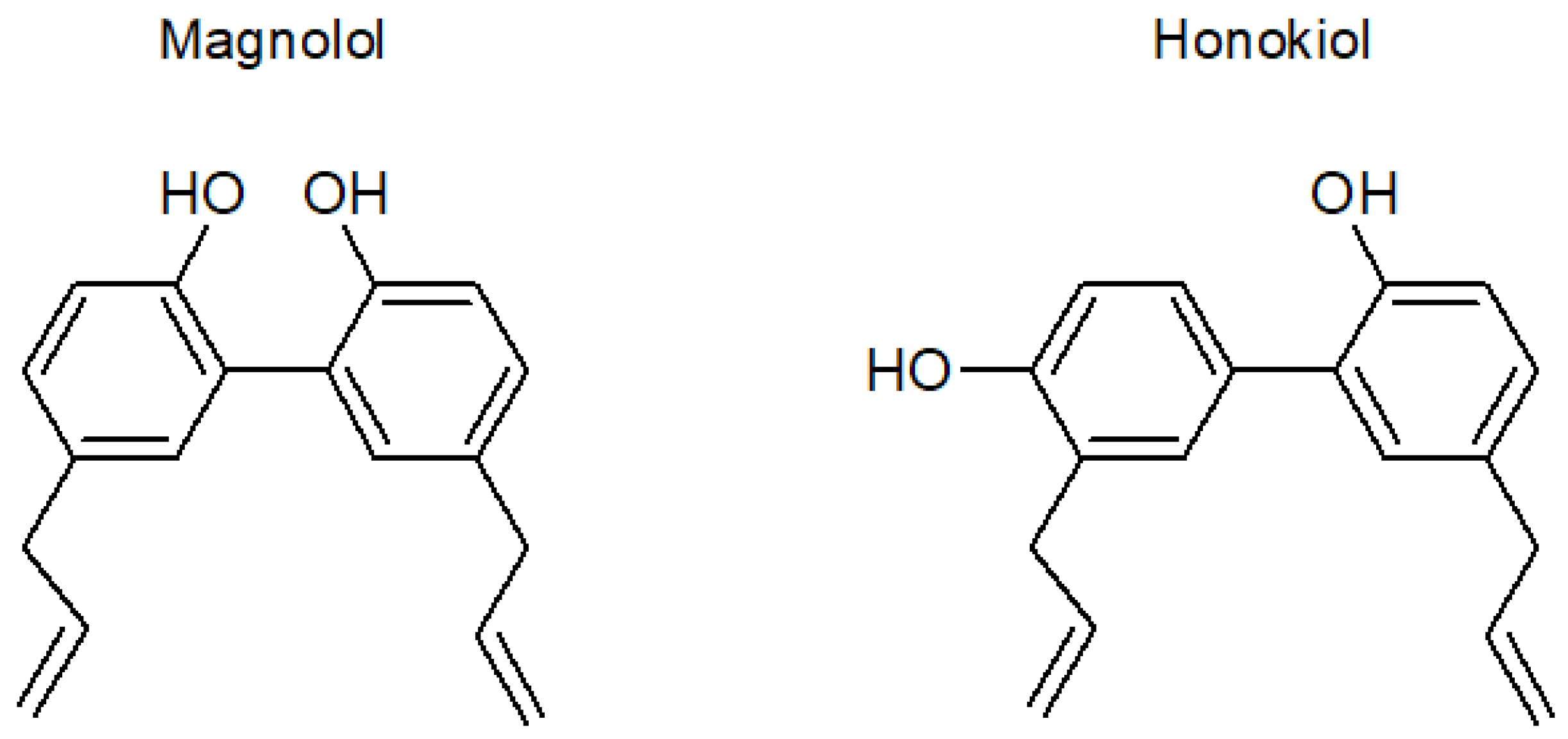
Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
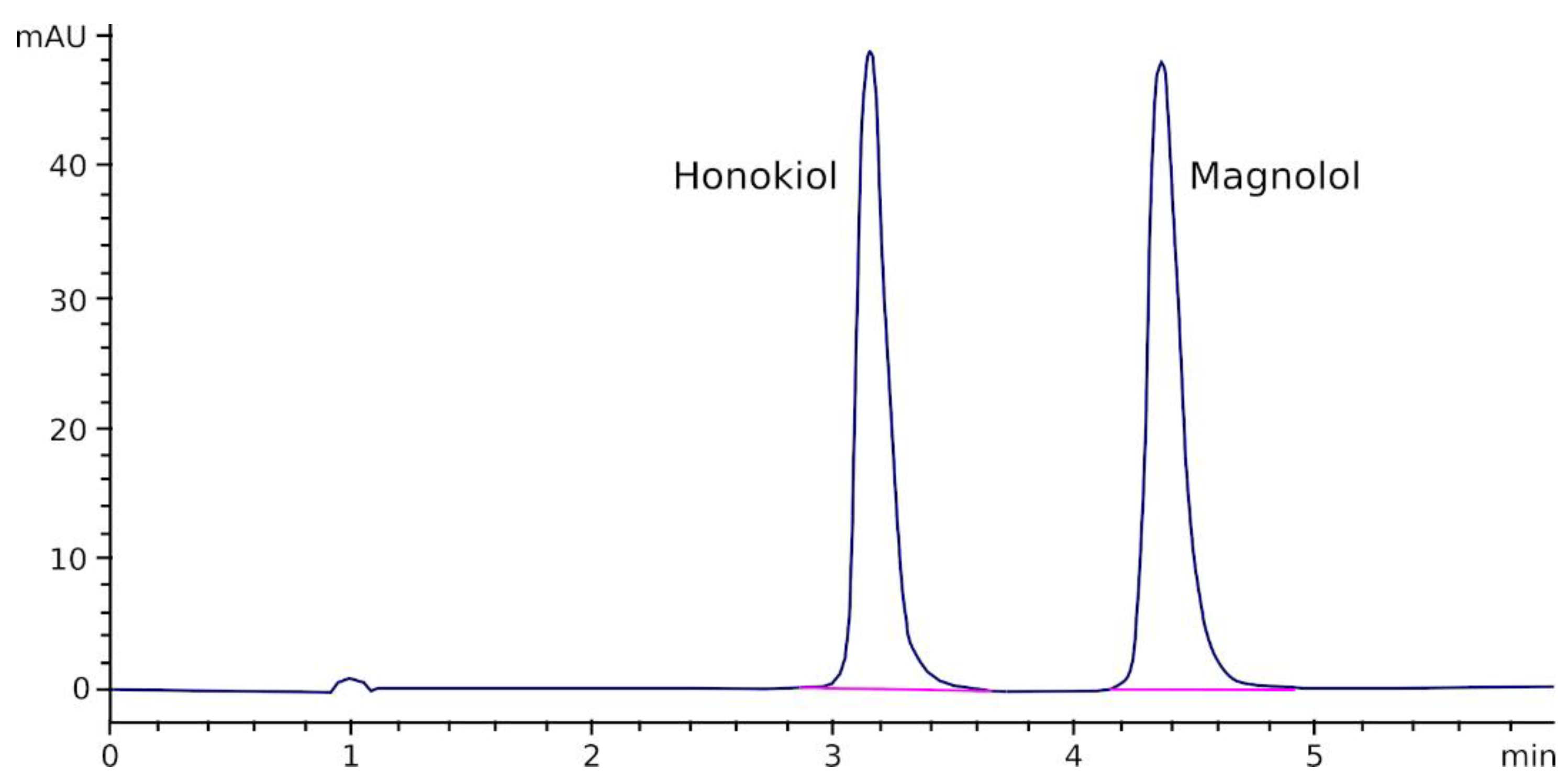
2.2. Analytical Method
2.3. Method Validation
2.4. Determination of Solubility in Water
2.5. Determination of Octanol-Water Distribution Coefficients
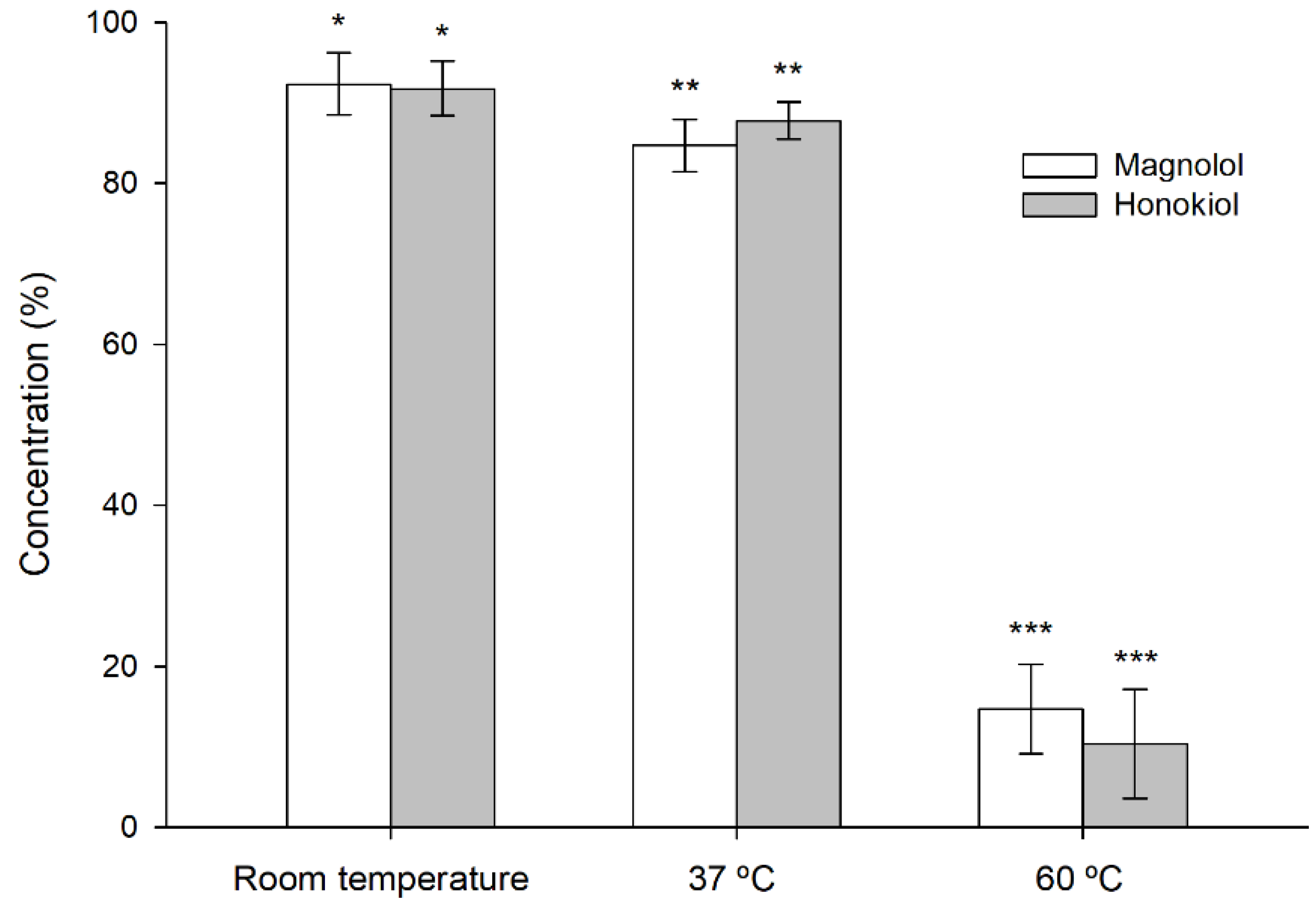
2.6. Forced-Degradation (Stress Testing) Studies
2.7. Liposome Preparation
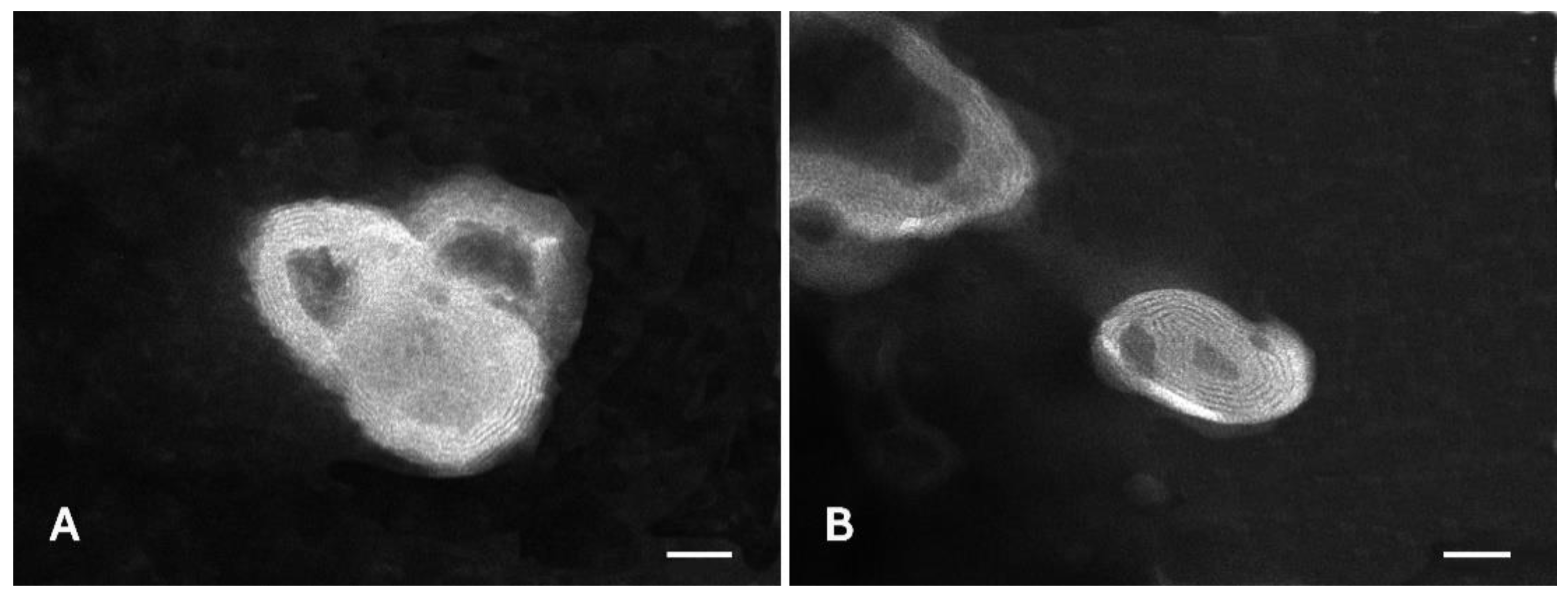
2.8. Liposome Characterization
2.9. Stability Studies of Honokiol-Loaded Liposomes
2.10. Statistical Analysis
3. Results
3.1. Assay Validation
3.2. Solubility and Partition Coefficient
3.3. Stability of Magnolol and Honokiol
3.4. Liposome Preparation and Characterization
3.5. Stability of Honokiol-Loaded Liposomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, K.H.; Han, M.H.; Lee, H.; Ahn, J.M.; Han, S.B.; Han, G.; Lee, K.; Park, S.K.; Kim, H.M. Antiinflammatory activity of methanol extract isolated from stem bark ofMagnolia kobus. Phytother. Res. 2008, 22, 883–888. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Z.; He, Y.; Gu, C.; Zhu, K.; Li, S.; Li, Y.; Lu, X.; Liu, J.; Zhang, N.; et al. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018, 196, 69–76. [Google Scholar] [CrossRef]
- Liou, K.T.; Shen, Y.C.; Chen, C.F.; Tsao, C.M.; Tsai, S.K. The anti-inflammatory effect of honokiol on neutrophils: Mechanisms in the inhibition of reactive oxygen species production. Eur. J. Pharmacol. 2003, 475, 19–27. [Google Scholar] [CrossRef]
- Lu, S.H.; Hsu, W.L.; Chen, T.H.; Chou, T.C. Activation of Nrf2/HO-1signaling pathway involves the anti-inflammatory activity of magnolol in Porphyromonas gingivalis lipopolysaccharide-stimulated mouse RAW 264.7 macrophages. Int. Immunopharmacol. 2015, 29, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liao, K.; Wang, D. Effects of Magnolol and Honokiol on Adhesion, Yeast-Hyphal Transition, and Formation of Biofilm by Candida albicans. PLoS ONE 2015, 10, e0117695. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Jung, E.; Park, Y.; Kim, K.; Park, B.; Jung, K.; Park, E.; Kim, J.; Park, D. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur. J. Pharmacol. 2004, 496, 189–195. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.Q. Comparison of antioxidant abilities of magnolol and honokiol to scavenge radicals and to protect DNA. Biochimie 2011, 93, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Kuo, H.C.; Lee, K.F.; Tsai, T.H. Magnolol protects neurons against ischemia injury via the downregulation of p38/MAPK, CHOP and nitrotyrosine. Toxicol. Appl. Pharmacol. 2014, 279, 294–302. [Google Scholar] [CrossRef]
- Parray, H.A.; Lone, J.; Park, J.P.; Choi, J.W.; Yun, J.W. Magnolol promotes thermogenesis and attenuates oxidative stress in 3T3-L1 adipocytes. Nutrition 2018, 50, 82–90. [Google Scholar] [CrossRef]
- Hoi, C.P.; Ho, Y.P.; Baum, L.; Chow, A.H. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother. Res. 2010, 24, 1538–1542. [Google Scholar] [CrossRef] [PubMed]
- Pyo, M.K.; Lee, Y.; Yun-Choi, H.S. Anti-platelet effect of the constituents isolated from the barks and fruits of Magnolia obovata. Arch. Pharm. Res. 2002, 25, 325–328. [Google Scholar] [CrossRef]
- Kuribara, H.; Kishi, E.; Hattori, N.; Okada, M.; Maruyama, Y. The Anxiolytic Effect of Two Oriental Herbal Drugs in Japan Attributed to Honokiol from Magnolia Bark. J. Pharm. Pharmacol. 2000, 52, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yi, L.T.; Pan, Y.; Wang, X.; Li, Y.C.; Li, J.M.; Wang, C.P.; Kong, L.D. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2008, 32, 715–725. [Google Scholar] [CrossRef]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Huang, X.; Shi, W.; Zhang, R.; Chen, M.; Huang, H.; Wu, L. Insights on the Multifunctional Activities of Magnolol. BioMed. Res. Int. 2019, 2019, 1847130. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.H.; Kim, Y.K.; Min, B.S.; Na, M.K.; Rhee, Y.H.; Lee, J.P.; Bae, K.H. Antifungal activity of magnolol and honokiol. Arch. Pharm. Res. 2000, 23, 46–49. [Google Scholar] [CrossRef]
- Jiang, Y.; Vaysse, J.; Gilard, V.; Balayssac, S.; Déjean, S.; Malet-Martino, M.; David, B.; Fiorini, C.; Barbin, Y. Quality Assessment of Commercial Magnoliae Officinalis Cortex by 1H-NMR-based Metabolomics and HPLC Methods. Phytochem. Anal. 2012, 23, 387–395. [Google Scholar] [CrossRef]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef]
- Ding, P.; Shen, H.; Wang, J.; Ju, J. Improved oral bioavailability of magnolol by using a binary mixed micelle system. Artif. Cells Nanomed. Biotechnol. 2018, 46, 668–674. [Google Scholar] [CrossRef]
- Han, M.; Yu, X.; Guo, Y.; Wang, Y.; Kuang, H.; Wang, X. Honokiol nanosuspensions: Preparation, increased oral bioavailability and dramatically enhanced biodistribution in the cardio-cerebro-vascular system. Colloids Surf. B Biointerfaces 2014, 116, 114–120. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Wang, L.; Zu, Y.; Wang, S.; Liu, P.; Zhao, X. Preparation of honokiol nanoparticles by liquid antisolvent precipitation technique, characterization, pharmacokinetics, and evaluation of inhibitory effect on HepG2 cells. Int. J. Nanomed. 2018, 13, 5469–5483. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Hou, Y.C.; Liao, T.Y.; Tsai, S.Y. Enhancing the bioavailability of magnolol in rabbits using melting solid dispersion with polyvinylpyrrolidone. Drug Dev. Ind. Pharm. 2014, 40, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Du, G.H. Magnolol and Honokiol. In Natural Small Molecule Drugs from Plants; Springer Nature Singapore Pte Ltd.: Singapore; People’s Medical Publishing House: Beijing, China, 2018; pp. 709–712. [Google Scholar]
- Coimbra, M.; Isacchi, B.; Van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Felice, B.; Prabhakaran, M.P.; Rodriguez, A.P.; Ramakrishna, S. Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 41, 178–195. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Usach, I.; Margarucci, E.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Petretto, G.L.; Manconi, M.; Peris, J.E. Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections. Nanomaterials 2020, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Petretto, G.; D’Hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus essential oil extraction, characterization and incorporation in phospholipid vesicles for the antioxidant/antibacterial treatment of oral cavity diseases. Colloids Surf. B Biointerfaces 2018, 171, 115–122. [Google Scholar] [CrossRef]
- USP—The United States Pharmacopeia. USP 35-NF 30. Second Supplement, Buffer Solutions; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2012. [Google Scholar]
- European Pharmacopoeia. European Pharmacopoeia 7.0; Council of Europe: Strasbourg, France, 2011. [Google Scholar]
- Zhijuan, B.; Lin, D.; Zhaotao, M.; Zhigang, M.; Zhongtao, D. Determination of the Dissociation Constants of Magnolol and Honokiol by UV Spectrophotometry. J. Yunnan Univ. Nat. Sci. Ed. 2004, 26, 66–69. [Google Scholar]
- Avdeef, A. Solubility of sparingly-soluble ionizable drugs. Adv. Drug Deliv. Rev. 2007, 59, 568–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Hu, S.C.; Yen, F.L.; Hsu, L.F.; Lee, I.T.; Lin, Z.C.; Tsai, M.H.; Huang, C.L.; Liang, C.J.; Chiang, Y.C. Magnolol Nanoparticles Exhibit Improved Water Solubility and Suppress TNF-α-Induced VCAM-1 Expression in Endothelial Cells. J. Biomed. Nanotechnol. 2017, 13, 255–268. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.; Wang, L.; Wang, L.; Zhao, X. Highly Water-Soluble Solid Dispersions of Honokiol: Preparation, Solubility, and Bioavailability Studies and Anti-Tumor Activity Evaluation. Pharmaceutics 2019, 11, 573. [Google Scholar] [CrossRef]
- Wang, Z.; Perumalsamy, H.; Wang, X.; Ahn, Y.-J. Toxicity and possible mechanisms of action of honokiol from Magnolia denudata seeds against four mosquito species. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]





| Nominal Concentration (µg/mL) | Magnolol | Honokiol | ||||
|---|---|---|---|---|---|---|
| Observed Concentration (µg/mL) | RSD (%) | Bias (%) | Observed Concentration (µg/mL) | RSD (%) | Bias (%) | |
| 1 | 1.08 | 1.0 | 7.9 | 1.09 | 1.9 | 8.6 |
| 10 | 10.70 | 1.3 | 7.0 | 10.41 | 1.2 | 4.1 |
| 50 | 49.32 | 3.0 | −1.4 | 49.78 | 1.8 | −0.4 |
| 75 | 76.37 | 0.4 | 1.8 | 78.15 | 0.5 | 4.2 |
| Aqueous Phase | Log Do/w | |
|---|---|---|
| Magnolol | Honokiol | |
| pH 1.2 | 4.50 ± 0.10 | 4.48 ± 0.05 |
| pH 4.5 | 4.55 ± 0.11 | 4.50 ± 0.08 |
| pH 6.8 | 4.48 ± 0.06 | 4.48 ± 0.04 |
| pH 7.4 | 4.30 ± 0.08 | 4.28 ± 0.05 |
| H2O | 4.07 ± 0.09 | 4.27 ± 0.06 |
| Sample | MD (nm) | PI | ZP (mV) |
|---|---|---|---|
| Empty liposomes | 222 ± 7 | 0.38 | −7.0 ± 0.4 |
| Honokiol-loaded liposomes | 175 ± 3 | 0.17 | −11.0 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usach, I.; Alaimo, A.; Fernández, J.; Ambrosini, A.; Mocini, S.; Ochiuz, L.; Peris, J.-E. Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties. Pharmaceutics 2021, 13, 224. https://doi.org/10.3390/pharmaceutics13020224
Usach I, Alaimo A, Fernández J, Ambrosini A, Mocini S, Ochiuz L, Peris J-E. Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties. Pharmaceutics. 2021; 13(2):224. https://doi.org/10.3390/pharmaceutics13020224
Chicago/Turabian StyleUsach, Iris, Alessandro Alaimo, Juan Fernández, Alessandro Ambrosini, Sara Mocini, Lacramioara Ochiuz, and José-Esteban Peris. 2021. "Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties" Pharmaceutics 13, no. 2: 224. https://doi.org/10.3390/pharmaceutics13020224
APA StyleUsach, I., Alaimo, A., Fernández, J., Ambrosini, A., Mocini, S., Ochiuz, L., & Peris, J.-E. (2021). Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties. Pharmaceutics, 13(2), 224. https://doi.org/10.3390/pharmaceutics13020224







