Population Pharmacokinetics and Pharmacodynamic Target Attainment of Isavuconazole against Aspergillus fumigatus and Aspergillus flavus in Adult Patients with Invasive Fungal Diseases: Should Therapeutic Drug Monitoring for Isavuconazole Be Considered as Mandatory as for the Other Mold-Active Azoles?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population Pharmacokinetic Modeling
2.3. Monte Carlo Simulation Analysis and Probability of Target Attainment
2.4. Statistics
2.5. Ethics
3. Results
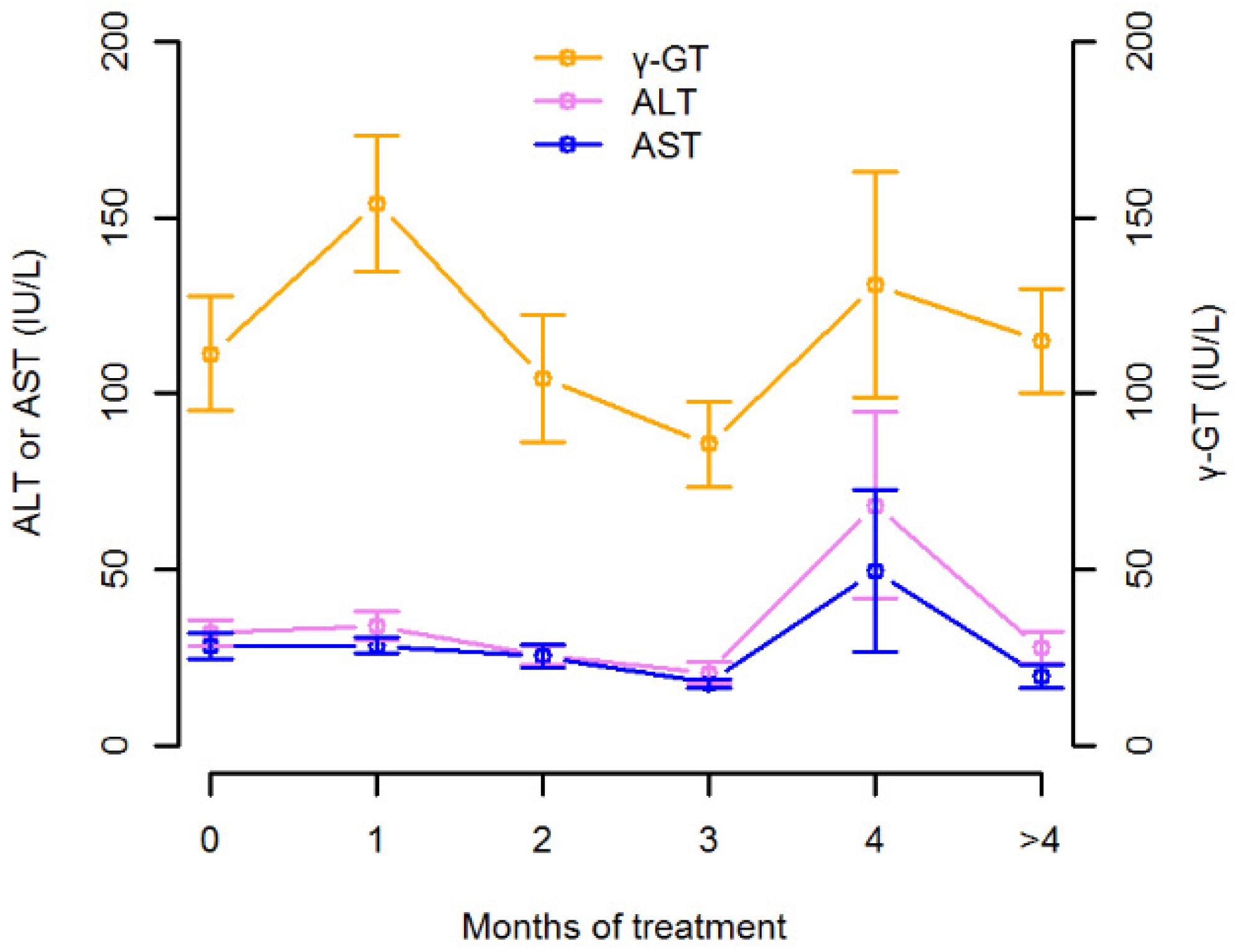
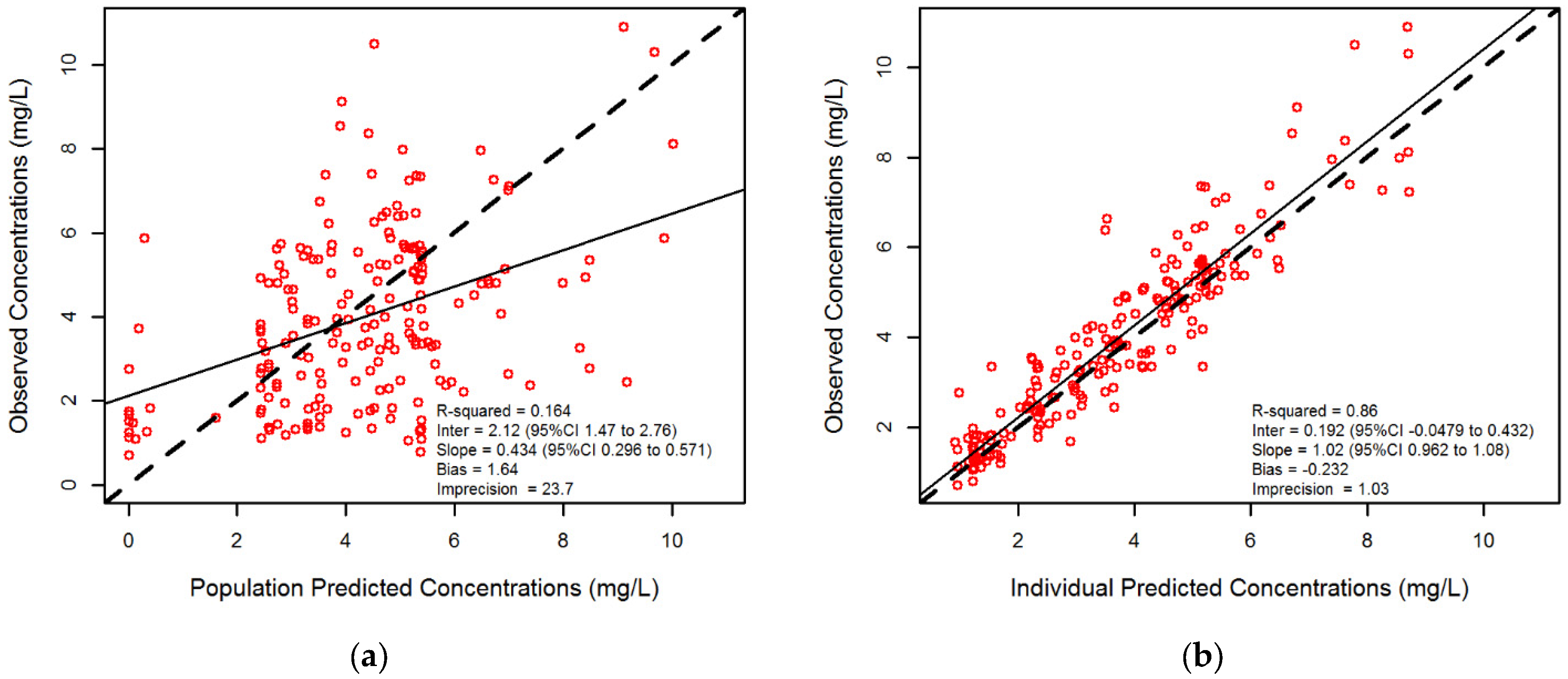
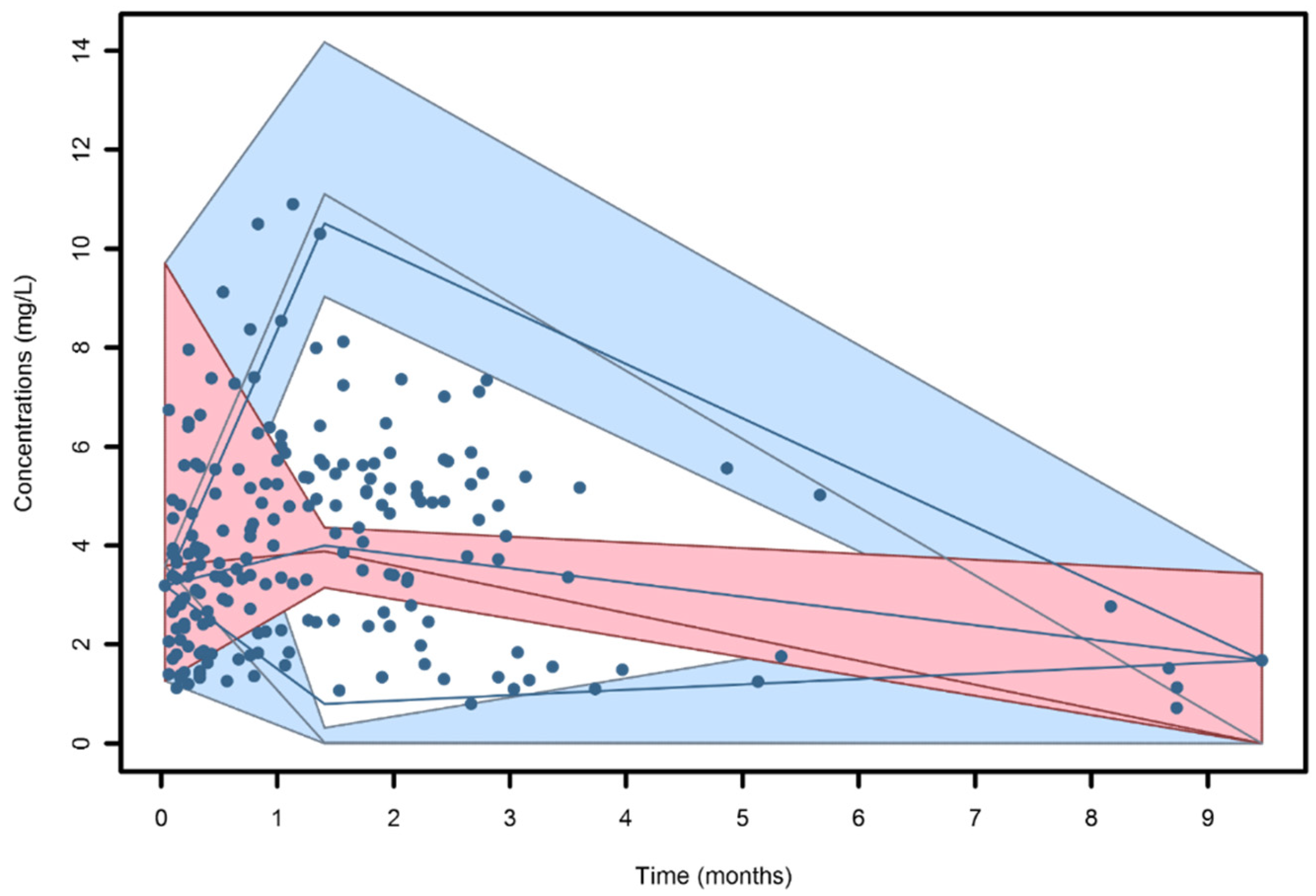
3.1. Isavuconazole Measurements and Regression Analysis
3.2. Population Pharmacokinetic Modeling
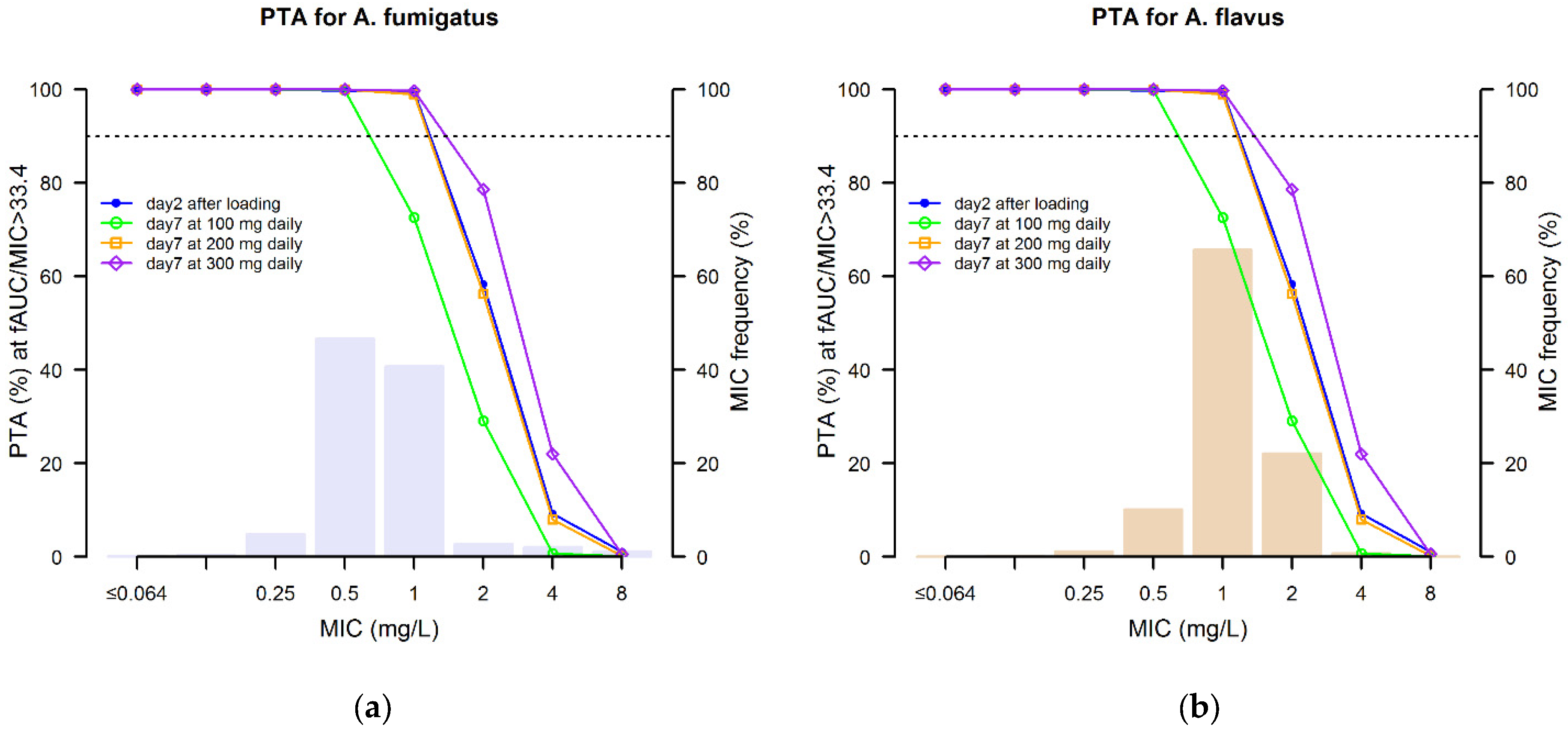
3.3. Monte Carlo Simulation and Probability of Target Attainment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornely, O.A.; Lass-Florl, C.; Lagrou, K.; Arsic-Arsenijevic, V.; Hoenigl, M. Improving outcome of fungal diseases—Guiding experts and patients towards excellence. Mycoses 2017, 60, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. In Human Fungal Pathogen Identification; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1508, pp. 17–65. [Google Scholar]
- Kauffman, C.A.; Freifeld, A.G.; Andes, D.R.; Baddley, J.W.; Herwaldt, L.; Walker, R.C.; Alexander, B.D.; Anaissie, E.J.; Benedict, K.; Ito, J.I.; et al. Endemic fungal infections in solid organ and hematopoietic cell transplant recipients enrolled in the Transplant-Associated Infection Surveillance Network (TRANSNET). Transpl. Infect. Dis. 2014, 16, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Taccone, F.S.; Van den Abeele, A.M.; Bulpa, P.; Misset, B.; Meersseman, W.; Cardoso, T.; Paiva, J.A.; Blasco-Navalpotro, M.; De Laere, E.; Dimopoulos, G.; et al. Epidemiology of invasive aspergillosis in critically ill patients: Clinical presentation, underlying conditions, and outcomes. Crit. Care 2015, 19, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nivoix, Y.; Velten, M.; Letscher-Bru, V.; Moghaddam, A.; Natarajan-Ame, S.; Fohrer, C.; Lioure, B.; Bilger, K.; Lutun, P.; Marcellin, L.; et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin. Infect. Dis. 2008, 47, 1176–1184. [Google Scholar] [CrossRef] [Green Version]
- Montagna, M.T.; Lovero, G.; Coretti, C.; Martinelli, D.; Delia, M.; De Giglio, O.; Caira, M.; Puntillo, F.; D’Antonio, D.; Venditti, M.; et al. SIMIFF study: Italian fungal registry of mold infections in hematological and non-hematological patients. Infection 2014, 42, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Florl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Rybak, J.M.; Marx, K.R.; Nishimoto, A.T.; Rogers, P.D. Isavuconazole: Pharmacology, Pharmacodynamics, and Current Clinical Experience with a New Triazole Antifungal Agent. Pharmacotherapy 2015, 35, 1037–1051. [Google Scholar] [CrossRef]
- Pettit, N.N.; Carver, P.L. Isavuconazole: A New Option for the Management of Invasive Fungal Infections. Ann. Pharmacother. 2015, 49, 825–842. [Google Scholar] [CrossRef]
- Shirley, M.; Scott, L.J. Isavuconazole: A Review in Invasive Aspergillosis and Mucormycosis. Drugs 2016, 76, 1647–1657. [Google Scholar] [CrossRef]
- Stott, K.E.; Hope, W.W. Therapeutic drug monitoring for invasive mould infections and disease: Pharmacokinetic and pharmacodynamic considerations. J. Antimicrob. Chemother. 2017, 72, i12–i18. [Google Scholar] [CrossRef]
- Andes, D.; Kovanda, L.; Desai, A.; Kitt, T.; Zhao, M.; Walsh, T.J. Isavuconazole Concentration in Real-World Practice: Consistency with Results from Clinical Trials. Antimicrob. Agents Chemother. 2018, 62, e00585-18. [Google Scholar] [CrossRef] [Green Version]
- Furfaro, E.; Signori, A.; Di Grazia, C.; Dominietto, A.; Raiola, A.M.; Aquino, S.; Ghiggi, C.; Ghiso, A.; Ungaro, R.; Angelucci, E.; et al. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J. Antimicrob. Chemother. 2019, 74, 2341–2346. [Google Scholar] [CrossRef]
- Mesini, A.; Cangemi, G.; Palmisani, E.; Dufour, C.; Castagnola, E. Hepatic veno-occlusive disease during isavuconazole administration. J. Chemother. 2018, 30, 63–64. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Isavuconazole and Aspergillus spp.: Rationale for the Clinical Breakpoints, Version 2.0. 2020. Available online: http://www.eucast.org (accessed on 16 June 2020).
- Pea, F.; Krause, R.; Muller, C.; Hennart, B.; Richardson, M.; Meinitzer, A.; Wiesen, M.H.J.; Wiktorowicz, T.; Spickermann, J.; Henriksen, A.S. Interlaboratory Analysis of Isavuconazole Plasma Concentration Assays Among European Laboratories. Ther. Drug Monit. 2019, 41, 657–664. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic Address, e.e.e.; Clinical Practice Guideline Panel, C.; Panel, m.; Representative, E.G.B. EASL Clinical Practice Guidelines: Drug-Induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [Green Version]
- Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers (accessed on 16 June 2020).
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [PubMed]
- Neely, M.N.; van Guilder, M.G.; Yamada, W.M.; Schumitzky, A.; Jelliffe, R.W. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther. Drug Monit. 2012, 34, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Seyedmousavi, S.; Bruggemann, R.J.; Meis, J.F.; Melchers, W.J.; Verweij, P.E.; Mouton, J.W. Pharmacodynamics of isavuconazole in an Aspergillus fumigatus mouse infection model. Antimicrob. Agents Chemother. 2015, 59, 2855–2866. [Google Scholar] [CrossRef] [Green Version]
- The European Committee on Antimicrobial Susceptibility Testing-EUCAST. Available online: https://www.eucast.org/mic_distributions_and_ecoffs (accessed on 16 June 2020).
- Desai, A.; Schmitt-Hoffmann, A.H.; Mujais, S.; Townsend, R. Population Pharmacokinetics of Isavuconazole in Subjects with Mild or Moderate Hepatic Impairment. Antimicrob. Agents Chemother. 2016, 60, 3025–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovanda, L.L.; Marty, F.M.; Maertens, J.; Desai, A.V.; Lademacher, C.; Engelhardt, M.; Lu, Q.; Hope, W.W. Impact of Mucositis on Absorption and Systemic Drug Exposure of Isavuconazole. Antimicrob. Agents Chemother. 2017, 61, e00101-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovanda, L.L.; Desai, A.V.; Lu, Q.; Townsend, R.W.; Akhtar, S.; Bonate, P.; Hope, W.W. Isavuconazole Population Pharmacokinetic Analysis Using Nonparametric Estimation in Patients with Invasive Fungal Disease (Results from the VITAL Study). Antimicrob. Agents Chemother. 2016, 60, 4568–4576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Venkataramanan, R.; Rivosecchi, R.M.; Tang, C.; Marini, R.V.; Shields, R.K.; Clancy, C.J.; Nguyen, M.H. Population Pharmacokinetics of Intravenous Isavuconazole in Solid-Organ Transplant Recipients. Antimicrob. Agents Chemother. 2020, 64, e01728-19. [Google Scholar] [CrossRef]
- Kovanda, L.L.; Petraitiene, R.; Petraitis, V.; Walsh, T.J.; Desai, A.; Bonate, P.; Hope, W.W. Pharmacodynamics of isavuconazole in experimental invasive pulmonary aspergillosis: Implications for clinical breakpoints. J. Antimicrob. Chemother. 2016, 71, 1885–1891. [Google Scholar] [CrossRef] [Green Version]
- Risum, M.; Vestergaard, M.B.; Weinreich, U.M.; Helleberg, M.; Vissing, N.H.; Jorgensen, R. Therapeutic Drug Monitoring of Isavuconazole: Serum Concentration Variability and Success Rates for Reaching Target in Comparison with Voriconazole. Antibiotics 2021, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Desai, A.; Han, D.; Howieson, C.; Kato, K.; Akhtar, S.; Kowalski, D.; Lademacher, C.; Lewis, W.; Pearlman, H.; et al. Pharmacokinetic Assessment of Drug-Drug Interactions of Isavuconazole with the Immunosuppressants Cyclosporine, Mycophenolic Acid, Prednisolone, Sirolimus, and Tacrolimus in Healthy Adults. Clin. Pharmacol. Drug Dev. 2017, 6, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, T.; Desai, A.; Han, D.; Kato, K.; Kowalski, D.; Akhtar, S.; Lademacher, C.; Kovanda, L.; Townsend, R. Pharmacokinetic Interaction Between Isavuconazole and a Fixed-Dose Combination of Lopinavir 400 mg/Ritonavir 100 mg in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2017, 6, 93–101. [Google Scholar] [CrossRef]
- Townsend, R.; Dietz, A.; Hale, C.; Akhtar, S.; Kowalski, D.; Lademacher, C.; Lasseter, K.; Pearlman, H.; Rammelsberg, D.; Schmitt-Hoffmann, A.; et al. Pharmacokinetic Evaluation of CYP3A4-Mediated Drug-Drug Interactions of Isavuconazole with Rifampin, Ketoconazole, Midazolam, and Ethinyl Estradiol/Norethindrone in Healthy Adults. Clin. Pharmacol. Drug Dev. 2017, 6, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Schmitt-Hoffmann, A.; Roos, B.; Maares, J.; Heep, M.; Spickerman, J.; Weidekamm, E.; Brown, T.; Roehrle, M. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 2006, 50, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Borman, A.M.; Hughes, J.M.; Oliver, D.; Fraser, M.; Sunderland, J.; Noel, A.R.; Johnson, E.M. Lessons from isavuconazole therapeutic drug monitoring at a United Kingdom Reference Center. Med. Mycol. 2020, 58, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Otu, A.; Moore, C.B.; Richardson, M.D.; Rautemaa-Richardson, R. Isavuconazole Therapeutic Drug Monitoring during Long-Term Treatment for Chronic Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2020, 65, e01511-20. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]

| Variable | Median or Count | Range or % |
|---|---|---|
| Age (years) | 61.5 | 51.3–72.0 |
| Gender (male/female) | 31/19 | 62/38 |
| Body weight (kg) | 65.0 | 55.5–71.5 |
| Albumin (g/L) | 35.0 | 28.4–40.0 |
| Total bilirubin (mg/dL) | 0.28 | 0.2–0.4 |
| Gamma-glutamyltransferase (IU/L) | 70.0 | 42.0–173.0 |
| Alanine-aminotransferase (IU/L) | 21.0 | 15.0–38.0 |
| Aspartate-aminotransferase (IU/L) | 20.0 | 15.0–31.0 |
| Type of infections | ||
| Invasive pulmonary aspergillosis | 40 | 80.0 |
| Invasive fusariosis | 2 | 4.0 |
| Cerebral mucormycosis | 1 | 2.0 |
| Scedosporium osteomyelitis | 1 | 2.0 |
| Aspergillus brain abscess | 1 | 2.0 |
| Invasive fungal disease, not specified | 5 | 10.0 |
| Underlying disease | ||
| Oncohematological malignancy | 25 | 50.0 |
| Nosocomial pneumonia | 11 | 22.0 |
| Immunosuppression° | 9 | 18.0 |
| Other | 5 | 10.0 |
| Isavuconazole treatment | ||
| First-line or switch from other azoles | 45/5 | 90/10 |
| Dose (mg) | 200 | 200–200 |
| Total number of Ctrough | 175 | |
| Ctrough (mg/L) | 3.68 | 2.07–5.38 |
| Total number of Cpeak | 24 | |
| Cpeak (mg/L) | 4.67 | 3.78–5.96 |
| Number of TDM instances | 2.0 | 1.0–4.0 |
| Treatment duration (days) * | 48.0 | 19.0–91.0 |
| Clinical outcome at end of treatment * | ||
| Successful treatment | 32 | 68.1 |
| Treatment failure | 12 | 25.5 |
| Dead for other reasons | 3 | 6.4 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Unstandardized β-Coefficient (95% CI) | p-Value | Unstandardized β-Coefficient (95% CI) | p-Value |
| Age (years) | 0.037 (0.066–0.007) | 0.022 | 0.037 (0.061−0.013) | <0.001 |
| Weight (kg) | −0.029 (0.006−0.064) | 0.106 | ||
| Gender (male vs. female) | 0.099 (6.986−6.788) | 0.977 | ||
| Dose/kg daily (mg/kg) | 0.815 (1.164–0.466) | 0.010 | 0.402 (0.819−0.016) | 0.067 |
| Days from starting therapy (days) | 0.001 (0.007−0.005) | 0.747 | ||
| Albumin (g/L) | 0.034 (0.087−0.019) | 0.214 | ||
| Total bilirubin (mg/dL) | −0.346 (0.034−0.726) | 0.078 | ||
| ALT (IU/L) | −0.001 (0.007−0.009) | 0.730 | ||
| AST (IU/L) | −0.008 (-0.002–0.004) | 0.230 | ||
| γ-GT (IU/L) | 0.003 (0.005–0.001) | 0.022 | −0.0004 (0.002−0.002) | 0.751 |
| Cotreatment with CYP3A4 inhibitors | 2.39 (3.337–1.443) | 0.039 | 2.154 (3.248–1.060) | 0.018 |
| CL (L/h) | Ka (h−1) | Fos (%) | Q (L/h) | V (L) | Vp (L) | |
|---|---|---|---|---|---|---|
| Mean | 1.52 | 22.64 | 0.95 | 16.78 | 89.50 | 735.24 |
| SD | 0.97 | 3.54 | 0.07 | 18.35 | 42.38 | 633.89 |
| CV (%) | 64.03 | 15.66 | 7.42 | 109.37 | 47.35 | 86.22 |
| Median | 1.33 | 22.64 | 1.00 | 5.08 | 102.58 | 385.93 |
| LD | MD of 100 mg Daily | MD of 200 mg Daily | MD of 300 mg Daily | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isavuconazole Ctrough (mg/L) | Day 2 | Day 7 | Day 14 | Day 21 | Day 28 | Day 60 | Day 7 | Day 14 | Day 21 | Day 28 | Day 60 | Day 7 | Day 14 | Day 21 | Day 28 | Day 60 |
| <1.0 | 1.7 | 21.7 | 16.4 | 12.9 | 12.0 | 11.7 | 4.1 | 1.8 | 1.3 | 1.0 | 1.1 | 0.8 | 0.2 | 0.2 | 0.1 | 0.1 |
| 1.0–5.13 | 85.2 | 76.4 | 81.5 | 84.6 | 83.8 | 81.1 | 84.3 | 80.4 | 73.6 | 71.3 | 59.7 | 76.9 | 60.6 | 48.6 | 46.9 | 26.6 |
| >5.13 | 13.1 | 1.9 | 2.1 | 2.5 | 4.2 | 7.2 | 11.6 | 17.8 | 25.1 | 27.7 | 39.2 | 22.3 | 39.2 | 51.2 | 53.0 | 73.2 |
| Aspergillus fumigatus | Aspergillus flavus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isavuconazole Dosing Regimens | Day 2 | Day 7 | Day 14 | Day 21 | Day 28 | Day 60 | Day 2 | Day 7 | Day 14 | Day 21 | Day 28 | Day 60 |
| LD + MD of 100 mg daily | 94.7 | 82.7 | 84.5 | 88.8 | 89.9 | 89.5 | 90.0 | 65.6 | 67.9 | 75.4 | 76.2 | 78.3 |
| LD + MD of 200 mg daily | 94.7 | 94.5 | 95.4 | 95.8 | 96.2 | 96.6 | 90.0 | 90.2 | 92.4 | 94.4 | 96.6 | 96.8 |
| LD + MD of 300 mg daily | 94.7 | 95.8 | 96.7 | 97.2 | 97.3 | 97.9 | 90.0 | 94.5 | 98.1 | 98.9 | 98.9 | 99.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojutti, P.G.; Carnelutti, A.; Lazzarotto, D.; Sozio, E.; Candoni, A.; Fanin, R.; Tascini, C.; Pea, F. Population Pharmacokinetics and Pharmacodynamic Target Attainment of Isavuconazole against Aspergillus fumigatus and Aspergillus flavus in Adult Patients with Invasive Fungal Diseases: Should Therapeutic Drug Monitoring for Isavuconazole Be Considered as Mandatory as for the Other Mold-Active Azoles? Pharmaceutics 2021, 13, 2099. https://doi.org/10.3390/pharmaceutics13122099
Cojutti PG, Carnelutti A, Lazzarotto D, Sozio E, Candoni A, Fanin R, Tascini C, Pea F. Population Pharmacokinetics and Pharmacodynamic Target Attainment of Isavuconazole against Aspergillus fumigatus and Aspergillus flavus in Adult Patients with Invasive Fungal Diseases: Should Therapeutic Drug Monitoring for Isavuconazole Be Considered as Mandatory as for the Other Mold-Active Azoles? Pharmaceutics. 2021; 13(12):2099. https://doi.org/10.3390/pharmaceutics13122099
Chicago/Turabian StyleCojutti, Pier Giorgio, Alessia Carnelutti, Davide Lazzarotto, Emanuela Sozio, Anna Candoni, Renato Fanin, Carlo Tascini, and Federico Pea. 2021. "Population Pharmacokinetics and Pharmacodynamic Target Attainment of Isavuconazole against Aspergillus fumigatus and Aspergillus flavus in Adult Patients with Invasive Fungal Diseases: Should Therapeutic Drug Monitoring for Isavuconazole Be Considered as Mandatory as for the Other Mold-Active Azoles?" Pharmaceutics 13, no. 12: 2099. https://doi.org/10.3390/pharmaceutics13122099
APA StyleCojutti, P. G., Carnelutti, A., Lazzarotto, D., Sozio, E., Candoni, A., Fanin, R., Tascini, C., & Pea, F. (2021). Population Pharmacokinetics and Pharmacodynamic Target Attainment of Isavuconazole against Aspergillus fumigatus and Aspergillus flavus in Adult Patients with Invasive Fungal Diseases: Should Therapeutic Drug Monitoring for Isavuconazole Be Considered as Mandatory as for the Other Mold-Active Azoles? Pharmaceutics, 13(12), 2099. https://doi.org/10.3390/pharmaceutics13122099







