Impact of CNS Diseases on Drug Delivery to Brain Extracellular and Intracellular Target Sites in Human: A “WHAT-IF” Simulation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. LeiCNS-PK3.0 Model
2.2. Drug Parameters
2.3. Selection of Pathophysiological Parameters Values
2.4. LeiCNS-PK3.0 Simulations and Data Analysis
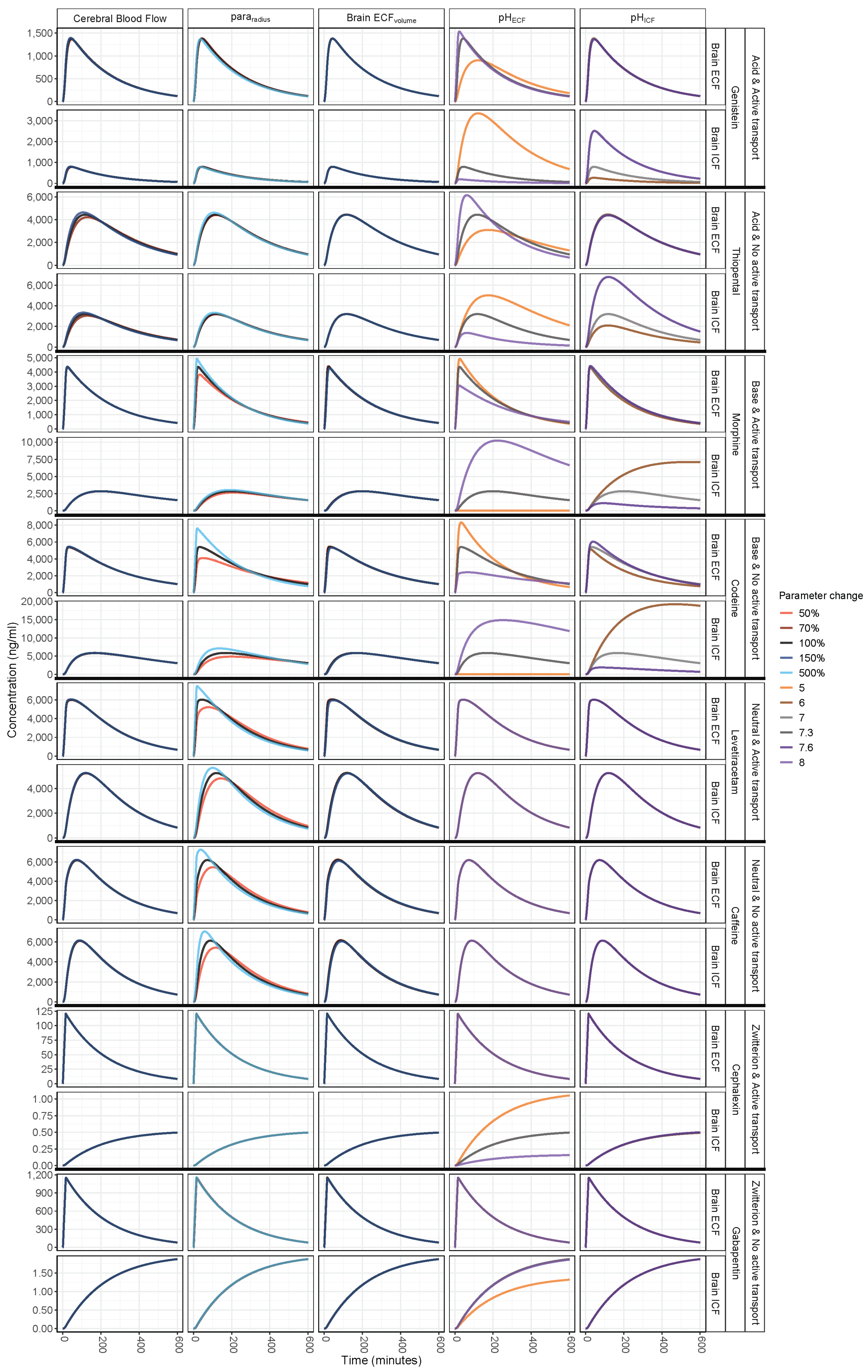
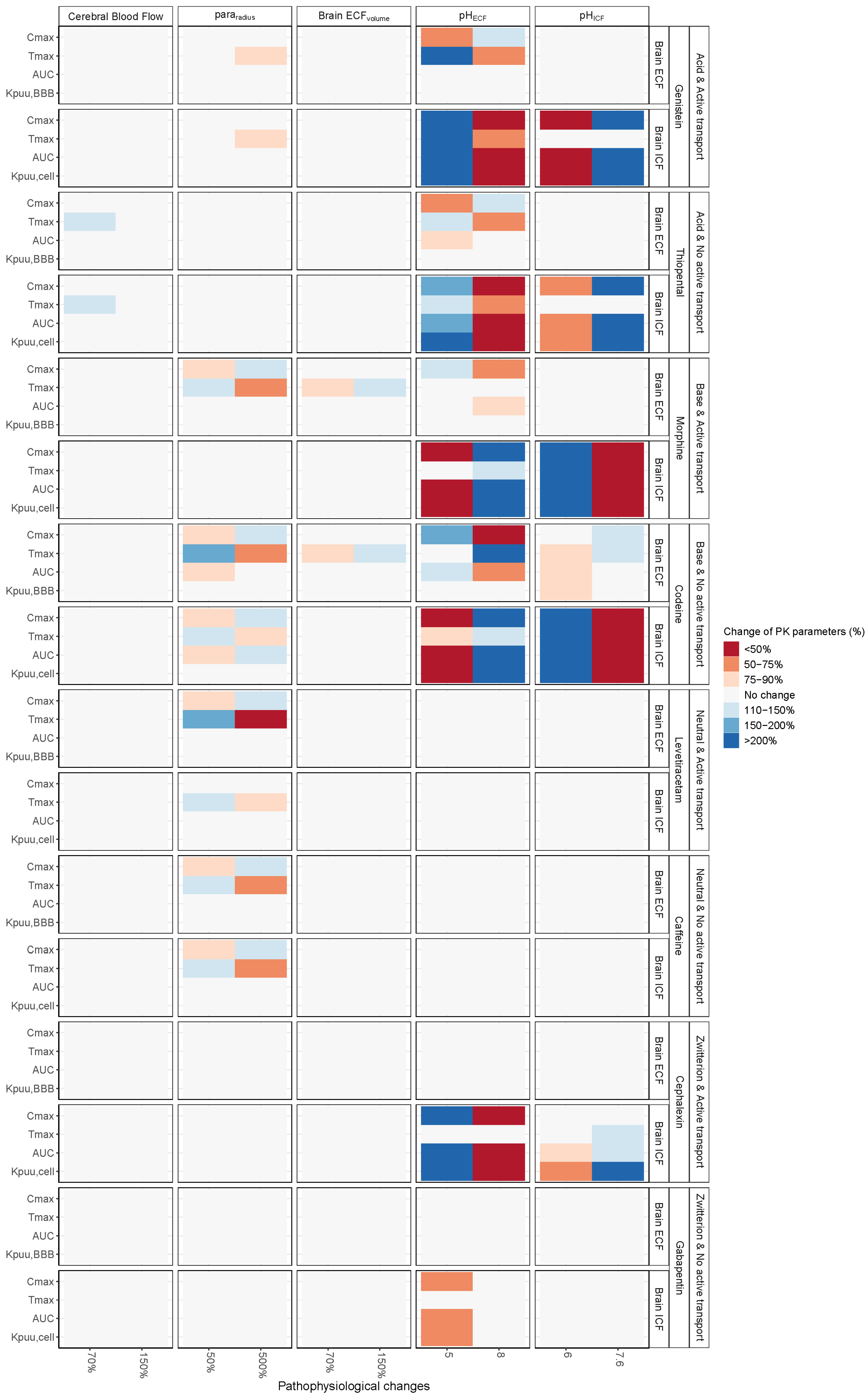
3. Results
3.1. Increased Passive Transport via Widened Pararadius
3.2. pHECF and pHICF are Key Factors of Drug Distribution in BrainECF and BrainICF
3.3. BrainECF Volume and CBF Have a Very Modest Effect on Rate of BBB Drug Transport
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hammarlund-Udenaes, M.; Fridén, M.; Syvänen, S.; Gupta, A. On the rate and extent of drug delivery to the brain. Pharm. Res. 2008, 25, 1737–1750. [Google Scholar] [CrossRef] [PubMed]
- De Lange, E.C.M. The Physiological Characteristics and Transcytosis Mechanisms of the Blood-Brain Barrier (BBB). Curr. Pharm. Biotechnol. 2012, 13, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Loryan, I.; Sinha, V.; Mackie, C.; Van Peer, A.; Drinkenburg, W.H.; Vermeulen, A.; Heald, D.; Hammarlund-Udenaes, M.; Wassvik, C.M. Molecular properties determining unbound intracellular and extracellular brain exposure of CNS drug candidates. Mol. Pharm. 2015, 12, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Ketharanathan, N.; Yamamoto, Y.; Rohlwink, U.K.; Wildschut, E.D.; Mathôt, R.A.A.; De Lange, E.C.M.; De Wildt, S.N.; Argent, A.C.; Tibboel, D.; Figaji, A.A. Combining Brain Microdialysis and Translational Pharmacokinetic Modeling to Predict Drug Concentrations in Pediatric Severe Traumatic Brain Injury: The Next Step Toward Evidence-Based Pharmacotherapy? J. Neurotrauma 2019, 36, 111–117. [Google Scholar] [CrossRef]
- Bouw, R.; Ederoth, P.; Lundberg, J.; Ungerstedt, U.; Nordström, C.-H.; Hammarlund-Udenaes, M. Increased blood–brain barrier permeability of morphine in a patient with severe brain lesions as determined by microdialysis. Acta Anaesthesiol. Scand. 2001, 45, 390–392. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Westerhout, J.; Van Den Berg, D.; Hartman, R.; Danhof, M.; De Lange, E.C.M. Prediction of methotrexate CNS distribution in different species—Influence of disease conditions. Eur. J. Pharm. Sci. 2014, 57, 11–24. [Google Scholar] [CrossRef]
- Castaneda-Hernandez, G.; Hoyo-Vadillo, C.; Herrera, J.E. Differences in nifedipine concentration-effect relationship between capsule and slow release tablet administration. Int. J. Clin. Pharmacol. Ther. 1995, 33, 56–60. [Google Scholar]
- Kuepfer, L.; Niederalt, C.; Wendl, T.; Schlender, J.F.; Willmann, S.; Lippert, J.; Block, M.; Eissing, T.; Teutonico, D. Applied Concepts in PBPK Modeling: How to Build a PBPK/PD Model. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 516–531. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Välitalo, P.A.; Wong, Y.C.; Huntjens, D.R.; Proost, J.H.; Vermeulen, A.; Krauwinkel, W.; Beukers, M.W.; Kokki, H.; Kokki, M.; et al. Prediction of human CNS pharmacokinetics using a physiologically-based pharmacokinetic modeling approach. Eur. J. Pharm. Sci. 2018, 112, 168–179. [Google Scholar] [CrossRef]
- Westerhout, J.; Ploeger, B.; Smeets, J.; Danhof, M.; De Lange, E.C.M. Physiologically based pharmacokinetic modeling to investigate regional brain distribution kinetics in rats. AAPS J. 2012, 14, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.A.A.; Loo, C.F.; Elassaiss-Schaap, J.; De Lange, E.C.M. Lumbar cerebrospinal fluid-to-Brain extracellular fluid surrogacy is context-specific: Insights from LeiCNS-PK3.0 simulations. J Pharmacokinet Pharmacodyn. Under review.
- Yamamoto, Y.; Välitalo, P.; Huntjens, D.; Proost, J.; Vermeulen, A.; Krauwinkel, W.; Beukers, M.; van den Berg, D.; Hartman, R.; Wong, Y.; et al. Predicting drug concentration-time profiles in multiple relevant CNS compartments using a comprehensive physiologically-based pharmacokinetic model. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Syková, E. The extracellular space in the CNS: Its regulation, volume and geometry in normal and pathological neuronal function. Neuroscientist 1997, 3, 28–41. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Shinohara, M.; Shinohara, M.; Yamazaki, A.; Murray, M.E.; Liesinger, A.M.; Heckman, M.G.; Lesser, E.R.; Parisi, J.E.; Petersen, R.C.; et al. Selective loss of cortical endothelial tight junction proteins during Alzheimer’s disease progression. Brain 2019, 142, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Costea, L.; Mészáros; Bauer, H.; Bauer, H.C.; Traweger, A.; Wilhelm, I.; Farkas, A.E.; Krizbai, I.A. The blood–brain barrier and its intercellular junctions in age-related brain disorders. Int. J. Mol. Sci. 2019, 20, 5472. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Meabon, J.S.; Cline, M.M.; Bullock, K.M.; Raskind, M.A.; Peskind, E.R.; Banks, W.A.; Cook, D.G. Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Roher, A.E.; Debbins, J.P.; Malek-Ahmadi, M.; Chen, K.; Pipe, J.G.; Maze, S.; Belden, C.; Maarouf, C.L.; Thiyyagura, P.; Mo, H.; et al. Cerebral blood flow in Alzheimer’s disease. Vasc. Health Risk Manag. 2012, 8, 599–611. [Google Scholar] [CrossRef]
- van Es, A.C.G.M.; van der Grond, J.; ten Dam, V.H.; de Craen, A.J.M.; Blauw, G.J.; Westendorp, R.G.J.; Admiraal-Behloul, F.; van Buchem, M.A. Associations between total cerebral blood flow and age related changes of the brain. PLoS ONE 2010, 5, e9825. [Google Scholar] [CrossRef]
- Laitio, R.M.; Kaisti, K.K.; Langsjö, J.W.; Aalto, S.; Salmi, E.; Maksimow, A.; Aantaa, R.; Oikonen, V.; Sipila, H.; Parkkola, R.; et al. Effects of xenon anesthesia on cerebral blood flow in neurosurgical humans. Anesthesiology 2007, 106, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Zygun, D.A.; Johnston, A.J.; Steiner, L.A.; Al-rawi, P.G.; Chatfield, D.O.T.; Shepherd, E.; Kirkpatrick, P.J.; Hutchinson, P.J.; Menon, D.K. Extracellular Brain pH and Outcome following Severe Traumatic Brain Injury. J. Neurotrauma 2004, 21, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Remzso, G.; Németh, J.; Varga, V.; Kovács, V.; TóthSzuki, V.; Kaila, K.; Voipio, J.; Domoki, F. Brain interstitial pH changes in the subacute phase of hypoxic-ischemic encephalopathy in newborn pigs. PLoS ONE 2020, 15, e0240643. [Google Scholar] [CrossRef]
- Rehncrona, S. Brain acidosis. Ann. Emerg. Med. 1985, 14, 770–776. [Google Scholar] [CrossRef]
- Lyros, E.; Ragoschke-Schumm, A.; Kostopoulos, P.; Sehr, A.; Backens, M.; Kalampokini, S.; Decker, Y.; Lesmeister, M.; Liu, Y.; Reith, W.; et al. Normal brain aging and Alzheimer’s disease are associated with lower cerebral pH: An in vivo histidine 1H-MR spectroscopy study. Neurobiol. Aging 2020, 87, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2017, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Manchester, J.; Walkup, G.; Rivin, O.; You, Z. Evaluation of pka Estimation Methods on 211 Druglike Compounds. J. Chem. Inf. Model. 2010, 50, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Mannhold, R.; Poda, G.I.; Ostermann, C.; Tetko, I.V. Calculation of Molecular Lipophilicity: State-of-the-Art and Comparison of LogP Methods on More Than 96,000 Compounds. J. Pharm. Sci. 2009, 98, 861–893. [Google Scholar] [CrossRef]
- Summerfield, S.G.; Read, K.; Begley, D.J.; Obradovic, T.; Hidalgo, I.J.; Coggon, S.; Lewis, A.V.; Porter, R.A.; Jeffrey, P. Central Nervous System Drug Disposition: The Relationship between in Situ Brain Permeability and Brain Free Fraction. J. Pharmacol. Exp. Ther. 2007, 322, 205–213. [Google Scholar] [CrossRef]
- Nagaya, Y.; Nozaki, Y.; Takenaka, O.; Watari, R.; Kusano, K.; Yoshimura, T.; Kusuhara, H. Investigation of utility of cerebrospinal fluid drug concentration as a surrogate for interstitial fluid concentration using microdialysis coupled with cisternal cerebrospinal fluid sampling in wild-type and Mdr1a(−/−) rats. Drug Metab. Pharmacokinet. 2016, 31, 57–66. [Google Scholar] [CrossRef]
- Campagne, O.; Davis, A.; Zhong, B.; Nair, S.; Haberman, V.; Patel, Y.T.; Janke, L.; Roussel, M.F.; Stewart, C.F. CNS Penetration of Cyclophosphamide and Metabolites in Mice Bearing Group 3 Medulloblastoma and Non-tumor Bearing Mice. J. Pharm. Pharm. Sci. 2019, 22, 612–629. [Google Scholar] [CrossRef] [PubMed]
- Al-Majdoub, Z.M.; Al Feteisi, H.; Achour, B.; Warwood, S.; Neuhoff, S.; Rostami-Hodjegan, A.; Barber, J. Proteomic Quantification of Human Blood–Brain Barrier SLC and ABC Transporters in Healthy Individuals and Dementia Patients. Mol. Pharm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Al Feteisi, H.; Al-Majdoub, Z.M.; Achour, B.; Couto, N.; Rostami-Hodjegan, A.; Barber, J. Identification and quantification of blood–brain barrier transporters in isolated rat brain microvessels. J. Neurochem. 2018, 146, 670–685. [Google Scholar] [CrossRef]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Uchida, Y.; Tachikawa, M.; Inoue, T.; Ohtsuki, S.; Terasaki, T. Quantitative Atlas of Blood–Brain Barrier Transporters, Receptors, and Tight Junction Proteins in Rats and Common Marmoset. J. Pharm. Sci. 2013, 102, 3343–3355. [Google Scholar] [CrossRef]
- Shawahna, R.; Uchida, Y.; Declèves, X.; Ohtsuki, S.; Yousif, S.; Dauchy, S.; Jacob, A.; Chassoux, F.; Daumas-Duport, C.; Couraud, P.O.; et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol. Pharm. 2011, 8, 1332–1341. [Google Scholar] [CrossRef]
- Kodaira, H.; Kusuhara, H.; Fuse, E.; Ushiki, J.; Sugiyama, Y. Quantitative investigation of the brain-to-cerebrospinal fluid unbound drug concentration ratio under steady-state conditions in rats using a pharmacokinetic model and scaling factors for active efflux transporters. Drug Metab. Dispos. 2014, 42, 983–989. [Google Scholar] [CrossRef]
- De Lange, E.C.M.; vd Berg, D.J.; Bellanti, F.; Voskuyl, R.A.; Syvänen, S. P-glycoprotein protein expression versus functionality at the blood-brain barrier using immunohistochemistry, microdialysis and mathematical modeling. Eur. J. Pharm. Sci. 2018, 124, 61–70. [Google Scholar] [CrossRef]
- Chu, X.; Bleasby, K.; Evers, R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin. Drug Metab. Toxicol. 2013, 9, 237–252. [Google Scholar] [CrossRef]
- Li, M.; Yuan, H.; Li, N.; Song, G.; Zheng, Y.; Baratta, M.; Hua, F.; Thurston, A.; Wang, J.; Lai, Y. Identification of interspecies difference in efflux transporters of hepatocytes from dog, rat, monkey and human. Eur. J. Pharm. Sci. 2008, 35, 114–126. [Google Scholar] [CrossRef]
- Booth-Genthe, C.L.; Louie, S.W.; Carlini, E.J.; Li, B.; Leake, B.F.; Eisenhandler, R.; Hochman, J.H.; Mei, Q.; Kim, R.B.; Rushmore, T.H.; et al. Development and characterization of LLC-PK1 cells containing Sprague-Dawley rat Abcb1a (Mdr1a): Comparison of rat P-glycoprotein transport to human and mouse. J. Pharmacol. Toxicol. Methods 2006, 54, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Nordström, C.H.; Koskinen, L.O.; Olivecrona, M. Aspects on the physiological and biochemical foundations of neurocritical care. Front. Neurol. 2017, 8, 1–24. [Google Scholar] [CrossRef] [PubMed]
- De Lange, E.C.M.; Hesselink, M.B.; Danhof, M.; de Boer, A.G.; Breimer, D.D. The Use of Intracerebral Microdialysis to Determine Changes in Blood-Brain Barrier Transport Characteristics. Pharm. Res. 1995, 12, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Arieff, A.I.; Kerian, A.; Massry, S.G.; DeLima, J. Intracellular pH of brain: Alterations in acute respiratory acidosis and alkalosis. Am. J. Physiol. 1976, 230, 804–812. [Google Scholar] [CrossRef][Green Version]
- Fidler, M.; Hallow, M.; Wilkins, J.; Wang, W. RxODE: Facilities for Simulating from ODE-Based Models. R package version 0.7.2-5. 2018. Available online: https://CRAN.R-project.org/package=RxODE.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Courchesne, E.; Chisum, H.J.; Townsend, J.; Cowles, A.; Covington, J.; Egaas, B.; Harwood, M.; Hinds, S.; Press, G.A. Normal Brain Development and Aging: Quantitative Analysis at in Vivo MR Imaging in Healthy Volunteers. Radiology 2000, 216, 672–682. [Google Scholar] [CrossRef]
- Filipek, P.A.; Richelme, C.; Kennedy, D.N.; Caviness, V.S. The Young Adult Human Brain: An MRI-based Morphometric Analysis. Cereb. Cortex 1994, 4, 344–360. [Google Scholar] [CrossRef]
- Gur, R.C.; Mozley, P.D.; Resnick, S.M.; Gottlieb, G.L.; Kohn, M.; Zimmerman, R.A.; Herman, G.T.; Atlas, S.; Grossman, R.; Berretta, D. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 1991, 88, 2845–2849. [Google Scholar] [CrossRef]
- Peters, M.; Jäncke, L.; Staiger, J.F.; Schlaug, G.; Huang, Y.; Steinmetz, H. Unsolved Problems in Comparing Brain Sizes in Homo Sapiens. Brain Cogn. 1998, 37, 254–285. [Google Scholar] [CrossRef][Green Version]
- Lei, Y.; Han, H.; Yuan, F.; Javeed, A.; Zhao, Y. The brain interstitial system: Anatomy, modeling, in vivo measurement, and applications. Prog. Neurobiol. 2017, 157, 230–246. [Google Scholar] [CrossRef]
- Miyajima, M.; Arai, H. Evaluation of the Production and Absorption of Cerebrospinal Fluid. Neurol. Med. Chir. (Tokyo) 2015, 55, 647–656. [Google Scholar] [CrossRef]
- Nicholson, C. Diffusion and related transport mechanisms in brain tissue. Rep. Prog. Phys. 2001, 64, 815–884. [Google Scholar] [CrossRef]
- Nicholson, C.; Kamali-Zare, P.; Tao, L. Brain Extracellular Space as a Diffusion Barrier. Comput. Vis. Sci. 2011, 14, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Hrabětová, S.; Nicholson, C. Diffusion of Epidermal Growth Factor in Rat Brain Extracellular Space Measured by Integrative Optical Imaging. J. Neurophysiol. 2004, 92, 3471–3481. [Google Scholar] [CrossRef] [PubMed]
- Weibel, E.R.; Stäubli, W.; Gnägi, H.R.; Hess, F.A. Correlated Morphometric and Biochemical Studies on the Liver Cell: I. Morphometric Model, Stereologic Methods, and Normal Morphometric Data for Rat Liver. J. Cell Biol. 1969, 42, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.P.; Delp, M.D.; Lindstedt, S.L.; Rhomberg, L.R.; Beliles, R.P. Physiological Parameter Values for Physiologically Based Pharmacokinetic Models. Toxicol. Ind. Health 1997, 13, 407–484. [Google Scholar] [CrossRef]
- Hu, Z.-Y.; Lu, J.; Zhao, Y. A physiologically based pharmacokinetic model of alvespimycin in mice and extrapolation to rats and humans. Br. J. Pharmacol. 2014, 171, 2778–2789. [Google Scholar] [CrossRef]
- Allen, J.S.; Damasio, H.; Grabowski, T.J. Normal neuroanatomical variation in the human brain: An MRI-volumetric study. Am. J. Phys. Anthropol. 2002, 118, 341–358. [Google Scholar] [CrossRef]
- Barra, V.; Frenoux, E.; Boire, J.-Y. Automatic volumetric measurement of lateral ventricles on magnetic resonance images with correction of partial volume effects. J. Magn. Reson. Imaging 2002, 15, 16–22. [Google Scholar] [CrossRef]
- Erdogan, A.L.I.R.; Dane, S.; Aydin, M.D.; Özdikici, M.; Diyarbakirli, S. Sex and Handedness Differences in Size of Cerebral Ventricles of Normal Subjects. Int. J. Neurosci. 2004, 114, 67–73. [Google Scholar] [CrossRef]
- Lamers, M.; Klein, W.; Góraj, B. Normal Values of Ventricular Volume and Cerebrospinal Fluid (CSF) Circulation in Healthy Subjects. Available online: https://posterng.netkey.at/esr/viewing/index.php?module=viewing_poster&doi=10.1594/ecr2010/C-2729 (accessed on 1 November 2020).
- Trimarchi, F.; Bramanti, P.; Marino, S.; Milardi, D.; Di Mauro, D.; Ielitro, G.; Valenti, B.; Vaccarino, G.; Milazzo, C.; Cutroneo, G. MRI 3D lateral cerebral ventricles in living humans: Morphological and morphometrical age-, gender-related preliminary study. Anat. Sci. Int. 2013, 88, 61–69. [Google Scholar] [CrossRef]
- Whitney, N.; Sun, H.; Pollock, J.M.; Ross, D.A. The human foramen magnum—Normal anatomy of the cisterna magna in adults. Neuroradiology 2013, 55, 1333–1339. [Google Scholar] [CrossRef]
- Conn, P.M. Neuroscience in Medicine; Springer Science & Business Media: Totowa, NJ, USA, 2003; ISBN 978-1-59259-371-2. [Google Scholar]
- Parviz, J. Surgical Anatomy of the Head and Neck; Harvard University Press: Cambridge, MA, USA, 2011; ISBN 978-0-674-41783-0. [Google Scholar]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef]
- Lassen, N.A. Normal Average Value of Cerebral Blood Flow in Younger Adults is 50 mL/100 g/min. J. Cereb. Blood Flow Metab. 1985, 5, 347–349. [Google Scholar] [CrossRef]
- Madsen, P.L.; Holm, S.; Herning, M.; Lassen, N.A. Average Blood Flow and Oxygen Uptake in the Human Brain during Resting Wakefulness: A Critical Appraisal of the Kety—Schmidt Technique. J. Cereb. Blood Flow Metab. 1993, 13, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, M.J.; Melzer, T.R.; Horwood, L.J.; Woodward, L.J.; Darlow, B.A. Altered grey matter volume, perfusion and white matter integrity in very low birthweight adults. NeuroImage Clin. 2019, 22, 101780. [Google Scholar] [CrossRef] [PubMed]
- Kimelberg, H.K. Water homeostasis in the brain: Basic concepts. Neuroscience 2004, 129, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Cserr, H.F. Physiology of the choroid plexus. Physiol. Rev. 1971. [Google Scholar] [CrossRef] [PubMed]
- Edsbagge, M.; Tisell, M.; Jacobsson, L.; Wikkelso, C. Spinal CSF absorption in healthy individuals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1450–R1455. [Google Scholar] [CrossRef]
- Lumenta, C.B.; Di Rocco, C.; Haase, J.; Mooij, J.J.A. Neurosurgery; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-79565-0. [Google Scholar]
- Wright, E.M. Transport processes in the formation of the cerebrospinal fluid. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 1978; Volume 83, pp. 1–34. ISBN 978-3-540-35785-8. [Google Scholar]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Crone, C. The Permeability of Capillaries in Various Organs as Determined by Use of the ‘Indicator Diffusion’ Method. Acta Physiol. Scand. 1963, 58, 292–305. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H. Blood-Brain Barrier in Drug Discovery: Optimizing Brain Exposure of CNS Drugs and Minimizing Brain Side Effects for Peripheral Drugs; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-1-118-78835-6. [Google Scholar]
- Gao, H.; Gao, X. Brain Targeted Drug Delivery Systems: A Focus on Nanotechnology and Nanoparticulates; Academic Press: Cambridge, MA, USA, 2018; ISBN 978-0-12-814002-4. [Google Scholar]
- Gross, P.M.; Sposito, N.M.; Pettersen, S.E.; Fenstermacher, J.D. Differences in Function and Structure of the Capillary Endothelium in Gray Matter, White Matter and a Circumventricular Organ of Rat Brain. J. Vasc. Res. 1986, 23, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Redzic, Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: Similarities and differences. Fluids Barriers CNS 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S. Blood-Spinal Cord and Brain Barriers in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 978-0-08-052822-9. [Google Scholar]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 1–22. [Google Scholar] [CrossRef]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef]
- Spector, R.; Keep, R.F.; Snodgrass, S.R.; Smith, Q.R.; Johanson, C.E. A balanced view of choroid plexus structure and function: Focus on adult humans. Exp. Neurol. 2015, 267, 78–86. [Google Scholar] [CrossRef]
- Andrade-Moraes, C.H.; Oliveira-Pinto, A.V.; Castro-Fonseca, E.; da Silva, C.G.; Guimarães, D.M.; Szczupak, D.; Parente-Bruno, D.R.; Carvalho, L.R.B.; Polichiso, L.; Gomes, B.V.; et al. Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain 2013, 136, 3738–3752. [Google Scholar] [CrossRef]
- Azevedo, F.A.C.; Carvalho, L.R.B.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.L.; Leite, R.E.P.; Filho, W.J.; Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef]
- Bakker, A.C.; Webster, P.; Jacob, W.A.; Andrews, N.W. Homotypic fusion between aggregated lysosomes triggered by elevated [Ca2+]i in fibroblasts. J. Cell Sci. 1997, 110, 2227–2238. [Google Scholar]
- Bandyopadhyay, D.; Cyphersmith, A.; Zapata, J.A.; Kim, Y.J.; Payne, C.K. Lysosome transport as a function of lysosome diameter. PLoS ONE 2014, 9, e86847. [Google Scholar] [CrossRef]
- Demers-Lamarche, J.; Guillebaud, G.; Tlili, M.; Todkar, K.; Bélanger, N.; Grondin, M.; Nguyen, A.P.; Michel, J.; Germain, M. Loss of Mitochondrial Function Impairs Lysosomes. J. Biol. Chem. 2016, 291, 10263–10276. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Y.E. Electron microscopy of lysosome-rich fractions from rat thymus isolated by density-gradient centrifugation before and after whole-body x-irradiation. J. Cell Biol. 1962, 13, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ren, D. Lysosomal Physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef]
- Cornford, M.E.; Landaw, E.M.; Hyman, S.; Cornford, E.M.; Delgado-Escueta, A.V. Interictal Seizure Resections Show Two Configurations of Endothelial Glut1 Glucose Transporter in the Human Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 1998, 18, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Sarin, H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes. Res. 2010, 2, 14. [Google Scholar] [CrossRef]
- Monteiro, J.N.; Goraksha, S.U. ‘ROSE concept’ of fluid management: Relevance in neuroanaesthesia and neurocritical care. J. Neuroanaesth. Crit. Care 2017, 4, 10–16. [Google Scholar] [CrossRef]
- Haas, T.L.; Duling, B.R. Morphology Favors an Endothelial Cell Pathway for Longitudinal Conduction within Arterioles. Microvasc. Res. 1997, 53, 113–120. [Google Scholar] [CrossRef]
- Schulze, C.; Firth, J.A. Interendothelial junctions during blood-brain barrier development in the rat: Morphological changes at the level of individual tight junctional contacts. Dev. Brain Res. 1992, 69, 85–95. [Google Scholar] [CrossRef]
- Atherton, J.C. Acid-base balance: Maintenance of plasma pH. Anaesth. Intensive Care Med. 2003, 4, 419–422. [Google Scholar] [CrossRef]
- Fridén, M.; Bergström, F.; Wan, H.; Rehngren, M.; Ahlin, G.; Hammarlund-Udenaes, M.; Bredberg, U. Measurement of unbound drug exposure in brain: Modeling of pH partitioning explains diverging results between the brain slice and brain homogenate methods. Drug Metab. Dispos. 2011, 39, 353–362. [Google Scholar] [CrossRef]
- Siesjö, B.K. Symposium on acid-base homeostasis. The regulation of cerebrospinal fluid pH. Kidney Int. 1972, 1, 360–374. [Google Scholar] [CrossRef] [PubMed]
- De Lange, E.C.M. Utility of CSF in translational neuroscience. J. Pharmacokinet. Pharmacodyn. 2013, 40, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Cosolo, W.C.; Martinello, P.; Louis, W.J.; Christophidis, N. Blood-brain barrier disruption using mannitol: Time course and electron microscopy studies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1989, 256, 443–447. [Google Scholar] [CrossRef]
- Ederoth, P.; Tunblad, K.; Bouw, R.; Lundberg, C.J.F.; Ungerstedt, U.; Nordström, C.H.; Hammarlund-Udenaes, M. Blood-brain barrier transport of morphine in patients with severe brain trauma. Br. J. Clin. Pharmacol. 2004, 57, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Brophy, G.M.; Mazzeo, A.T.; Brar, S.; Alves, O.L.; Bunnell, K.; Gilman, C.; Karnes, T.; Hayes, R.L.; Bullock, R. Exposure of Cyclosporin A in Whole Blood, Cerebral Spinal Fluid, and Brain Extracellular Fluid Dialysate in Adults with Traumatic Brain Injury. J. Neurotrauma 2013, 30, 1484–1489. [Google Scholar] [CrossRef]
- Vink, R.; McIntosh, T.K.; Weiner, M.W.; Faden, A.I. Effects of traumatic brain injury on cerebral high-energy phosphates and pH: A 31P magnetic resonance spectroscopy study. J. Cereb. Blood Flow Metab. 1987, 7, 563–571. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef]
- Hue, C.D.; Cho, F.S.; Cao, S.; Nicholls, R.E.; Vogel, E.W.; Sibindi, C.; Arancio, O.; Dale Bass, C.R.; Meaney, D.F.; Morrison, B. Time Course and Size of Blood-Brain Barrier Opening in a Mouse Model of Blast-Induced Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1202–1211. [Google Scholar] [CrossRef]
- Readnower, R.D.; Chavko, M.; Adeeb, S.; Conroy, M.D.; Pauly, J.R.; McCarron, R.M.; Sullivan, P.G. Increase in Blood Brain Barrier Permeability, Oxidative Stress, and Activated Microglia in a Rat Model of Blast Induced Traumatic Brain Injury. J. Neurosci. Res. 2010, 88, 3530–3539. [Google Scholar] [CrossRef]
- Höcht, C.; Lazarowski, A.; Gonzalez, N.N.; Auzmendi, J.; Opezzo, J.A.W.; Bramuglia, G.F.; Taira, C.A.; Girardi, E. Nimodipine restores the altered hippocampal phenytoin pharmacokinetics in a refractory epileptic model. Neurosci. Lett. 2007, 413, 168–172. [Google Scholar] [CrossRef]
- Van Vliet, E.A.; Araújo, S.D.C.; Redeker, S.; Van Schaik, R.; Aronica, E.; Gorter, J.A. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain 2007, 130, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Siesjo, B.K.; von Hanwehr, R.; Nergelius, G.; Nevander, G.; Ingvar, M. Extra- and intracellular pH in the brain during seizures and in the recovery period following the arrest of seizure activity. J. Cereb. Blood Flow Metab. 1985, 5, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Baltes, S.; Gastens, A.M.; Fedrowitz, M.; Potschka, H.; Kaever, V.; Löscher, W. Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology 2007, 52, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Sarin, H.; Kanevsky, A.S.; Wu, H.; Brimacombe, K.R.; Fung, S.H.; Sousa, A.A.; Auh, S.; Wilson, C.M.; Sharma, K.; Aronova, M.A.; et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J. Transl. Med. 2008, 6, 1–15. [Google Scholar] [CrossRef]
- Lim, H.; Albatany, M.; Martínez-Santiesteban, F.; Bartha, R.; Scholl, T.J. Longitudinal Measurements of Intra- and Extracellular pH Gradient in a Rat Model of Glioma. Tomography 2018, 4, 46–54. [Google Scholar] [CrossRef]
- Hao, G.; Xu, Z.P.; Li, L. Manipulating extracellular tumour pH: An effective target for cancer therapy. RSC Adv. 2018, 8, 22182–22192. [Google Scholar] [CrossRef]
- Strbian, D.; Durukan, A.; Pitkonen, M.; Marinkovic, I.; Tatlisumak, E.; Pedrono, E.; Abo-Ramadan, U.; Tatlisumak, T. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience 2008, 153, 175–181. [Google Scholar] [CrossRef]
- Nedergaard, M.; Kraig, R.P.; Tanabe, J.; Pulsinelli, W.A. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am. J. Physiol. 1991, 260, R581–R588. [Google Scholar] [CrossRef]
- Hurn, P.D.; Traystman, R.J. pH-Associated brain injury in cerebral ischemia and circulatory arrest. J. Intensive Care Med. 1996, 11, 205–218. [Google Scholar] [CrossRef]
- Gustafsson, S.; Sehlin, D.; Lampa, E.; Hammarlund-Udenaes, M.; Loryan, I. Heterogeneous drug tissue binding in brain regions of rats, Alzheimer’s patients and controls: Impact on translational drug development. Sci. Rep. 2019, 9, 5308. [Google Scholar] [CrossRef]
- Fjell, A.M.; McEvoy, L.; Holland, D.; Dale, A.M.; Walhovd, K.B. Brain changes in older adults at very low risk for Alzheimer’s disease. J. Neurosci. 2013, 33, 8237–8242. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value | Range | Reference | |
|---|---|---|---|---|
| Volumes (mL) | Total brain | 1250 | 1110–1380 | [47,48,49,50] |
| Brain extracellular fluid (brainECF) | 253 1 | 217–300 | [51,52,53,54,55] | |
| Brain intracellular fluid (brainICF) | 1000 1 | calculated | ||
| Brain cell lysosomes (VLYS) | 12.5 2 | [56] | ||
| Brain microvasculature | 45 3 | 37–50 | [53,57,58] | |
| Lateral ventricles | 20 | 11–16 | [59,60,61,62,63] | |
| 3rd and 4th ventricles | 3 | 2.3–3.7 | [61,62] | |
| Cisterna magna | 1 | [64] | ||
| Subarachnoid space | 116 | 110–116 | [65,66,67] | |
| Flows (mL/min) | Cerebral blood flow (CBF) | 689 | 644–722 | [68,69,70] |
| Brain ECF bulk flow | 0.2 4 | [71] | ||
| CSF flow | 0.42 | 0.28–0.68 | [67,72,73,74,75] | |
| Surface areas (cm2) | Blood–brain barrier (SABBB) | 150,000 | 140 × 103−360 × 103 | [76,77,78,79,80,81,82,83,84] |
| Blood CSF barrier (SABCSFB) | 15,000 5 | [85,86] | ||
| Brain cell membrane (SABCM) | 2,666,520 6 | [87,88] | ||
| Lysosomes membrane | 1,980,260 7 | [89,90,91,92,93] | ||
| Width (µm) | Blood brain barrier | 0.5 | 0.2–0.4 | [81,94] |
| Blood CSF barrier | ||||
| Number | Total brain cells (Nbr,cells) | 1.71× 1011 8 | [87,88] | |
| Paracellular pore radius (µm) | Blood–brain barrier (pararadius) | 0.0007 | 0.0007–0.0009 | [10,13,95,96] |
| Blood CSF barrier | 0.0027 | [10,13,95] | ||
| Effective surface area (%) | BBB Transcellular transport | 99.8 | [13,97,98] | |
| BCSFB Transcellular transport | 99.8 | |||
| BBB paracellular transport | 0.004 9 | [10,95] | ||
| BCSFB paracellular transport | 0.016 9 | |||
| pH | Plasma and brain MV | 7.4 | [99] | |
| Brain extracellular fluid (pHECF) | 7.3 | [100] | ||
| Cerebrospinal fluid | 7.3 | [101] | ||
| Brain cells (pHICF) | 7 | [100] | ||
| Brain cell lysosomes | 5 | [100] | ||
| Drug | Mwt | logP | Drug Ion Class | pka | pkb | Kpuu,ECF | Kpuu,LV | Kpuu,CM | BCRP | p-gp | OAT3 | MRP4 | CLp | CLT,ef | CLT,in |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caffeine | 194.2 | −0.07 | Neutral | NA | −0.92 | 0.96 1 | 0.96 1 | 0.96 1 | X | - | - | - | 48.9 | 4.28 | 2.38 |
| Cephalexin | 347.4 | 0.65 | Zwitterion | 3.26 | 7.23 | 0.015 1 | 0.015 1 | 0.015 1 | - | - | X | - | 37.4 | 2736 | <0.01 |
| Codeine | 299.4 | 1.39 | Base | 13.8 | 9.19 | 1 1 | 1 1 | 1 1 | - | - | - | - | 40.1 | 0.71 | 0.89 |
| Gabapentin | 171.2 | 1.25 | Zwitterion | 4.63 | 9.91 | 0.13 1 | 0.13 1 | 0.13 1 | - | - | - | - | 51.9 | 347 | <0.01 |
| Genistein | 270.2 | 3.04 | Acid | 6.55 | −5.3 | 0.04 1 | 0.041 | 0.04 1 | X | X | - | - | 42.3 | 1557 | 245 |
| Levetiracetam | 170.2 | −0.64 | Neutral | 16.1 | −1.6 | 0.31 1 | 0.31 1 | 0.31 1 | - | X | - | X | 52.0 | 3.73 | 0.69 |
| Morphine | 285.3 | 0.87 | Base | 10.3 | 9.12 | 0.23 2 | 0.23 2 | 0.23 2 | - | X | - | - | 41.0 | 30.2 | 0.34 |
| Thiopental | 242.3 | 2.85 | Acid | 7.2 | −3 | 0.9 1 | 0.9 1 | 0.9 1 | - | - | - | - | 44.2 | 569 | 508 |
| Disease | Parameter | Value | References |
|---|---|---|---|
| Alzheimer’s | BBB permeability | ↔ (86–150,000 Da) | [107] |
| pHECF | ↓ (0.01 pH unit/decade) | [25] | |
| pHICF | |||
| Brain tumors | pararadius | ↑ (800%) | [114] |
| pHECF | ↓ (0.6 pH unit) | [115,116] | |
| pHICF | ↑ (0.3 pH unit) | [115,116] | |
| TBI | BBB permeability | ↑ (up to 160,000 Da) | [107,108,109] |
| pHECF | ↓ (0.3 pH unit) | [22] | |
| pHICF | ↓ (0.1 pH unit) | [106] | |
| Ischemia | BBB permeability | ↑ (up to 70,000 Da) | [117] |
| pHECF | ↓ (1.4 pH unit) | [118] | |
| pHICF | ↓ (2 pH unit) | [23,24,119] | |
| Epilepsy | BBB permeability | ↑ (albumin and up to 70,000 Da) | [111] |
| pHECF | ↓ (0.5 pH unit) | [112] | |
| pHICF | ↓ (0.3 pH unit) | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, M.A.A.; de Lange, E.C.M. Impact of CNS Diseases on Drug Delivery to Brain Extracellular and Intracellular Target Sites in Human: A “WHAT-IF” Simulation Study. Pharmaceutics 2021, 13, 95. https://doi.org/10.3390/pharmaceutics13010095
Saleh MAA, de Lange ECM. Impact of CNS Diseases on Drug Delivery to Brain Extracellular and Intracellular Target Sites in Human: A “WHAT-IF” Simulation Study. Pharmaceutics. 2021; 13(1):95. https://doi.org/10.3390/pharmaceutics13010095
Chicago/Turabian StyleSaleh, Mohammed A. A., and Elizabeth C. M. de Lange. 2021. "Impact of CNS Diseases on Drug Delivery to Brain Extracellular and Intracellular Target Sites in Human: A “WHAT-IF” Simulation Study" Pharmaceutics 13, no. 1: 95. https://doi.org/10.3390/pharmaceutics13010095
APA StyleSaleh, M. A. A., & de Lange, E. C. M. (2021). Impact of CNS Diseases on Drug Delivery to Brain Extracellular and Intracellular Target Sites in Human: A “WHAT-IF” Simulation Study. Pharmaceutics, 13(1), 95. https://doi.org/10.3390/pharmaceutics13010095





