296. [Google Scholar] [CrossRef]
- Seavey, M.M.; Lu, L.D.; Stump, K.L.; Wallace, N.H.; Ruggeri, B.A. Novel, orally active, proteasome inhibitor, delanzomib (CEP-18770), ameliorates disease symptoms and glomerulonephritis in two preclinical mouse models of SLE. Int. Immunopharmacol. 2012, 12, 257–270. [Google Scholar] [CrossRef]
- Reese, S.R.; Wilson, N.A.; Huang, G.; Redfield, R.R., 3rd; Zhong, W.; Djamali, A. Calcineurin Inhibitor Minimization with Ixazomib, an investigational proteasome inhibitor, for the prevention of antibody mediated rejection in a preclinical model. Transplantation 2015, 99, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Carugo, A.; Genovese, G. Targeting proteostasis and autophagy in SMARCB1-deficient malignancies: Where next? Oncotarget 2019, 10, 3979–3981. [Google Scholar] [CrossRef]
- Adams, J. The proteasome: A suitable antineoplastic target. Nat. Rev. Cancer 2004, 4, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Neubert, K.; Meister, S.; Moser, K.; Weisel, F.; Maseda, D.; Amann, K.; Wiethe, C.; Winkler, T.H.; Kalden, J.R.; Manz, R.A.; et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat. Med. 2008, 14, 748–755. [Google Scholar] [CrossRef] [PubMed]
- FDA. Clinical Pharmacology Biopharmaceutics Review(s) for NINLARO® (Ixazomib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208462Orig1s000ClinPharmR.pdf (accessed on 29 July 2020).
- Shah, J.; Usmani, S.; Stadtmauer, E.A.; Rifkin, R.M.; Berenson, J.R.; Berdeja, J.G.; Lyons, R.M.; Klippel, Z.; Chang, Y.L.; Niesvizky, R. Oprozomib, pomalidomide, and Dexamethasone in Patients With Relapsed and/or Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, 570–578.e571. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Bensinger, W.I.; Zimmerman, T.M.; Reeder, C.B.; Berenson, J.R.; Berg, D.; Hui, A.M.; Gupta, N.; Di Bacco, A.; Yu, J.; et al. Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood 2014, 124, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.J.; Aujay, M.A.; Bennett, M.K.; Dajee, M.; Demo, S.D.; Fang, Y.; Ho, M.N.; Jiang, J.; Kirk, C.J.; Laidig, G.J.; et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047). J. Med. Chem. 2009, 52, 3028–3038. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Xu, Y.; Gore, L.; Harvey, R.D.; Mita, A.; Papadopoulos, K.P.; Wang, Z.; Cutler, R.E., Jr.; Pinchasik, D.E.; Tsimberidou, A.M. Physiologically-based pharmacokinetic modelling to predict oprozomib CYP3A drug-drug interaction potential in patients with advanced malignancies. Br. J. Clin. Pharmacol. 2019, 85, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Sanchez, E.; Li, M.; Li, J.; Wang, C.; Chen, H.; Jones-Bolin, S.; Hunter, K.; Ruggeri, B.; Berenson, J.R. CEP-18770 (delanzomib) in combination with dexamethasone and lenalidomide inhibits the growth of multiple myeloma. Leuk. Res. 2012, 36, 1422–1427. [Google Scholar] [CrossRef]
- FDA. Label Revision for NINLARO® (Ixazomib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208462s006lbl.pdf (accessed on 29 July 2020).
- Vanwert, A.L.; Srimaroeng, C.; Sweet, D.H. Organic anion transporter 3 (oat3/slc22a8) interacts with carboxyfluoroquinolones, and deletion increases systemic exposure to ciprofloxacin. Mol. Pharmacol. 2008, 74, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sweet, D.H. Interaction of natural dietary and herbal anionic compounds and flavonoids with human organic anion transporters 1 (SLC22A6), 3 (SLC22A8), and 4 (SLC22A11). Evid. Based Complement Altern. Med. 2013, 2013, 612527. [Google Scholar] [CrossRef]
- Leestemaker, Y.; de Jong, A.; Witting, K.F.; Penning, R.; Schuurman, K.; Rodenko, B.; Zaal, E.A.; van de Kooij, B.; Laufer, S.; Heck, A.J.R.; et al. Proteasome activation by small molecules. Cell Chem. Biol. 2017, 24, 725–736.e727. [Google Scholar] [CrossRef] [PubMed]
- Kors, S.; Geijtenbeek, K.; Reits, E.; Schipper-Krom, S. Regulation of proteasome activity by (Post-)transcriptional mechanisms. Front. Mol. Biosci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Njomen, E.; Tepe, J.J. Proteasome activation as a new therapeutic approach to target proteotoxic disorders. J. Med. Chem. 2019, 62, 6469–6481. [Google Scholar] [CrossRef]
- Trippier, P.C.; Zhao, K.T.; Fox, S.G.; Schiefer, I.T.; Benmohamed, R.; Moran, J.; Kirsch, D.R.; Morimoto, R.I.; Silverman, R.B. Proteasome activation is a mechanism for pyrazolone small molecules displaying therapeutic potential in amyotrophic lateral sclerosis. ACS Chem. Neurosci. 2014, 5, 823–829. [Google Scholar] [CrossRef]
- Jones, C.L.; Njomen, E.; Sjogren, B.; Dexheimer, T.S.; Tepe, J.J. Small molecule enhancement of 20S proteasome activity targets intrinsically disordered proteins. ACS Chem. Biol. 2017, 12, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Villar, S.R.; Brandoni, A.; Anzai, N.; Endou, H.; Torres, A.M. Altered expression of rat renal cortical OAT1 and OAT3 in response to bilateral ureteral obstruction. Kidney Int. 2005, 68, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Liang, Z.; Zhang, J.; You, G. Oral Proteasomal Inhibitors Ixazomib, Oprozomib, and Delanzomib Upregulate the Function of Organic Anion Transporter 3 (OAT3): Implications in OAT3-Mediated Drug-Drug Interactions. Pharmaceutics 2021, 13, 314. https://doi.org/10.3390/pharmaceutics13030314
Fan Y, Liang Z, Zhang J, You G. Oral Proteasomal Inhibitors Ixazomib, Oprozomib, and Delanzomib Upregulate the Function of Organic Anion Transporter 3 (OAT3): Implications in OAT3-Mediated Drug-Drug Interactions. Pharmaceutics. 2021; 13(3):314. https://doi.org/10.3390/pharmaceutics13030314
Chicago/Turabian StyleFan, Yunzhou, Zhengxuan Liang, Jinghui Zhang, and Guofeng You. 2021. "Oral Proteasomal Inhibitors Ixazomib, Oprozomib, and Delanzomib Upregulate the Function of Organic Anion Transporter 3 (OAT3): Implications in OAT3-Mediated Drug-Drug Interactions" Pharmaceutics 13, no. 3: 314. https://doi.org/10.3390/pharmaceutics13030314
APA StyleFan, Y., Liang, Z., Zhang, J., & You, G. (2021). Oral Proteasomal Inhibitors Ixazomib, Oprozomib, and Delanzomib Upregulate the Function of Organic Anion Transporter 3 (OAT3): Implications in OAT3-Mediated Drug-Drug Interactions. Pharmaceutics, 13(3), 314. https://doi.org/10.3390/pharmaceutics13030314




