Therapeutic Potential of Mesenchymal Stem Cells and Their Products in Lung Diseases—Intravenous Administration versus Inhalation
Abstract
1. Introduction
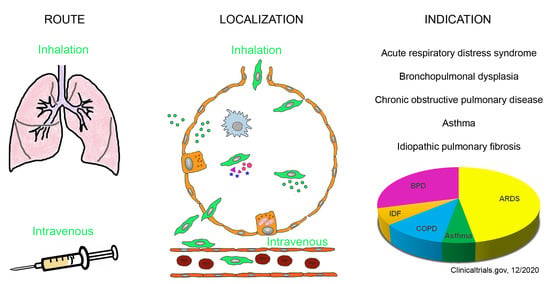
2. Pulmonary Indications for the Use of MSCs and MSC-Derived Products
2.1. Description of Diseases
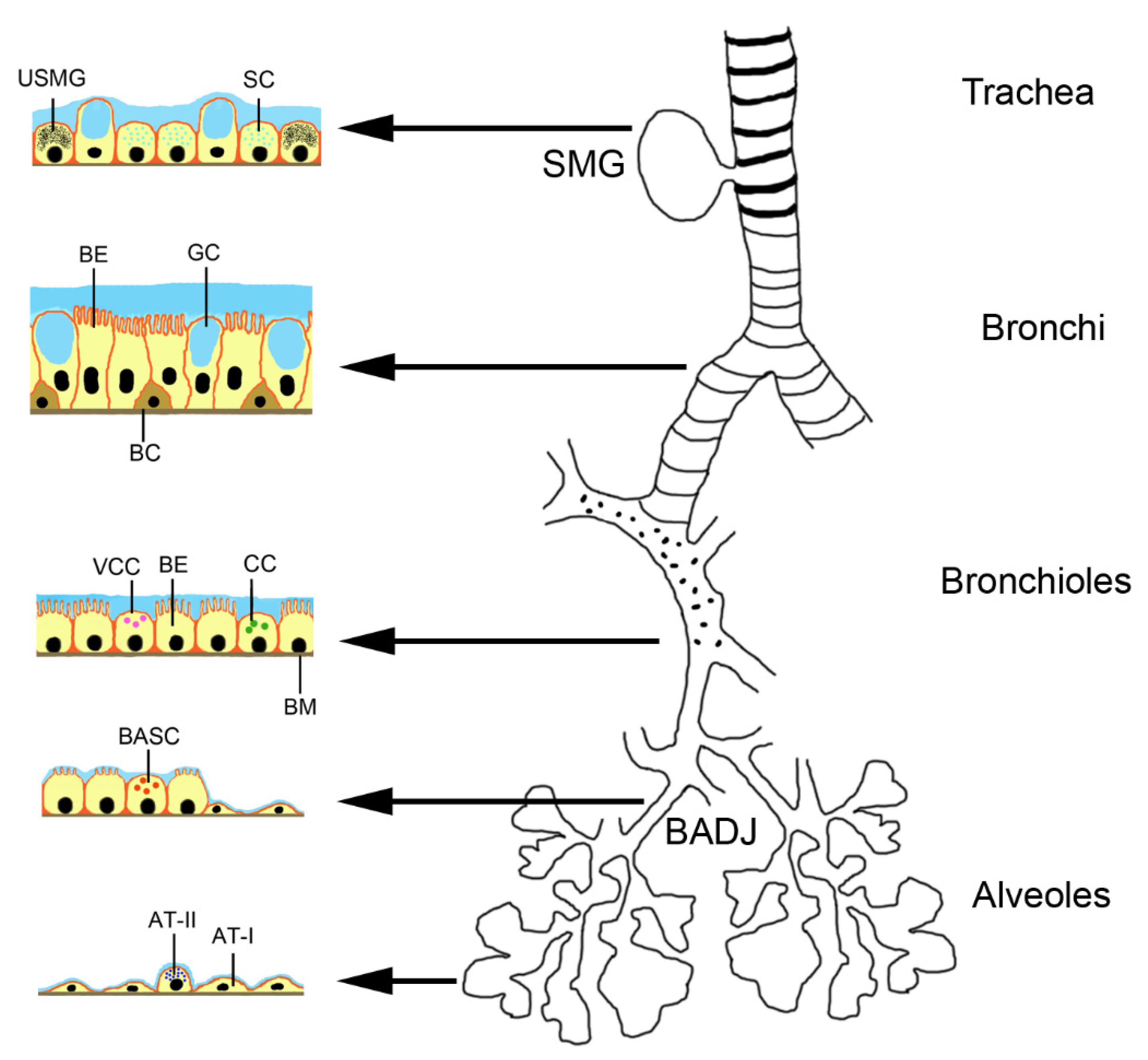
2.2. Stem Cells in the Lungs
3. Types of MSCs and MSC-Derived Products
3.1. Biological Characteristics of MSCs
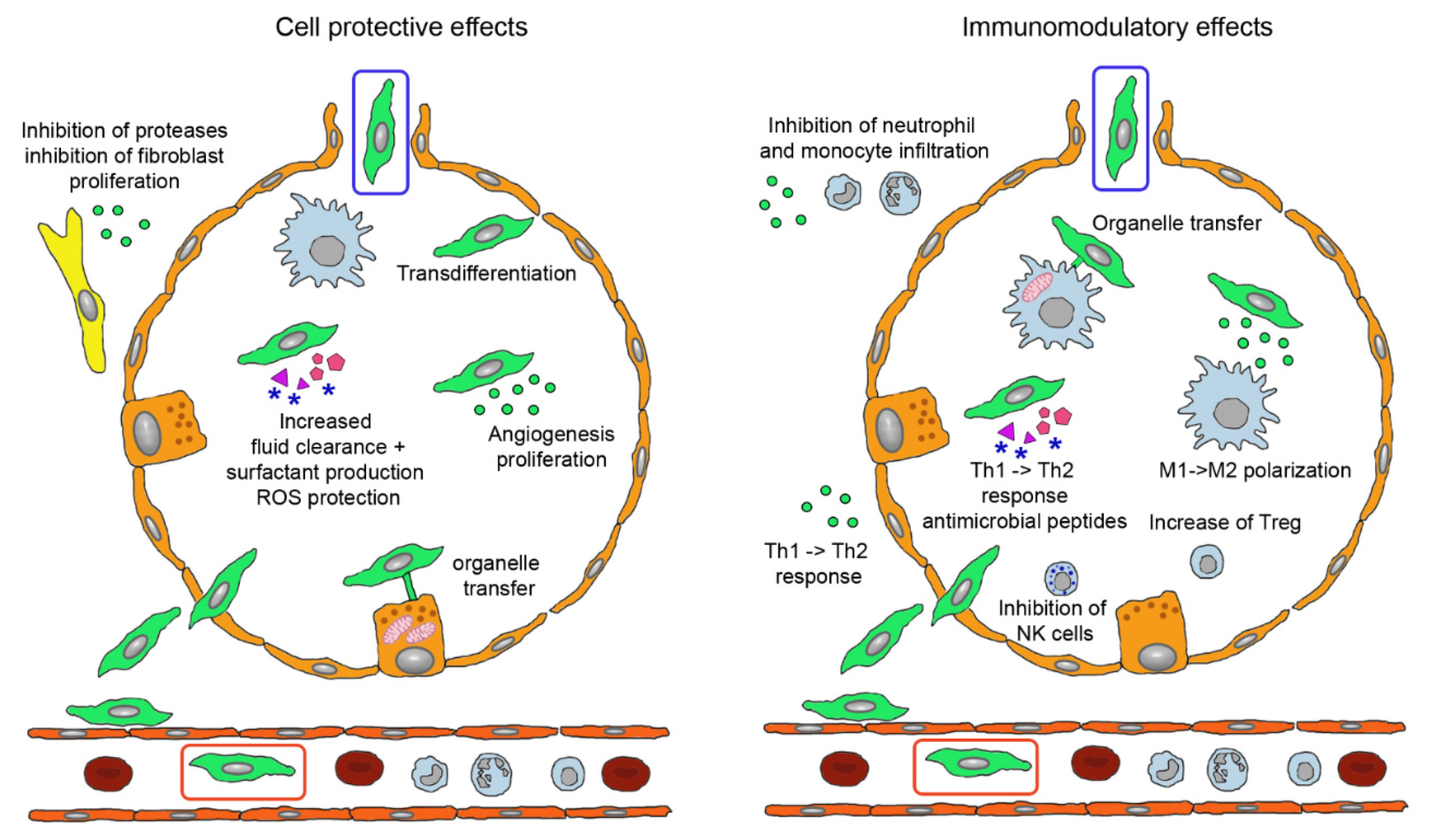
3.2. Modes of Action of MSCs
3.3. MSC-Derived Products
3.4. Differences in the Actions of MSCs and EVs
3.5. Therapeutic Use of MSCs and MSC-Derived Products in Pulmonary Diseases
3.5.1. Clinical Trials with MSCs
3.5.2. Studies on the Efficacy of MSC-Derived Products
4. Intravenous versus Inhalation Route of Delivery of MSCs and MSC-Derived Products
5. Administration of MSCs and MSC-Derived Products
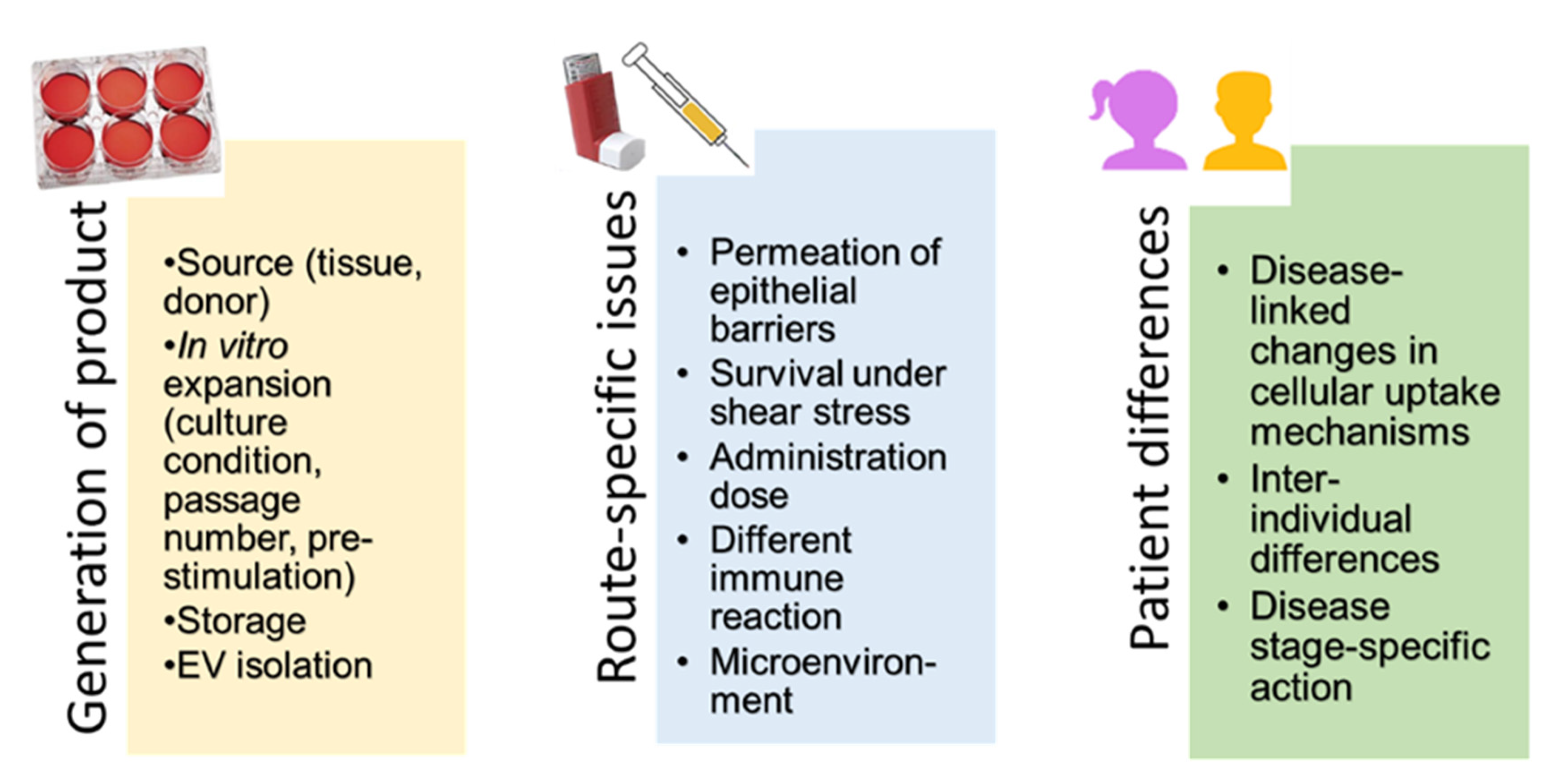
5.1. Production of MSCs and MSC-Derived EVs as Medicinal Products
5.2. Methods for Topical Administration to the Lungs
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brave, H.; MacLoughlin, R. State of the Art Review of Cell Therapy in the Treatment of Lung Disease and the Potential for Aerosol Delivery. Int. J. Mol. Sci. 2020, 21, 6435. [Google Scholar] [CrossRef]
- Patrikoski, M.; Mannerström, B.; Miettinen, S. Perspectives for Clinical Translation of Adipose Stromal/Stem Cells. Stem Cells Int. 2019, 2019, 1–21. [Google Scholar] [CrossRef]
- Stemcellresearchfacts. Available online: https://www.stemcellresearchfacts.org/stem-cell-treatments (accessed on 2 November 2020).
- Wilson, A.; Webster, A.; Genever, P. Nomenclature and heterogeneity: Consequences for the use of mesenchymal stem cells in regenerative medicine. Regen. Med. 2019, 14, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-L.; Ji, H.-L.; Zhao, R.-Z. Stem cell therapy for COVID-19 and other respiratory diseases: Global trends of clinical trials. World J. Stem Cells 2020, 12, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? STEM CELLS Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging Roles of Exosomes in Normal and Pathological Conditions: New Insights for Diagnosis and Therapeutic Applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Sueblinvong, V.; Weiss, D.J. Stem cells and cell therapy approaches in lung biology and diseases. Transl. Res. 2010, 156, 188–205. [Google Scholar] [CrossRef]
- Quaderi, S.; Hurst, J.R. The unmet global burden of COPD. Glob. Heal. Epidemiol. Genom. 2018, 3, e4. [Google Scholar] [CrossRef]
- Geiger, S.; Hirsch, D.; Hermann, F.G. Cell therapy for lung disease. Eur. Respir. Rev. 2017, 26, 170044. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Carroll, C.; Bhandari, V. BPD Following Preterm Birth: A Model for Chronic Lung Disease and a Substrate for ARDS in Childhood. Front. Pediatr. 2016, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K. Tuberculosis: A Global Health Problem. J. Health Popul. Nutr. 2010, 28, 111–113. [Google Scholar] [CrossRef]
- Bauer, T.T.; Ewig, S.; Rodloff, A.C.; Mueller, E. Acute Respiratory Distress Syndrome and Pneumonia: A Comprehensive Review of Clinical Data. Clin. Infect. Dis. 2006, 43, 748–756. [Google Scholar] [CrossRef]
- Matthay, M.; Zemans, R.L. The Acute Respiratory Distress Syndrome: Pathogenesis and Treatment. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 147–163. [Google Scholar] [CrossRef]
- Zambon, M.; Vincent, J.-L. Mortality Rates for Patients With Acute Lung Injury/ARDS Have Decreased Over Time. Chest 2008, 133, 1120–1127. [Google Scholar] [CrossRef]
- Happle, C.; Lachmann, N.; Ackermann, M.; Mirenska, A.; Göhring, G.; Thomay, K.; Mucci, A.; Hetzel, M.; Glomb, T.; Suzuki, T.; et al. Pulmonary Transplantation of Human Induced Pluripotent Stem Cell–derived Macrophages Ameliorates Pulmonary Alveolar Proteinosis. Am. J. Respir. Crit. Care Med. 2018, 198, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Pranke, I.; Golec, A.; Hinzpeter, A.; Edelman, A.; Sermet-Gaudelus, I. Emerging Therapeutic Approaches for Cystic Fibrosis. From Gene Editing to Personalized Medicine. Front. Pharmacol. 2019, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Kadyk, L.C.; DeWitt, N.D.; Gomperts, B.N. Proceedings: Regenerative Medicine for Lung Diseases: A CIRM Workshop Report. Stem Cells Transl. Med. 2017, 6, 1823–1828. [Google Scholar] [CrossRef]
- Freitag, A.; Mazurek, H.; Mejza, F. Cystic Fibrosis. In McMaster Textbook of Internal Medicine; Medycyna Praktyczna: Kraków, Poland, 2019. [Google Scholar]
- Barkauskas, C.E.; Chung, M.-I.; Fioret, B.; Gao, X.; Katsura, H.; Hogan, B.L.M. Lung organoids: Current uses and future promise. Development 2017, 144, 986–997. [Google Scholar] [CrossRef]
- Li, F.; He, J.; Wei, J.; Cho, W.C.; Liu, X. Diversity of Epithelial Stem Cell Types in Adult Lung. Stem Cells Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, N.; Nor, S.N.M.; Mohamed, M.S. Global Human Embryonic Stem Cell Laws and Policies and Their Influence on Stem Cell Tourism. Biotechnol. Law Rep. 2018, 37, 255–269. [Google Scholar] [CrossRef]
- Masterson, C.H.; Curley, G.F.; Laffey, J.G. Modulating the distribution and fate of exogenously delivered MSCs to enhance therapeutic potential: Knowns and unknowns. Intensiv. Care Med. Exp. 2019, 7, 41. [Google Scholar] [CrossRef]
- Chou, H.; Lin, W.; Chen, C. Human mesenchymal stem cells attenuate pulmonary hypertension induced by prenatal lipopolysaccharide treatment in rats. Clin. Exp. Pharmacol. Physiol. 2016, 43, 906–914. [Google Scholar] [CrossRef]
- Kim, E.S.; Chang, Y.S.; Choi, S.J.; Kim, J.K.; Yoo, H.S.; Ahn, S.Y.; Sung, D.K.; Kim, S.Y.; Park, Y.R.; Park, W.S. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir. Res. 2011, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Tolar, J.; Nauta, A.J.; Osborn, M.J.; Mortari, A.P.; McElmurry, R.T.; Bell, S.; Xia, L.; Zhou, N.; Riddle, M.; Schroeder, T.M.; et al. Sarcoma Derived from Cultured Mesenchymal Stem Cells. Stem Cells 2007, 25, 371–379. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.-B.; Song, Y.-P.; Han, Z.C. Safety of Mesenchymal Stem Cells for Clinical Application. Stem Cells Int. 2012, 2012, 1–4. [Google Scholar] [CrossRef]
- Semedo, P.; Burgos-Silva, M.; Donizetti-Oliviera, C.; Olsen Saraiva Camara, N. How do Mesenchymal Stem Cells Repair. In Stem Cells in Clinic and Research; Gholamrezanezhad, A., Ed.; InTech Open: Rijeka, Croatia, 2011. [Google Scholar]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate of Culture-Expanded Mesenchymal Stem Cells in The Microvasculature. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Luk, C.; Dahlke, M.; Hoogduijn, M.J. The Life and Fate of Mesenchymal Stem Cells. Front. Immunol. 2014, 5, 148. [Google Scholar] [CrossRef]
- Von Bahr, L.; Batsis, I.; Moll, G.; Hägg, M.; Szakos, A.; Sundberg, B.; Uzunel, M.; Ringden, O.; Le Blanc, K. Analysis of Tissues Following Mesenchymal Stromal Cell Therapy in Humans Indicates Limited Long-Term Engraftment and No Ectopic Tissue Formation. Stem Cells 2012, 30, 1575–1578. [Google Scholar] [CrossRef]
- Spees, J.L.; Olson, S.D.; Ylostalo, J.; Lynch, P.J.; Smith, J.; Perry, A.; Peister, A.; Wang, M.Y.; Prockop, D.J. Differentiation, cell fusion and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc. Natl. Acad. Sci. USA 2003, 100, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, L.; Sarukhan, A.; Dander, E.; Castor, M.G.M.; Cibella, J.; Soldani, C.; E Trovato, A.; Ploia, C.; Luca, G.; Calvitti, M.; et al. Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia 2012, 27, 500–503. [Google Scholar] [CrossRef]
- Leibacher, J.; Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res. Ther. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed]
- Yen, B.L.; Yen, M.-L.; Wang, L.; Liu, K.; Sytwu, H. Current status of mesenchymal stem cell therapy for immune/inflammatory lung disorders: Gleaning insights for possible use in COVID -19. Stem Cells Transl. Med. 2020, 9, 1163–1173. [Google Scholar] [CrossRef]
- Pinky; Gupta, S.; Krishnakumar, V.; Sharma, Y.; Dinda, A.K.; Mohanty, S. Mesenchymal Stem Cell Derived Exosomes: A Nano Platform for Therapeutics and Drug Delivery in Combating COVID-19. Stem Cell Rev. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Khalaj, K.; Figueira, R.L.; Antounians, L.; Lauriti, G.; Zani, A. Systematic review of extracellular vesicle-based treatments for lung injury: Are EVs a potential therapy for COVID-19? J. Extracell. Vesicles 2020, 9, 1795365. [Google Scholar] [CrossRef]
- Broekman, W.; Khedoe, P.P.S.J.; Schepers, K.; Roelofs, H.; Stolk, J.; Hiemstra, P.S. Mesenchymal stromal cells: A novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax 2018, 73, 565–574. [Google Scholar] [CrossRef]
- Laffey, J.G.; Matthay, M.A. Fifty Years of Research in ARDS. Cell-based Therapy for Acute Respiratory Distress Syndrome. Biology and Potential Therapeutic Value. Am. J. Respir. Crit. Care Med. 2017, 196, 266–273. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Antebi, B.; Batchinsky, A.I.; Cancio, L.C. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir. Res. 2018, 19, 1–14. [Google Scholar] [CrossRef]
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through “pharmaceuticalization” for the best formulation. J. Control Release 2019, 309, 11–24. [Google Scholar] [CrossRef]
- Canham, M.A.; Campbell, J.D.M.; Mountford, J.C. The use of mesenchymal stromal cells in the treatment of coronavirus disease 2019. J. Transl. Med. 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- Agarwal, Y.; Mukherjee, A.; Kumar, D.P.; Chatterjee, P.; Nag, V.; Malhotra, P. Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020, 371. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Agarwal, S.; Clauss, M.; Britt, N.S.; Dhillon, N.K. Extracellular vesicles: Novel communicators in lung diseases. Respir. Res. 2020, 21, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; Del Boccio, P.; Vergara, D.; Vergara, D.; Miscia, S.; et al. Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int. J. Mol. Sci. 2020, 21, 2514. [Google Scholar] [CrossRef]
- Gebara, N.; Rossi, A.; Skovronova, R.; Aziz, J.M.; Asthana, A.; Bussolati, B. Extracellular Vesicles, Apoptotic Bodies and Mitochondria: Stem Cell Bioproducts for Organ Regeneration. Curr. Transplant. Rep. 2020, 7, 105–113. [Google Scholar] [CrossRef]
- Holtzman, J.; Lee, H. Emerging role of extracellular vesicles in the respiratory system. Exp. Mol. Med. 2020, 52, 887–895. [Google Scholar] [CrossRef]
- Bartel, S.; Deshane, J.; Wilkinson, T.; Gabrielsson, S. Extracellular Vesicles as Mediators of Cellular Cross Talk in the Lung Microenvironment. Front. Med. 2020, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Lanyu, Z.; Feilong, H. Emerging role of extracellular vesicles in lung injury and inflammation. Biomed. Pharmacother. 2019, 113, 108748. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Mammadova, R.; Juarez, A.P.R.; Bokka, R.; Trepiccione, F.; Capasso, G. COVID-19 and Extracellular Vesicles: An Intriguing Interplay. Kidney Blood Press. Res. 2020, 45, 661–670. [Google Scholar] [CrossRef] [PubMed]
- McVey, M.J.; Maishan, M.; Blokland, K.E.C.; Bartlett, N.W.; Kuebler, W.M. Extracellular vesicles in lung health, disease and therapy. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L977–L989. [Google Scholar] [CrossRef]
- Park, K.-S.; Bandeira, E.; Shelke, G.V.; Lässer, C.; Lötvall, J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: Challenges and opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef]
- Al-Khawaga, S.; Abdelalim, E.M. Potential application of mesenchymal stem cells and their exosomes in lung injury: An emerging therapeutic option for COVID-19 patients. Stem Cell Res. Ther. 2020, 11, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.R.; Miyazawa, B.Y.; Gibb, S.L.; Deng, X.; Togaratti, P.P.; Croze, R.H.; Srivastava, A.K.; Trivedi, A.; Matthay, M.; Holcomb, J.B.; et al. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J. Trauma Acute Care Surg. 2018, 84, 245–256. [Google Scholar] [CrossRef]
- Willis, G.R.; Mitsialis, S.A.; Kourembanas, S. “Good things come in small packages”: Application of exosome-based therapeutics in neonatal lung injury. Pediatr. Res. 2018, 83, 298–307. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Jafari, D.; Shajari, S.; Jafari, R.; Mardi, N.; Gomari, H.; Ganji, F.; Moghadam, M.F.; Samadikuchaksaraei, A. Designer Exosomes: A New Platform for Biotechnology Therapeutics. BioDrugs 2020, 34, 567–586. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Sveiven, S.N.; Nordgren, T.M. Lung-resident mesenchymal stromal cells are tissue-specific regulators of lung homeostasis. Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L197–L210. [Google Scholar] [CrossRef]
- Ricciardi, M.; Malpeli, G.; Bifari, F.; Bassi, G.; Pacelli, L.; Kamdje, A.H.N.; Chilosi, M.; Krampera, M. Comparison of Epithelial Differentiation and Immune Regulatory Properties of Mesenchymal Stromal Cells Derived from Human Lung and Bone Marrow. PLoS ONE 2012, 7, e35639. [Google Scholar] [CrossRef]
- Xunian, Z.; Kalluri, R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020, 111, 3100–3110. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020, 1–14. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Dabbous, M.; Liang, S.; Qiu, T.; Toumi, M. Chinese Clinical Studies for Pharmacological Treatments of Coronavirus Disease 2019 (COVID-19). Preprints 2020. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Saadeldin, I.M.; Ahmad, A.; Kumar, D.; Azhar, E.I.; Siddiqui, A.J.; Kurdi, B.; Sajini, A.A.; Alrefaei, A.F.; Jahan, S. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes as Immunomodulatory Agents for COVID-19 Patients. Stem Cells Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Heathman, T.R.J.; Nienow, A.; McCall, M.J.; Coopman, K.; Kara, B.; Hewitt, C.J. The translation of cell-based therapies: Clinical landscape and manufacturing challenges. Regen. Med. 2015, 10, 49–64. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Davies, J.E.; Walker, J.T.; Keating, A. Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2017, 6, 1620–1630. [Google Scholar] [CrossRef]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Tesei, A. The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis. Int. J. Mol. Sci. 2019, 20, 3876. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Navarro, K.J.; Padilla-Camberos, E.; Díaz, N.F.; Miranda-Altamirano, A.; Díaz-Martínez, N.E. Human Mesenchymal Stem Cells: The Present Alternative for High-Incidence Diseases, Even SARS-Cov-2. Stem Cells Int. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Majolo, F.; Da Silva, G.L.; Vieira, L.; Timmers, L.F.S.M.; Laufer, S.; Goettert, M.I. Review of Trials Currently Testing Stem Cells for Treatment of Respiratory Diseases: Facts Known to Date and Possible Applications to COVID-19. Stem Cell Rev. Rep. 2020, 1–12. [Google Scholar] [CrossRef]
- Papait, A.; Cargnoni, A.; Sheleg, M.; Silini, A.R.; Kunis, G.; Ofir, R.; Parolini, O. Perinatal Cells: A Promising COVID-19 Therapy? Front. Bioeng. Biotechnol. 2021, 8. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W.I.; Park, W.S. Mesenchymal Stem Cells for Bronchopulmonary Dysplasia: Phase 1 Dose-Escalation Clinical Trial. J. Pediatr. 2014, 164, 966–972.e6. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Hare, J.M.; Bu, G.; Mallea, J.M.; Pascual, J.M.; Caplan, A.I.; Kurtzberg, J.; Zubair, A.C.; Kubrova, E.; et al. Cell-based therapy to reduce mortality from COVID -19: Systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem cells Transl. Med. 2020, 9, 1007–1022. [Google Scholar] [CrossRef]
- 83% Survival in COVID-19 Patients with Moderate/Severe Acute Respiratory Distress Syndrome Treated in New York with Mesoblast’s Cell Therapy Remestemcel-L. Available online: https://www.globenewswire.com/news-release/2020/04/24/2021558/0/en/83-Survival-in-COVID-19-Patients-with-Moderate-Severe-Acute-Respiratory-Distress-Syndrome-Treated-in-New-York-with-Mesoblast-s-Cell-Therapy-Remestemcel-L.html (accessed on 2 December 2020).
- Emukah, C.; Dittmar, E.; Naqvi, R.; Martinez, J.; Corral, A.; Moreira, A.; Moreira, A. Mesenchymal stromal cell conditioned media for lung disease: A systematic review and meta-analysis of preclinical studies. Respir. Res. 2019, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, S.; Zhang, Y. Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin-induced acute lung injury in mice through secretion of exosome. Int. J. Clin. Exp. Med. 2015, 8, 3825–3832. [Google Scholar]
- Monsel, A.; Zhu, Y.-G.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.-J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell–derived Microvesicles in Severe Pneumonia in Mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336. [Google Scholar] [CrossRef]
- FDA. A Review of the Latest FDA Communication Regarding Exosomes. Available online: Https://Cdn2.Hubspot.Net/Hubfs/4723748/Fda%20public%20safety%20notification%20on%20exosome%20products%20-%2012.11.2019.Pdf?Fbclid=Iwar1suxsieiejjcdgcbnp46v6bmfshef9wdw_38q89ml5gwyiudtsud0ij-S (accessed on 2 December 2020).
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Genengnews. Organicell Regenerative Medicine–Zofin™ (Organcell™ Flow). Available online: https://www.genengnews.com/covid-19-candidates/covid-19-too-soon-to-tell/organicell-regenerative/ (accessed on 2 November 2020).
- FDA. Direct Biologics Granted Expanded Access by FDA for ExoFlo™ in the Treatment of COVID-19. Available online: https://www.finanzen.at/nachrichten/aktien/direct-biologics-granted-expanded-access-by-fda-for-exoflo-in-the-treatment-of-covid-19-1029676601 (accessed on 2 November 2020).
- Directbiologics. Growth Factors. Available online: https://directbiologics.com/growth-factors/ (accessed on 2 November 2020).
- Fleig, S.V.; Humphreys, B.D. Rationale of Mesenchymal Stem Cell Therapy in Kidney Injury. Nephron Clin. Pr. 2014, 127, 75–80. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Lo, H.-Y.; Cheng, S.-P.; Chang, K.-T.; Lin, X.-F.; Lee, S.-P.; Ma, J.; Kuo, M.-L.; Hsieh, M.-F.; Chan, C.-K. Therapeutic Efficacy of Subcutaneous and Intraperitoneal Injections of a Single Dose of Human Umbilical Mesenchymal Stem Cells in Acute and Chronic Colitis in a Mouse Model. J. Med Biol. Eng. 2019, 40, 82–90. [Google Scholar] [CrossRef]
- Garcia, O.; Carraro, G.; Navarro, S.; Bertoncello, I.; McQualter, J.L.; Driscoll, B.; Jesudason, E.; Warburton, D. Cell-based therapies for lung disease. Br. Med Bull. 2012, 101, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Benseler, V.; Kroemer, H.; Popp, F.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Dorland, Y.L.; Cornelissen, A.S.; Kuijk, C.; Tol, S.; Hoogenboezem, M.; Van Buul, J.D.; Nolte, M.A.; Voermans, C.; Huveneers, S. Nuclear shape, protrusive behaviour and in vivo retention of human bone marrow mesenchymal stromal cells is controlled by Lamin-A/C expression. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly and the Promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef]
- Kim, J.; Guenthart, B.; O’Neill, J.D.; Dorrello, N.V.; Bacchetta, M.; Vunjak-Novakovic, G. Controlled delivery and minimally invasive imaging of stem cells in the lung. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- McIntyre, L.A.; Moher, D.; Fergusson, D.A.; Sullivan, K.J.; Mei, S.H.J.; Lalu, M.; Marshall, J.; MacLeod, M.; Griffin, G.; Grimshaw, J.; et al. Efficacy of Mesenchymal Stromal Cell Therapy for Acute Lung Injury in Preclinical Animal Models: A Systematic Review. PLoS ONE 2016, 11, e0147170. [Google Scholar] [CrossRef]
- Harrell, C.R.; Sadikot, R.; Pascual, J.; Fellabaum, C.; Jankovic, M.G.; Jovicic, N.U.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases: Current Understanding and Future Perspectives. Stem Cells Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Paspaliaris, V.; Koliakos, G.; Ntolios, P.; Bouros, E.; Oikonomou, A.; Zissimopoulos, A.; Boussios, N.; Dardzinski, B.; Gritzalis, D.; et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J. Transl. Med. 2013, 11, 171. [Google Scholar] [CrossRef]
- Ntolios, P.; Manoloudi, E.; Tzouvelekis, A.; Bouros, E.; Steiropoulos, P.; Anevlavis, S.; Bouros, D.; Froudrarakis, M. Longitudinal outcomes of patients enrolled in a phase Ib clinical trial of the adipose-derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Clin. Respir. J. 2018, 12, 2084–2089. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Feng, X.-M.; Abbott, J.; Fang, X.-H.; Hao, Q.; Monsel, A.; Qu, J.-M.; Matthay, M.A.; Lee, J.W. Human Mesenchymal Stem Cell Microvesicles for Treatment ofEscherichia coliEndotoxin-Induced Acute Lung Injury in Mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef]
- Worthington, E.N.; Hagood, J.S. Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int. J. Mol. Sci. 2020, 21, 2318. [Google Scholar] [CrossRef]
- Rubio, A.P.D.; Martínez, J.; Palavecino, M.; Fuentes, F.; López, C.M.S.; Marcilla, A.; Pérez, O.E.; Piuri, M. Transcytosis of Bacillus subtilis extracellular vesicles through an in vitro intestinal epithelial cell model. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Carobolante, G.; Mantaj, J.; Ferrari, E.; Vllasaliu, D. Cow Milk and Intestinal Epithelial Cell-Derived Extracellular Vesicles as Systems for Enhancing Oral Drug Delivery. Pharmaceutics 2020, 12, 226. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, X.; Hao, J.; Xu, G.; Zhang, W. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration. Cell Transplant. 2020, 29. [Google Scholar] [CrossRef]
- Kim, M.; Bae, Y.K.; Um, S.; Kwon, J.H.; Kim, G.-H.; Choi, S.J.; Oh, W.; Jin, H.J. A Small-Sized Population of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Shows High Stemness Properties and Therapeutic Benefit. Stem Cells Int. 2020, 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Absher, P.M. Enrichment and Isolation Techniques for Animal Cell Types. In Encyclopedia of Industrial Biotechnology; Wiley online Library: Hoboken, NJ, USA, 2010. [Google Scholar]
- Gomzikova, M.O.; James, V.; Rizvanov, A. Therapeutic Application of Mesenchymal Stem Cells Derived Extracellular Vesicles for Immunomodulation. Front. Immunol. 2019, 10, 2663. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.-K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; A Horn, P.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Rohde, E.; Pachler, K.; Gimona, M. Manufacturing and characterization of extracellular vesicles from umbilical cord–derived mesenchymal stromal cells for clinical testing. Cytotherapy 2019, 21, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, B.H. ID2008 Aerosol-based cell delivery as an innovative treatment for lung diseases. Biomed. Res. Ther. 2017, 4, 41. [Google Scholar] [CrossRef][Green Version]
- Halim, N.S.S.; Ch’Ng, E.S.; Kardia, E.; Ali, S.A.; Radzi, R.; Yahaya, B.H. Aerosolised Mesenchymal Stem Cells Expressing Angiopoietin-1 Enhances Airway Repair. Stem Cell Rev. Rep. 2018, 15, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, S. Vibrating mesh nebulisers–can greater drug delivery to the airways and lungs improve respiratory outcomes. Eur. Respir. Pulmon. Dis. 2018, 4, 33–43. [Google Scholar] [CrossRef]
- Woods, N.; MacLoughlin, R. Defining a Regulatory Strategy for ATMP/Aerosol Delivery Device Combinations in the Treatment of Respiratory Disease. Pharmaceutics 2020, 12, 922. [Google Scholar] [CrossRef]
- Averyanov, A.V.; Konoplyannikov, A.G.; Antonov, N.S.; Osipova, G.L.; Vasil’Eva, O.S.; Sakharova, M.G.; Tatarskii, A.R.; Kobylyansky, V.I. Survival of Mesenchymal Stem Cells in Different Methods of Nebulization. Bull. Exp. Biol. Med. 2018, 164, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Averyanov, A.; Konoplyannikov, A.; Zabozlaev, F.; Danilevskaya, O.; Konoplyannikov, M.; Kuzovlev, O.; Kulagina, N.; Koroleva, I. Comparative effects of inhaled and intravenous mesenchymal stem cells in bleomycin-induced pulmonary fibrosis in rabbits. Eur. Respir. J. 2013, 42, 226. [Google Scholar]
- Saparov, A.; Ogay, V.; Nurgozhin, T.; Jumabay, M.; Chen, W.C.W. Preconditioning of Human Mesenchymal Stem Cells to Enhance Their Regulation of the Immune Response. Stem Cells Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.D.; Horgan, E.; Ali, A.; Masterson, C.; Laffey, J.G.; MacLoughlin, R.; O’Toole, D. Nebulized Mesenchymal Stem Cell Derived Conditioned Medium Retains Antibacterial Properties Against Clinical Pathogen Isolates. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Dinh, P.-U.C.; Paudel, D.; Brochu, H.; Popowski, K.D.; Gracieux, M.C.; Cores, J.; Huang, K.; Hensley, M.T.; Harrell, E.; Vandergriff, A.C.; et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, W.; Kim, S.Y.; McClements, L. Can Stem Cells Beat COVID-19: Advancing Stem Cells and Extracellular Vesicles Toward Mainstream Medicine for Lung Injuries Associated With SARS-CoV-2 Infections. Front. Bioeng. Biotechnol. 2020, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Wang, H.; Bian, L.; Huang, J.; Wu, D.; Fei, F.; Zhang, R.; Chen, Y.; Xia, J. Nebulization Therapy for COVID-19 Pneumonia with Embryonic Mesenchymal Stem Cells-Derived Exosomes. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Li, Z.; Niu, S.; Guo, B.; Gao, T.; Wang, L.; Wang, Y.; Wang, L.; Tan, Y.; Wu, J.; Hao, J. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif. 2020, 53, e12939. [Google Scholar] [CrossRef] [PubMed]
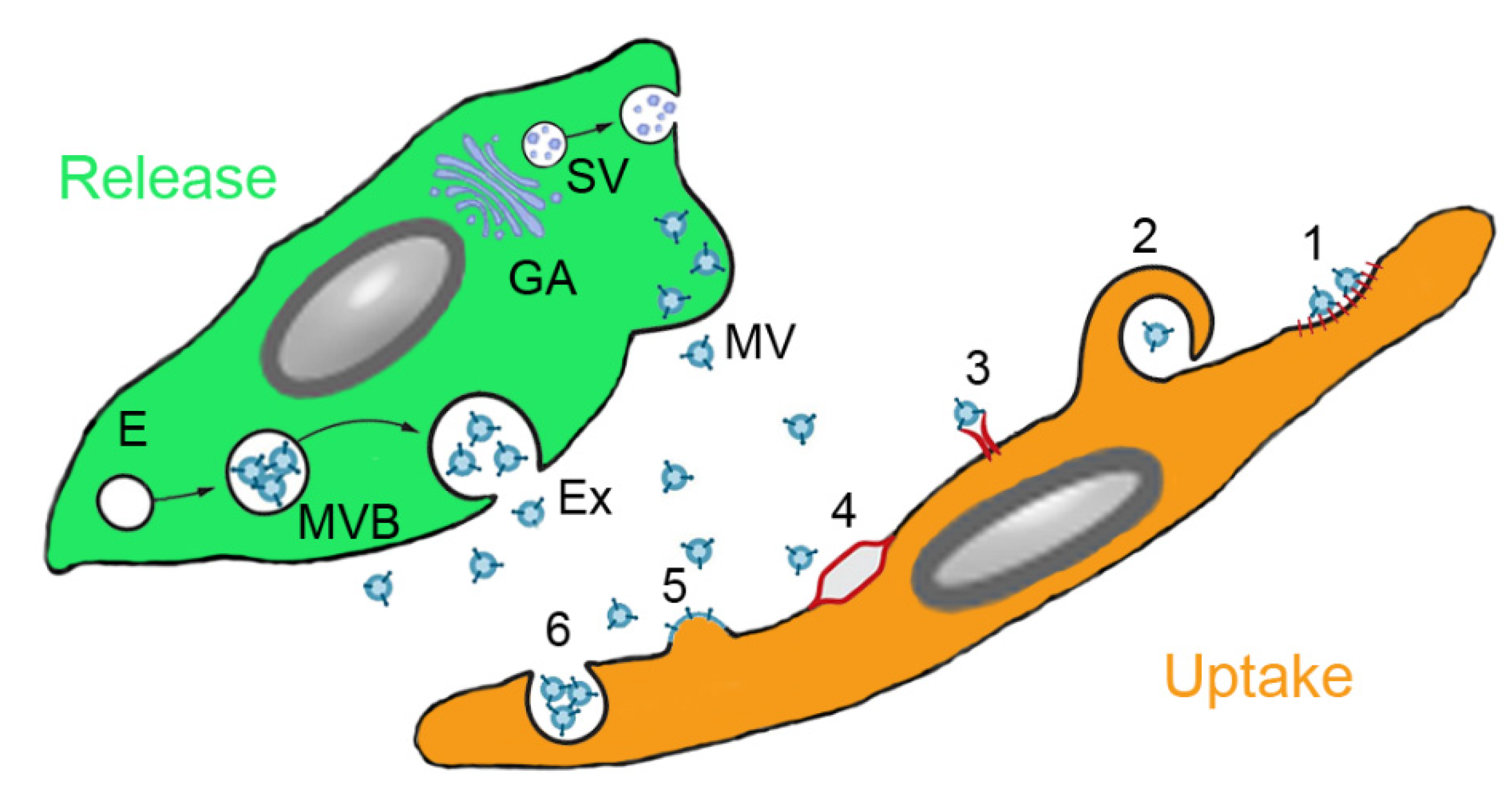
| Type of Extracellular vesicle | Type of Secretion of Vesicle | Size of Vesicle | Surface Marker of the Extracellular Vesicle | Content of the Extracellular Vesicles | Uptake by Target Cell |
|---|---|---|---|---|---|
| Apoptotic bodies | Membrane blebbing | 500–2000 nm | Phosphatidylserine, calreticulin, calnexin | Proteins, lipids, nuclear fragments, organelles | Phagocytosis |
| Microvesicles | Blebbing | 100–1000 nm | Integrins, selectins, CD40 | Proteins, lipids, ncRNAs, mRNA | Fusion |
| Exosomes | Exocytosis from multivesicular bodies | 40–100 nm | Tetraspanins (CD9, CD63, CD81), ESCRT, TSG101, flotillin, annexin | Membrane and cytoplasmic proteins, lipids, ncRNAs, mRNA, MHC molecules, receptors | Endocytosis |
| Year of Publication | Number of Trials Analysed | % of (Specific) Pulmonary Diseases in the Analysed Trials | Reference |
|---|---|---|---|
| 2015 | 339 (all indications) | 3 (pulmonary diseases) | [74] |
| 2015 | 516 (all indications) | 6 (pulmonary diseases) | [6] |
| 2016 | 493 (all indications) | 5 (pulmonary diseases) | [75] |
| 2017 | 109 (all indications) | 10 (pulmonary diseases) | [76] |
| 2019 | 49 (pulmonary diseases) | BPD (37), IPF (14), ARDS (29), asthma (4), COPD (4) | [77] |
| 2020 | 767 (all indications) | 7 (pulmonary diseases) | [78] |
| 2020 | 62 (COVID-19) | N.a. | [79] |
| 2020 | 73 (ARDS, COVID-19) | ARDS (57), COVID-19 (22), ARDS/COVID-19 (21) | [1] |
| 2020 | 16 (COVID-19) | N.a. | [80] |
| 2020 | 83 (pulmonary diseases) | COVID-19 (47), pulmonary fibrosis (10), ARDS (10), asthma (2), COPD (7) | [81] |
| 2020 | 68 (pulmonary diseases) | COVID-19 (45), ARDS (15), COPD (15), asthma (3), IPF (9) | [39] |
| Product | Content | Source of Variation | Comments |
|---|---|---|---|
| Stem cells (e.g., mesenchymal stem cells) | Viable cells | Donor, tissue source, composition of culture media, cell density, passage number, pre-culture conditions | Tissue source and pre-conditioning can be reported |
| Conditioned media | Soluble proteins, extracellular vesicles | As for stem cells plus culture time until collection, volume and composition of the collection medium | Isolation method can be standardized. There are no guidelines for characterization of the conditioned media |
| EVs/exosomes | Cytokines, growth factors, signaling lipids, mRNAs, regulatory miRs | As for conditioned media plus isolation method and storage condition of EVs | Recommendations for characterization of exosomes are available |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröhlich, E. Therapeutic Potential of Mesenchymal Stem Cells and Their Products in Lung Diseases—Intravenous Administration versus Inhalation. Pharmaceutics 2021, 13, 232. https://doi.org/10.3390/pharmaceutics13020232
Fröhlich E. Therapeutic Potential of Mesenchymal Stem Cells and Their Products in Lung Diseases—Intravenous Administration versus Inhalation. Pharmaceutics. 2021; 13(2):232. https://doi.org/10.3390/pharmaceutics13020232
Chicago/Turabian StyleFröhlich, Eleonore. 2021. "Therapeutic Potential of Mesenchymal Stem Cells and Their Products in Lung Diseases—Intravenous Administration versus Inhalation" Pharmaceutics 13, no. 2: 232. https://doi.org/10.3390/pharmaceutics13020232
APA StyleFröhlich, E. (2021). Therapeutic Potential of Mesenchymal Stem Cells and Their Products in Lung Diseases—Intravenous Administration versus Inhalation. Pharmaceutics, 13(2), 232. https://doi.org/10.3390/pharmaceutics13020232