A Physiologically Based Pharmacokinetic Model for In Vivo Alpha Particle Generators Targeting Neuroendocrine Tumors in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. 212Pb-SSTA-PBPK Model Structure
2.2. Model Evaluation, Parameters Estimation and Sensitivity Analyses
2.3. Dosimetry and Simulations
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kunikowska, J.; Królicki, L. Targeted α-Emitter Therapy of Neuroendocrine Tumors. Semin. Nucl. Med. 2020, 50, 171–176. [Google Scholar] [CrossRef]
- Sgouros, G. Dosimetry, Radiobiology and Synthetic Lethality: Radiopharmaceutical Therapy (RPT) With Alpha-Particle-Emitters. Semin. Nucl. Med. 2020, 50, 124–132. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Stallons, T.A.R.; Saidi, A.; Tworowska, I.; Delpassand, E.S.; Torgue, J.J. Preclinical Investigation of 212Pb-DOTAMTATE for Peptide Receptor Radionuclide Therapy in a Neuroendocrine Tumor Model. Mol. Cancer Ther. 2019, 18, 1012–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, H.; Cederkrantz, E.; Bäck, T.; Divgi, C.; Elgqvist, J.; Himmelman, J.; Horvath, G.; Jacobsson, L.; Jensen, H.; Lindegren, S.; et al. Intraperitoneal α-Particle Radioimmunotherapy of Ovarian Cancer Patients: Pharmacokinetics and Dosimetry of 211At-MX35 F(ab′)2—A Phase I Study. J. Nucl. Med. 2009, 50, 1153–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and Imaging of 212Pb-TCMC-Trastuzumab after Intraperitoneal Administration in Ovarian Cancer Patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M. Dose Escalation and Dosimetry of First-in-Human α Radioimmunotherapy with 212Pb-TCMC-Trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgqvist, J.; Frost, S.; Pouget, J.-P.; Albertsson, P. The potential and hurdles of targeted alpha therapy—clinical trials and beyond. Front. Oncol. 2014, 3, 324. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Rousseau, J.; Jaraquemada-Peláez, M.d.G.; Wang, X.; Robertson, A.; Radchenko, V.; Schaffer, P.; Lin, K.-S.; Bénard, F.; Orvig, C. 225Ac-H4py4pa for Targeted Alpha Therapy. Bioconjug. Chem. 2021, 32, 1348–1363. [Google Scholar] [CrossRef] [PubMed]
- Mengshi, L.; Edwin, A.S.; Dongyoul, L.; Daniel, M.; Anthony, J.D.; Keith, R.O.; Stephen, G.; Roy, C.; Saed, M.; Brian, E.Z.; et al. 203/212Pb Theranostic Radiopharmaceuticals for Image-guided Radionuclide Therapy for Cancer. Curr. Med. Chem. 2020, 27, 7003–7031. [Google Scholar] [CrossRef]
- Brechbiel, M.W. Bifunctional chelates for metal nuclides. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 166–173. [Google Scholar] [PubMed]
- Thiele, N.A.; Wilson, J.J. Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm. 2018, 33, 336–348. [Google Scholar] [CrossRef]
- Begum, N.J.; Glatting, G.; Wester, H.-J.; Eiber, M.; Beer, A.J.; Kletting, P. The effect of ligand amount, affinity and internalization on PSMA-targeted imaging and therapy: A simulation study using a PBPK model. Sci. Rep. 2019, 9, 20041. [Google Scholar] [CrossRef]
- Begum, N.J.; Glatting, G.; Eiber, M.; Beer, A.J.; Kletting, P. An in silico study on the effect of the radionuclide half-life on PET/CT imaging with PSMA-targeting radioligands. Nuklearmedizin 2021, 60, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Franco, L.D.; Glatting, G.; Prasad, V.; Weber, W.A.; Beer, A.J.; Kletting, P. Effect of Tumor Perfusion and Receptor Density on Tumor Control Probability in 177Lu-DOTATATE Therapy: An In Silico Analysis for Standard and Optimized Treatment. J. Nucl. Med. 2021, 62, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kletting, P.; Kull, T.; Maaß, C.; Malik, N.; Luster, M.; Beer, A.J.; Glatting, G. Optimized peptide amount and activity for 90Y-Labeled DOTATATE therapy. J. Nucl. Med. 2016, 57, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Barrett, P.H.; Bell, B.M.; Cobelli, C.; Golde, H.; Schumitzky, A.; Vicini, P.; Foster, D.M. SAAM II: Simulation, Analysis, and Modeling Software for tracer and pharmacokinetic studies. Metabolism 1998, 47, 484–492. [Google Scholar] [CrossRef]
- Bates, C.M.; Kegg, H.; Grady, S. Expression of somatostatin receptors 1 and 2 in the adult mouse kidney. Regul. Pept. 2004, 119, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Storch, D.; Behe, M.; Walter, M.A.; Chen, J.H.; Powell, P.; Mikolajczak, R. Evaluation of [99mTc/EDDA/HYNIC0]octreotide derivatives compared with [111In-DOTA0,Tyr3,Thr8]octreotide and [111In-DTPA0]octreotide: Does tumor or pancreas uptake correlate with the rate of internalization? J. Nucl. Med. 2005, 46, 1561–1569. [Google Scholar]
- Hunyady, B.l.; Hipkin, R.W.; Schonbrunn, A.; Mezey, E.v. Immunohistochemical Localization of Somatostatin Receptor SST2A in the Rat Pancreas. Endocrinology 1997, 138, 2632–2635. [Google Scholar] [CrossRef]
- Borie, R.; Fabre, A.; Prost, F.; Marchal-Somme, J.; Lebtahi, R.; Marchand-Adam, S.; Aubier, M.; Soler, P.; Crestani, B. Activation of somatostatin receptors attenuates pulmonary fibrosis. Thorax 2008, 63, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schupp, G.; Daniel, H.; Eakins, G.W.; Jensen, E.N. Transition Intensities in the Tl208 Beta Decay, the Bi212 ->Po212 Decay Scheme, and the Bi212 Branching Ratio. Phys. Rev. 1960, 120, 189–198. [Google Scholar] [CrossRef]
- Zaid, N.R.R.; Kletting, P.; Beer, A.J.; Rozgaja Stallons, T.A.; Torgue, J.J.; Glatting, G. Mathematical modeling of in vivo alpha particle generators and chelator stability. Cancer Biother. Radiopharm. 2021. [Google Scholar] [CrossRef]
- Davies, B.; Morris, T. Physiological Parameters in Laboratory Animals and Humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef]
- Riches, A.C.; Sharp, J.G.; Thomas, D.B.; Smith, S.V. Blood volume determination in the mouse. J. Physiol. 1973, 228, 279–284. [Google Scholar] [CrossRef]
- Brown, R.P.; Delp, M.D.; Lindstedt, S.L.; Rhomberg, L.R.; Beliles, R.P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 1997, 13, 407–484. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Wittrup, K.D. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol. Cancer Ther. 2009, 8, 2861–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabkin, R.; Dahl, D.C. Renal Uptake and Disposal of Proteins and Peptides. In Biological Barriers to Protein Delivery; Andus, K.L., Raub, T.L., Eds.; Pharmaceutical Biotechnology; Springer Science+Business Media, LLC: New York, NY, USA, 1993; Volume 4, pp. 299–338. [Google Scholar]
- Zaid, N.R.R.; Kletting, P.; Winter, G.; Beer, A.J.; Glatting, G. A Whole-Body Physiologically Based Pharmacokinetic Model for Alpha Particle Emitting Bismuth in Rats. Cancer Biother. Radiopharm. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.K.; Betts, A.M. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J. Pharmacokinet. Pharmacodyn. 2011, 39, 67–86. [Google Scholar] [CrossRef]
- Delaney, M.A.; Kowalewska, J.; Treuting, P.M. 16—Urinary System. In Comparative Anatomy and Histology, 2nd ed.; Treuting, P.M., Dintzis, S.M., Montine, K.S., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 275–301. [Google Scholar]
- Letts, R.F.R.; Zhai, X.-Y.; Bhikha, C.; Grann, B.L.; Blom, N.B.; Thomsen, J.S.; Rubin, D.M.; Christensen, E.I.; Andreasen, A. Nephron morphometry in mice and rats using tomographic microscopy. Am. J. Physiol. Renal Physiol. 2017, 312, F210–F229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrow, R.A.; Coupland, R.E. Blood flow to the adrenal gland of the rat: Its distribution between the cortex and the medulla before and after haemorrhage. J. Anat. 1987, 155, 51–61. [Google Scholar] [PubMed]
- Groothuis, D.R. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro-Oncology 2000, 2, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Hipkin, R.W.; Friedman, J.; Clark, R.B.; Eppler, C.M.; Schonbrunn, A. Agonist-induced desensitization, internalization, and phosphorylation of the sst2A somatostatin receptor. J. Biol. Chem. 1997, 272, 13869–13876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, J.A.; Kaur, R.; Dodgeon, I.; Edwardson, J.M.; Humphrey, P.P.A. Fates of endocytosed somatostatin sst2 receptors and associated agonists. Biochem. J. 1998, 336, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.S.; Konijnenberg, M.W.; Daniels, T.; Nysus, M.; Makvandi, M.; de Blois, E.; Breeman, W.A.; Atcher, R.W.; de Jong, M.; Norenberg, J.P. Improved safety and efficacy of 213Bi-DOTATATE-targeted alpha therapy of somatostatin receptor-expressing neuroendocrine tumors in mice pre-treated with L-lysine. EJNMMI Res. 2016, 6, 83. [Google Scholar] [CrossRef] [Green Version]
- Kimura, N.; Schindler, M.; Kasai, N.; Kimura, I. Immunohistochemical localization of somatostatin receptor type 2A in rat and human tissues. Endocr. J. 2001, 48, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Miederer, M.; Henriksen, G.; Alke, A.; Mossbrugger, I.; Quintanilla-Martinez, L.; Senekowitsch-Schmidtke, R.; Essler, M. Preclinical Evaluation of the α-Particle Generator Nuclide 225Ac for Somatostatin Receptor Radiotherapy of Neuroendocrine Tumors. Clin. Cancer Res. 2008, 14, 3555–3561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geenen, L.; Nonnekens, J.; Konijnenberg, M.; Baatout, S.; De Jong, M.; Aerts, A. Overcoming nephrotoxicity in peptide receptor radionuclide therapy using [177Lu]Lu-DOTA-TATE for the treatment of neuroendocrine tumours. Nucl. Med. Biol. 2021, 102, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maack, T.; Johnson, V.; Kau, S.T.; Figueiredo, J.; Sigulem, D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: A review. Kidney Int. 1979, 16, 251–270. [Google Scholar] [CrossRef] [Green Version]
- Vegt, E.; Eek, A.; Oyen, W.J.; de Jong, M.; Gotthardt, M.; Boerman, O.C. Albumin-derived peptides efficiently reduce renal uptake of radiolabelled peptides. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Barone, R.; Van Der Smissen, P.; Devuyst, O.; Beaujean, V.; Pauwels, S.; Courtoy, P.J.; Jamar, F. Endocytosis of the somatostatin analogue, octreotide, by the proximal tubule-derived opossum kidney (OK) cell line. Kidney Int. 2005, 67, 969–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behr, T.M.; Goldenberg, D.M.; Becker, W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: Present status, future prospects and limitations. Eur. J. Nucl. Med. 1998, 25, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Behr, T.M.; Sharkey, R.M.; Juweid, M.E.; Blumenthal, R.D.; Dunn, R.M.; Griffiths, G.L.; Bair, H.J.; Wolf, F.G.; Becker, W.S.; Goldenberg, D.M. Reduction of the renal uptake of radiolabeled monoclonal antibody fragments by cationic amino acids and their derivatives. Cancer Res. 1995, 55, 3825–3834. [Google Scholar] [PubMed]
- Kletting, P.; Schimmel, S.; Kestler, H.A.; Hänscheid, H.; Luster, M.; Fernández, M.; Bröer, J.H.; Nosske, D.; Lassmann, M.; Glatting, G. Molecular radiotherapy: The NUKFIT software for calculating the time-integrated activity coefficient. Med. Phys. 2013, 40, 102504. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G. MIRD Pamphlet No. 22 (Abridged): Radiobiology and Dosimetry of a-Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konijnenberg, M.W.; Bijster, M.; Krenning, E.P.; De Jong, M. A stylized computational model of the rat for organ dosimetry in support of preclinical evaluations of peptide receptor radionuclide therapy with 90Y, 111In, or 177Lu. J. Nucl. Med. 2004, 45, 1260–1269. [Google Scholar] [PubMed]
- Banerjee, S.R.; Lisok, A.; Minn, I.; Josefsson, A.; Kumar, V.; Brummet, M.; Boinapally, S.; Brayton, C.; Mease, R.C.; Sgouros, G.; et al. Preclinical Evaluation of 213Bi- and 225Ac-Labeled Low-Molecular-Weight Compounds for Radiopharmaceutical Therapy of Prostate Cancer. J. Nucl. Med. 2021, 62, 980–988. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Minn, I.; Kumar, V.; Josefsson, A.; Lisok, A.; Brummet, M.; Chen, J.; Kiess, A.P.; Baidoo, K.; Brayton, C.; et al. Preclinical Evaluation of 203/212Pb-Labeled Low-Molecular-Weight Compounds for Targeted Radiopharmaceutical Therapy of Prostate Cancer. J. Nucl. Med. 2020, 61, 80–88. [Google Scholar] [CrossRef]
- Norenberg, J.P.; Krenning, B.J.; Konings, I.R.H.M.; Kusewitt, D.F.; Nayak, T.K.; Anderson, T.L.; de Jong, M.; Garmestani, K.; Brechbiel, M.W.; Kvols, L.K. 213Bi-[DOTA0,Tyr3]Octreotide Peptide Receptor Radionuclide Therapy of Pancreatic Tumors in a Preclinical Animal Model. Clin. Cancer Res. 2006, 12, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Steiniger, B.S. Human spleen microanatomy: Why mice do not suffice. Immunology 2015, 145, 334–346. [Google Scholar] [CrossRef]
- Elliott, D.E.; Li, J.; Blum, A.M.; Metwali, A.; Patel, Y.C.; Weinstock, J.V. SSTR2A is the dominant somatostatin receptor subtype expressed by inflammatory cells, is widely expressed and directly regulates T cell IFN-gamma release. Eur. J. Immunol. 1999, 29, 2454–2463. [Google Scholar] [CrossRef]
- Vegt, E.; Melis, M.; Eek, A.; de Visser, M.; Brom, M.; Oyen, W.J.; Gotthardt, M.; de Jong, M.; Boerman, O.C. Renal uptake of different radiolabelled peptides is mediated by megalin: SPECT and biodistribution studies in megalin-deficient mice. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 623–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vegt, E.; de Jong, M.; Wetzels, J.F.M.; Masereeuw, R.; Melis, M.; Oyen, W.J.G.; Gotthardt, M.; Boerman, O.C. Renal Toxicity of Radiolabeled Peptides and Antibody Fragments: Mechanisms, Impact on Radionuclide Therapy, and Strategies for Prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Caruano, A.L.; Lewis, M.R.; Meyer, L.A.; VanderWaal, R.P.; Anderson, C.J. Subcellular localization of radiolabeled somatostatin analogues: Implications for targeted radiotherapy of cancer. Cancer Res. 2003, 63, 6864–6869. [Google Scholar] [PubMed]
- Waser, B.; Tamma, M.L.; Cescato, R.; Maecke, H.R.; Reubi, J.C. Highly efficient in vivo agonist-induced internalization of sst2 receptors in somatostatin target tissues. J. Nucl. Med. 2009, 50, 936–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
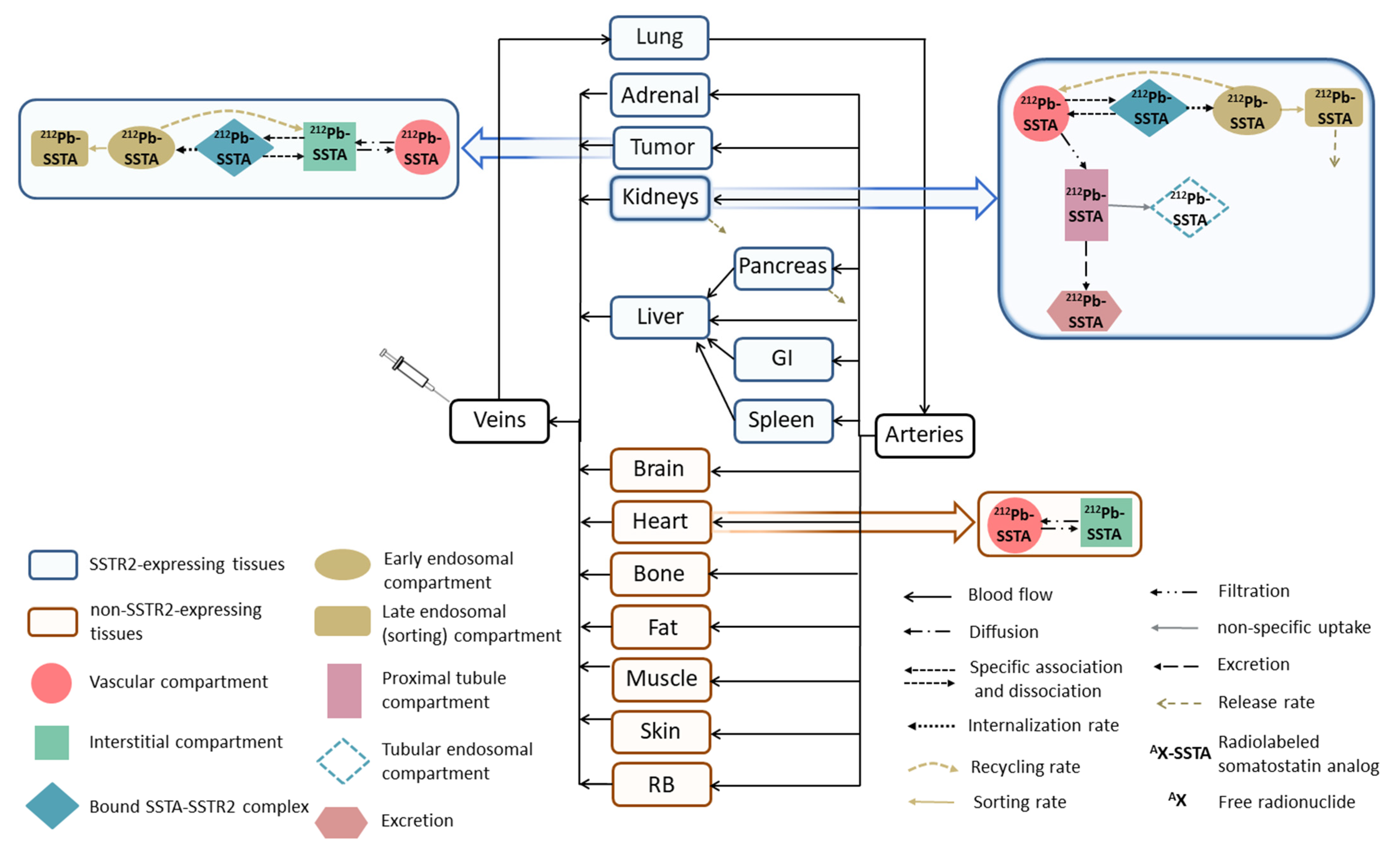
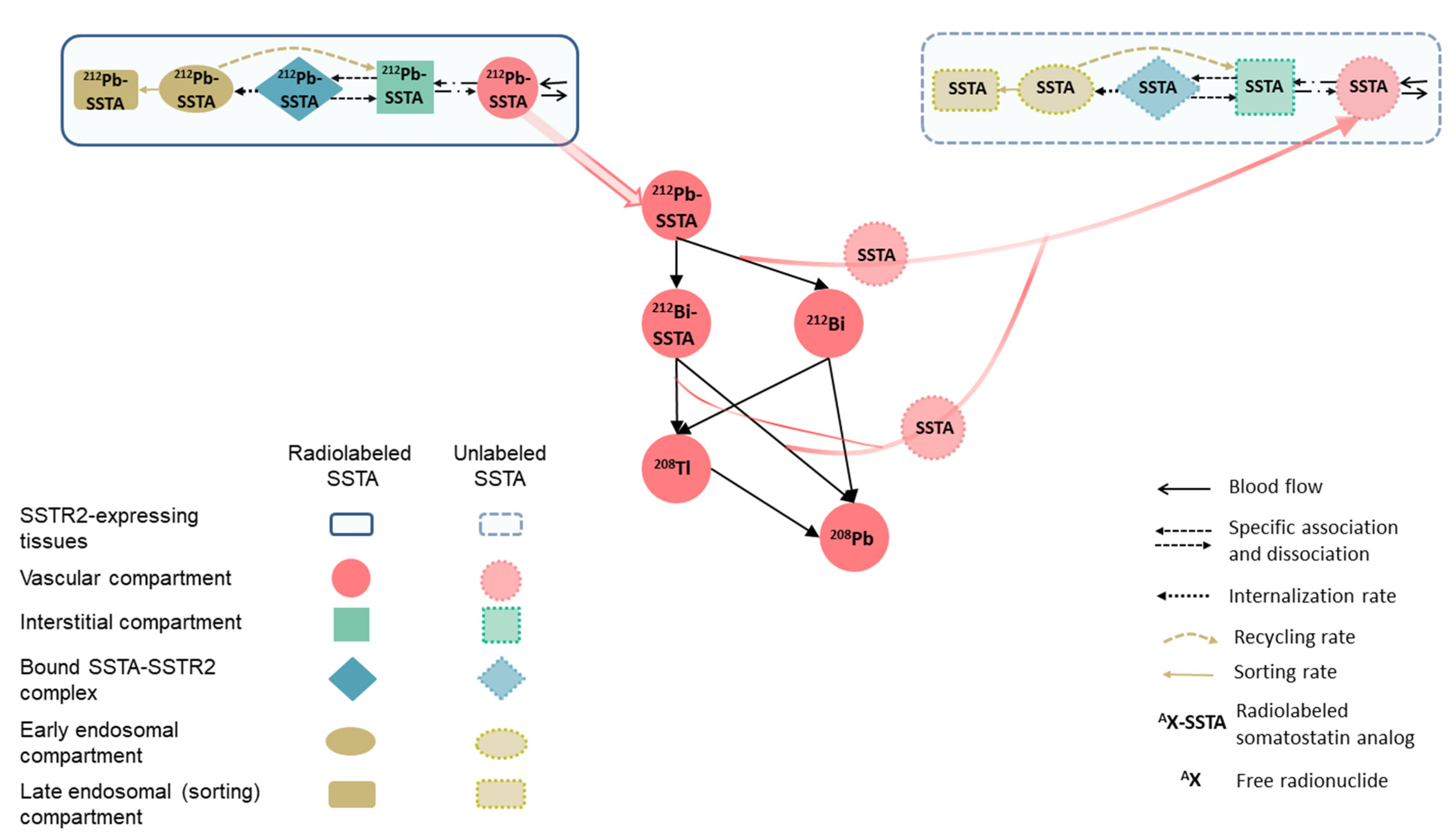
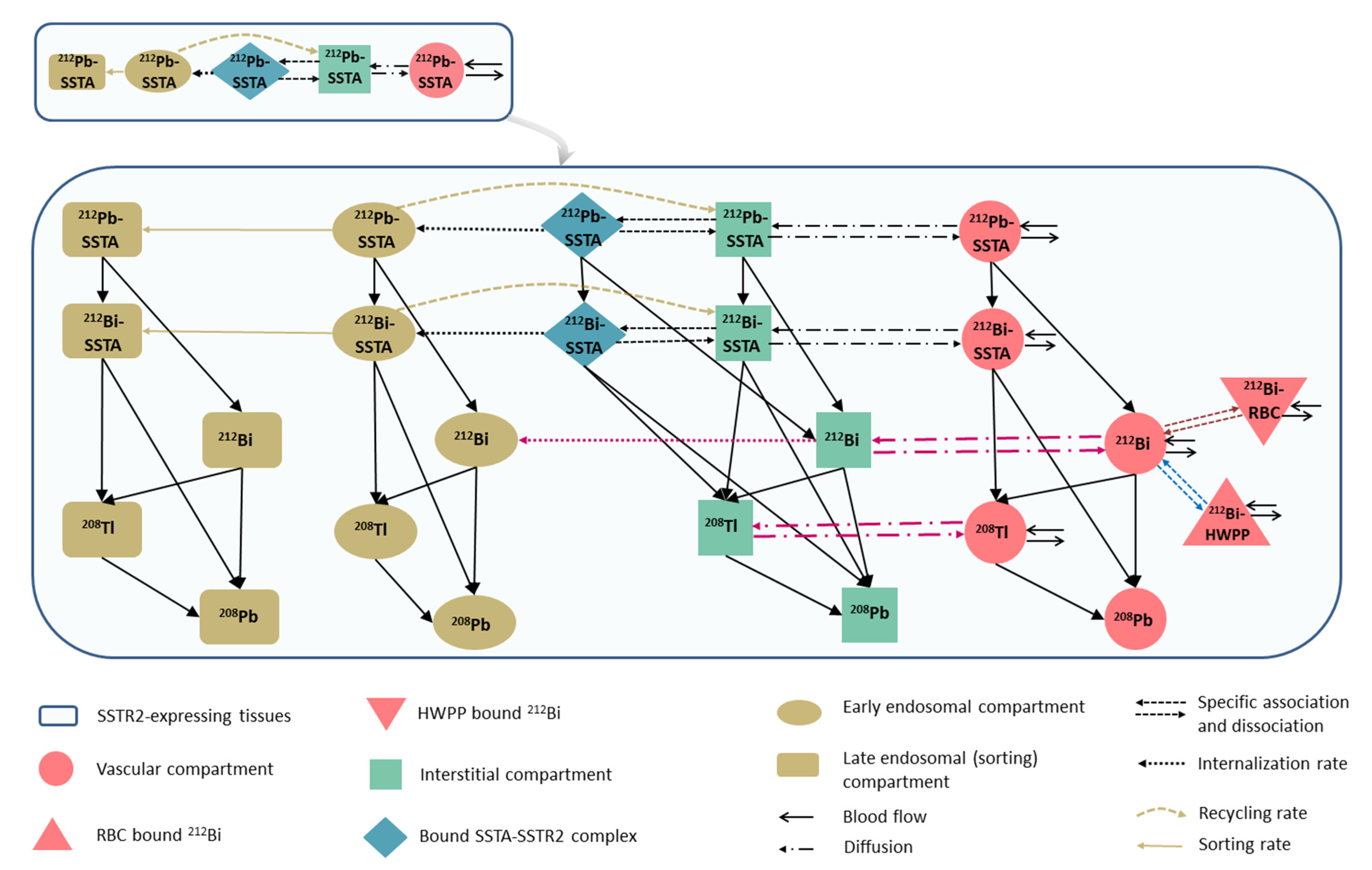
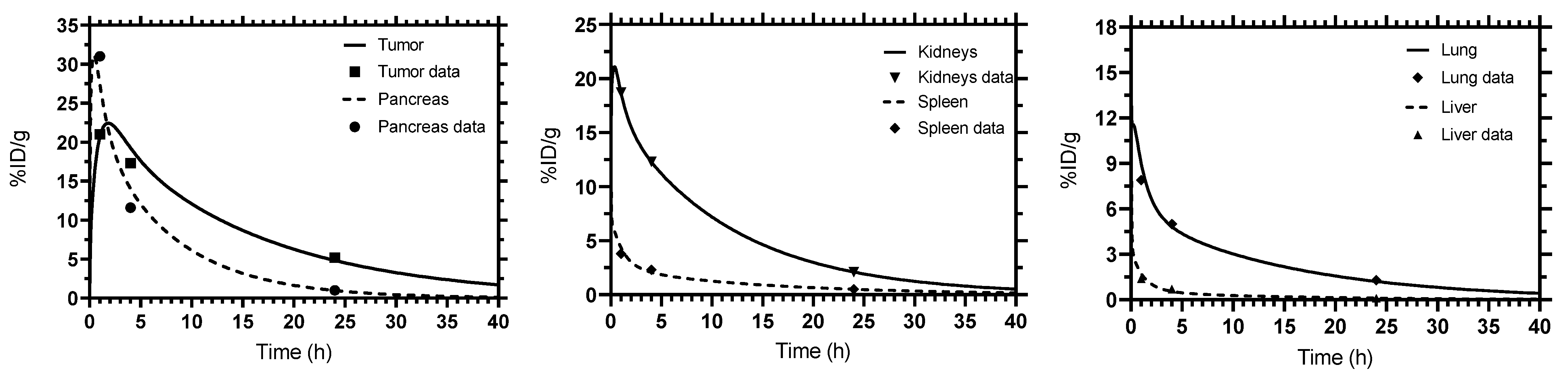
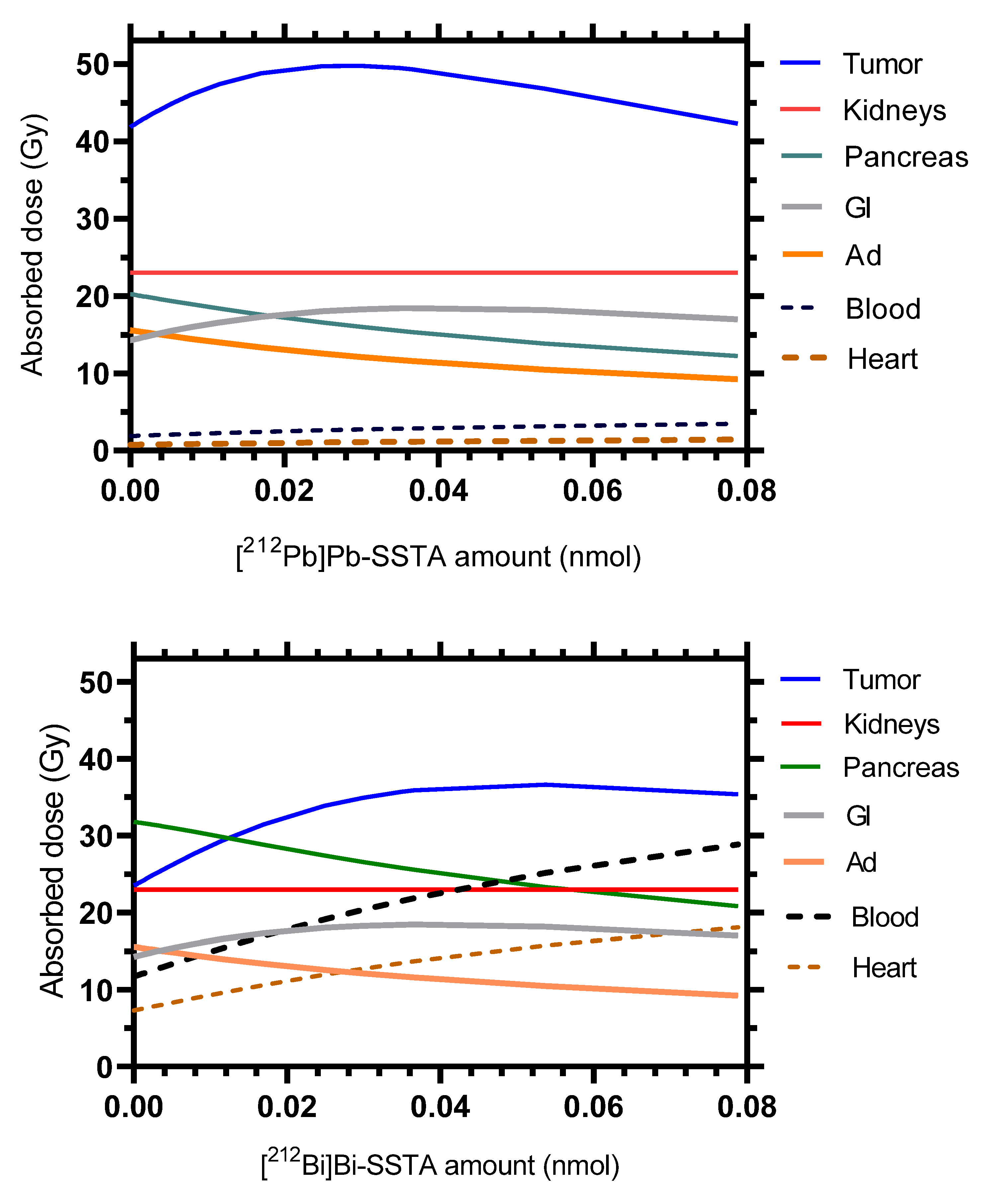
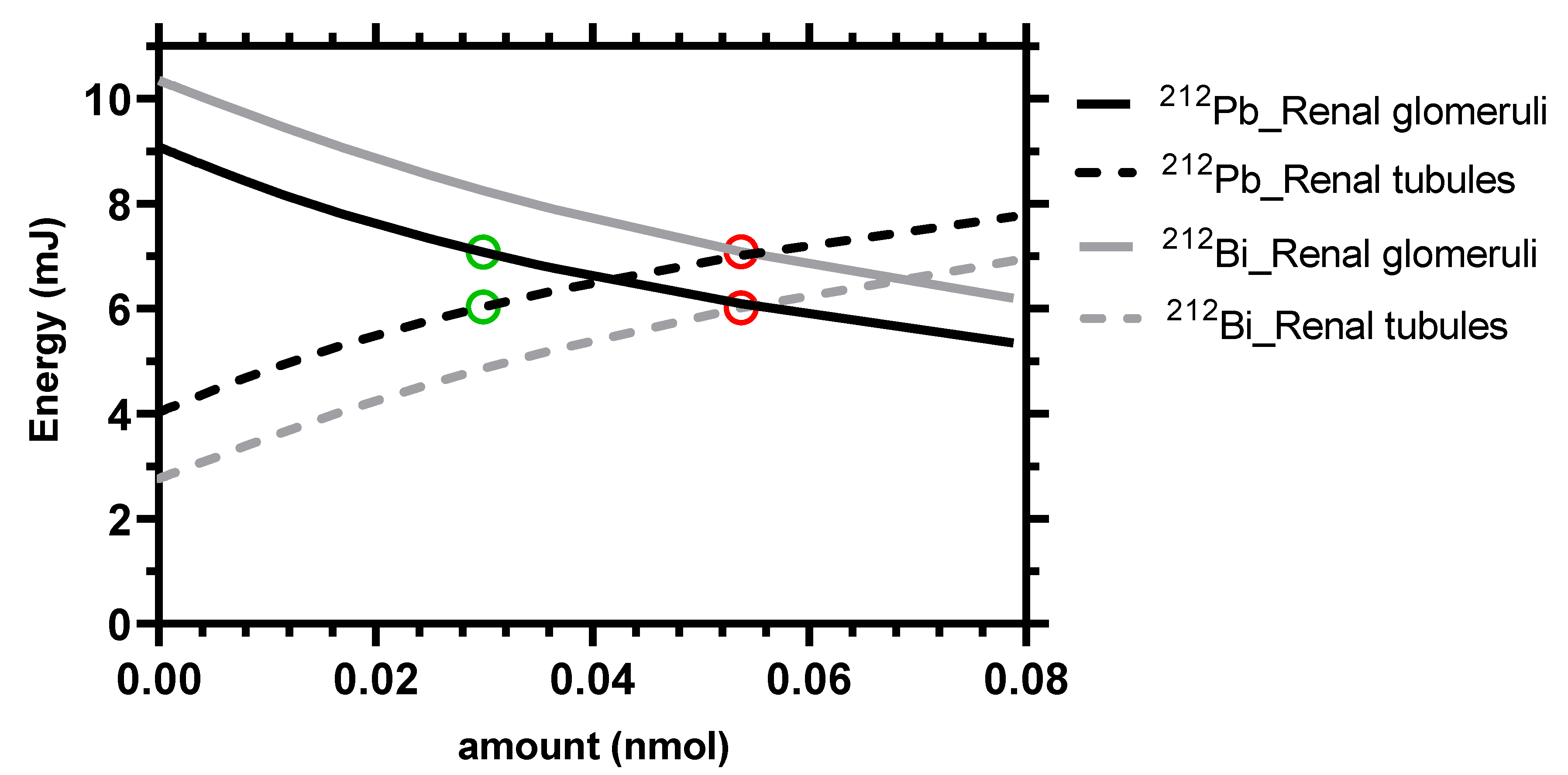
| Parameter (Unit) | Tissue | SAAM II | Simbiology |
|---|---|---|---|
| Estimated Value | |||
| SSTR2 density (nmol·L−1) | Kidneys | 3.52 ± 0.07 | 3.52 |
| Liver | 0.17 ± 0.04 | 0.16 | |
| Spleen | 0.73 ± 0.07 | 0.73 | |
| Lung | 1.77 ± 0.14 | 1.77 | |
| Pancreas | 6.18 ± 0.94 | 6.18 | |
| Tumor | 11.73 ± 2.97 | 11.73 | |
| Perfusion (mL·min−1·g−1) | Tumor | 0.09 ± 0.03 | 0.09 |
| Sorting rate (min−1) | SSTR2-expressing tissues | 0.0076 ± 0.0003 | 0.0076 |
| Release rate (min−1) | Pancreas | 0.0011 ± 0.0002 | 0.0011 |
| Kidneys | 0.000380 ± 0.000001 | 0.000380 | |
| Tissue | ADC (Gy/MBq) | Contribution to the Total Absorbed Dose per Tissue (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conjugated Radionuclides | Free Radionuclides | |||||||||
| 212Pb | 212Bi | 212Bi | 212Po | 212Bi | 208Tl | 212Bi | 212Po | |||
| Beta | Alpha | Beta | Alpha | |||||||
| SSTR2-expressing tissues | Tumor | 248 | 5 | 11 | 18 | 45 | 2 | 6 | 3 | 8 |
| Kidneys | 134 | 6 | 11 | 18 | 45 | 2 | 6 | 3 | 8 | |
| Pancreas | 116 | 6 | 11 | 17 | 44 | 2 | 6 | 4 | 9 | |
| Liver | 9 | 5 | 9 | 14 | 35 | 5 | 6 | 7 | 19 | |
| Lung | 65 | 6 | 11 | 18 | 45 | 2 | 6 | 3 | 8 | |
| Spleen | 28 | 6 | 11 | 17 | 44 | 2 | 6 | 4 | 10 | |
| Ad | 89 | 6 | 11 | 18 | 45 | 2 | 6 | 3 | 9 | |
| GI | 85 | 5 | 11 | 18 | 45 | 2 | 6 | 3 | 9 | |
| Non-SSTR2-expressing tissues | Skin | 1.8 | 9 | 11 | 16 | 42 | 2 | 6 | 4 | 9 |
| Bone | 2.7 | 3 | 3 | 5 | 14 | 10 | 7 | 16 | 41 | |
| Muscle | 0.6 | 11 | 11 | 18 | 46 | 1 | 7 | 2 | 4 | |
| Heart | 3.6 | 2 | 14 | 23 | 58 | 0 | 1 | 0 | 1 | |
| Brain | 0.1 | 12 | 11 | 17 | 43 | 2 | 6 | 3 | 7 | |
| Fat | 1.0 | 9 | 9 | 14 | 36 | 1 | 28 | 1 | 3 | |
| RB | 1.0 | 11 | 11 | 18 | 45 | 1 | 6 | 2 | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaid, N.R.R.; Kletting, P.; Winter, G.; Prasad, V.; Beer, A.J.; Glatting, G. A Physiologically Based Pharmacokinetic Model for In Vivo Alpha Particle Generators Targeting Neuroendocrine Tumors in Mice. Pharmaceutics 2021, 13, 2132. https://doi.org/10.3390/pharmaceutics13122132
Zaid NRR, Kletting P, Winter G, Prasad V, Beer AJ, Glatting G. A Physiologically Based Pharmacokinetic Model for In Vivo Alpha Particle Generators Targeting Neuroendocrine Tumors in Mice. Pharmaceutics. 2021; 13(12):2132. https://doi.org/10.3390/pharmaceutics13122132
Chicago/Turabian StyleZaid, Nouran R. R., Peter Kletting, Gordon Winter, Vikas Prasad, Ambros J. Beer, and Gerhard Glatting. 2021. "A Physiologically Based Pharmacokinetic Model for In Vivo Alpha Particle Generators Targeting Neuroendocrine Tumors in Mice" Pharmaceutics 13, no. 12: 2132. https://doi.org/10.3390/pharmaceutics13122132
APA StyleZaid, N. R. R., Kletting, P., Winter, G., Prasad, V., Beer, A. J., & Glatting, G. (2021). A Physiologically Based Pharmacokinetic Model for In Vivo Alpha Particle Generators Targeting Neuroendocrine Tumors in Mice. Pharmaceutics, 13(12), 2132. https://doi.org/10.3390/pharmaceutics13122132







