Preparation and Optimization of Garlic Oil/Apple Cider Vinegar Nanoemulsion Loaded with Minoxidil to Treat Alopecia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Estimation of Required Hydrophilic Lipophilic Balance (RHLB) for Garlic Oil in Apple Cider Vinegar
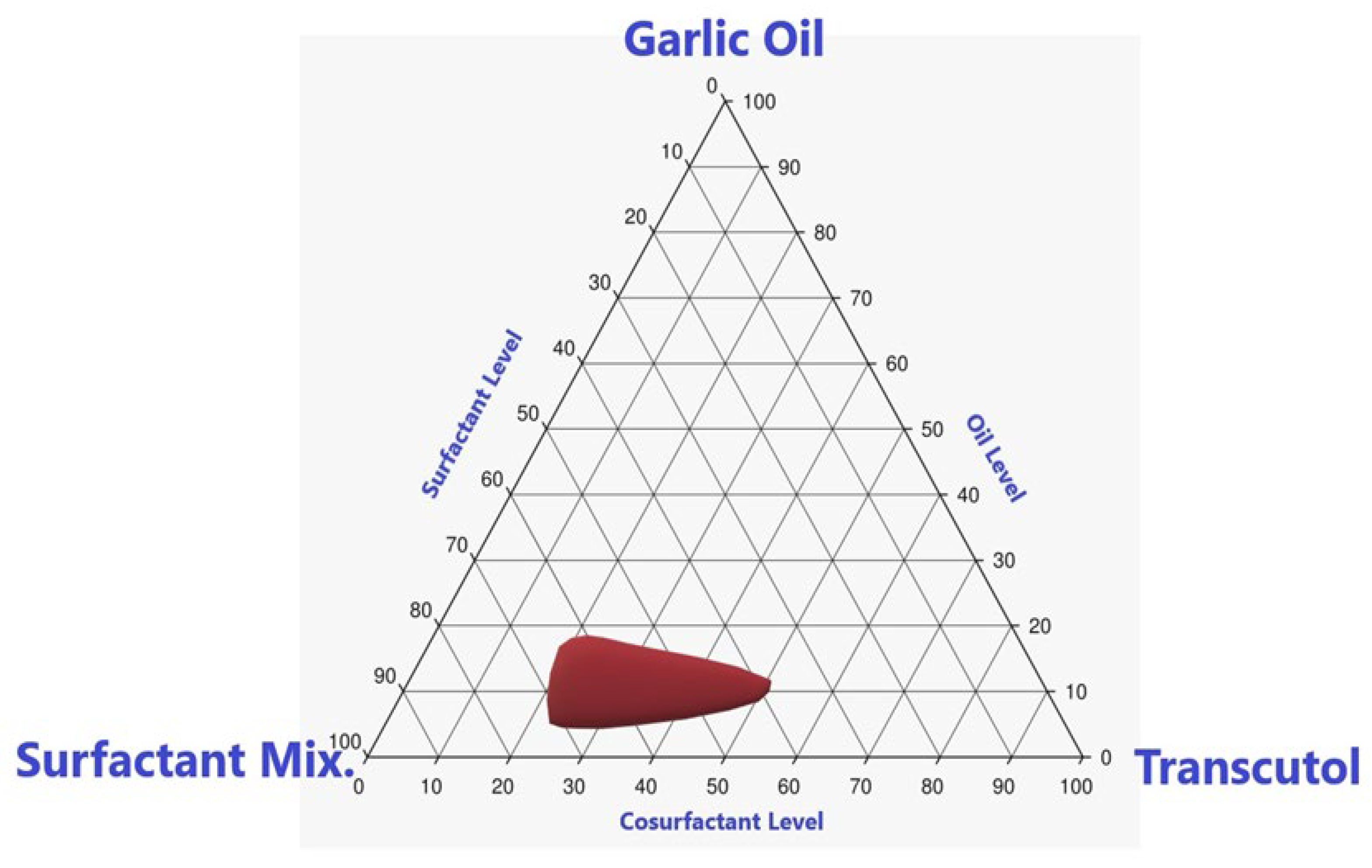
2.3. Pseudoternary Phase Diagram Construction
2.4. Preparation and Optimization of the MX-Loaded GO-APCV Nanoemulsion
2.5. Preparation of MX-GO-APCV Nanoemulsion
2.6. Evaluation of the MX-GO-APCV Nanoemulsion
2.6.1. Determination of MX-GO-APCV Nanoemulsion Droplet Size
2.6.2. Ex Vivo Skin Permeation Study of MX-GO-APCV Nanoemulsion
2.6.3. Assessment of MIC of the Prepared MX-GO-APCV Nanoemulsion against P. acnes
2.7. Optimization of the MX-GO-APCV Nanoemulsion
2.8. Preparation and Evaluation of the Optimized MX-GO-APCV Nanoemulsion
2.9. Stability Index Determination
Observation on Phase Separation
2.10. Analysis of Permeation Parameters and Kinetic Modeling
3. Results and Discussion
3.1. Estimation of Required Hydrophilic Lipophilic Balance (RHLB) for GO in APCV
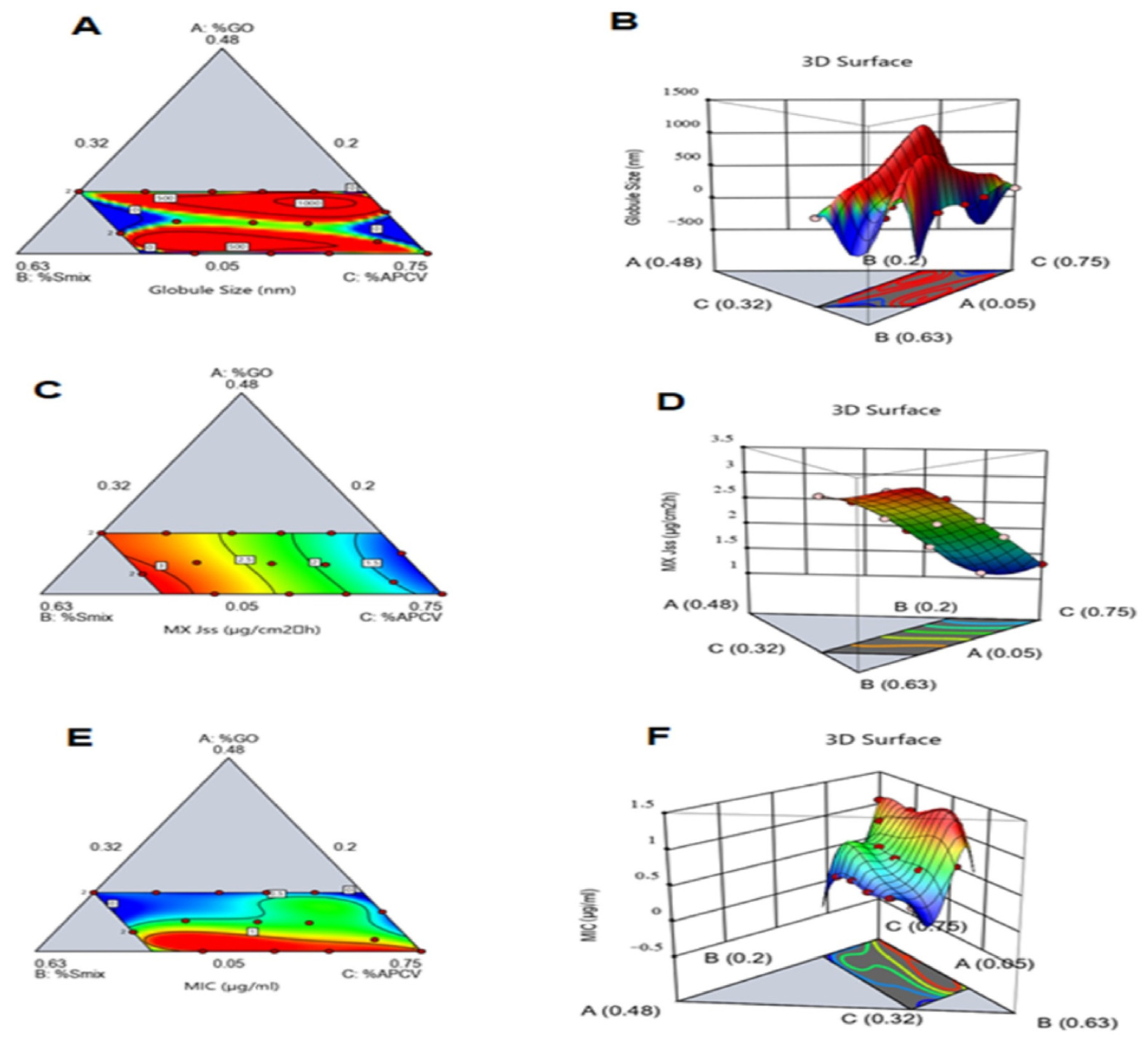
3.2. Evaluation of the MX-GO-APCV Nanoemulsion
3.2.1. Determination of MX-GO-APCV Nanoemulsion Droplet Size
1006AB(A − B) + 3.078 × 1006AC(A − C) + 22,757.47BC(B − C) − 1.110 × 1007A2BC − 2.963 × 1006AB2C − 3.067 ×
1006ABC2 + 1.323 × 1006AB(A − B)2 + 1.024 × 1006AC(A − C)2 + 14,427.31BC(B − C)2
3.2.2. Ex Vivo Skin Permeation Study of MX-GO-APCV Nanoemulsion
3.2.3. Assessment of MIC of the Prepared MX-GO-APCV Nanoemulsion against P. acnes
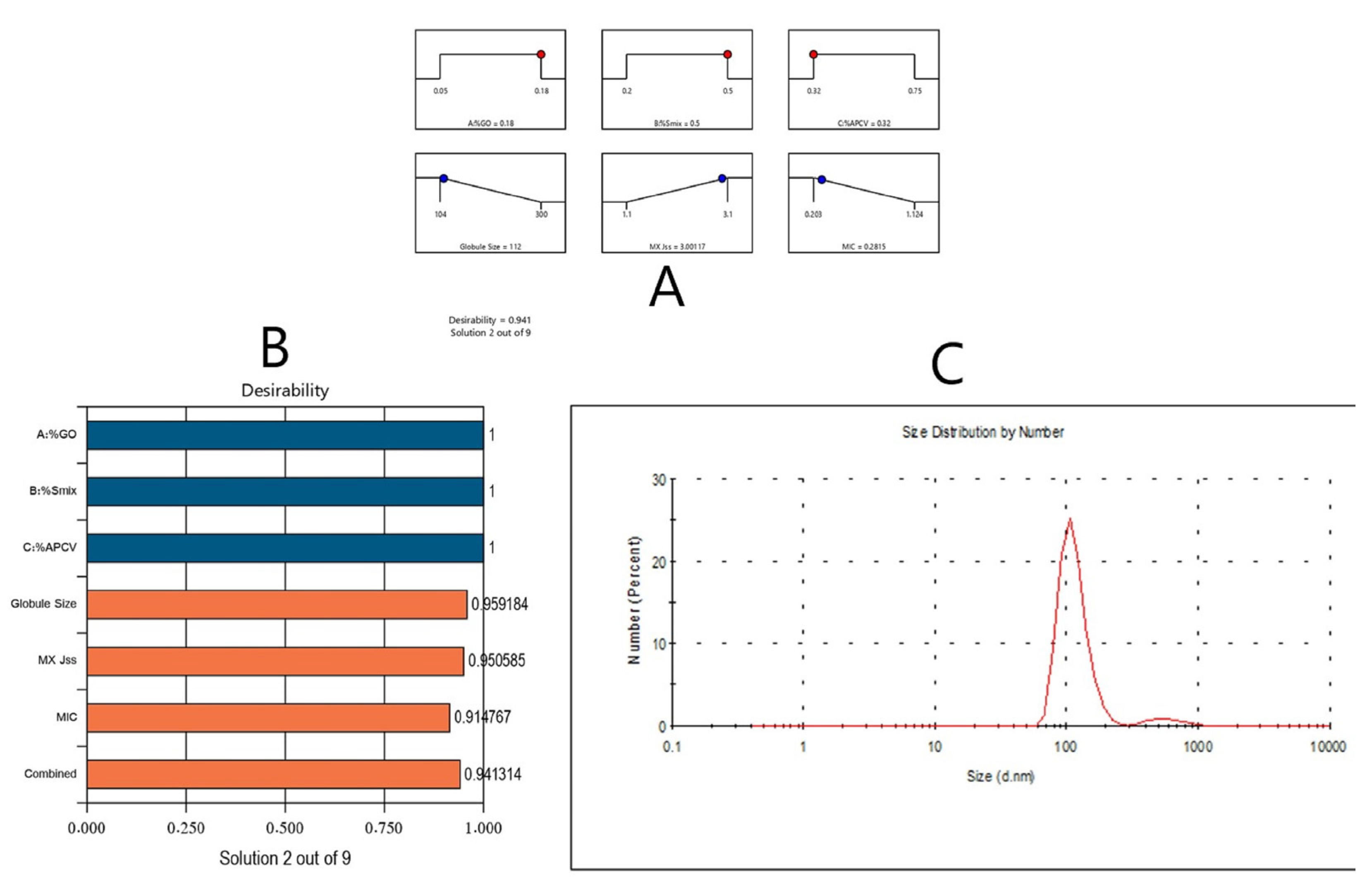
3.3. Optimization of MX-GO-APCV Nanoemulsion Formulations
3.4. Evaluation of the Optimized Formulation
3.4.1. Determination of Stability Index
3.4.2. Analysis of Permeation Parameters of Optimum Formulation
3.4.3. Kinetic Analysis of the Permeation Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Epstein, E. Evidence-based treatment of alopecia areata. J. Am. Acad. Dermatol. 2001, 45, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Dawn, G.; Kumar, B. Profile of alopecia areata in northern India. Int. J. Dermatol. 1996, 35, 22–27. [Google Scholar] [CrossRef]
- Strober, B.E.; Siu, K.; Alexis, A.F.; Kim, G.; Washenik, K.; Sinha, A.; Shupack, J.L. Etanercept does not effectively treat moderate to severe alopecia areata: An open-label study. J. Am. Acad. Dermatol. 2005, 52, 1082–1084. [Google Scholar] [CrossRef]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Dai, L.R.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- McElwee, K.J.; Boggess, D.; Olivry, T.; Oliver, R.F.; Whiting, D.; Tobin, D.J.; Bystryn, J.-C.; King, L.E., Jr.; Sundberg, J.P. Comparison of alopecia areata in human and nonhuman mammalian species. Pathobiology 1998, 66, 90–107. [Google Scholar] [CrossRef]
- Wang, E.; Lee, J.S.; Hee, T.H. Is propionibacterium acnes associated with hair casts and alopecia? Int. J. Trichology 2012, 4, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Iwata, C.; Akimoto, N.; Sato, T. Augmentation of lipogenesis by 15 deoxy-Ä12,14—prostaglandin J2 in hamster sebaceous glands: Identification of cytochrome P450-mediated by 15 deoxy-Ä12,14 -prostaglandin J2 production. J. Investig. Dermatol. 2005, 125, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüggerman, H. Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. J. Cutan. Med. Surg. 2005, 24, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W.; Conte, E.T.; Leavitt, M.L.; Nafz, M.A.; Scroeter, A.L. Cutaneous immunopathology of androgenetic alopecia. J. Am. Osteopath Assoc. 1991, 91, 765–771. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Dev. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [Green Version]
- Mura, S.; Manconi, M.; Sinico, C.; Valenti, D.; Fadda, A.M. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of monoxidil. Int. J. Pharm. 2009, 380, 72–79. [Google Scholar] [CrossRef]
- Aronson, J.K. Minoxidil. In Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions; Aronson, J.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 2354–2356. [Google Scholar]
- Pavithran, K. Erythema multiforme following topical minoxidil. Indian J. Dermatol. Venerol. Leprol. 1993, 59, 313–314. [Google Scholar]
- Wagner, L.; Kenreigh, C. Minoxidil. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., David, B.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–5. [Google Scholar]
- Padois, K.; Cantiéni, C.; Bertholle, V.; Bardel, C.; Pirot, F.; Falson, F. Solid lipid nanoparticles suspension versus commercial solutions for dermal delivery of minoxidil. Int. J. Pharm. 2011, 416, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar]
- Satyal, P.; Craft, J.D.; Dosoky, N.S.; Setzer, W.N. The Chemical Compositions of the Volatile Oils of Garlic (Allium sativum) and Wild Garlic (Allium vineale). Foods 2017, 6, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, H.S.; Chizzola, R.; Ramadan, A.A.; Edris, A.E. Chemical composition and antimicrobial activity of garlic essential oils evaluated in organic solvent, emulsifying, and self-microemulsifying water based delivery systems. Food Chem. 2017, 221, 196–204. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Rnault, I.; Haffner, T.; Siess, M.H.; Vollmar, A.; Kahane, R.; Auger, J. Analytical method for appreciation of garlic therapeutic potential and for validation of a new formulation. J. Pharm. Biomed Anal. 2005, 37, 963–970. [Google Scholar] [CrossRef]
- Piyasena, P.; Rayner, M.; Bartlett, F.M.; Lu, X.; McKellar, R.C. Characterization of Apples and Apple Cider Produced by a Guelph Area Orchard. LWT—Food Sci. Technol. 2002, 35, 622–627. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Le Quéré, J.M.; Bauduin, R.; Symoneaux, R.; Le Bourvellec, C.; Baron, A. Modulating polyphenolic composition and organoleptic properties of apple juices by manipulating the pressing conditions. Food Chem. 2010, 124, 117–125. [Google Scholar] [CrossRef]
- Yagnik, D.; Serafin, V.J.; Shah, A. Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression. Sci. Rep. 2018, 8, 1732. [Google Scholar] [CrossRef]
- Tang, S.C.; Yang, J.H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef] [Green Version]
- Salem, H.F.; Nafady, M.M.; Ewees, M.G.E.; Hassan, H.; Khallaf, R.A. Rosuvastatin calcium-based novel nanocubic vesicles capped with silver nanoparticles-loaded hydrogel for wound healing management: Optimization employing Box-Behnken design: In vitro and in vivo assessment. J. Liposome Res. 2021, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; El-Menshawe, S.F.; Khallaf, R.A.; Rabea, Y.K. A novel transdermal nanoethosomal gel of lercanidipine HCl for treatment of hypertension: Optimization using Box-Benkhen design, in vitro and in vivo characterization. Drug Deliv. Transl. Res. 2020, 10, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, R.A.; Aboud, H.M.; Sayed, O.M. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. J. Liposome Res. 2020, 30, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Sindi, A.M.; Maira, Y.H.; Khallaf, R.A. Oral gel loaded by ethotransfersomes of antifungal drug for oral thrush: Preparation, characterization, and assessment of antifungal activity. J. Drug Del. Sci. Technol. 2021, 66, 102841. [Google Scholar] [CrossRef]
- Hosny, K.M.; Alhakamy, N.A.; Sindi, A.M.; Khallaf, R.A. Coconut Oil Nanoemulsion Loaded with a Statin Hypolipidemic Drug for Management of Burns: Formulation and In Vivo Evaluation. Pharmaceutics 2020, 12, 1061. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Hassan, A.H.; Bakhaidar, R.B.; Abualsunun, W.A.; Almehmady, A.M.; Khames, A.; Rizg, W.Y. Development of omega-3 loxoprofen-loaded nanoemulsion to limit the side effect associated with NSAIDs in treatment of tooth pain. Drug Deliv. 2021, 28, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Khallaf, R.A.; Asfour, H.Z.; Rizg, W.Y.; Alhakamy, N.A.; Sindi, A.M.; Alkhalidi, H.M.; Abualsunun, W.A.; Bakhaidar, R.B.; Almehmady, A.M.; et al. Development and Optimization of Cinnamon Oil Nanoemulgel for Enhancement of Solubility and Evaluation of Antibacterial, Antifungal and Analgesic Effects against Oral Microbiota. Pharmaceutics 2021, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.; Asfour, H.; Rizg, W.; Alhakamy, N.A.; Sindi, A.; Alkhalidi, H.; Abualsunun, W.; Bakhaidar, R.; Almehmady, A.M.; Akeel, S.; et al. Formulation, Optimization, and Evaluation of Oregano Oil Nanoemulsions for the Treatment of Infections Due to Oral Microbiota. Int. J. Nanomed. 2021, 16, 5465–5478. [Google Scholar] [CrossRef] [PubMed]
- Gregg Stetsko Manager. Statistical Experimental Design and its Application to Pharmaceutical Development Problems. Drug Dev. Indust Pharm. 1986, 12, 1109–1123. [Google Scholar] [CrossRef] [Green Version]
- Hosny, K.M.; Rizg, W.Y.; Khallaf, R.A. Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics 2020, 12, 263. [Google Scholar] [CrossRef] [Green Version]
- Alkhalidia, H.M.; Naguib, G.H.; Kurakula, M.; Hamed, M.T.; Attar, M.H.; Almatrook, Z.H.; Aldryhim, A.Y.; Bahmdan, R.H.; Khallaf, R.A.; el Sisi, A.M.; et al. In vitro and preclinical assessment of factorial design based nanoethosomal transdermal film formulation of mefenamic acid to overcome barriers to its use in relieving pain and inflammation. J. Drug Del. Sci. Technol. 2018, 48, 450–456. [Google Scholar] [CrossRef]
- Long, Y.; Huang, W.; Wang, Q.; Yang, G. Green synthesis of garlic oil nanoemulsion using ultrasonication technique and its mechanism of antifungal action against Penicillium italicum. Ultrason. Sonochem. 2020, 64, 104970. [Google Scholar] [CrossRef] [PubMed]
- Purkait, A.; Worede, R.E.; Baral, D.; Hazra, D.K. Development of nanoemulsion formulation of mustard oil, its chemical characterization and evaluation against post harvest anthracnose pathogens. Indian Phytopathol. 2020, 73, 449–460. [Google Scholar] [CrossRef]
- Abou-Taleb, H.A.; Khallaf, R.A.; Abdel-Aleem, J.A. Intranasal niosomes of nefopam with improved bioavailability: Preparation, optimization, and In-Vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 3501–3516. [Google Scholar] [CrossRef] [Green Version]
- Zillich, O.V.; Schweiggert-Weisz, U.; Hasenkopf, K.; Eisner, P.; Kerscher, M. Release and in vitro skin permeation of polyphenols from cosmetic emulsions. Int. J. Cosmet. Sci. 2013, 35, 491–501. [Google Scholar] [CrossRef]
- Kumar, R.; Mishra, A.K.; Dubey, N.K.; Tripathi, Y.B. Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int. J. Food Microbiol. 2007, 115, 159–164. [Google Scholar] [CrossRef]
- Mapunya, M.B.; Hussein, A.A.; Rodriguez, B.; Lall, N. Tyrosinase activity of Greyia flanaganii (Bolus) constituents. Phytomedicine 2011, 18, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar]
- Shin, S.C.; Cho, C.W.; Oh, I.J. Effects of non-ionic surfactants as permeation enhancers towards piroxicam from the poloxamer gel through rat skins. Int. J. Pharm. 2001, 222, 199–203. [Google Scholar] [CrossRef]
- Lu, X.; Rasco, B.A.; Jabal, J.M.; Aston, D.E.; Lin, M.; Konkel, M.E. Investigating antibacterial effects of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni by using Fourier transform infrared spectroscopy, Raman spectroscopy, and electron microscopy. Appl. Environ. Microbiol. 2011, 77, 5257–5269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casella, S.; Leonardi, M.; Melai, B.; Fratini, F.; Pistelli, L. The role of diallyl sulfides and dipropyl sulfides in the In Vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytother. Res. 2013, 27, 380–383. [Google Scholar] [CrossRef]
- Hajheydari, Z.; Jamshidi, M.; Akbari, J.; Mohammadpour, R. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: A double-blind randomized controlled study. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 29–32. [Google Scholar] [CrossRef]
- Abdelbary, A.; Salem, H.F.; Khallaf, R.A.; Ali, A.M. Mucoadhesive niosomal in situ gel for ocular tissue targeting: In Vitro and In Vivo evaluation of lomefloxacin hydrochloride. Pharm. Dev. Technol. 2017, 22, 409–417. [Google Scholar] [CrossRef]
- Glover, R.E.; Smith, R.R.; Jones, M.V.; Simon, K.; Jackson, S.K.; Rowlands, C.C. An EPR investigation of surfactant action on bacterial membranes. FEMS Microbiol. Lett. 1999, 177, 57–62. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, Y.; Zhang, H.; Liu, P.; Yao, J.; Yao, P.; Chen, J.; Duan, J. Development of essential oils as skin permeation enhancers: Penetration enhancement effect and mechanism of action. Pharm. Biol. 2017, 55, 1592–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Independent Variables | Dependent Variables | Constraints | ||
|---|---|---|---|---|
| −1 | +1 | |||
| GO % (A) | 5 | 18 | Droplet Size(Y1) (nm) | Minimum |
| Smix % (B) | 20 | 50 | MX Jss (Y2) (μg/cm2 h) | Maximum |
| APCV % (C) | 32 | 75 | MIC (Y3) (μg/mL) | Minimum |
| RHLB | Tween 20 Ratio | Span 20 Ratio | Droplet Size of Formed GO-APCV Emulsion |
|---|---|---|---|
| 12 | 0.57 | 0.43 | 550 ± 40 nm |
| 12.5 | 0.52 | 0.48 | 435 ± 34 nm |
| 13 | 0.45 | 0.55 | 280 ± 28 nm |
| 13.5 | 0.39 | 0.61 | 210 ± 20 nm |
| 14 | 0.33 | 0.67 | 295 ± 27 nm |
| 14.5 | 0.27 | 0.73 | 360 ± 36 nm |
| 15 | 0.20 | 0.80 | 410 ± 33 nm |
| 15.5 | 0.14 | 0.86 | 470 ± 41 nm |
| 16 | 0.085 | 0.915 | 580 ± 49 nm |
| Component 1 | Component 2 | Component 3 | Y1 | Y2 | Y3 | ||
|---|---|---|---|---|---|---|---|
| Run | A: %GO | B: %Smix | C: %APCV | Globule Size | MX Jss | MIC | PDI |
| (nm) | (μg/cm2 h) | (μg/mL) | |||||
| 1 | 0.0756 | 0.2392 | 0.685 | 270 | 1.5 | 0.854 | 0.12 |
| 2 | 0.05 | 0.4436 | 0.5063 | 104 | 2.9 | 1.124 | 0.17 |
| 3 | 0.18 | 0.3603 | 0.4596 | 120 | 2.4 | 0.203 | 0.23 |
| 4 | 0.18 | 0.4305 | 0.3894 | 154 | 2.8 | 0.266 | 0.3 |
| 5 | 0.18 | 0.3078 | 0.5121 | 253 | 2.1 | 0.261 | 0.19 |
| 6 | 0.1369 | 0.2 | 0.663 | 300 | 1.1 | 0.318 | 0.22 |
| 7 | 0.05 | 0.2 | 0.75 | 147 | 1.3 | 1.075 | 0.25 |
| 8 | 0.05 | 0.3637 | 0.5862 | 115 | 2.4 | 1.101 | 0.18 |
| 9 | 0.1173 | 0.4291 | 0.4535 | 169 | 2.8 | 0.552 | 0.09 |
| 10 | 0.18 | 0.5 | 0.32 | 114 | 3 | 0.282 | 0.31 |
| 11 | 0.0925 | 0.5 | 0.4074 | 105 | 3.1 | 0.702 | 0.3 |
| 12 | 0.18 | 0.5 | 0.32 | 110 | 3 | 0.281 | 0.26 |
| 13 | 0.05 | 0.3028 | 0.6471 | 141 | 2 | 1.083 | 0.11 |
| 14 | 0.0925 | 0.5 | 0.4074 | 106 | 3.1 | 0.702 | 0.13 |
| 15 | 0.18 | 0.2537 | 0.5662 | 280 | 1.7 | 0.206 | 0.29 |
| 16 | 0.05 | 0.2 | 0.75 | 146 | 1.3 | 1.074 | 0.21 |
| 17 | 0.115 | 0.35 | 0.535 | 216 | 2.3 | 0.563 | 0.25 |
| 18 | 0.1127 | 0.2936 | 0.5936 | 253 | 1.9 | 0.622 | 0.3 |
| Solution | GO % | Smix % | APCV % | Droplet Size (nm) | MX Jss (μg/cm2 h) | MIC (μg/mL) | Desirability |
|---|---|---|---|---|---|---|---|
| Predicated value | 0.180 | 0.500 | 0.320 | 112 | 3.001 | 0.281 | 0.941 |
| Experimental value | 0.180 | 0.500 | 0.320 | 110 | 3 | 0.275 | 0.941 |
| Permeation Parameters | Optimized Formulation | Optimized Formulation Prepared with Oleic Acid Instead of GO | Optimized Formulation Prepared with Distilled Water Instead of APCV | MX Aqueous Suspension |
|---|---|---|---|---|
| Cumulative amount permeated, Q, (μg/cm2) a | 2315 ± 243 | 1511 ± 112 | 1894 ± 192 | 711 ± 87 |
| Cumulative percentage permeated | 41.6% | 27.2% | 34.1% | 12.7% |
| Steady state flux, Jss, (μg/cm2·h) b | 3 ± 0.15 | 2.1 ± 0.11 | 2.4 ± 0.21 | 1.11 ± 0.12 |
| Permeability coefficient, P, (cm/h) c | 2.142 × 10−4 | 1.5 × 10−4 | 1.714 × 10−4 | 0.785 × 10−4 |
| Diffusion coefficient, D, (cm2/h) d | 8.11 × 10−5 | 5.93 × 10−5 | 6.71 × 10−5 | 5.66 × 10−5 |
| Enhancement factor (EF) e | 3.25 | 2.12 | 2.66 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizg, W.Y.; Hosny, K.M.; Elgebaly, S.S.; Alamoudi, A.J.; Felimban, R.I.; Tayeb, H.H.; Alharbi, M.; Bukhary, H.A.; Abualsunun, W.A.; Almehmady, A.M.; et al. Preparation and Optimization of Garlic Oil/Apple Cider Vinegar Nanoemulsion Loaded with Minoxidil to Treat Alopecia. Pharmaceutics 2021, 13, 2150. https://doi.org/10.3390/pharmaceutics13122150
Rizg WY, Hosny KM, Elgebaly SS, Alamoudi AJ, Felimban RI, Tayeb HH, Alharbi M, Bukhary HA, Abualsunun WA, Almehmady AM, et al. Preparation and Optimization of Garlic Oil/Apple Cider Vinegar Nanoemulsion Loaded with Minoxidil to Treat Alopecia. Pharmaceutics. 2021; 13(12):2150. https://doi.org/10.3390/pharmaceutics13122150
Chicago/Turabian StyleRizg, Waleed Y., Khaled M. Hosny, Samar S. Elgebaly, Abdulmohsin J. Alamoudi, Raed I. Felimban, Hossam H. Tayeb, Majed Alharbi, Haitham A. Bukhary, Walaa A. Abualsunun, Alshaimaa M. Almehmady, and et al. 2021. "Preparation and Optimization of Garlic Oil/Apple Cider Vinegar Nanoemulsion Loaded with Minoxidil to Treat Alopecia" Pharmaceutics 13, no. 12: 2150. https://doi.org/10.3390/pharmaceutics13122150
APA StyleRizg, W. Y., Hosny, K. M., Elgebaly, S. S., Alamoudi, A. J., Felimban, R. I., Tayeb, H. H., Alharbi, M., Bukhary, H. A., Abualsunun, W. A., Almehmady, A. M., & Khallaf, R. A. (2021). Preparation and Optimization of Garlic Oil/Apple Cider Vinegar Nanoemulsion Loaded with Minoxidil to Treat Alopecia. Pharmaceutics, 13(12), 2150. https://doi.org/10.3390/pharmaceutics13122150






