A 3D-Printed Polymer–Lipid-Hybrid Tablet towards the Development of Bespoke SMEDDS Formulations
Abstract
:1. Introduction
2. Materials and Methods
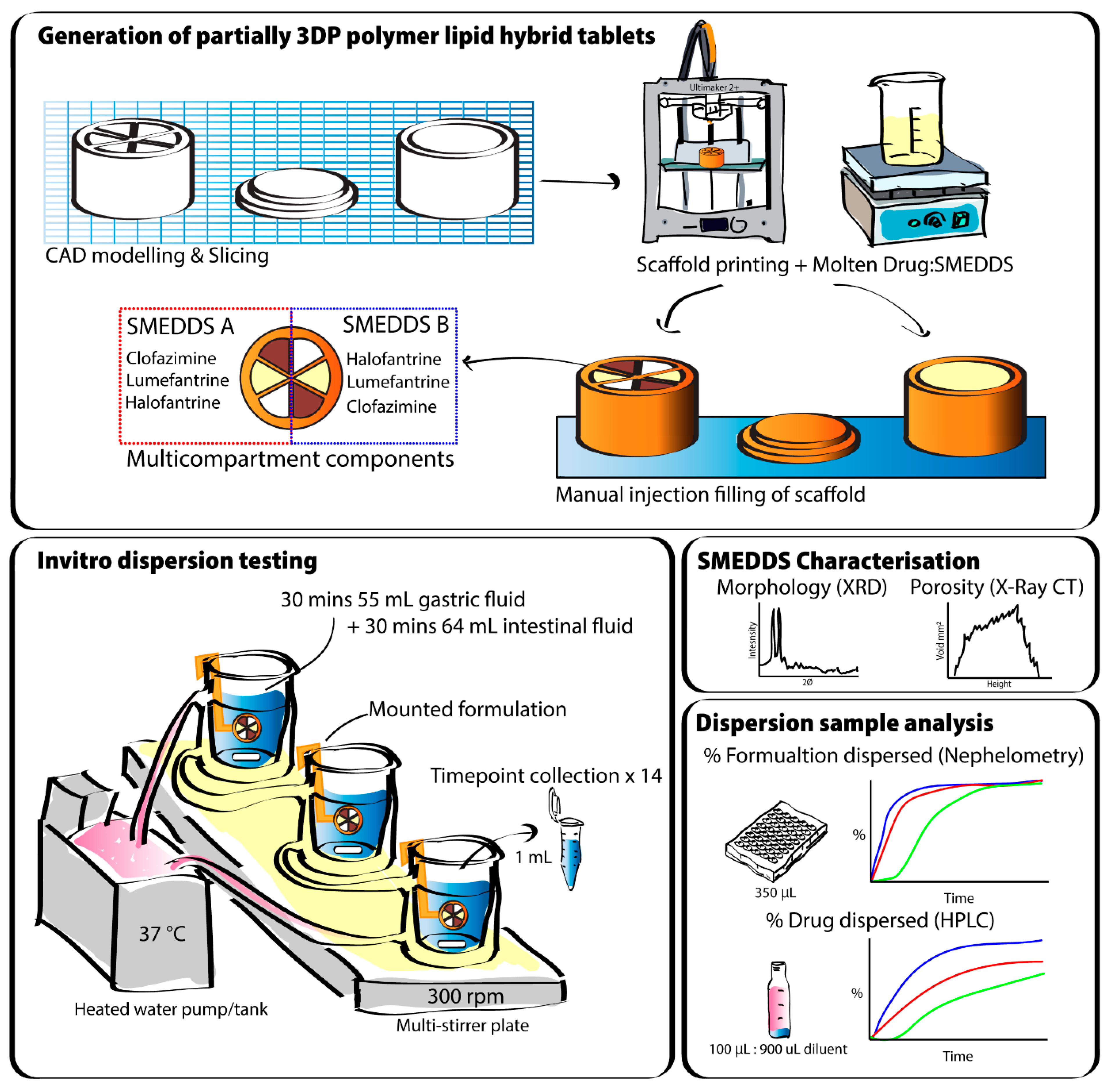
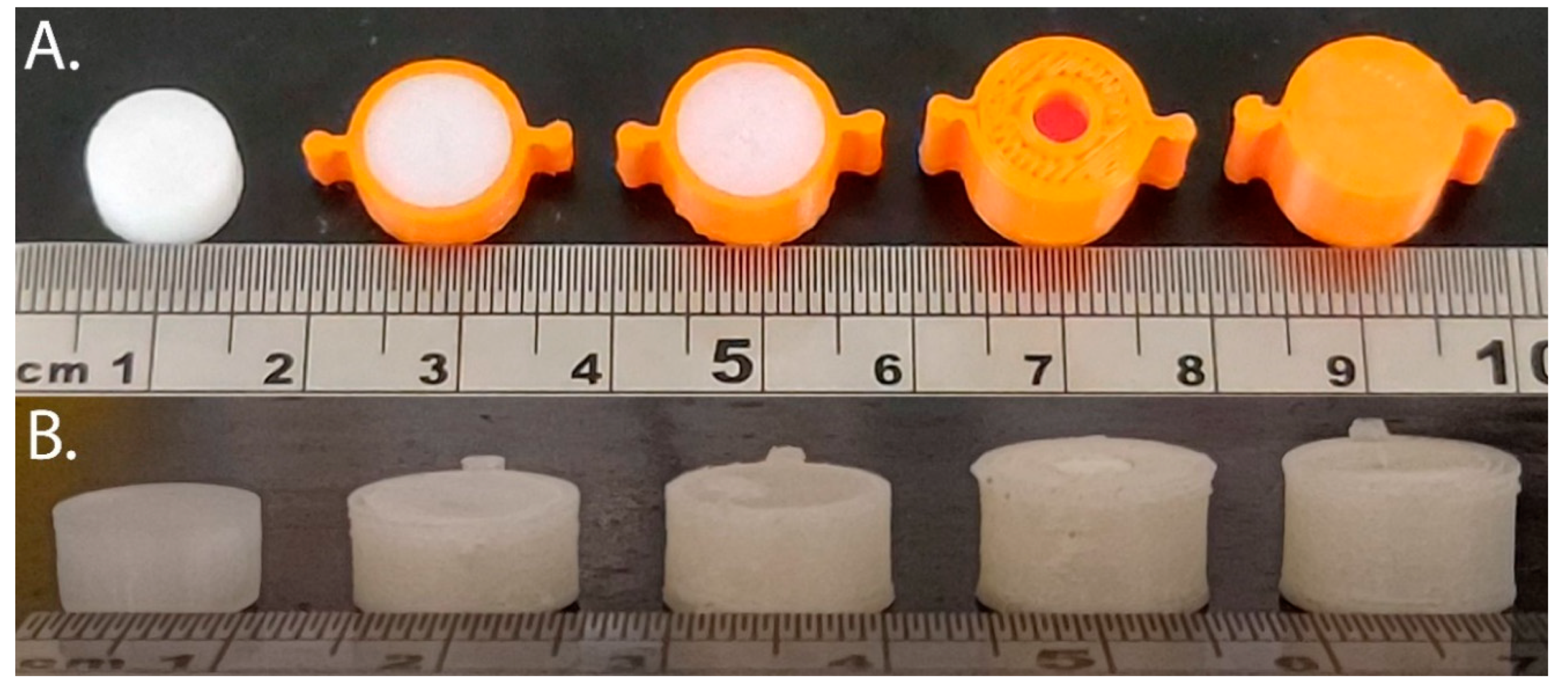
2.1. 3D Printing PLA and PVOH Scaffolds
2.2. Preparing Lipid Filled 3DP Scaffolds
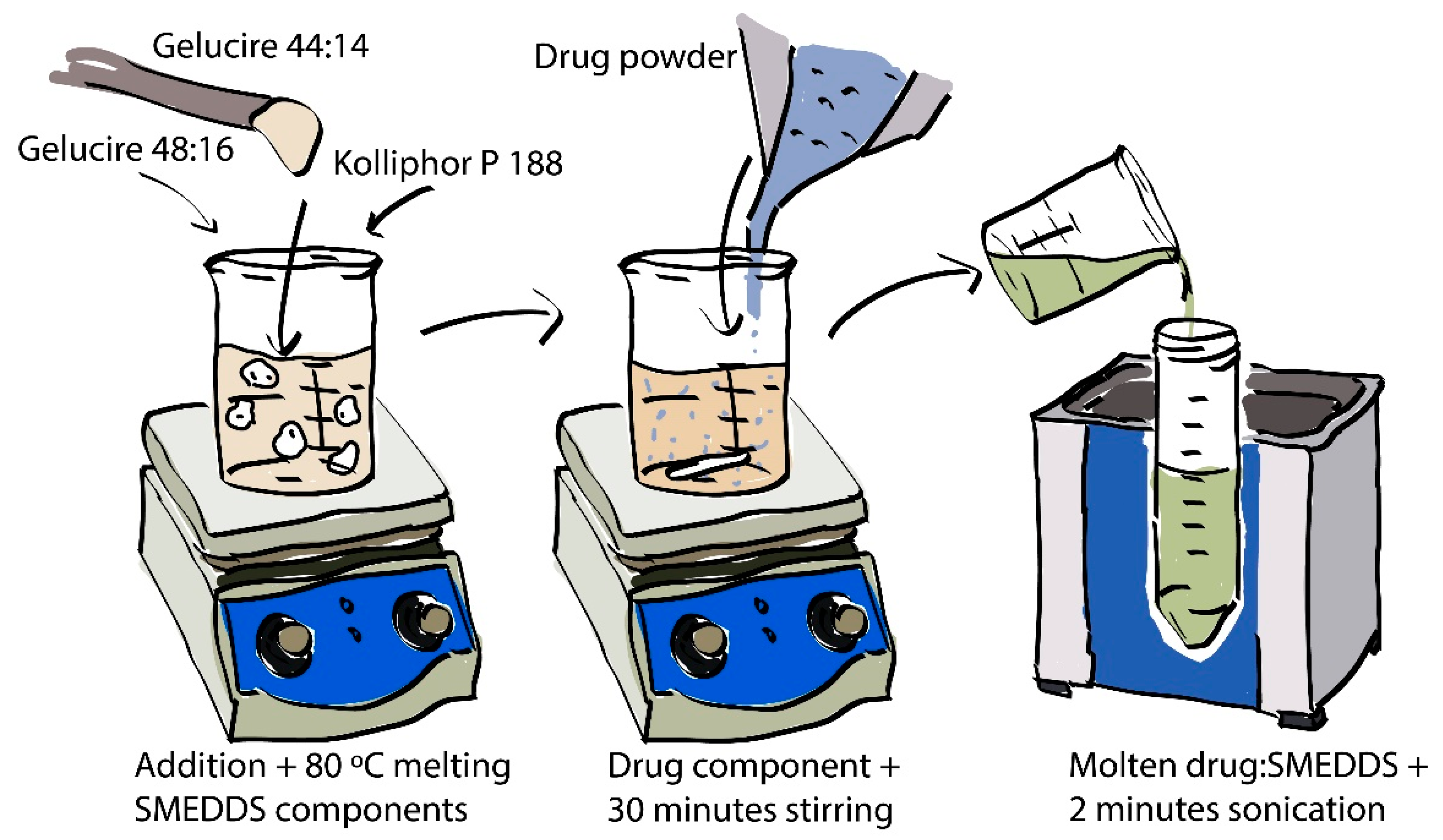
2.2.1. Preparation of Drug-Loaded SMEDDS
2.2.2. Filling 3DP Scaffolds with Molten Lipid Formulation
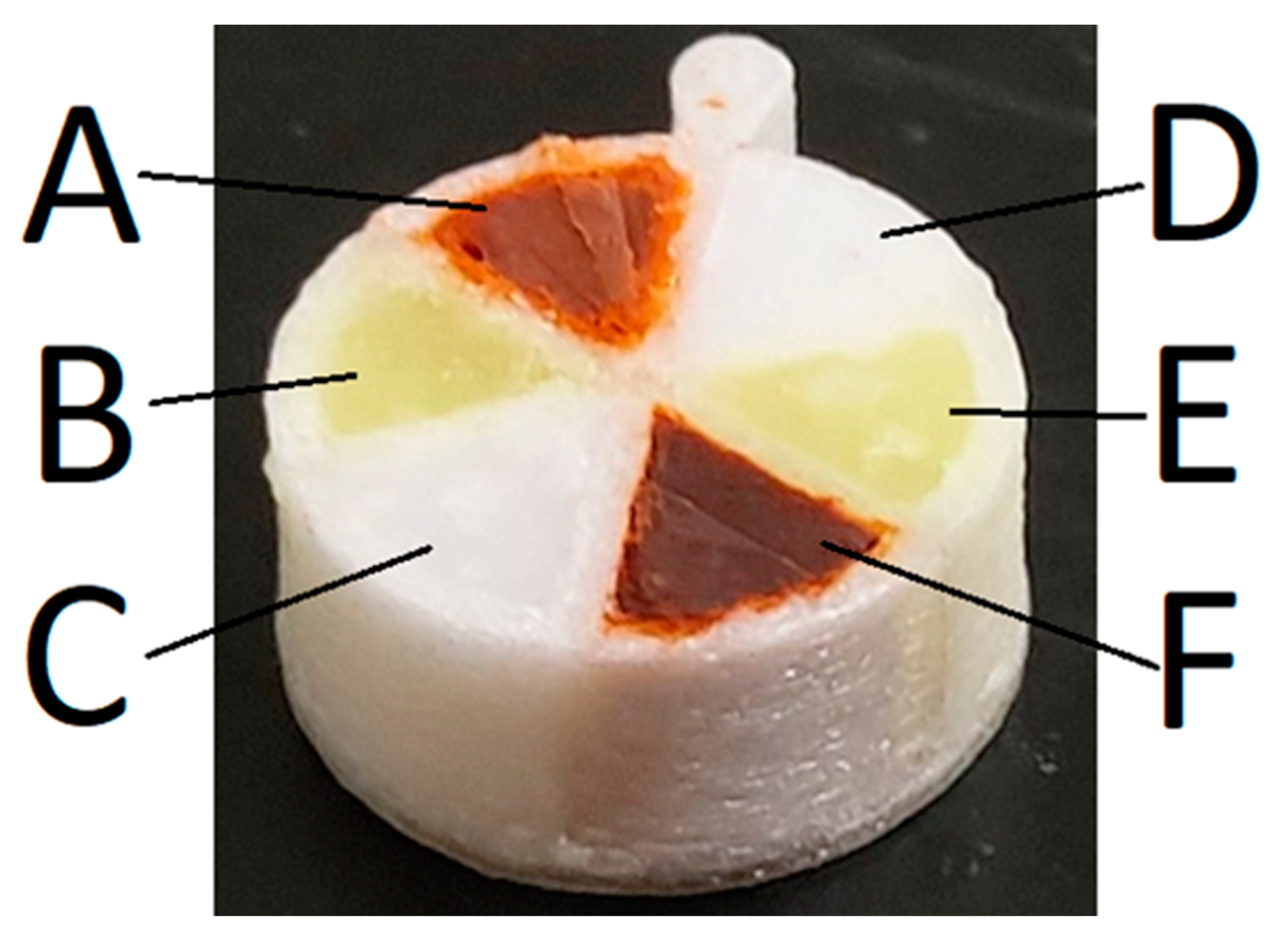
2.2.3. Filling Multicompartment Scaffolds with Molten Lipid Formulation
2.2.4. Preparation of Mixed Drug and Formulation Pellets
2.3. Dispersion Studies in Simulated Gastric to Intestinal Media
2.4. Dispersion Kinetics of SMEDDS Studied Using Nephelometry
2.5. Quantification of Drug in Dispersion Media—UPLC
2.6. Solid State Characterisation - X-ray Diffractometer (XRD)
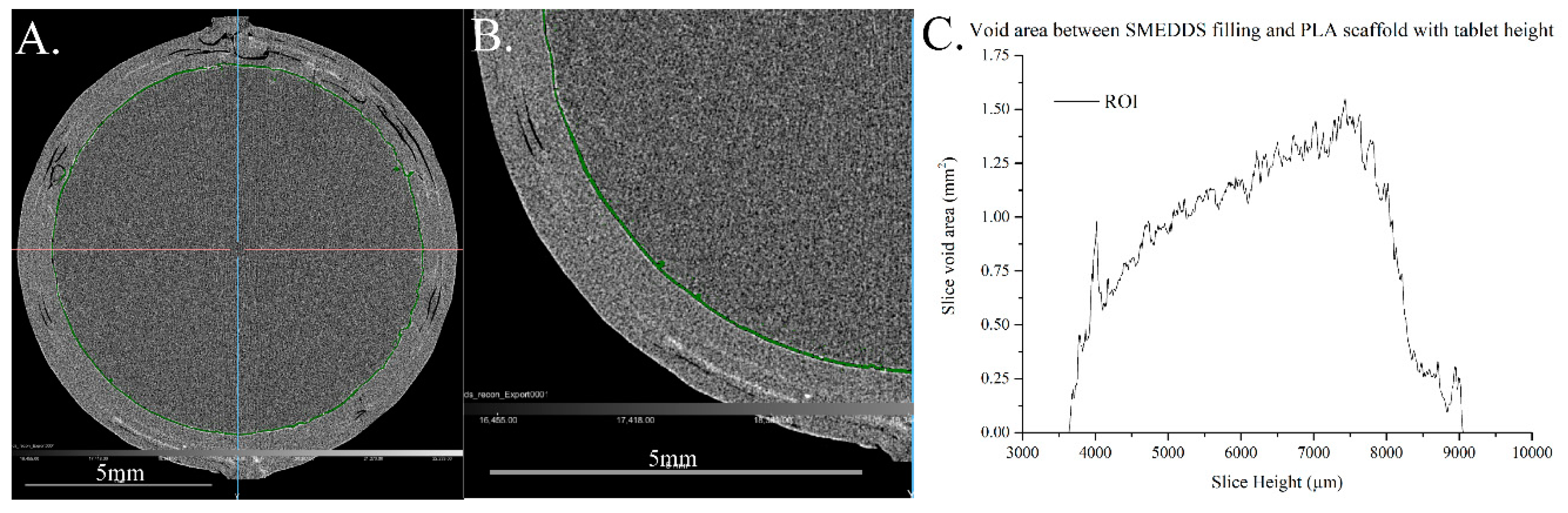
2.7. X-ray CT Imaging Analysis
2.8. Cryo-TEM Imaging Analysis
3. Results
3.1. Characterisation of SMEDDS
3.2. Characterisation of Filling of the Single Compartment Scaffolds Using X-ray CT
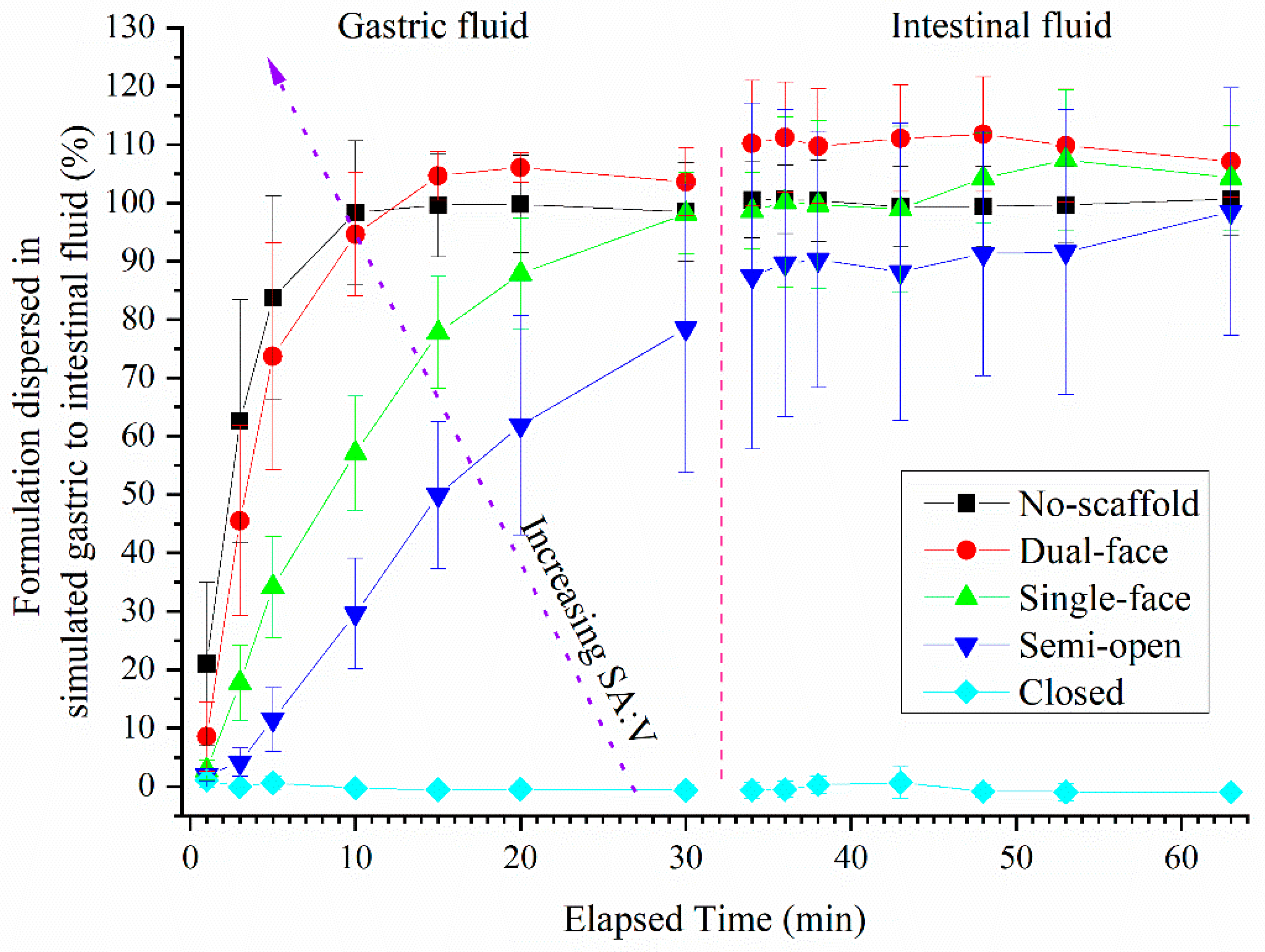
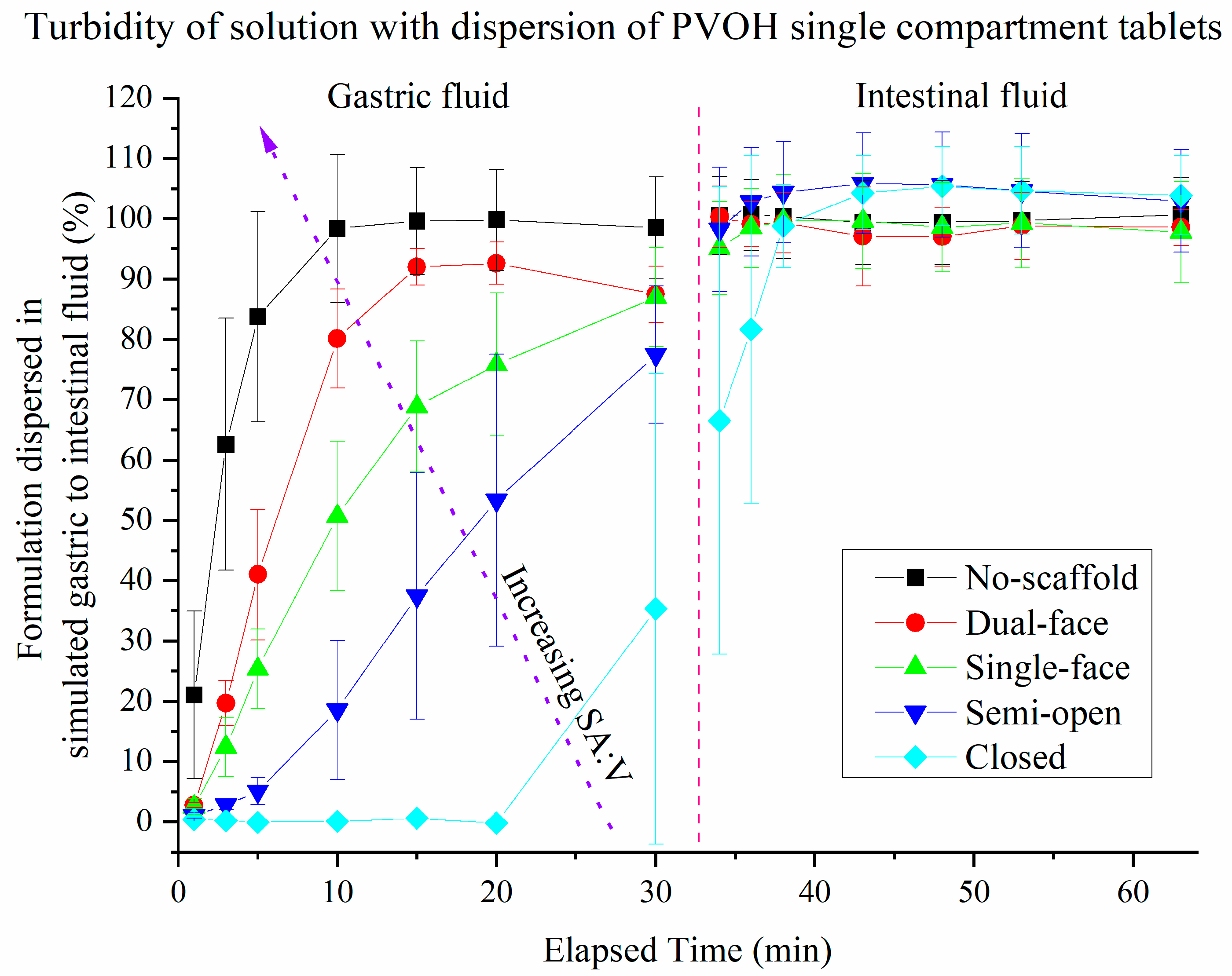
3.3. Dispersion of Drug-Loaded SMEDDS Formulations from Single Compartment Scaffolds Using Turbidity
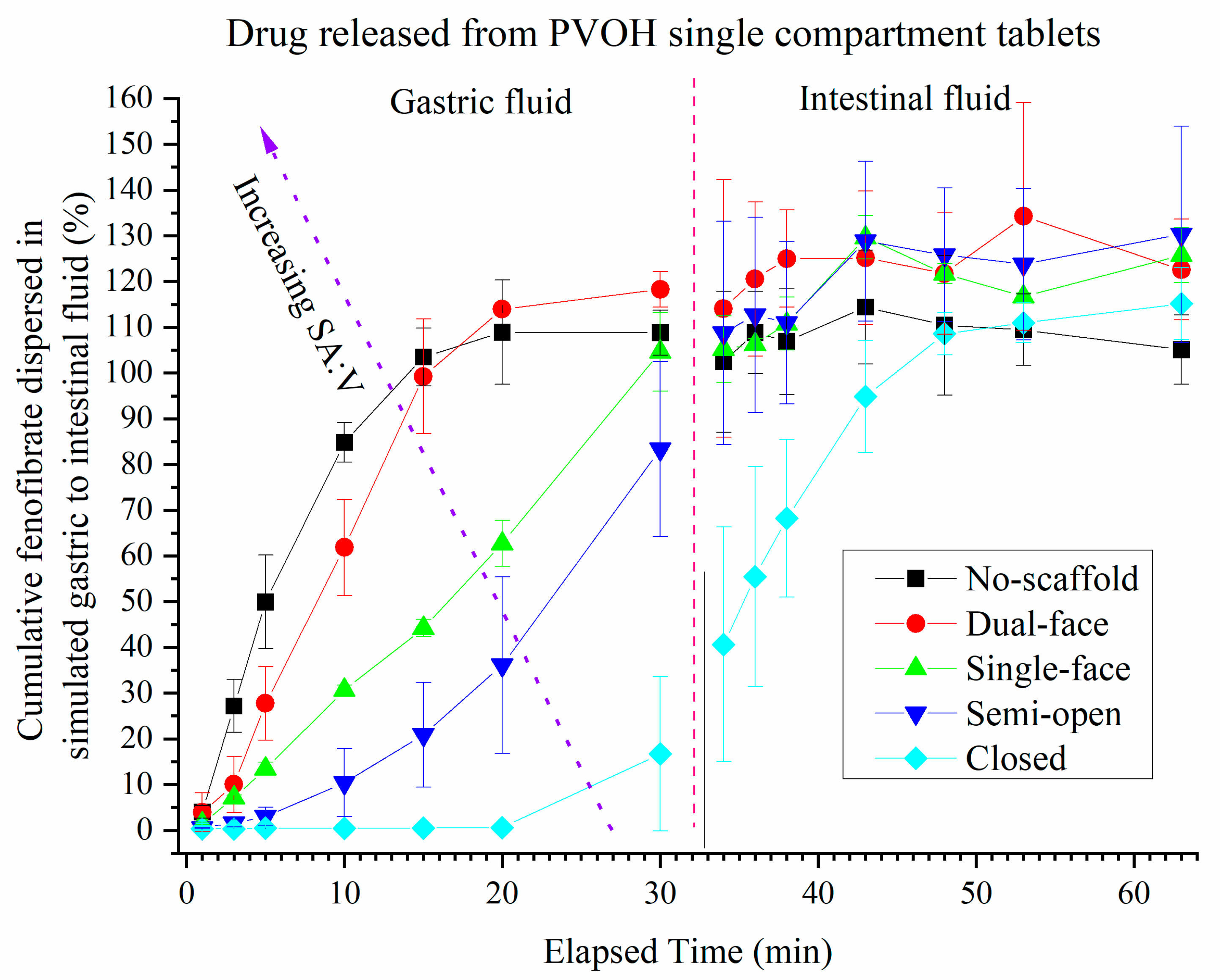
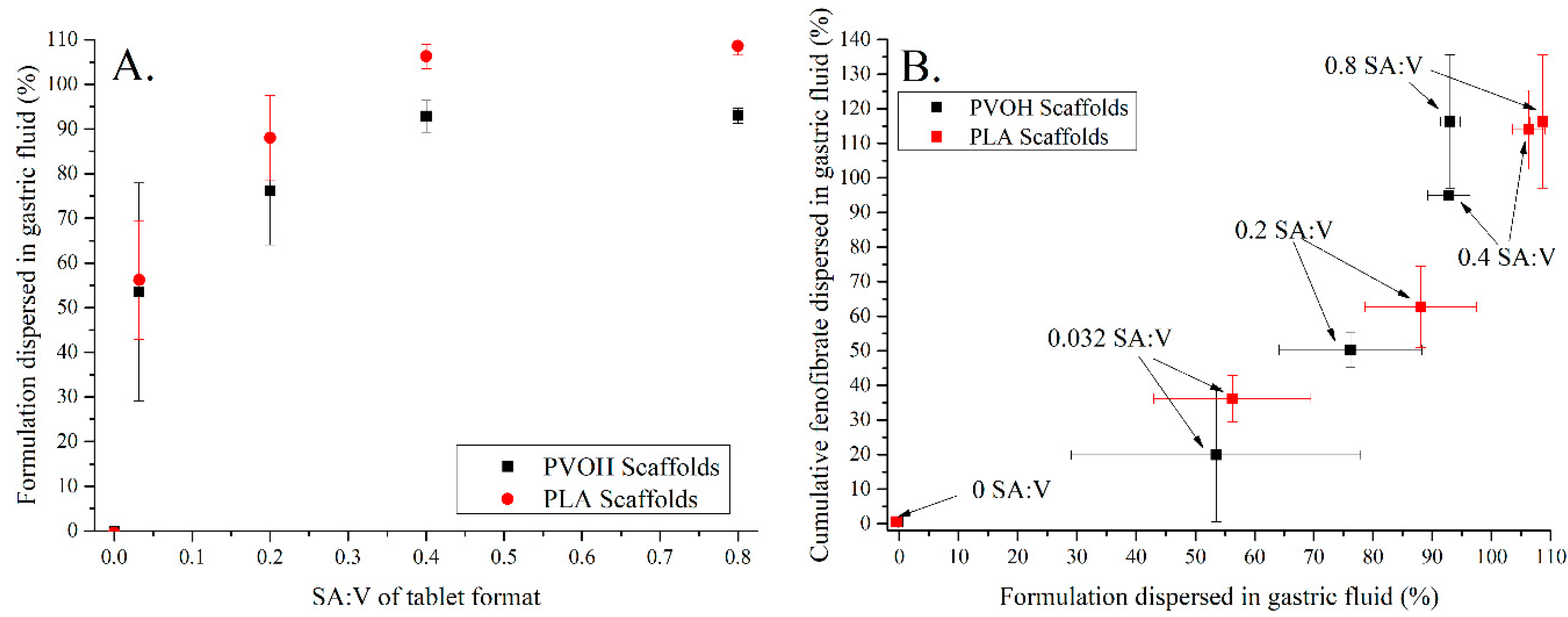
3.4. Release of Drug (FEN) from Single Compartment Tablets
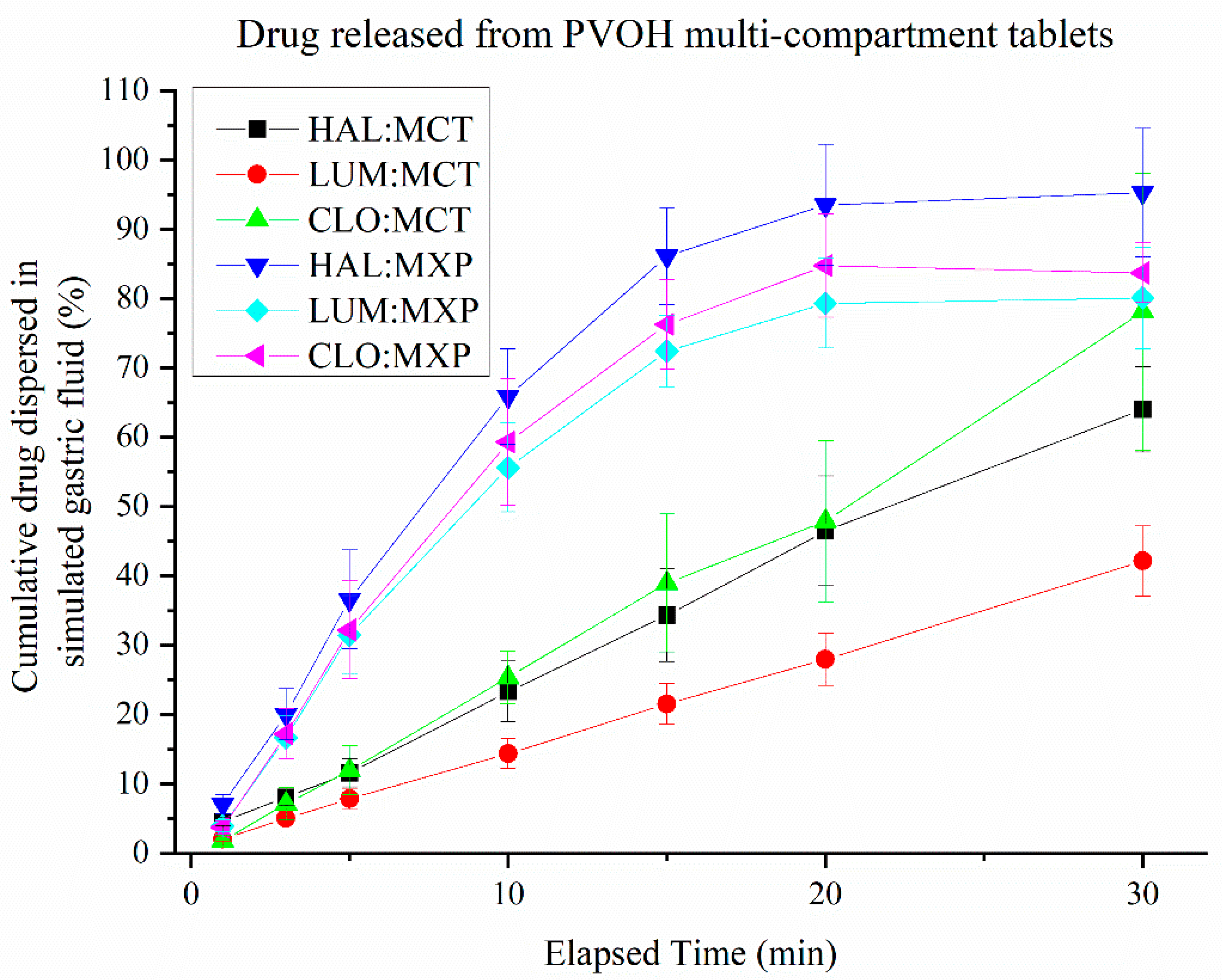
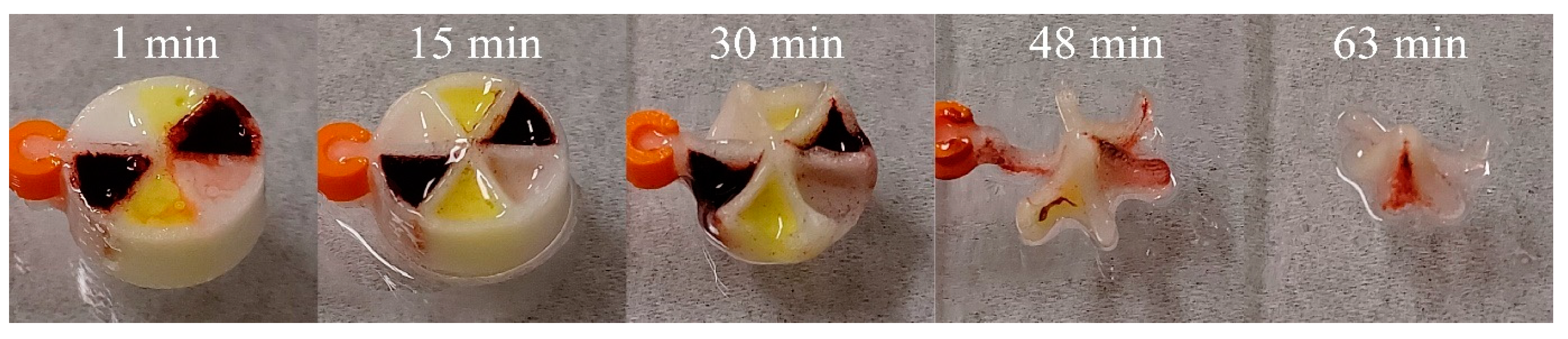
3.5. Release of Drugs from Multi-Compartment Tablets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polasek, T.M.; Kirkpatrick, C.M.J.; Rostami-Hodjegan, A. Precision dosing to avoid adverse drug reactions. Ther. Adv. Drug Saf. 2019, 10, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, M.; Jee, J.; Silva, S.; Blomgren, C.; Pontinha, V.M.; Dixon, D.L.; Van Tassel, B.; Bortner, M.J.; Williams, C.; Gilmer, E.; et al. Wijesinghe, Additive manufacturing of pharmaceuticals for precision medicine applications: A review of the promises and perils in implementation. Addit. Manuf. 2018, 23, 319–328. [Google Scholar]
- Ginsburg, G.S.; Phillips, K.A. Precision Medicine: From Science to Value. Health Aff. 2018, 37, 694–701. [Google Scholar] [CrossRef]
- Milton, J.C.; Jackson, S.H. Inappropriate polypharmacy: Reducing the burden of multiple medication. Clin. Med. 2007, 7, 514–517. [Google Scholar] [CrossRef]
- Cohen, J.S. Ways to minimize adverse drug reactions—Individualized doses and common sense are key. Postgrad. Med. 1999, 106, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Habib, W.A.; Alanizi, A.S.; Abdelhamid, M.M.; Alanizi, F.K. Accuracy of tablet splitting: Comparison study between hand splitting and tablet cutter. Saudi Pharm. J. 2014, 22, 454–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3D printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- El Aita, I.; Breitkreutz, J.; Quodbach, J. On-demand manufacturing of immediate release levetiracetam tablets using pressure-assisted microsyringe printing. Eur. J. Pharm. Biopharm. 2019, 134, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Ayyoubi, S.; Cerda, J.R.; Fernández-García, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef]
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised dosing: Printing a dose of one’s own medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Robles-Martinez, P.; Xu, X.Y.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Chandekar, A.; Dinesh, K.M.; Sharma, S.; Saraogi, G.K.; Gupta, U.; Gupta, G. 3D Printing Technology: A New Milestone in the Development of Pharmaceuticals. Curr. Pharm. Des. 2019, 25, 937–945. [Google Scholar] [CrossRef]
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a Shell-Core Delayed Release Tablet Using Dual FDM 3D Printing for Patient-Centred Therapy. Pharm. Res. 2017, 34, 427–437. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials-Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Formulation of self-emulsifying drug delivery systems. Adv. Drug Deliv. Rev. 1997, 25, 47–58. [Google Scholar] [CrossRef]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An Overview of 3D Printing Technologies for Soft Materials and Potential Opportunities for Lipid-based Drug Delivery Systems. Pharm. Res. 2019, 36, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, A.; Mader, K. Preparation and characterization of a self-emulsifying pellet formulation. Eur. J. Pharm. Biopharm. 2007, 66, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. A Proof of Concept for 3D Printing of Solid Lipid-Based Formulations of Poorly Water-Soluble Drugs to Control Formulation Dispersion Kinetics. Pharm. Res. 2019, 36, 102. [Google Scholar] [CrossRef]
- Chatzitaki, A.-T.; Tsongas, K.; Tzimtzimis, E.K.; Tzetzis, D.; Bouropoulos, N.; Barmpalexis, P.; Eleftheriadis, G.K.; Fatouros, D.G. 3D printing of patient-tailored SNEDDS-based suppositories of lidocaine. J. Drug Deliv. Sci. Technol. 2021, 61, 102292. [Google Scholar] [CrossRef]
- Wald, N.J.; Law, M.R. A strategy to reduce cardiovascular disease by more than 80%. BMJ 2003, 326, 1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellano, J.M.; Sanz, G.; Penalvo, J.L.; Bansilal, S.; Fernandez-Ortiz, A.; Alvarez, L.; Guzman, L.; Linares, J.C.; Garcia, F.; D’Aniello, F.; et al. A Polypill Strategy to Improve Adherence Results from the FOCUS Project. J. Am. Coll. Cardiol. 2014, 64, 2071–2082. [Google Scholar] [CrossRef] [Green Version]
- Goh, W.J.; Tan, S.X.; Pastorin, G.; Ho, P.C.L.; Hu, J.; Lim, S.H. 3D Printing of Four-in-One Oral Polypill with Multiple Release Profiles for Personalized Delivery of Caffeine and Vitamin B Analogues. Int. J. Pharm. 2021, 598, 120360. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; Isreb, A.; Isreb, M.; Forbes, R.T.; Oga, E.F.; Alhnan, M.A. Additive Manufacturing of a Point-of-Care “Polypill:” Fabrication of Concept Capsules of Complex Geometry with Bespoke Release against Cardiovascular Disease. Adv. Healthc. Mater. 2020, 9, 2000236. [Google Scholar] [CrossRef] [PubMed]
- Smith, R. The polypill story from a ringside seat. J. R. Soc. Med. 2009, 102, 509–512. [Google Scholar] [CrossRef]
- Markl, D.; Zeitler, J.A.; Rasch, C.; Michaelsen, M.H.; Müllertz, A.; Rantanen, J.; Rades, T.; Bøtker, J. Analysis of 3D Prints by X-ray Computed Microtomography and Terahertz Pulsed Imaging. Pharm. Res. 2017, 34, 1037–1052. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Soares, C.; Gollwitzer, V.; Habashy, R.; Timmins, P.; Alhnan, M.A. On demand manufacturing of patient-specific liquid capsules via co-ordinated 3D printing and liquid dispensing. Eur. J. Pharm. Sci. 2018, 118, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Object Research Systems. Dragonfly Pro: Easy to Use Visualization and Analysis Software with Python Scripting Capability. 2020. Available online: https://info.dragonfly-pro.com/home.html (accessed on 10 March 2021).
- Gioumouxouzis, C.I.; Tzimtzimis, E.; Katsamenis, O.L.; Dourou, A.; Markopoulou, C.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. Fabrication of an osmotic 3D printed solid dosage form for controlled release of active pharmaceutical ingredients. Eur. J. Pharm. Sci. 2020, 143, 105176. [Google Scholar] [CrossRef]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control Release 2018, 269, 355–363. [Google Scholar] [CrossRef]
- Webster, R.; Castellano, J.M.; Onuma, O.K. Putting polypills into practice: Challenges and lessons learned. Lancet 2017, 389, 1066–1074. [Google Scholar] [CrossRef]
- Nashed, N.; Lam, M.; Nokhodchi, A. A comprehensive overview of extended release oral dosage forms manufactured through hot melt extrusion and its combination with 3D printing. Int. J. Pharm. 2021, 596, 120237. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Makanga, M.; Krudsood, S. The clinical efficacy of artemether/lumefantrine (Coartem). Malar. J. 2009, 8 (Suppl. 1), S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryson, H.M.; Goa, K.L. Halofantrine. A review of its antimalarial activity, pharmacokinetic properties and therapeutic potential. Drugs 1992, 43, 236–258. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Salim, M.; Clulow, A.J.; Ramirez, G.; Pham, A.C.; Hawley, A. The impact of digestion is essential to the understanding of milk as a drug delivery system for poorly water soluble drugs. J. Control Release 2018, 292, 13–17. [Google Scholar] [CrossRef]
- Salim, M.; Ramirez, G.; Clulow, A.J.; Zhang, Y.; Ristroph, K.D.; Feng, J.; McManus, S.A.; Hawley, A.; Prud’homme, R.K.; Boyd, B.J. Solid-State Behavior and Solubilization of Flash Nanoprecipitated Clofazimine Particles during the Dispersion and Digestion of Milk-Based Formulations. Mol. Pharm. 2019, 16, 2755–2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, J.; Hawley, A.; Rades, T.; Boyd, B.J. In Situ Lipolysis and Synchrotron Small-Angle X-ray Scattering for the Direct Determination of the Precipitation and Solid-State Form of a Poorly Water-Soluble Drug during Digestion of a Lipid-Based Formulation. J. Pharm. Sci. 2016, 105, 2631–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [Green Version]

| Scheme | A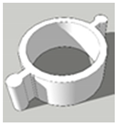 | B | C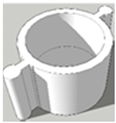 |
|---|---|---|---|
| Foot diameter (mm) | 12, 11.3 | 12, 11.3 | 12, 11.3 |
| Height (mm) | 5, 5 | 7, 5.6 | 8, 6.2 |
| Cavity depth (mm) | 5, 5 | 5, 5 | 6, 5.6 |
| Cavity diameter (mm) | 10, 10 | 10, 10 | 10, 10 |
| Wall thickness (mm) | 1, 0.65 | 1, 0.65 | 1, 0.65 |
| Printing time (min) | 2.5 | 5 | 5 |
| Scaffold Cover | A | B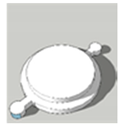 |
|---|---|---|
| Foot diameter (mm) | 12, 11.3 | 12, 11.3 |
| Overall height (mm) | 2, 1.2 | 2, 1.2 |
| Lip height (mm) | 1, 0.6 | 1, 0.6 |
| Lip thickness (mm) | 1, 0.65 | 1, 0.65 |
| Cavity diameter (mm) | 4, 4 | NA, NA |
| Printing time (min) | 2.5 | 2.5 |
| Multicompartment Scaffold (Constructed from PVOH) |  |
|---|---|
| Foot diameter (mm) | 12.4 |
| Wedge depth (mm) | 5 |
| Height (mm) | 5.6 |
| Wall thickness (mm) | 0.6 |
| Printing time (min) | 6.25 |
| Single Compartment Systems | |
|---|---|
| SMEDDS A | Component (% w/w) |
| Drug | FEN |
| Drug content | 7.0 |
| Gelucire® 44/14 | 46.5 |
| Gelucire® 48/16 | 23.3 |
| Kolliphor P 188 | 23.2 |
| Drug | SMEDDS A | SMEDDS B | ||||
|---|---|---|---|---|---|---|
| CLO | LUM | HAL | CLO | LUM | HAL | |
| Drug content | 7.0 | 7.0 | 3.5 | 7.0 | 7.0 | 3.5 |
| Gelucire® 44/14 | 46.5 | 46.5 | 48.3 | |||
| Gelucire® 48/16 | 23.3 | 23.3 | 24.1 | 93.0 | 93.0 | 96.5 |
| Kolliphor P 188 | 23.2 | 23.2 | 24.1 | |||
| Tablet Type | No-Scaffold | Dual-Face | Single-Face | Semi-Open | Closed |
|---|---|---|---|---|---|
| SA:V ratio (mm2 × µL−1) | 4:5 (0.800) | 2:5 (0.400) | 1:5 (0.200) | 4:125 (0.032) | 0:1 (0.000) |
| Component | Mass Required (g) |
|---|---|
| NaH2PO4 | 3.438 |
| NaOH pellets | 0.420 |
| NaCl | 6.186 |
| FaSSIF powder | 2.240 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barber, B.W.; Dumont, C.; Caisse, P.; Simon, G.P.; Boyd, B.J. A 3D-Printed Polymer–Lipid-Hybrid Tablet towards the Development of Bespoke SMEDDS Formulations. Pharmaceutics 2021, 13, 2107. https://doi.org/10.3390/pharmaceutics13122107
Barber BW, Dumont C, Caisse P, Simon GP, Boyd BJ. A 3D-Printed Polymer–Lipid-Hybrid Tablet towards the Development of Bespoke SMEDDS Formulations. Pharmaceutics. 2021; 13(12):2107. https://doi.org/10.3390/pharmaceutics13122107
Chicago/Turabian StyleBarber, Bryce W., Camille Dumont, Philippe Caisse, George P. Simon, and Ben J. Boyd. 2021. "A 3D-Printed Polymer–Lipid-Hybrid Tablet towards the Development of Bespoke SMEDDS Formulations" Pharmaceutics 13, no. 12: 2107. https://doi.org/10.3390/pharmaceutics13122107
APA StyleBarber, B. W., Dumont, C., Caisse, P., Simon, G. P., & Boyd, B. J. (2021). A 3D-Printed Polymer–Lipid-Hybrid Tablet towards the Development of Bespoke SMEDDS Formulations. Pharmaceutics, 13(12), 2107. https://doi.org/10.3390/pharmaceutics13122107








