Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets
Abstract
1. Introduction
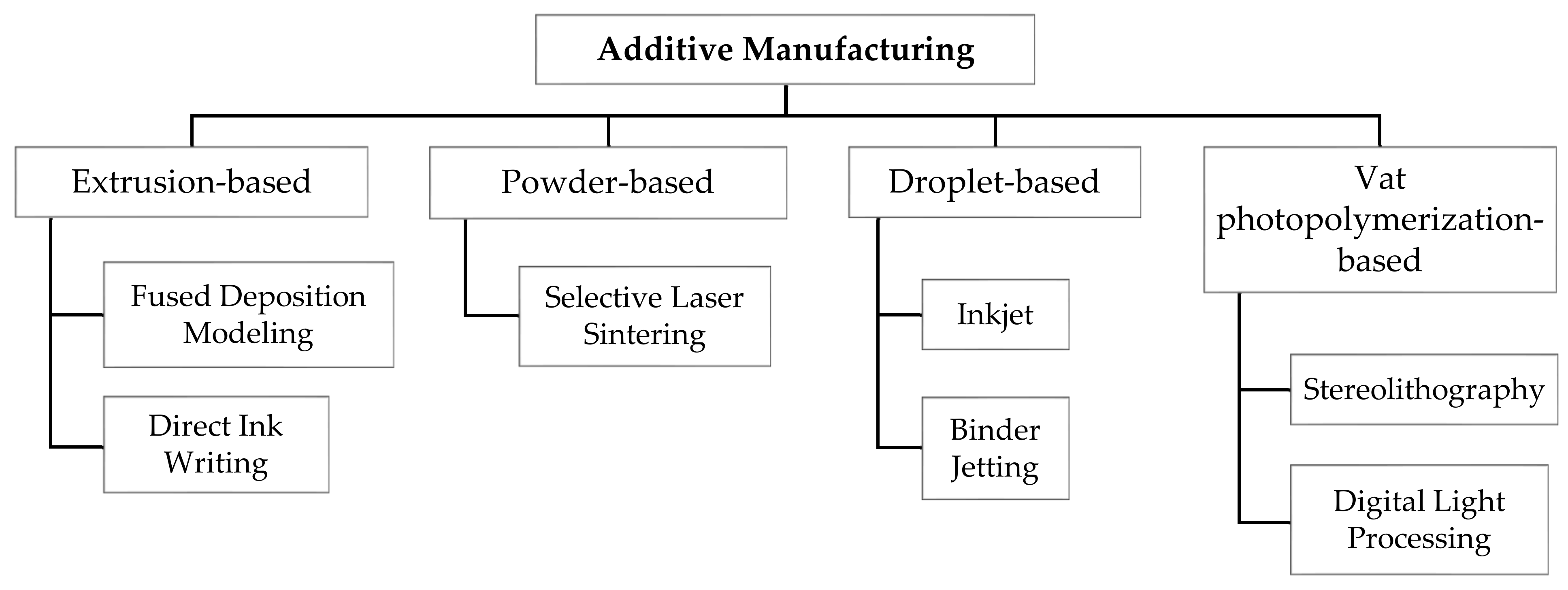
2. AM Technologies
3. Materials for 3D Printing Oral Tablets
3.1. Polymers
3.1.1. Cellulose-Based Polymers
3.1.2. Poly (Vinyl Alcohol)
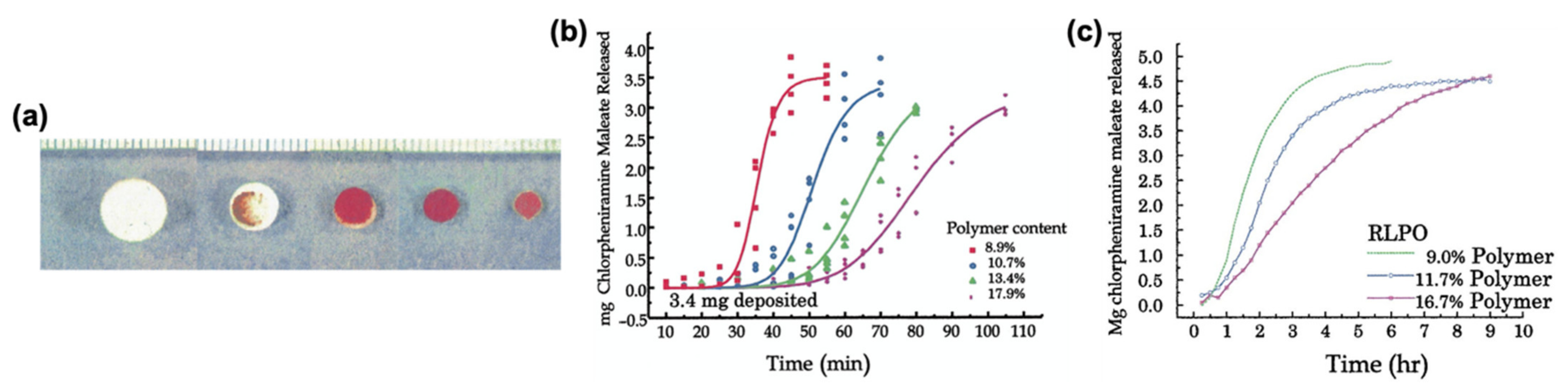
3.1.3. Eudragit
3.1.4. Polyvinylpyrrolidone
3.1.5. Polycaprolactone
3.1.6. Carbopol
3.1.7. Polyethylene Glycol
3.1.8. Polymer Blends/Mixtures
3.2. Additives
3.2.1. Plasticizers
3.2.2. Lubricants
3.2.3. Disintegrants
3.2.4. Binding Agents
3.2.5. Fillers
3.3. APIs
4. Tablet Printing Using AM Technologies
4.1. FDM-Printed Tablets
4.2. DIW-Printed Tablets
4.3. SLS-Printed Tablets
4.4. SLA-Printed Tablets
4.5. DLP-Printed Tablets
4.6. Inkjet-Printed Tablets
4.7. BJ-Printed Tablets
5. Challenges and Potential Strategies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darji, M.A.; Lalge, R.M.; Marathe, S.P.; Mulay, T.D.; Fatima, T.; Alshammari, A.; Lee, H.K.; Repka, M.A.; Narasimha Murthy, S. Excipient Stability in Oral Solid Dosage Forms: A Review. AAPS PharmSciTech 2018, 19, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Bharawaj, S.; Jain, V.; Sharma, S.; Jat, R.C.; Jain, S. Orally Disintegrating Tablets: A Review. Drug Invent. Today 2010, 2, 81–88. [Google Scholar]
- Panigrahi, R.; Behera, S.P.; Panda, C.S. A Review on Fast Dissolving Tablets. WebmedCentral Pharm. Sci. 2010, 1, 7–17. [Google Scholar]
- Pawar, P.B.; Mansuk, A.; Ramteke, K.; Sharma, Y.; Patil, S. Mouth dissolving tablet: A review. Int. J. Herb. Drug Res. 2011, 1, 22–29. [Google Scholar]
- Abebe, A.; Akseli, I.; Sprockel, O.; Kottala, N.; Cuitiño, A.M. Review of bilayer tablet technology. Int. J. Pharm. 2014, 461, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Hindiyeh, M.; Altalafha, T.; Al-Naerat, M.; Saidan, H.; Al-Salaymeh, A.; Sbeinati, L.; Saidan, M.N. Process modification of pharmaceutical tablet manufacturing operations: An eco-efficiency approach. Processes 2018, 6, 15. [Google Scholar] [CrossRef]
- Teżyk, M.; Milanowski, B.; Ernst, A.; Lulek, J. Recent progress in continuous and semi-continuous processing of solid oral dosage forms: A review. Drug Dev. Ind. Pharm. 2016, 42, 1195–1214. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Vervaet, C. Recent progress in continuous manufacturing of oral solid dosage forms. Int. J. Pharm. 2020, 579, 119194. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3D printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharm. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef]
- Liaw, C.-Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef] [PubMed]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-Z.; Gosselin, F.; Guerin, N.; Lanouette, A.-M.; Heuzey, M.-C.; Therriault, D. Solvent-cast three-dimensional printing of multifunctional microsystems. Small 2013, 9, 4118–4122. [Google Scholar] [CrossRef] [PubMed]
- Kruth, J.-P.; Mercelis, P.; Van Vaerenbergh, L.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 41101. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Thompson, A.B.; Tipton, C.; Juel, A.; Hazel, A.; Dowling, M. Sequential deposition of overlapping droplets to form a liquid line. J. Fluid Mech. 2014, 761, 261–281. [Google Scholar] [CrossRef]
- Shikhmurzaev, Y.; Sprittles, J. The coalescence of liquid drops in a viscous fluid: Interface formation model. arXiv 2014, arXiv:1406.5843. [Google Scholar]
- Tian, D.; Song, Y.; Jiang, L. Patterning of controllable surface wettability for printing techniques. Chem. Soc. Rev. 2013, 42, 5184–5209. [Google Scholar] [CrossRef]
- Ahn, B.Y.; Lewis, J.A. Amphiphilic silver particles for conductive inks with controlled wetting behavior. Mater. Chem. Phys. 2014, 148, 686–691. [Google Scholar] [CrossRef]
- Gokuldoss, P.K.; Kolla, S.; Eckert, J. Additive manufacturing processes: Selective laser melting, electron beam melting and binder jetting—Selection guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Taormina, G.; Sciancalepore, C.; Messori, M.; Bondioli, F. 3D printing processes for photocurable polymeric materials: Technologies, materials, and future trends. J. Appl. Biomater. Funct. Mater. 2018, 16, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.F.; Lees, I.; Maclean, D. Polymers as drugs-Advances in therapeutic applications of polymer binding agents. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3146–3157. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Pattinson, S.W.; Hart, A.J. Additive Manufacturing of Cellulosic Materials with Robust Mechanics and Antimicrobial Functionality. Adv. Mater. Technol. 2017, 2, 1600084. [Google Scholar] [CrossRef]
- Paggi, R.A.; Salmoria, G.V.; Ghizoni, G.B.; Back, H.M.; Gindri, I.M. Structure and mechanical properties of 3D-printed cellulose tablets by fused deposition modeling. Int. J. Adv. Manuf. Technol. 2018, 100, 2767–2774. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef]
- Kempin, W.; Franz, C.; Koster, L.C.; Schneider, F.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur. J. Pharm. Biopharm. 2017, 115, 84–93. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Loreti, G.; Maroni, A.; Gazzaniga, A.; Zema, L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 360–367. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef] [PubMed]
- Borujeni, S.H.; Mirdamadian, S.Z.; Varshosaz, J.; Taheri, A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose 2019, 27, 1573–1589. [Google Scholar] [CrossRef]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef] [PubMed]
- Arafat, B.; Wojsz, M.; Isreb, A.; Forbes, R.T.; Isreb, M.; Ahmed, W.; Arafat, T.; Alhnan, M.A. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 2018, 118, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef]
- Jamroz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials-Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef]
- Dürig, T.; Karan, K. Binders in Wet Granulation. In Handbook of Pharmaceutical Wet Granulation; Academic Press: Cambridge, MA, USA, 2019; pp. 317–349. [Google Scholar]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.; Mohamad, A.B.; Al-Amiery, A.A. Properties and Applications of Polyinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Konta, A.A.; Garcia-Pina, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Muppalaneni, S.; Omidian, H. Polyvinyl Alcohol in Medicine and Pharmacy: A Perspective. J. Dev. Drugs 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Libros Digitales-Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef] [PubMed]
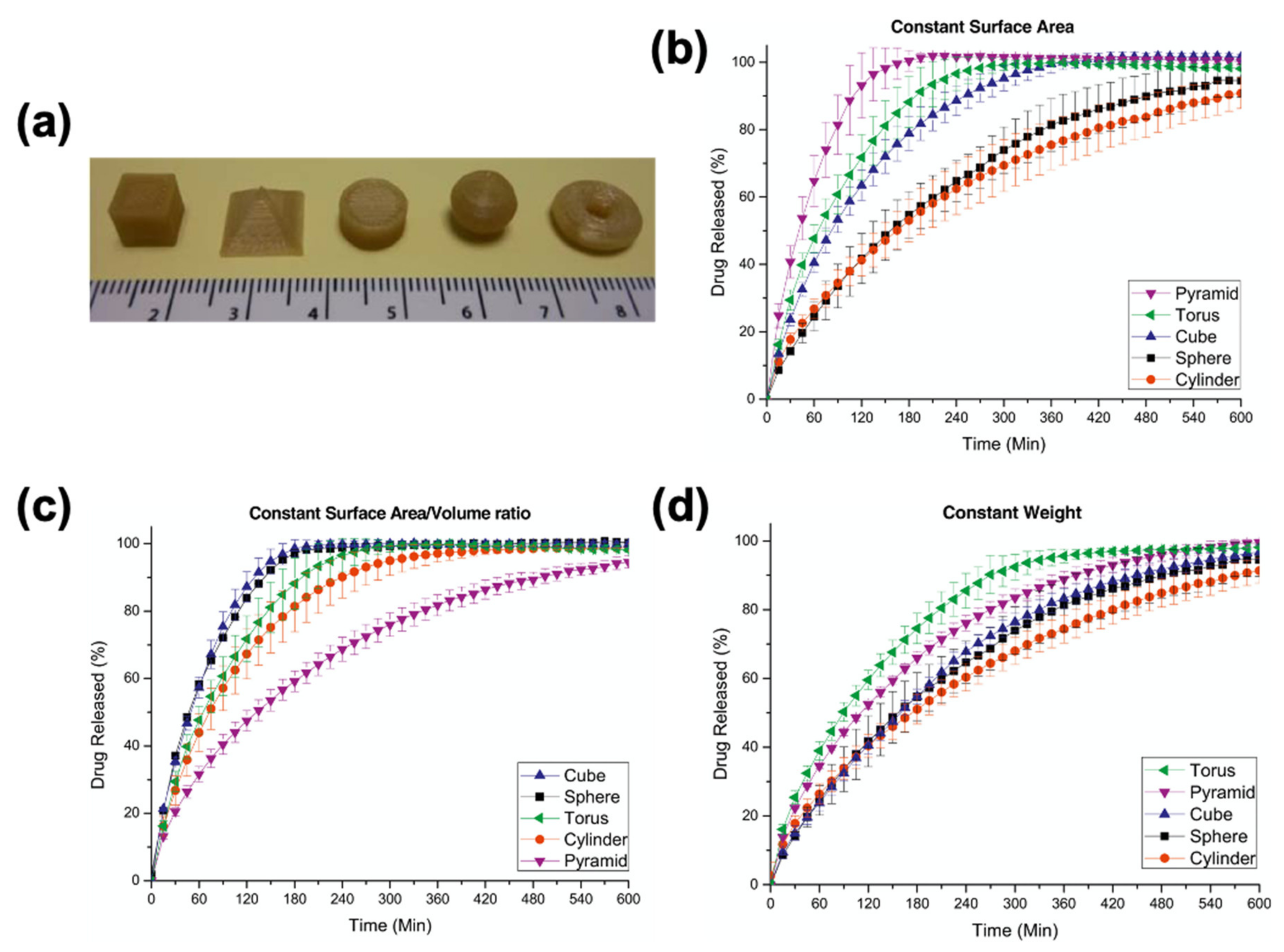
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Kobayashi, M.; Martínez-Pacheco, R.; Gaisford, S.; Basit, A.W. Fused-filament 3D printing of drug products: Microstructure analysis and drug release characteristics of PVA-based caplets. Int. J. Pharm. 2016, 514, 290–295. [Google Scholar] [CrossRef]
- Goyanes, A.; Chang, H.; Sedough, D.; Hatton, G.B.; Wang, J.; Buanz, A.; Gaisford, S.; Basit, A.W. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int. J. Pharm. 2015, 496, 414–420. [Google Scholar] [CrossRef]
- Jamroz, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef]
- Li, Q.; Wen, H.; Jia, D.; Guan, X.; Pan, H.; Yang, Y.; Yu, S.; Zhu, Z.; Xiang, R.; Pan, W. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int. J. Pharm. 2017, 525, 5–11. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Baklavaridis, A.; Katsamenis, O.L.; Markopoulou, C.K.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Fukushige, K.; Ogawa, E.; Hayashi, N.; Ozeki, T. 3D Printing Factors Important for the Fabrication of Polyvinylalcohol Filament-Based Tablets. Biol. Pharm. Bull. 2017, 40, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.S.C.; Lim, L.Y. Drug release from heat-treated polyvinyl alcohol films. Drug Dev. Ind. Pharm. 1992, 18, 1895–1906. [Google Scholar] [CrossRef]
- Nair, S.S. Polyvinyl alcohol based transdermal devices for enhanced skin permeation. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2019, 5, 81–89. [Google Scholar]
- Sivaraman, A.; Ganti, S.; Nguyen, H.; Birk, G.; Wieber, A.; Lubda, D.; Banga, A. Development and evaluation of a polyvinyl alcohol based topical gel. J. Drug Deliv. Sci. Technol. 2017, 39, 210–216. [Google Scholar] [CrossRef]
- Khaled, S.A.; Alexander, M.R.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Nikam, V.K. Eudragit a versatile polymer: A review. Pharmacologyonline 2011, 1, 152–164. [Google Scholar]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef]
- Patra, C.N.; Priya, R.; Swain, S. Pharmaceutical significance of Eudragit: A review. Future J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Verma, P.; Gupta, R.N.; Jha, A.K.; Pandey, R. Development, in vitro and in vivo characterization of Eudragit RL 100 nanoparticles for improved ocular bioavailability of acetazolamide. Drug Deliv. 2013, 20, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Ye, T.; Chen, F.; Yu, S.; Chen, J.; Yang, X.; Yang, N.; Zhang, J.; Liu, J.; et al. Nanostructured lipid carrier surface modified with Eudragit RS 100 and its potential ophthalmic functions. Int. J. Nanomed. 2014, 9, 4305–4315. [Google Scholar]
- Diarraa, M.; Pourroy, G.; Boymond, C.; Muster, D. Fluoride controlled release tablets for intrabuccal use. Biomaterials 2003, 24, 1293–1300. [Google Scholar] [CrossRef]
- Hao, S.; Wang, B.; Wang, Y.; Zhu, L.; Wang, B.; Guo, T. Preparation of Eudragit L 100-55 enteric nanoparticles by a novel emulsion diffusion method. Colloids Surf B Biointerfaces 2013, 108, 127–133. [Google Scholar] [CrossRef]
- Tang, J.; Xu, N.; Ji, H.; Liu, H.; Wang, Z.; Wu, L. Eudragit nanoparticles containing genistein: Formulation, development, and bioavailability assessment. Int. J. Nanomed. 2011, 6, 2429–2435. [Google Scholar]
- Momoh, M.A.; Kenechukwu, F.C.; Adedokun, M.O.; Odo, C.E.; Attama, A.A. Pharmacodynamics of diclofenac from novel Eudragit entrapped microspheres. Drug Deliv. 2014, 21, 193–203. [Google Scholar] [CrossRef][Green Version]
- Quinteros, D.A.; Manzo, R.H.; Allemandi, D.A. Design of a colonic delivery system based on cationic polymethacrylate (Eudragit E100)-mesalamine complexes. Drug Deliv. 2010, 17, 208–213. [Google Scholar] [CrossRef]
- Yoo, J.W.; Giri, N.; Lee, C.H. pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int. J. Pharm. 2011, 40, 262–267. [Google Scholar] [CrossRef]
- Verma, P.R.P.; Iyer, S.S. Transdermal Delivery of Propranolol Using Mixed Grades of Eudragit- Design and In Vitro and In Vivo Evaluation. Drug Dev. Ind. Pharm. 2000, 26, 471–476. [Google Scholar] [CrossRef]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a Shell-Core Delayed Release Tablet Using Dual FDM 3D Printing for Patient-Centred Therapy. Pharm. Res. 2017, 34, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Katstra, W.E.; Palazzolo, R.D.; Rowe, C.W.; Giritlioglu, B.; Teung, P.; Cima, M.J. Oral dosage forms fabricated by Three Dimensional Printing. J. Control. Release 2000, 66, 1–9. [Google Scholar] [CrossRef]
- Rowe, C.W.; Katstra, W.E.; Palazzolo, R.D.; Giritlioglu, B.; Teung, P.; Cima, M.J. Multimechanism oral dosage forms fabricated by three dimensional printing. J. Control. Release 2000, 66, 11–17. [Google Scholar] [CrossRef]
- Awasthi, R.; Manchanda, S.; Das, P.; Velu, V.; Malipeddi, H.; Pabreja, K.; Pinto, T.D.J.A.; Gupta, G.; Dua, K. Poly(vinylpyrrolidone). In Engineering of Biomaterials for Drug Delivery Systems; Woodhead Publishing: Cambridge, UK, 2018; pp. 255–272. [Google Scholar]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.W.; Alhnan, M.A. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm. Res. 2018, 35, 124. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Alexander, M.R.; Irvine, D.J.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. Extrusion 3D Printing of Paracetamol Tablets from a Single Formulation with Tunable Release Profiles Through Control of Tablet Geometry. AAPS PharmSciTech 2018, 19, 3403–3413. [Google Scholar] [CrossRef]
- Wilts, E.M.; Ma, D.; Bai, Y.; Williams, C.B.; Long, T.E. Comparison of Linear and 4-Arm Star Poly(vinyl pyrrolidone) for Aqueous Binder Jetting Additive Manufacturing of Personalized Dosage Tablets. ACS Appl. Mater. Interfaces 2019, 11, 23938–23947. [Google Scholar] [CrossRef]
- Mohabe, V.; Akhand, R.; Pathak, A.K. Preparation and Evaluation of Captopril Transdermal Patches. Bull. Pharm. Res. 2011, 1, 47–52. [Google Scholar]
- Sadashivaiah, R.; Dinesh, B.M.; Patil, U.A.; Desai, B.G.; Raghu, K.S. Design and in vitro evaluation of haloperidol lactate transdermal patches containing ethyl cellulose-povidone as film formers. Asian J. Pharm. 2008, 2, 43–49. [Google Scholar]
- Robinson, B.V.; Sullivan, F.M.; Borzelleca, J.F.; Schwartz, S.L. PVP-A Critical Review of The Kinetics and Toxicology of Polyvinylprrolidone; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Franco, P.; De Marco, I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Polymeric modification and its implication in drug delivery: Poly-epsilon-caprolactone (PCL) as a model polymer. Mol. Pharm. 2012, 9, 2365–2379. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Klauss, P.; Zepon, K.M.; Kanis, L.A. The effects of laser energy density and particle size in the selective laser sintering of polycaprolactone/progesterone specimens: Morphology and drug release. Int. J. Adv. Manuf. Technol. 2012, 66, 1113–1118. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Klauss, P.; Kanis, L.A. Laser Printing of PCL/Progesterone Tablets for Drug Delivery Applications in Hormone Cancer Therapy. Lasers Manuf. Mater. Process. 2017, 4, 108–120. [Google Scholar] [CrossRef]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.; Alayoubi, A.; Asfari, S.; Coburn, J.; Ghammraoui, B.; Aqueel, S.; Cruz, C.N.; Ashraf, M. Development of mechanistic models to identify critical formulation and process variables of pastes for 3D printing of modified release tablets. Int. J. Pharm. 2019, 555, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Cruise, G.M.; Scharp, D.S.; Hubbell, J.A. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials 1998, 19, 1287–1294. [Google Scholar] [CrossRef]
- Pelras, T.; Glass, S.; Scherzer, T.; Elsner, C.; Schulze, A.; Abel, B. Transparent Low Molecular Weight Poly(Ethylene Glycol) Diacrylate-Based Hydrogels as Film Media for Photoswitchable Drugs. Polymers 2017, 9, 639. [Google Scholar] [CrossRef]
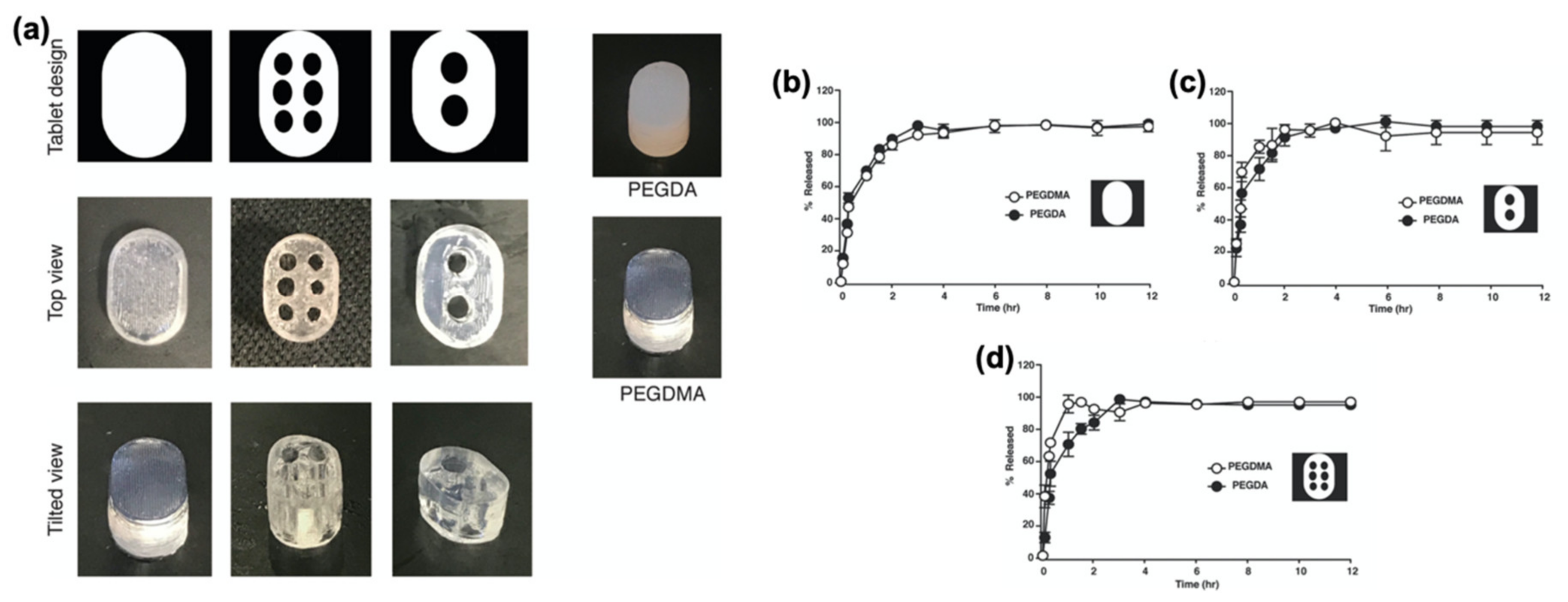
- Clark, E.A.; Alexander, M.R.; Irvine, D.J.; Roberts, C.J.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Hague, R.; Tuck, C.J.; Wildman, R.D. 3D printing of tablets using inkjet with UV photoinitiation. Int. J. Pharm. 2017, 529, 523–530. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Healy, A.V.; Fuenmayor, E.; Doran, P.; Geever, L.M.; Higginbotham, C.L.; Lyons, J.G. Additive Manufacturing of Personalized Pharmaceutical Dosage Forms via Stereolithography. Pharmaceutics 2019, 11, 645. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Krkobabić, M.; Medarević, D.; Cvijić, S.; Grujić, B.; Ibrić, S. Hydrophilic excipients in digital light processing (DLP) printing of sustained release tablets: Impact on internal structure and drug dissolution rate. Int. J. Pharm. 2019, 572, 118790. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Matthias, K.; Kolter, K. Evaluation of different polymers in 3D printing technologies. Am. Pharm. Rev. 2019, 22, 166–175. [Google Scholar]
- Ilyés, K.; Kovács, N.K.; Balogh, A.; Borbás, E.; Farkas, B.; Casian, T.; Marosi, G.; Tomuță, I.; Nagy, Z.K. The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique: Material considerations–printability–process modulation, with consecutive effects on in vitro release, stability and degradation. Eur. J. Pharm. Sci. 2019, 129, 110–123. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef]
- Shi, K.; Tan, D.K.; Nokhodchi, A.; Maniruzzaman, M. Drop-On-Powder 3D Printing of Tablets with an Anti-Cancer Drug, 5-Fluorouracil. Pharmaceutics 2019, 11, 150. [Google Scholar] [CrossRef]
- Raju, Y.P.; Jayasri, V.; Yasmeen, B.R.; Chowdary, V.H.; Satyanandam, S. Significance of Pharmaceutical Excipients—A Review. J. Innov. Trends Pharm. Sci. 2011, 2, 191–201. [Google Scholar]
- Chaudhari, S.P.; Patil, P.S. Pharmaceutical Excipients: A review. Int. J. Adv. Pharm. Biol. Chem. 2012, 1, 21–34. [Google Scholar]
- Poonam, V.; Sagar, G.; Abhishek, K.; Yuvraj, S. Remarkable Contribution of Natural Excipients in Finished Pharmaceutical Products. Int. J. Pharm. Sciences Rev. Res. 2018, 52, 7–14. [Google Scholar]
- Karolewicz, B. A review of polymers as multifunctional excipients in drug dosage form technology. Saudi Pharm. J. 2016, 24, 525–536. [Google Scholar] [CrossRef]
- Alvi, M.; Chatterjee, P. Excipients and Active Pharmaceutical Ingredients; Springer: New York, NY, USA, 2014; pp. 347–361. [Google Scholar]
- Laboulfie, F.; Hemati, M.; Lamure, A.; Diguet, S. Effect of the plasticizer on permeability, mechanical resistance and thermal behaviour of composite coating films. Powder Technol. 2013, 238, 14–19. [Google Scholar] [CrossRef]
- Kundu, J.; Patra, C.; Kundu, S.C. Design, fabrication and characterization of silk fibroin-HPMC-PEG blended films as vehicle for transmucosal delivery. Mater. Sci. Eng. C 2008, 28, 1376–1380. [Google Scholar] [CrossRef]
- Cadogan, D.F.; Howick, C.J. Plasticizers, in Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Bialecka-Florjanczyk, E.; Florjanczyk, Z. Solubility of Plasticizers, Polymers and Environmental Pollution. In Thermodynamics, Solubility and Environmental Issues; Elsevier: Amsterdam, The Netherlands, 2007; pp. 397–408. [Google Scholar]
- Mencik, P.; Přikryl, R.; Stehnová, I.; Melčová, V.; Kontárová, S.; Figalla, S.; Alexy, P.; Bočkaj, J. Effect of Selected Commercial Plasticizers on Mechanical, Thermal, and Morphological Properties of Poly(3-hydroxybutyrate)/Poly(lactic acid)/Plasticizer Biodegradable Blends for Three-Dimensional (3D) Print. Materials 2018, 11, 1893. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y. Lubricants in Pharmaceutical Solid Dosage Forms. Lubricants 2014, 2, 21–43. [Google Scholar] [CrossRef]
- Shaikh, R.; Croker, D.; O’Brien, D.; Walker, G. The Development of a Pharmaceutical Oral Solid Dosage forms. In Process Systems Engineering for Pharmaceutical Manufacturing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–65. [Google Scholar]
- Bowden, F.P.; Bowden, F.P.; Tabor, D. The Friction and Lubrication of Solids; Oxford University Press: Oxford, UK, 2001; Volume 1. [Google Scholar]
- Faghihnejad, A.; Zeng, H. Fundamentals of surface adhesion, friction, and lubrication. Polym. Adhes. Frict. Lubr. 2013, 1–57. [Google Scholar]
- Kanher, P.R. Lubricants in Pharmaceutical Solid Dosage Forms with Special Emphasis on Magnesium Stearate. World J. Pharm. Res. 2017, 131–146. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- El-Barghouthi, M.; Eftaiha, A.; Rashid, I.; Al-Remawi, M.; Badwan, A. A Novel Superdisintegrating Agent Made from Physically Modified Chitosan with Silicon Dioxide. Drug Dev. Ind. Pharm. 2008, 34, 373–383. [Google Scholar] [CrossRef]
- Alebiowu, G.; Itiola, O.A. Effects of starches on the mechanical properties of paracetamol tablet formulations. II. Sorghum and plantain starches as disintegrants. Acta Pharm. 2003, 53, 313–320. [Google Scholar]
- Quodbach, J.; Moussavi, A.; Tammer, R.; Frahm, J.; Kleinebudde, P. Tablet Disintegration Studied by High-Resolution Real-Time Magnetic Resonance Imaging. J. Pharm. Sci. 2014, 103, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.M.; Liew, C.V.; Heng, P.W.S. Review of Disintegrants and the Disintegration Phenomena. J. Pharm. Sci. 2016, 105, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Moreton, R.C. Disintegrants in Tableting, in Pharmaceutical Dosage Forms-Tablets; CRC Press: Boca Raton, FL, USA, 2008; pp. 233–266. [Google Scholar]
- Bele, M.H.; Derle, D.V. Effect of Sorbed Water on Disintegrant Performance of Four Brands of Polacrilin Potassium NF. Aaps Pharmscitech 2012, 13, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.M.; Er, P.X.; Liew, C.V.; Heng, P.W. Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a quality by design approach. AAPS PharmSciTech 2014, 15, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Shailendra, P.; Shikha, A.; Singh, L.B. Natural Binding Agents in Tablet Formulation. Int. J. Pharm. Biol. Arch. 2012, 3, 466–473. [Google Scholar]
- Yu, D.G.; Shen, X.X.; Branford-White, C.; Zhu, L.M.; White, K.; Yang, X.L. Novel oral fast-disintegrating drug delivery devices with predefined inner structure fabricated by Three-Dimensional Printing. J. Pharm. Pharm. 2009, 61, 323–329. [Google Scholar] [CrossRef]
- Abinusawa, A.; Tenjarla, S. Release of 5-Aminosalicylic Acid (5-ASA) from Mesalamine Formulations at Various pH Levels. Adv. Ther. 2015, 32, 477–484. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- McConnell, E.L.; Liu, F.; Basit, A.W. Colonic treatments and targets: Issues and opportunities. J. Drug Target 2009, 17, 335–363. [Google Scholar] [CrossRef]
- Heel, R.C.; Brogden, R.N.; Speight, T.M.; Avery, G.S. Captopril: A Preliminary Review of its Pharmacological Properties and Therapeutic Efficacy. Drugs 1980, 20, 409–452. [Google Scholar] [CrossRef]
- Parente, L. Deflazacort: Therapeutic index, relative potency and equivalent doses versus other corticosteroids. BMC Pharm. Toxicol. 2017, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Reddymasu, S.C.; Soykan, I.; McCallum, R.W. Domperidone: Review of pharmacology and clinical applications in gastroenterology. Am. J. Gastroenterol. 2007, 102, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Van Der Velden, V.H.J. Glucocorticoids: Mechanisms of action and anti-inflammatory potential in asthma. Mediat. Inflamm. 1998, 7, 229–237. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Shaqour, B.; Samaro, A.; Verleije, B.; Beyers, K.; Vervaet, C.; Cos, P. Production of Drug Delivery Systems Using Fused Filament Fabrication: A Systematic Review. Pharmaceutics 2020, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, R.; Zia, H. Hot-Melt Extrusion Technique: A Review. Iran. J. Pharm. Res. 2004, 3, 3–16. [Google Scholar]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Repka, M.A.; Majumdar, S.; Kumar Battu, S.; Srirangam, R.; Upadhye, S.B. Applications of hot-melt extrusion for drug delivery. Expert Opin. Drug Deliv. 2008, 5, 1357–1376. [Google Scholar] [CrossRef]
- Leong, K.F.; Chua, C.K.; Gui, W.S.; Verani, M. Building Porous Biopolymeric Microstructures for Controlled Drug Delivery Devices Using Selective Laser Sintering. Int. J. Adv. Manuf. Technol. 2006, 31, 483–489. [Google Scholar] [CrossRef]
- Cheah, C.M.; Leong, K.F.; Chua, C.K.; Low, K.H.; Quek, H.S. Characterization of microfeatures in selective laser sintered drug delivery devices. Proc. Inst. Mech. Eng. 2002, 216, 369–383. [Google Scholar] [CrossRef]
- Arcaute, K.; Mann, B.K.; Wicker, R.B. Stereolithography of Three-Dimensional Bioactive Poly(Ethylene Glycol) Constructs with Encapsulated Cells. Ann. Biomed. Eng. 2006, 34, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
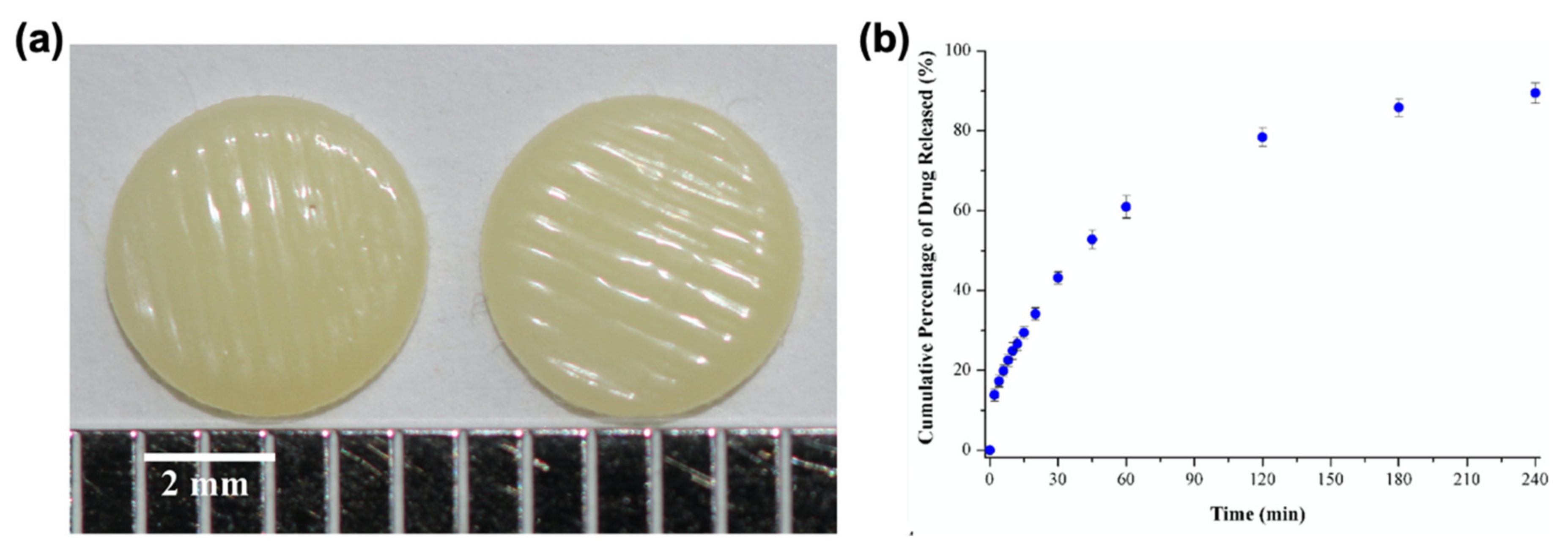
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control. Release 2017, 261, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.C.; Wang, T.; Derby, B. Inkjet delivery of glucose oxidase. Chem. Commun. 2010, 46, 5452–5454. [Google Scholar] [CrossRef]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Zhang, J.; Vo, A.Q.; Feng, X.; Bandari, S.; Repka, M.A. Pharmaceutical Additive Manufacturing: A Novel Tool for Complex and Personalized Drug Delivery Systems. AAPS PharmSciTech 2018, 19, 3388–3402. [Google Scholar] [CrossRef]
- Yu, D.G.; Zhu, L.M.; Branford-White, C.J.; Yang, X.L. Three-Dimensional Printing in Pharmaceutics- Promises and Problems. J. Pharm. Sci. 2008, 97, 3666–3690. [Google Scholar] [CrossRef]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef]
- Liang, K.; Brambilla, D.; Leroux, J.C. Is 3D Printing of Pharmaceuticals a Disruptor or Enabler? Adv. Mater. 2019, 31, 1805680. [Google Scholar] [CrossRef]
- Coogan, T.J.; Kazmer, D.O. In-line rheological monitoring of fused deposition modeling. J. Rheol. 2018, 63, 141–155. [Google Scholar] [CrossRef]
- Coogan, T.J.; Kazmer, D.O. Prediction of interlayer strength in material extrusion additive manufacturing. Addit. Manuf. 2020, 35, 101368. [Google Scholar] [CrossRef]
- Di Prima, M.; Coburn, J.; Hwang, D.; Kelly, J.; Khairuzzaman, A.; Ricles, L. Additively manufactured medical products—The FDA perspective. 3D Print. Med. 2016, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Osouli-Bostanabad, K.; Adibkia, K. Made-on-demand, complex and personalized 3D-printed drug products. BioImpacts 2018, 8, 77–79. [Google Scholar] [CrossRef] [PubMed]




| Drug | Effect on the Body | Reference |
|---|---|---|
| 4-ASA (4-Aminosalicylic acid) | Antibiotic primarily used to treat tuberculosis | [52] |
| 5-ASA (5-aminosalicylic acid or Mesalamine) | Anti-inflammatory | [137] |
| Aripiprazole | Antipsychotic | [49] |
| Aspirin | Reduces risk of blood clotting and reduces the risk of heart attacks and strokes | [138] |
| Atenolol | Used to treat hypertension and prevent heart attack | [138] |
| Budesonide | Treats inflammatory bowel disease | [139] |
| Caffeine | Stimulant to reduce fatigue | [45] |
| Captopril | Lowers blood pressure (for hypertension) | [140] |
| Deflazacort | Immunosuppressant and anti-inflammatory | [141] |
| Domperidone | Treats gastroparesis and other conditions causing chronic nausea and vomiting | [142] |
| Hydrochlorothiazide | Prevents absorption of too much salt and treats oedema | [138] |
| Paracetamol | Analgesic and Antipyretic | [59] |
| Pravastatin | Reduces blood cholesterol and triglyceride in hyperlipidemic patients | [138] |
| Prednisolone | Anti-inflammatory | [143] |
| Ramipril | Angiotensin (increases blood pressure) | [138] |
| Theophylline | Bronchodilator | [144] |
| Printing Technology | Polymer | Model Drug | Reference |
|---|---|---|---|
| FDM of hot melt extruded loaded filament | EC | Quinine | [30] |
| Carbamazepine | [33] | ||
| HPC | Paracetamol | [31] | |
| Itraconazole | [32] | ||
| Carbamazepine | [33] | ||
| Domperidone | [34] | ||
| Theophylline | [35,73] | ||
| PVA | Paracetamol | [45,46,47] | |
| Caffeine | [45,47] | ||
| Budesonide | [48] | ||
| Aripiprazole | [49] | ||
| Glipizide | [50] | ||
| Hydrochlorothiazide | [51] | ||
| Eudragit | Hydrochlorothiazide | [74] | |
| Theophylline | [73,76] | ||
| 5-ASA | [75] | ||
| Captopril | [75] | ||
| Prednisolone | [75] | ||
| PVP | Theophylline | [80] | |
| Dipyridamole | [80] | ||
| Pantoprazole sodium | [81] | ||
| FDM, API incorporated by soaking | PVA | 4-ASA | [52] |
| 5-ASA | [52] | ||
| Prednisolone | [53] | ||
| Curcumin | [54] | ||
| Fluorescein | [55] | ||
| Eudragit | Deflazacort | [96] | |
| PCL | Deflazacort | [96] | |
| DIW | HPMC | Atenolol | [138] |
| Pravastatin | [138] | ||
| Ramipril | [138] | ||
| Guaifenesin | [36] | ||
| Dipyridamole | [37] | ||
| PVP | Aspirin | [138] | |
| Hydrochlorothiazide | [138] | ||
| Paracetamol | [59,82] | ||
| Carbopol | Guaifenesin | [36] | |
| Binder jetting | Eudragit | Chlorpheniramine maleate | [77,78] |
| PVP | Paracetamol | [83,136] | |
| Inkjet printing | PEGDA | Ropinirole hydrochloride | [101] |
| SLS | PCL | Progesterone | [94,95] |
| SLA | PEGDA | Paracetamol | [102,103] |
| 4-ASA | [102] | ||
| Aspirin | [103] | ||
| Ibuprofen | [104] | ||
| DLP | PEGDA | Theophylline | [105] |
| Paracetamol | [106] | ||
| PEGDMA | Theophylline | [105] |
| Printing Technology | Advantages | Disadvantages |
|---|---|---|
| FDM | High drug loading Can print complex shapes Easy to adjust drug release profiles | Preprinting processes can take time Possible thermal degradation of APIs |
| DIW | High drug loading No risk of thermal degradation | Possible phase separation of drug formulations Hard to uniformly distribute APIs within the paste Drying of the tablet is required post printing |
| SLS | High resolution No need for preprinting processes | Possible degradation of APIs due to sintering |
| SLA | High resolution | Possible degradation of APIs due to laser projected onto the drug-loaded solution Additional post-printing processes needed, such as photocuring of the final product |
| DLP | High resolution | Possible degradation of APIs due to laser projected onto the drug-loaded solution Additional post-printing processes needed, such as photocuring of the final product |
| Inkjet | Versatility of the technology, can be used with heat or light-based approach | Cannot be used with high drug loading APIs can be affected by high shear rates during printing |
| BJ | Uniform final product | Highly porous final products lead to low mechanical properties Requires additional post-printing processes such as sintering |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abaci, A.; Gedeon, C.; Kuna, A.; Guvendiren, M. Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets. Pharmaceutics 2021, 13, 156. https://doi.org/10.3390/pharmaceutics13020156
Abaci A, Gedeon C, Kuna A, Guvendiren M. Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets. Pharmaceutics. 2021; 13(2):156. https://doi.org/10.3390/pharmaceutics13020156
Chicago/Turabian StyleAbaci, Alperen, Christina Gedeon, Anna Kuna, and Murat Guvendiren. 2021. "Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets" Pharmaceutics 13, no. 2: 156. https://doi.org/10.3390/pharmaceutics13020156
APA StyleAbaci, A., Gedeon, C., Kuna, A., & Guvendiren, M. (2021). Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets. Pharmaceutics, 13(2), 156. https://doi.org/10.3390/pharmaceutics13020156







