Multi-Component Hydrogel Beads Incorporated with Reduced Graphene Oxide for pH-Responsive and Controlled Co-Delivery of Multiple Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of rGO
2.3. Preparation of GL-DOX and GL-rGO-DOX Beads
2.4. Preparation of SA-5-FU and SA-rGO-5-FU Beads
2.5. Synthesis of SA-5-FU-rGO-GL-DOX Beads
2.6. Structural Characterization
2.7. Determination of the Encapsulation Efficiency (EE)
2.8. Evaluation of In Vitro Drug Release Profiles
2.9. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) Assay
2.10. Haemolysis Assay
2.11. Determination of Endogenous Reactive Oxygen Species (ROS) Production
3. Results
3.1. Structural and Morphological Characterization
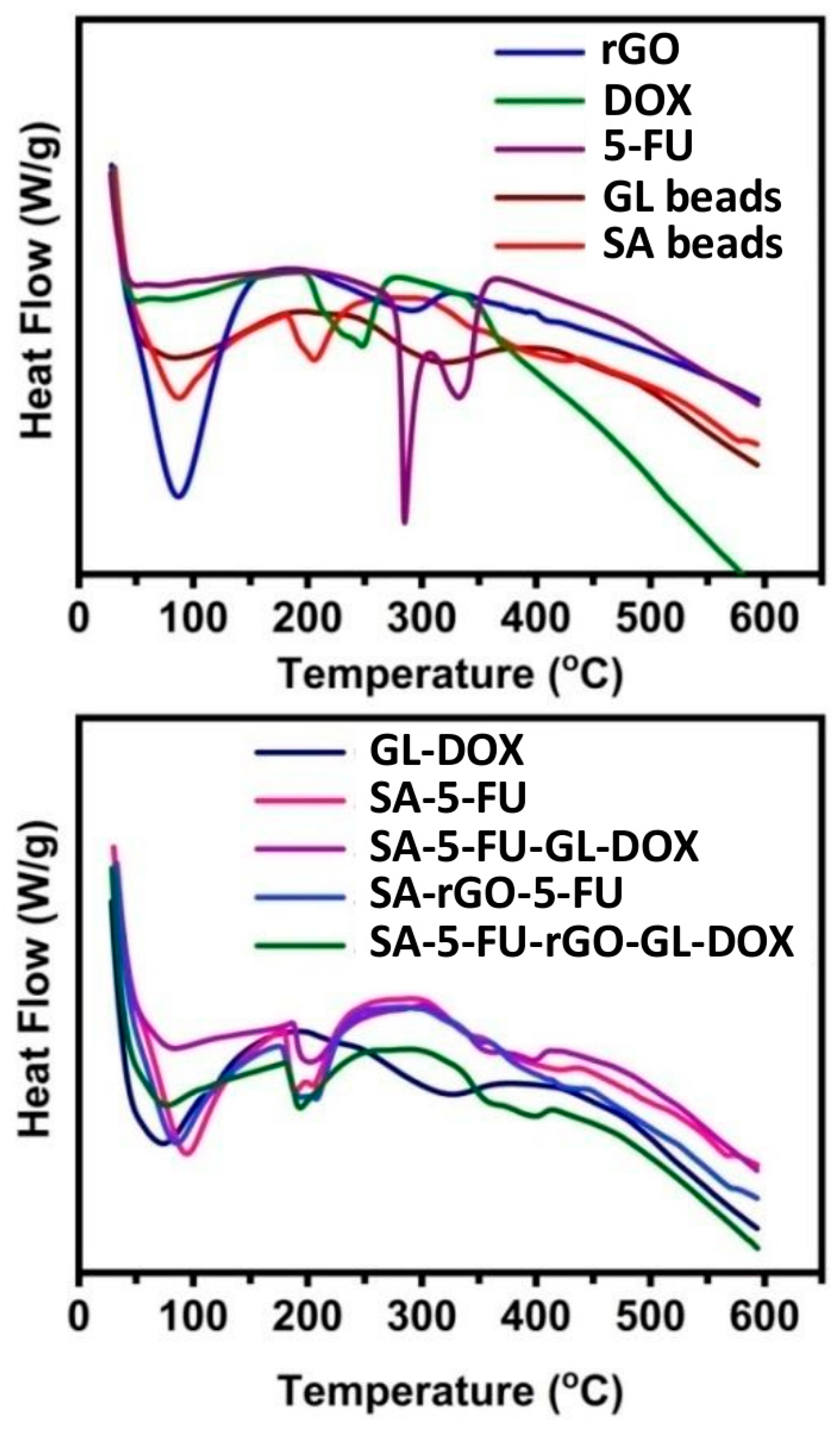
3.2. Thermal Analysis
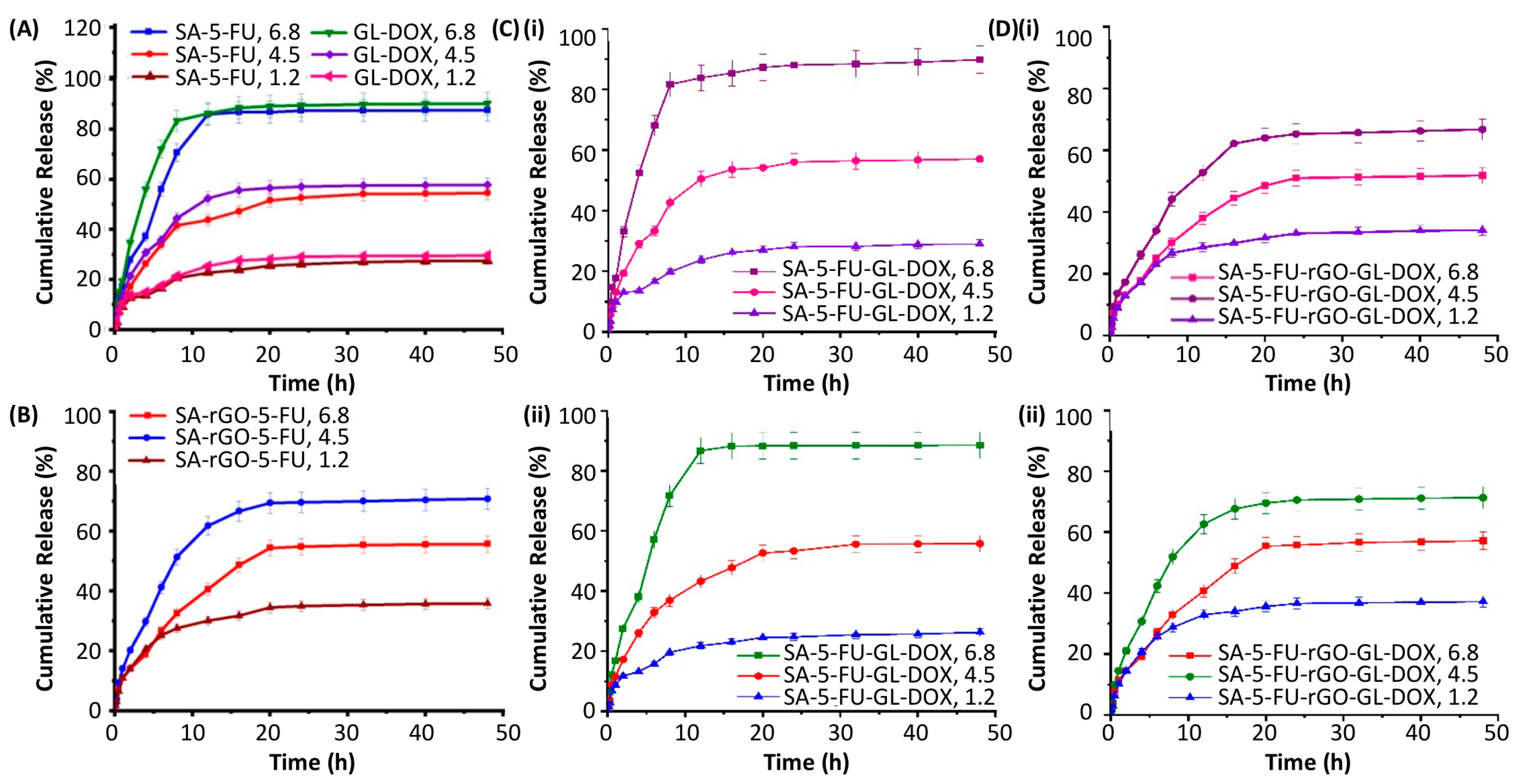
3.3. Drug Encapsulation and pH-Responsive Release
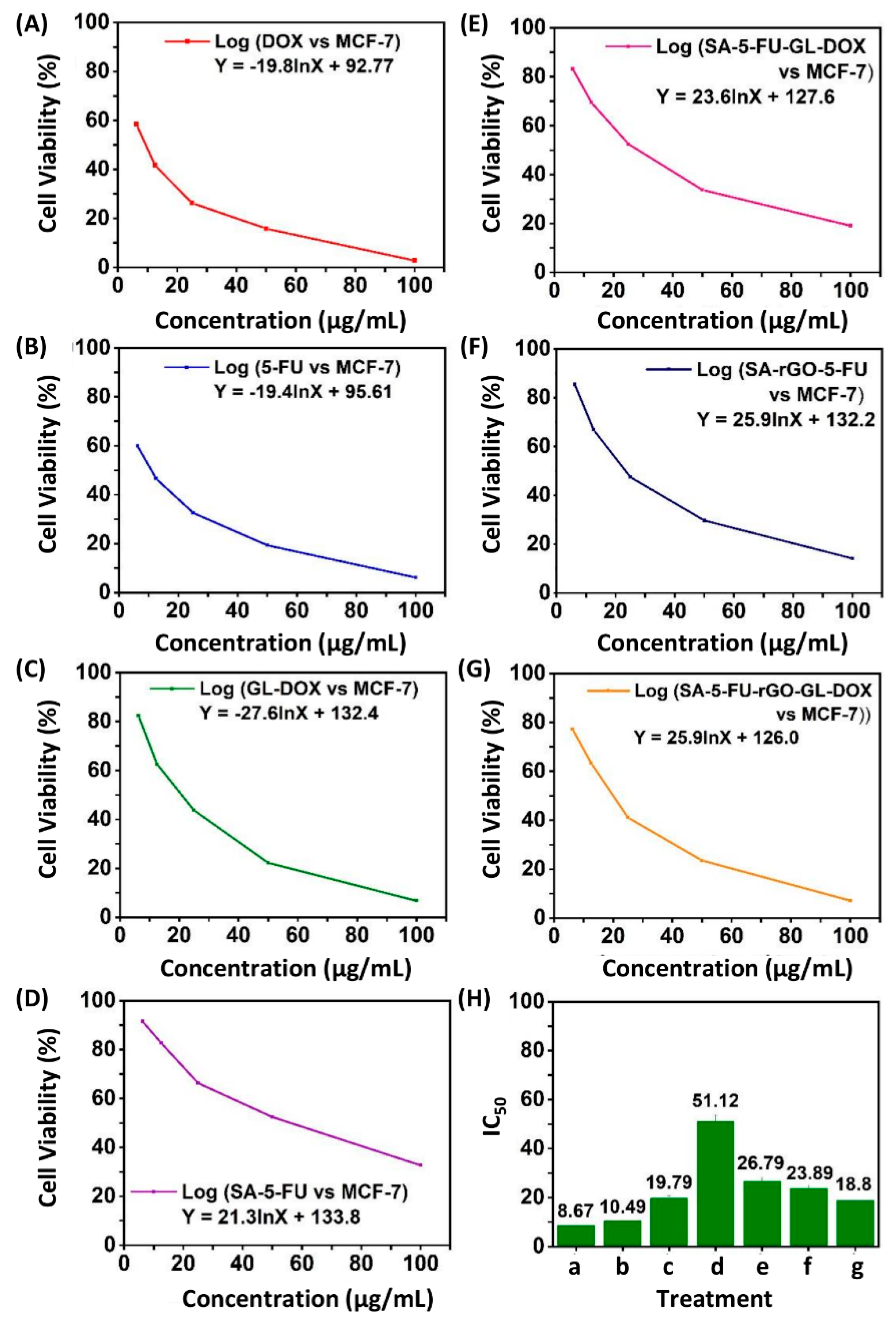
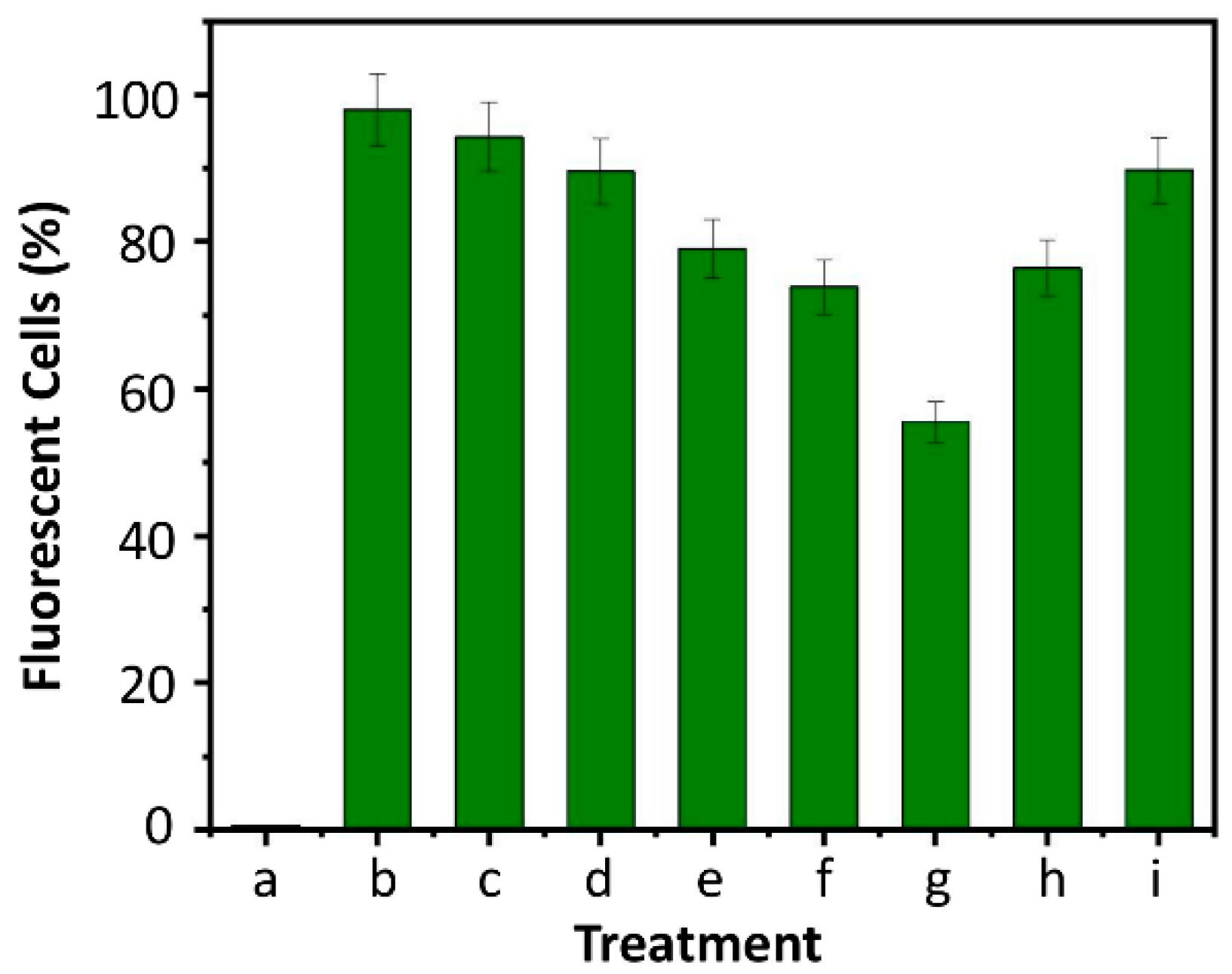
3.4. Evaluation of Haemolytic and Anti-Cancer Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef]
- Hughes, G.A. Nanostructure-mediated drug delivery. Nanomed.-Nanotechnol. Biol. Med. 2005, 1, 22–30. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.S.; Subha, M.; Jithendra, T.; Madhavi, C.; Rao, K.C. Fabrication of Gelatin/Karaya gum blend microspheres for the controlled release of Distigmine bromide. J. Drug Deliv. Ther. 2019, 9, 1–11. [Google Scholar]
- Gattás-Asfura, K.M.; Weisman, E.; Andreopoulos, F.M.; Micic, M.; Muller, B.; Sirpal, S.; Pham, S.M.; Leblanc, R.M. Nitrocinnamate-functionalized gelatin: Synthesis and “smart” hydrogel formation via photo-cross-linking. Biomacromolecules 2005, 6, 1503–1509. [Google Scholar] [CrossRef]
- Curcio, M.; Spizzirri, U.G.; Iemma, F.; Puoci, F.; Cirillo, G.; Parisi, O.I.; Picci, N. Grafted thermo-responsive gelatin microspheres as delivery systems in triggered drug release. Eur. J. Pharm. Biopharm. 2010, 76, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin. Drug Deliv. 2015, 12, 1547–1563. [Google Scholar] [CrossRef]
- Lai, W.F.; Huang, E.; Lui, K.H. Alginate-based complex fibers with the Janus morphology for controlled release of co-delivered drugs. Asian J. Pharm. Sci. 2021, 16, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Susha, A.S.; Rogach, A.L. Multicompartment microgel beads for co-delivery of multiple drugs at individual release rates. ACS Appl. Mater. Interfaces 2016, 8, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Susha, A.S.; Rogach, A.L.; Wang, G.A.; Huang, M.J.; Hu, W.J.; Wong, W.T. Electrospray-mediated preparation of compositionally homogeneous core-shell hydrogel microspheres for sustained drug release. RSC Adv. 2017, 7, 44482–44491. [Google Scholar] [CrossRef]
- Sreekanth Reddy, O.; Subha, M.C.S.; Jithendra, T.; Madhavi, C.; Chowdoji Rao, K. Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. J. Pharm. Anal. 2020. [Google Scholar] [CrossRef]
- Reddy, O.S.; Subha, M.C.S.; Jithendra, T.; Madhavi, C.; Rao, K.C. Fabrication and characterization of smart karaya gum/sodium alginate semi-IPN microbeads for controlled release of D-penicillamine drug. Polym. Polym. Compos. 2020. [Google Scholar] [CrossRef]
- Cho, A.R.; Chun, Y.G.; Kim, B.K.; Park, D.J. Preparation of alginate-CaCl2 microspheres as resveratrol carriers. J. Mater. Sci. 2014, 49, 4612–4619. [Google Scholar] [CrossRef]
- Pandey, S.P.; Shukla, T.; Dhote, V.K.; Mishra, D.K.; Maheshwari, R.; Tekade, R.K. Use of polymers in controlled release of active agents. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: New York, NY, USA, 2019; pp. 113–172. [Google Scholar]
- Seifu, M.F.; Nath, L.K. Polymer-drug conjugates: Novel carriers for cancer chemotherapy. Polym.-Plast. Tech. Mater. 2019, 58, 158–171. [Google Scholar] [CrossRef]
- Nogueira, E.; Freitas, J.; Loureiro, A.; Nogueira, P.; Gomes, A.C.; Preto, A.; Carmo, A.M.; Moreira, A.; Cavaco-Paulo, A. Neutral PEGylated liposomal formulation for efficient folate-mediated delivery of MCL1 siRNA to activated macrophages. Colloid Surf. B-Biointerfaces 2017, 155, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ghiani, S.; Capozza, M.; Cabella, C.; Coppo, A.; Miragoli, L.; Brioschi, C.; Bonafè, R.; Maiocchi, A. In vivo tumor targeting and biodistribution evaluation of paramagnetic solid lipid nanoparticles for magnetic resonance imaging. Nanomed.-Nanotechnol. Biol. Med. 2017, 13, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, A.; Voulgari, E.; Diamanti, E.K.; Gournis, D.; Avgoustakis, K. Graphene oxide stabilized by PLA–PEG copolymers for the controlled delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2015, 93, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Lee, M.-Y.; Kong, W.H.; Do, I.H.; Hahn, S.K. Nanographene oxide–hyaluronic acid conjugate for target specific cancer drug delivery. RSC Adv. 2014, 4, 14197–14200. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, X.; Yi, X.; Huang, M.; Ning, P.; Liu, T.; Ge, C.; Chai, Z.; Liu, Z.; Yang, K. Radionuclide 131I labeled reduced graphene oxide for nuclear imaging guided combined radio- and photothermal therapy of cancer. Biomaterials 2015, 66, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Li, C.M. Restoring basal planes of graphene oxides for highly efficient loading and delivery of β-lapachone. Mol. Pharm. 2012, 9, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, D.; Kim, J.; Kim, T.-I.; Kim, W.J. Photothermally triggered cytosolic drug delivery via endosome disruption using a functionalized reduced graphene oxide. ACS Nano 2013, 7, 6735–6746. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, I.; Miyaji, H.; Takita, H.; Nishida, E.; Tsuji, M.; Fugetsu, B.; Sun, L.; Inoue, K.; Ibara, A.; Akasaka, T.; et al. Comparative study of bioactivity of collagen scaffolds coated with graphene oxide and reduced graphene oxide. Int. J. Nanomed. 2014, 9, 3363–3373. [Google Scholar] [PubMed]
- Sharma, H.; Mondal, S. Functionalized graphene oxide for chemotherapeutic drug delivery and cancer treatment: A promising material in nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zhang, Y.; Shi, X.; Du, X.; Wang, X.; Yu, A.; Zhai, G. Recent progress of functionalised graphene oxide in cancer therapy. J. Drug Target. 2019, 27, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, H.J.; Georgopapadakou, N.H. Anticancer therapeutics: “Addictive” targets, multi-targeted drugs, new drug combinations. Drug Resist. Updat. 2005, 8, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Vicent, M.J. Combination therapy: Opportunities and challenges for polymer–drug conjugates as anticancer nanomedicines. Adv. Drug Deliv. Rev. 2009, 61, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, J.; Griffin, J.P.; Behrman, R.E. Development of novel combination therapies. N. Engl. J. Med. 2011, 364, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Rogach, A.L.; Wong, W.T. One-pot synthesis of an emulsion-templated hydrogel-microsphere composite with tunable properties. Compos. Part A-Appl. Sci. Manuf. 2018, 113, 318–329. [Google Scholar] [CrossRef]
- Lai, W.F.; Lin, M.C. Chemotherapeutic drugs interfere with gene delivery mediated by chitosan-graft-poly(ethylenimine). PLoS ONE 2015, 10, e0126367. [Google Scholar] [CrossRef] [PubMed]
- Revathy, T.A.; Dhanavel, S.; Sivaranjani, T.; Narayanan, V.; Maiyalagan, T.; Stephen, A. Highly active graphene-supported palladium-nickel alloy nanoparticles for catalytic reduction of 4-nitrophenol. Appl. Surf. Sci. 2018, 449, 764–771. [Google Scholar] [CrossRef]
- Katz-Brull, R.; Margalit, P.; Bendel, P.; Degani, H. Choline metabolism in breast cancer; 2H-, 13C- and 31P-NMR studies of cells and tumors. Magn. Reson. Mater. Phy. 1998, 6, 44–52. [Google Scholar] [CrossRef]
- Lai, W.F.; Huang, E.; Wong, W.T. A gel-forming clusteroluminogenic polymer with tunable emission behavior as a sustained-release carrier enabling real-time tracking during bioactive agent delivery. Appl. Mater. Today 2020, 21, 100876. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Chintha, M.; Kashayi, C.R.; Venkata, K.R.K.S.; Subbarao, S.M.C. Gelatin-coated dual cross-linked sodium alginate/magnetite nanoparticle microbeads for controlled release of doxorubicin. ChemistrySelect 2020, 5, 10276–10284. [Google Scholar] [CrossRef]
- Piao, Y.; Chen, B. One-pot synthesis and characterization of reduced graphene oxide–gelatin nanocomposite hydrogels. RSC Adv. 2016, 6, 6171–6181. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, B.; Liu, J.; Zhang, P.; Li, Z.; Luan, Y. Green fabricated reduced graphene oxide: Evaluation of its application as nano-carrier for pH-sensitive drug delivery. Int. J. Pharm. 2015, 496, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Dhanavel, S.; Revathy, T.A.; Sivaranjani, T.; Sivakumar, K.; Palani, P.; Narayanan, V.; Stephen, A. 5-Fluorouracil and curcumin co-encapsulated chitosan/reduced graphene oxide nanocomposites against human colon cancer cell lines. Polym. Bull. (Berl.) 2020, 77, 213–233. [Google Scholar] [CrossRef]
- Prokhorov, E.; Barquera-Bibiano, Z.; Manzano-Ramírez, A.; Luna-Barcenas, G.; Kovalenko, Y.; Hernández-Landaverde, M.A.; Reyes, B.E.C.; Vargas, J.H. New insights in graphene oxide dielectric constant. Mater. Res. Express 2019, 6, 085622. [Google Scholar] [CrossRef]
- Depan, D.; Shah, J.; Misra, R.D.K. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312. [Google Scholar] [CrossRef]
- Sanchez-Ballester, N.M.; Soulairol, I.; Bataille, B.; Sharkawi, T. Flexible heteroionic calcium-magnesium alginate beads for controlled drug release. Carbohydr. Polym. 2019, 207, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Mumper, R.J.; Hoffman, A.S.; Puolakkainen, P.A.; Bouchard, L.S.; Gombotz, W.R. Calcium-alginate beads for the oral delivery of transforming growth factor-β1 (TGF-β1): Stabilization of TGF-β1 by the addition of polyacrylic-acid within acid-treated beads. J. Control. Release 1994, 30, 241–251. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Han, M.; Wang, X.; Du, J.; Zhang, H.; Li, W. Co-delivery of 5-fluorodeoxyuridine and doxorubicin via gold nanoparticle equipped with affibody-DNA hybrid strands for targeted synergistic chemotherapy of HER2 overexpressing breast cancer. Sci. Rep. 2020, 10, 22015. [Google Scholar] [CrossRef] [PubMed]





| Bead | EE (%) | |
|---|---|---|
| 5-FU | DOX | |
| SA-5-FU | 62.73 | N/A |
| GL-DOX | N/A | 69.55 |
| SA-rGO-5-FU | 73.38 | N/A |
| SA-5-FU-GL-DOX | 61.04 | 67.48 |
| SA-5-FU-rGO-GL-DOX | 71.62 | 73.15 |
| Bead | Drug | pH | Korsmeyer–Peppas | Zero-order | First-order | Higuchi | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | r2 | K0 | r2 | K1 | r2 | KH | r2 | |||
| SA-5-FU | 5-FU | 6.8 | 0.993 | 0.660 | 0.586 | 2.786 | 0.674 | 0.136 | 0.821 | 16.83 |
| 4.5 | 0.986 | 0.538 | 0.663 | 1.681 | 0.730 | 0.031 | 0.882 | 10.08 | ||
| 1.2 | 0.949 | 0.411 | 0.666 | 0.845 | 0.696 | 0.011 | 0.882 | 5.11 | ||
| GL-DOX | DOX | 6.8 | 0.975 | 0.531 | 0.515 | 2.899 | 0.651 | 0.203 | 0.765 | 17.80 |
| 4.5 | 0.994 | 0.538 | 0.612 | 1.821 | 0.669 | 0.038 | 0.846 | 11.02 | ||
| 1.2 | 0.974 | 0.406 | 0.651 | 0.923 | 0.679 | 0.012 | 0.871 | 05.60 | ||
| SA-rGO-5-FU | 5-FU | 6.8 | 0.981 | 0.584 | 0.738 | 1.692 | 0.784 | 0.030 | 0.919 | 9.961 |
| 4.5 | 0.988 | 0.630 | 0.647 | 2.204 | 0.725 | 0.060 | 0.868 | 13.19 | ||
| 1.2 | 0.974 | 0.468 | 0.618 | 1.116 | 0.658 | 0.016 | 0.851 | 06.77 | ||
| SA-5-FU-GL-DOX | 5-FU | 6.8 | 0.996 | 0.665 | 0.583 | 2.818 | 0.669 | 0.141 | 0.818 | 17.03 |
| 4.5 | 0.985 | 0.537 | 0.686 | 1.708 | 0.755 | 0.032 | 0.897 | 10.21 | ||
| 1.2 | 0.951 | 0.413 | 0.660 | 0.803 | 0.687 | 0.010 | 0.877 | 04.86 | ||
| DOX | 6.8 | 0.972 | 0.545 | 0.540 | 0.285 | 0.690 | 0.181 | 0.784 | 17.41 | |
| 4.5 | 0.994 | 0.556 | 0.637 | 1.777 | 0.697 | 0.035 | 0.863 | 10.71 | ||
| 1.2 | 0.947 | 0.402 | 0.683 | 0.890 | 0.712 | 0.011 | 0.891 | 05.37 | ||
| SA-5-FU-rGO-GL-DOX | 5-FU | 6.8 | 0.985 | 0.570 | 0.748 | 1.725 | 0.797 | 0.031 | 0.925 | 10.15 |
| 4.5 | 0.986 | 0.617 | 0.643 | 2.226 | 0.722 | 0.063 | 0.866 | 13.35 | ||
| 1.2 | 0.977 | 0.486 | 0.621 | 1.158 | 0.662 | 0.017 | 0.854 | 07.01 | ||
| DOX | 6.8 | 0.985 | 0.583 | 0.741 | 1.572 | 0.789 | 0.026 | 0.921 | 9.254 | |
| 4.5 | 0.981 | 0.604 | 0.695 | 2.047 | 0.764 | 0.047 | 0.898 | 12.15 | ||
| 1.2 | 0.981 | 0.509 | 0.633 | 1.054 | 0.670 | 0.014 | 0.861 | 6.370 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obireddy, S.R.; Lai, W.-F. Multi-Component Hydrogel Beads Incorporated with Reduced Graphene Oxide for pH-Responsive and Controlled Co-Delivery of Multiple Agents. Pharmaceutics 2021, 13, 313. https://doi.org/10.3390/pharmaceutics13030313
Obireddy SR, Lai W-F. Multi-Component Hydrogel Beads Incorporated with Reduced Graphene Oxide for pH-Responsive and Controlled Co-Delivery of Multiple Agents. Pharmaceutics. 2021; 13(3):313. https://doi.org/10.3390/pharmaceutics13030313
Chicago/Turabian StyleObireddy, Sreekanth Reddy, and Wing-Fu Lai. 2021. "Multi-Component Hydrogel Beads Incorporated with Reduced Graphene Oxide for pH-Responsive and Controlled Co-Delivery of Multiple Agents" Pharmaceutics 13, no. 3: 313. https://doi.org/10.3390/pharmaceutics13030313
APA StyleObireddy, S. R., & Lai, W.-F. (2021). Multi-Component Hydrogel Beads Incorporated with Reduced Graphene Oxide for pH-Responsive and Controlled Co-Delivery of Multiple Agents. Pharmaceutics, 13(3), 313. https://doi.org/10.3390/pharmaceutics13030313







