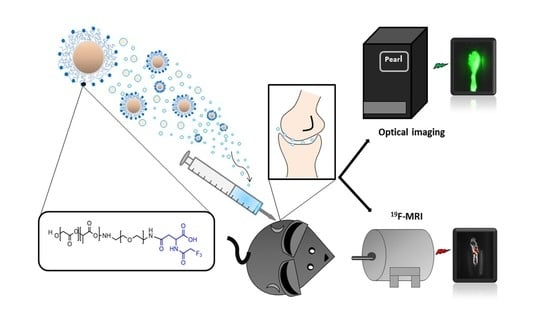
Novel Fluorinated Poly (Lactic-Co-Glycolic acid) (PLGA) and Polyethylene Glycol (PEG) Nanoparticles for Monitoring and Imaging in Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
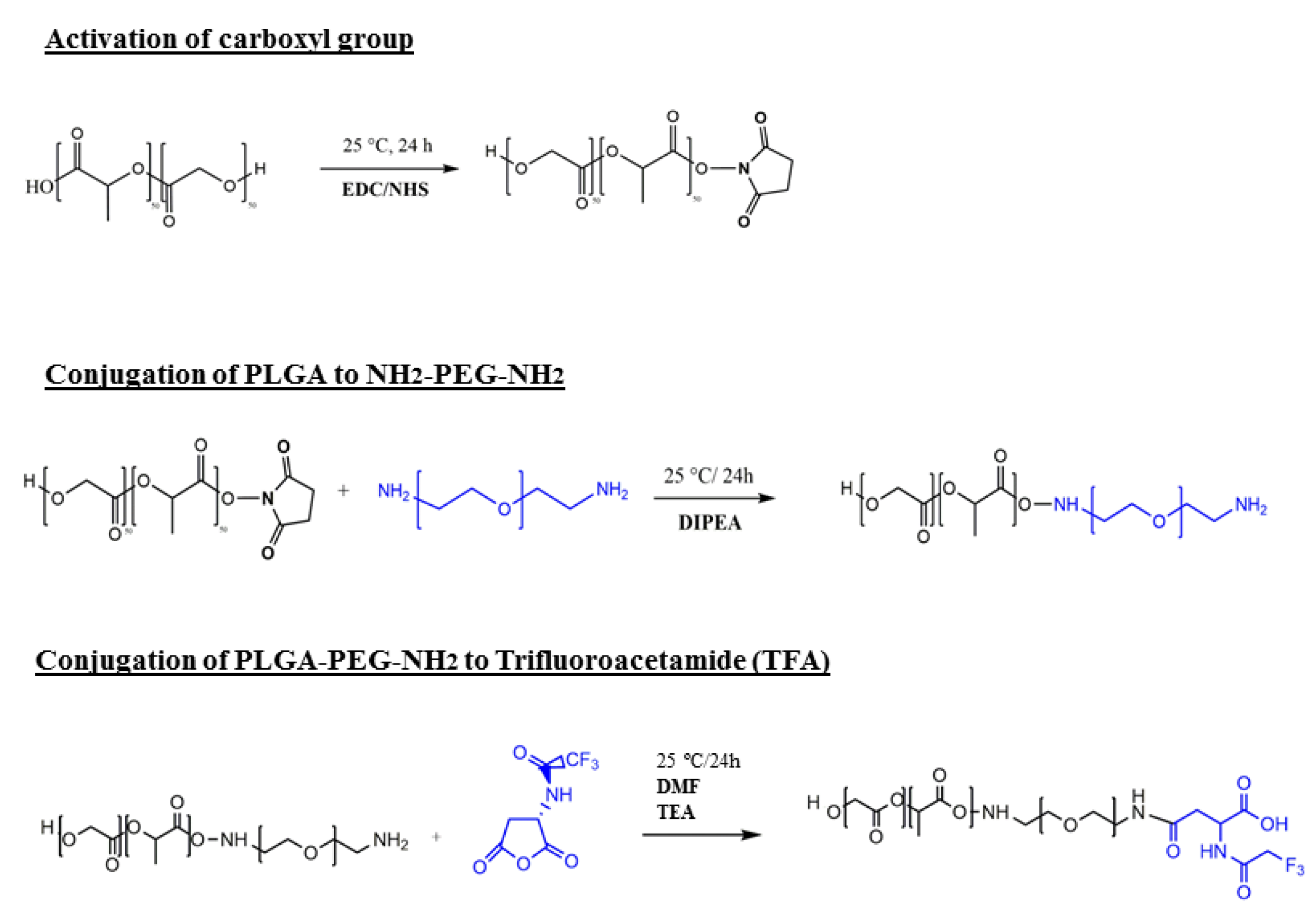
2.2. Copolymer Formulation
2.3. Preparation of NPs
Preparation of PLGA-PEG and PLGA-PEG-TFA NPs
2.4. Characterization of NPs
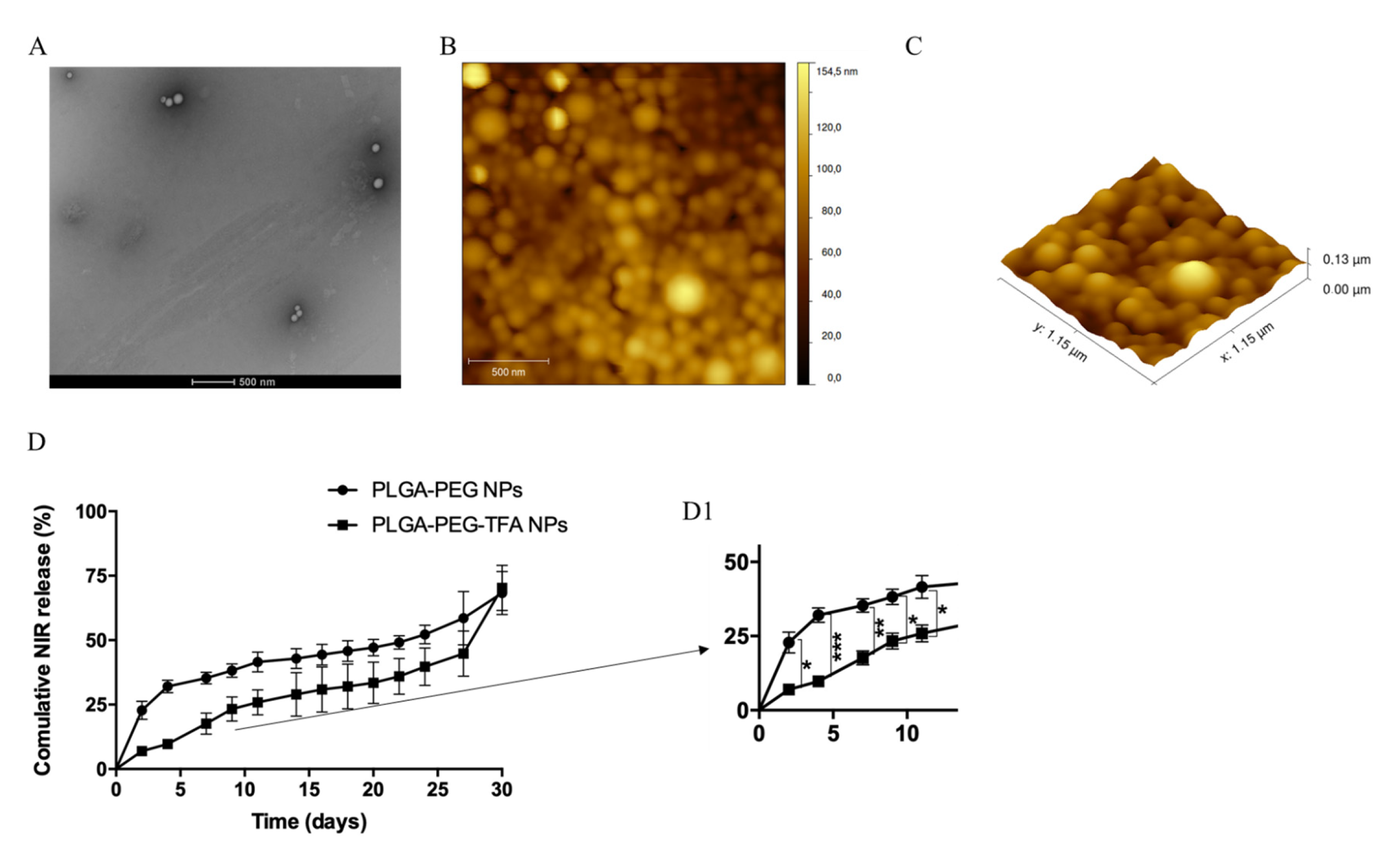
2.4.1. Morphology and Size Characterization
2.4.2. Encapsulation Efficiency Analysis of NIR Dye
2.4.3. Encapsulation Efficiency Analysis of FITC dye
2.4.4. Study of NIR Dye in PBS Released from NPs
2.4.5. Relaxivity Measurements
2.5. In Vitro Experiments
2.5.1. Cell Culture
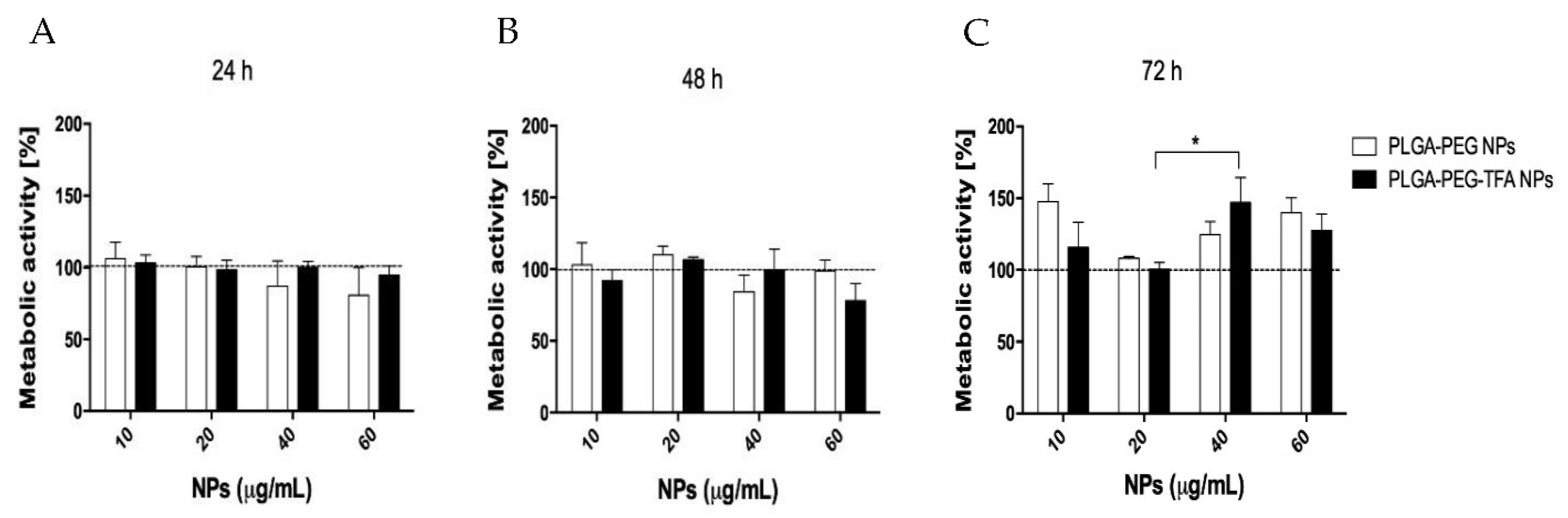
2.5.2. Cell Metabolic Assay (MTS)
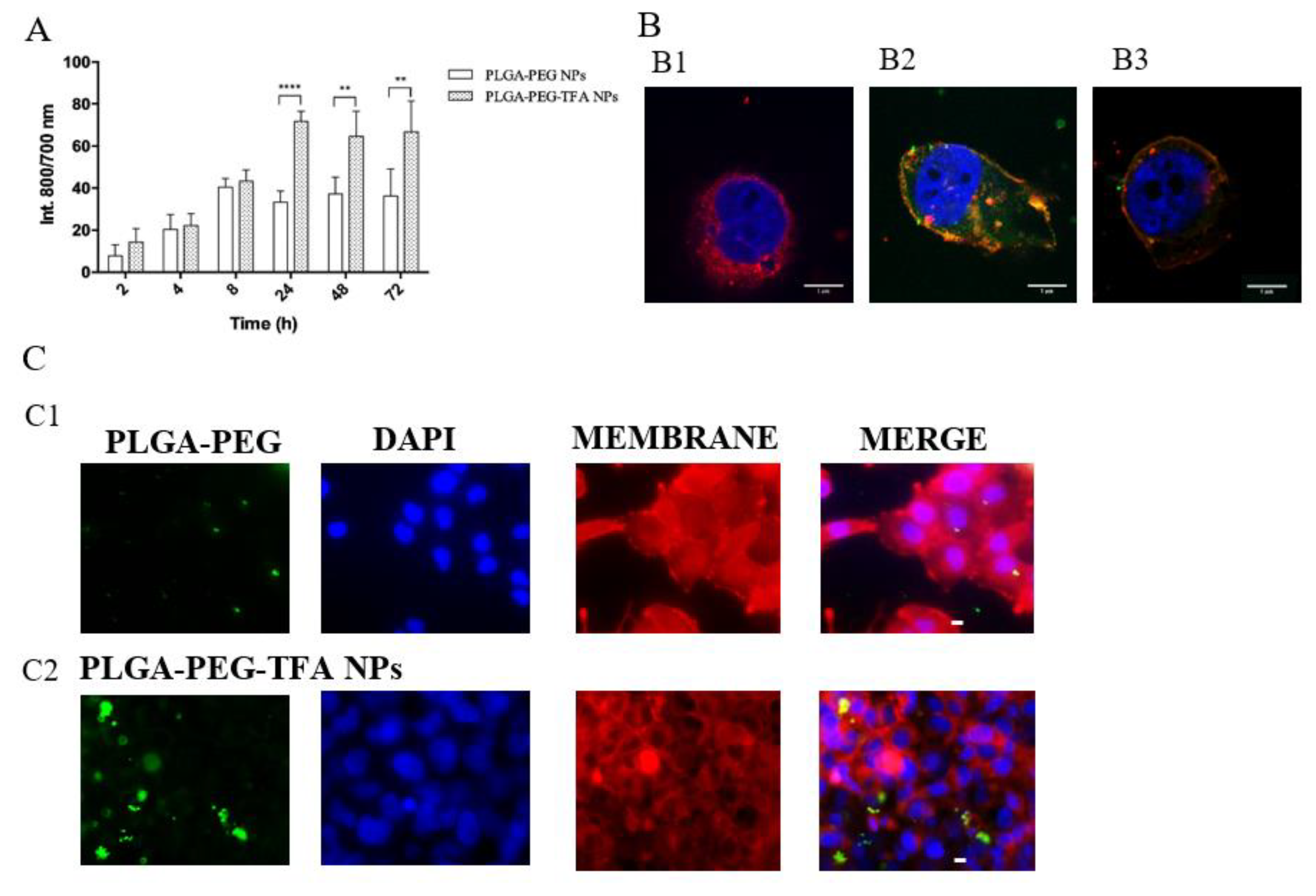
2.5.3. NP Uptake Study by Optical Imaging
2.5.4. NP Uptake Study by Confocal Microscopy
2.5.5. NP Uptake Studies by Fluorescence Microscopy
2.6. In Vivo and Ex Vivo NP Characterization
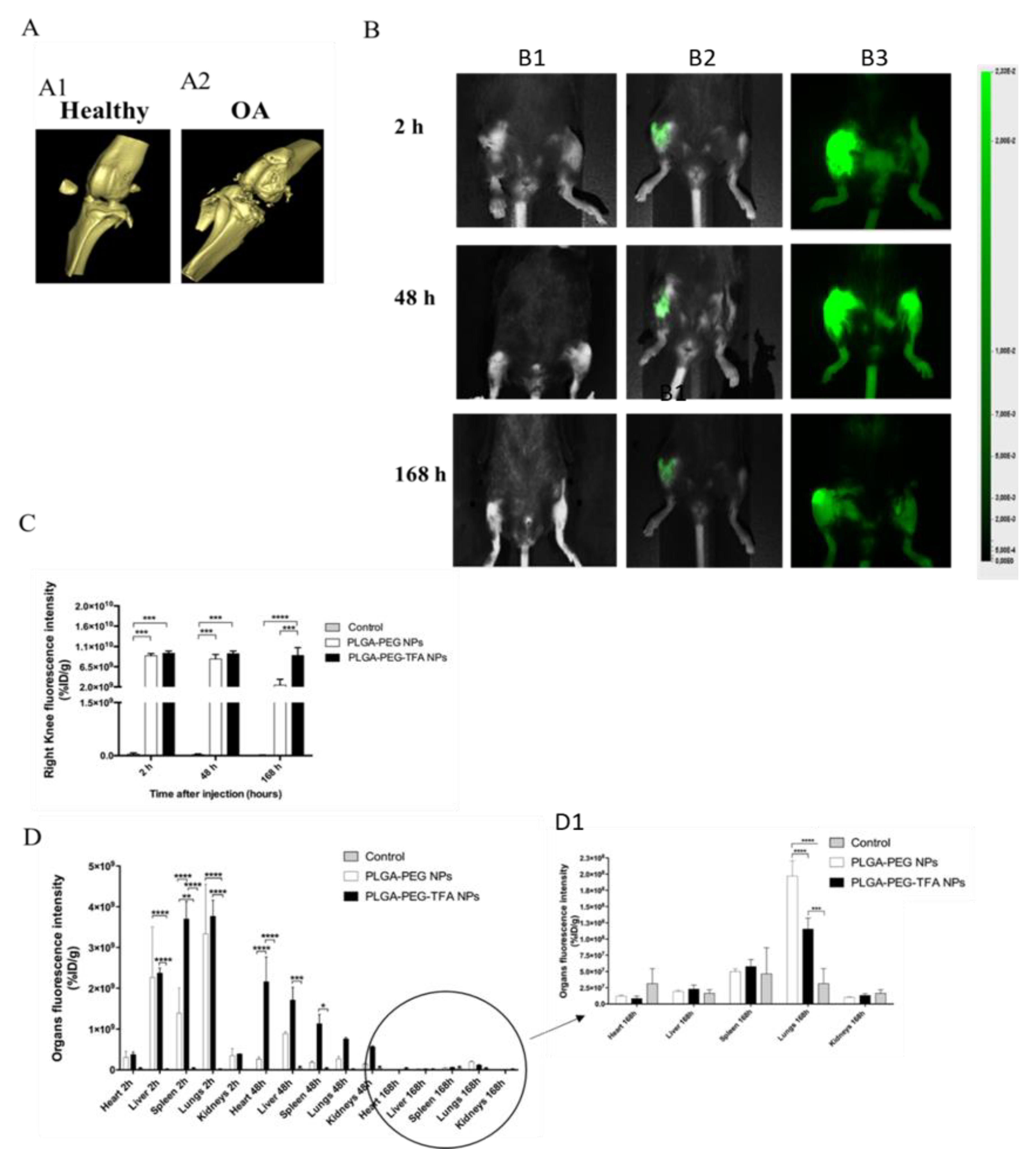
2.6.1. Optical Imaging
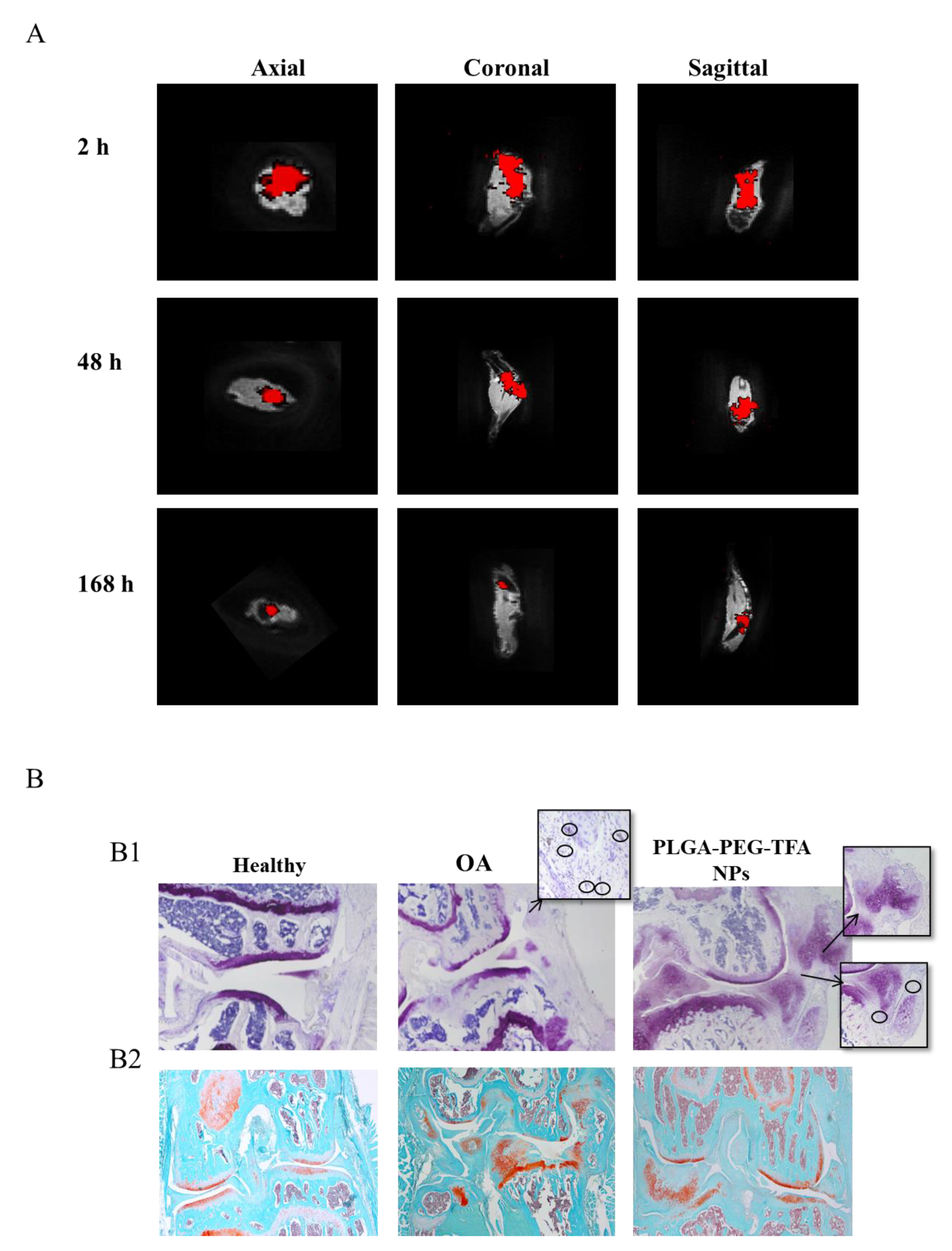
2.6.2. 19F-MRI
2.6.3. µ-. CT Scan
2.6.4. Histological Analysis
2.7. Statistical Analysis
3. Results and Discussion
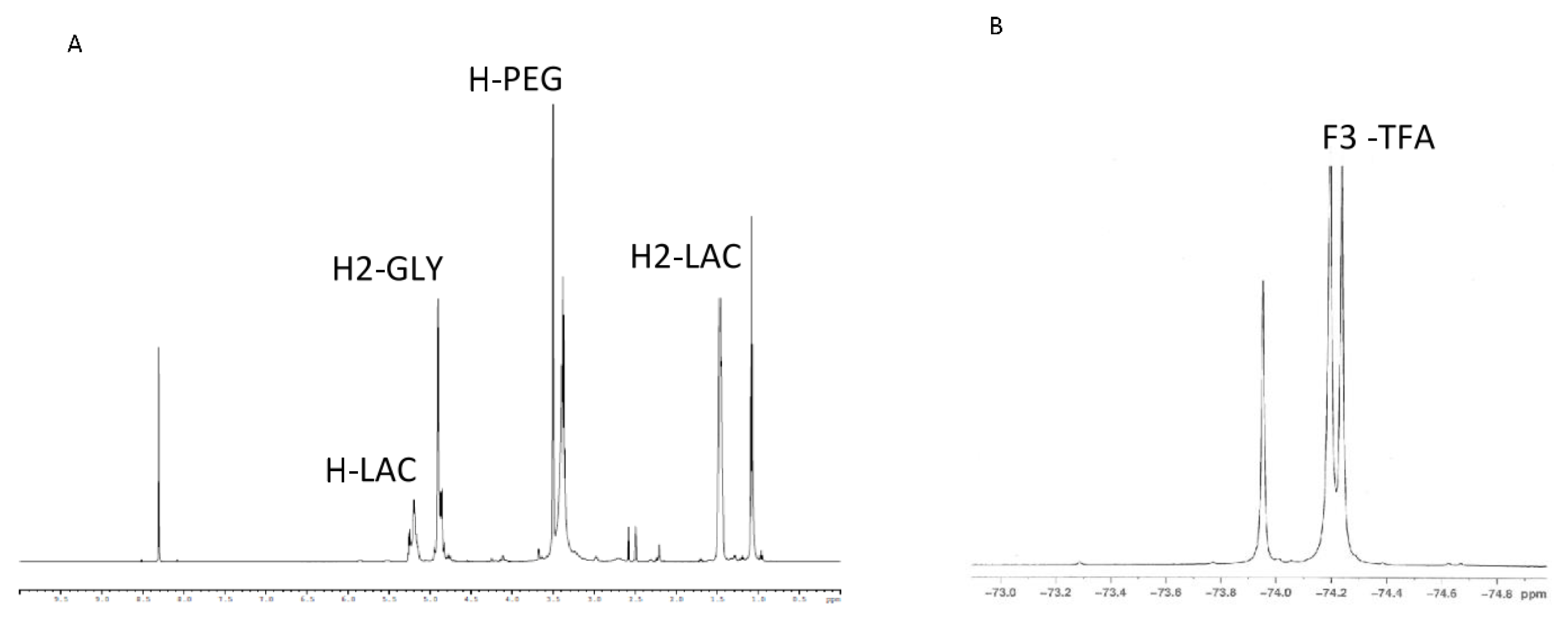
3.1. Synthesis and Characterization of the PLGA–PEG–TFA Copolymer
3.2. Preparation and Characterization of NPs
3.3. Characterization of Novel Polymeric NPs in the In Vitro Experiments
3.3.1. Cellular Metabolic Assay
3.3.2. NP Uptake by Chondrocytes
3.4. In Vivo and Ex Vivo Imaging of PLGA-PEG NPs Injected into a Mouse Model of Osteoarthritis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 201703856. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. The Epidemiology and Impact of Pain in Osteoarthritis. Osteoarthr. Cartil. 2014, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Rannou, F.; Pelletier, J.P.; Martel-Pelletier, J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2016, 45, S18–S21. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E. Pharmacologic and Non-Pharmacologic Treatment of Osteoarthritis. Curr. Treat. Options Rheumatol. 2016, 2, 111–125. [Google Scholar] [CrossRef]
- Wenham, C.Y.J.; Conaghan, P.G. Imaging the painful osteoarthritic knee joint: What have we learned? Nat. Clin. Pract. Rheumatol. 2009, 5, 149–158. [Google Scholar] [CrossRef]
- Garnero, P.; Peterfy, C.; Zaim, S.; Schoenharting, M. Bone marrow abnormalities on magnetic resonance imaging are associated with type II collagen degradation in knee osteoarthritis: A three-month longitudinal study. Arthritis Rheum. 2005, 52, 2822–2829. [Google Scholar] [CrossRef]
- Srinivas, M.; Cruz, L.J.; Bonetto, F.; Heerschap, A.; Figdor, C.G.; de Vries, I.J.M. Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using 19F MRI and optical imaging. Biomaterials 2010, 31, 7070–7077. [Google Scholar] [CrossRef]
- Koshkina, O.; Lajoinie, G.; Bombelli, F.B.; Swider, E.; Cruz, L.J.; White, P.B.; Schweins, R.; Dolen, Y.; van Dinther, E.A.W.; van Riessen, N.K.; et al. Multicore Liquid Perfluorocarbon-Loaded Multimodal Nanoparticles for Stable Ultrasound and 19 F MRI Applied to In Vivo Cell Tracking. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Cruz, L.J.; Tacken, P.J.; Zeelenberg, I.S.; Srinivas, M.; Bonetto, F.; Weigelin, B.; Eich, C.; de Vries, I.J.; Figdor, C.G. Tracking targeted bimodal nanovaccines: Immune responses and routing in cells, tissue, and whole organism. Mol. Pharm. 2014, 11, 4299–4313. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol.Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Rosalia, R.A.; Cruz, L.J.; van Duikeren, S.; Tromp, A.T.; Silva, A.L.; Jiskoot, W.; de Gruijl, T.; Löwik, C.; Oostendorp, J.; van der Burg, S.H.; et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 2015, 40, 88–97. [Google Scholar] [CrossRef]
- Zerrillo, L.; Que, I.; Vepris, O.; Morgado, L.N.; Chan, A.; Bierau, K.; Li, Y.; Galli, F.; Bos, E.; Censi, R.; et al. pH-responsive poly(lactide-co-glycolide) nanoparticles containing near-infrared dye for visualization and hyaluronic acid for treatment of osteoarthritis. J. Control. Release 2019, 309, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. Culture of immortalized chondrocytes and their use as models of chondrocyte function. Methods Mol. Med. 2004, 100, 37–52. [Google Scholar]
- Claassen, H.; Schicht, M.; Brandt, J.; Reuse, K.; Schädlich, R.; Goldring, M.B.; Guddat, S.S.; Thate, A.; Paulsen, F. C-28/I2 and T/C-28a2 chondrocytes as well as human primary articular chondrocytes express sex hormone and insulin receptors-Useful cells in study of cartilage metabolism. Ann. Anat. 2011, 193, 23–29. [Google Scholar] [CrossRef]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef]
- Yang, Q.; Lai, S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 1–11. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Ayala, V.; Herrera, A.P.; Latorre-Esteves, M.; Torres-Lugo, M.; Rinaldi, C. Effect of surface charge on the colloidal stability in vitro uptake of carboxymethyl dextran-coated iron oxide nanoparticles. J. Nanopartic. Res. 2013, 15, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Neogi, T. Clinical significance of bone changes in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Arms, L.; Smith, D.W.; Flynn, J.; Palmer, W.; Martin, A.; Woldu, A.; Hua, S. Advantages and Limitations of Current Techniques for Analyzing the Biodistribution of Nanoparticles. Front. Pharmacol. 2018, 9, 802. [Google Scholar] [CrossRef]
- Afrasiabi, M.; Noroozian, N. Advantages and Limitations of Functional Magnetic Resonance Imaging (fMRI) of the Human Visual Brain. In Horizons in Neuroscience Research Series; Costa, A., Villalba, E., Eds.; Nova Science Publishers: New York, NY, USA, 2015; Volume 17, pp. 65–72. [Google Scholar]
| NPs | Particle Size (nm) | PDI | ζ Potential (mV) | EE% (NIR) | EE% (FITC) |
|---|---|---|---|---|---|
| PLGA-PEG | 169.7 ± 5.4 | 0.30 ± 0.01 | −16.9 ± 3.6 | 57.6 | 26.3 |
| PLGA-PEG-TFA | 202.8 ± 1.7 | 0.20 ± 0.03 | −21.3 ± 1.8 | 44.4 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerrillo, L.; Gupta, K.B.S.S.; Lefeber, F.A.W.M.; Da Silva, C.G.; Galli, F.; Chan, A.; Veltien, A.; Dou, W.; Censi, R.; Di Martino, P.; et al. Novel Fluorinated Poly (Lactic-Co-Glycolic acid) (PLGA) and Polyethylene Glycol (PEG) Nanoparticles for Monitoring and Imaging in Osteoarthritis. Pharmaceutics 2021, 13, 235. https://doi.org/10.3390/pharmaceutics13020235
Zerrillo L, Gupta KBSS, Lefeber FAWM, Da Silva CG, Galli F, Chan A, Veltien A, Dou W, Censi R, Di Martino P, et al. Novel Fluorinated Poly (Lactic-Co-Glycolic acid) (PLGA) and Polyethylene Glycol (PEG) Nanoparticles for Monitoring and Imaging in Osteoarthritis. Pharmaceutics. 2021; 13(2):235. https://doi.org/10.3390/pharmaceutics13020235
Chicago/Turabian StyleZerrillo, Luana, Karthick Babu Sai Sankar Gupta, Fons A.W.M. Lefeber, Candido G. Da Silva, Federica Galli, Alan Chan, Andor Veltien, Weiqiang Dou, Roberta Censi, Piera Di Martino, and et al. 2021. "Novel Fluorinated Poly (Lactic-Co-Glycolic acid) (PLGA) and Polyethylene Glycol (PEG) Nanoparticles for Monitoring and Imaging in Osteoarthritis" Pharmaceutics 13, no. 2: 235. https://doi.org/10.3390/pharmaceutics13020235
APA StyleZerrillo, L., Gupta, K. B. S. S., Lefeber, F. A. W. M., Da Silva, C. G., Galli, F., Chan, A., Veltien, A., Dou, W., Censi, R., Di Martino, P., Srinivas, M., & Cruz, L. (2021). Novel Fluorinated Poly (Lactic-Co-Glycolic acid) (PLGA) and Polyethylene Glycol (PEG) Nanoparticles for Monitoring and Imaging in Osteoarthritis. Pharmaceutics, 13(2), 235. https://doi.org/10.3390/pharmaceutics13020235