Electrospun Fibers Loaded with Natural Bioactive Compounds as a Biomedical System for Skin Burn Treatment. A Review
Abstract
1. Introduction
2. Literature Reviewed
3. Categorization and Characterization of Electrospun Fibers
4. Materials Used for Production of Electrospun Fibers
4.1. Natural Materials
4.1.1. Chitosan (CH)
4.1.2. Collagen (COL)
4.1.3. Gelatin (GE)
4.1.4. Keratin
4.1.5. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PHBV)
4.1.6. Silk Fibroin (SF)
4.1.7. Sodium Alginate (SA)
4.2. Synthetic Materials
4.2.1. Poly(Ethylene Glycol) (PEG)
4.2.2. Poly(Lactic-co-Glycolic Acids) (PLGA)
4.2.3. Poly(L-Lactic Acid) (PLLA)
4.2.4. Poly(Vinyl Alcohol) (PVA)
4.2.5. Poly(Vinyl Pyrrolidone) (PVP)
4.2.6. Polycaprolactone (PCL)
4.2.7. Poly-D,L-Lactic Acid (PDLLA)
4.2.8. Polylactide (PLA)
4.2.9. Polyurethane (PU)
5. Natural Bioactive Compounds Used in Electrospun Fibers
5.1. Natural Antimicrobial Bioactive Compounds
5.1.1. Badger (Meles Meles) Oil
5.1.2. Olive (Olea Europaea L.) Oil
5.1.3. Chitosan (CH) & CH/l-Arginine (CH-Arg)
5.1.4. Curcumin (CU)
5.1.5. Antimicrobial Peptides (AMP) HHC36
5.1.6. Manuka Honey (MH)
5.1.7. ε-Polylysine (εPL)
5.1.8. Gymnema Sylvestre Extract
5.2. Natural Bioactive Compounds Producing Accelerated Burn Wound Healing
5.2.1. Bromelain (Br)
5.2.2. Microalga Spirulina (Arthrospira sp.)
5.2.3. Plasmid DNA Encoding Angiopoietin-1 (pAng)
5.2.4. Astragaloside IV
5.2.5. α-Lactalbumin (ALA)
5.2.6. Fibrin
5.2.7. Actinidin
5.2.8. Quercetin/Rutin
5.3. Natural Bioactive Compounds with Antimicrobial Effects for Burn Wound Healing
5.3.1. Lavender Essential Oil (Lavandula Angustifolia)
5.3.2. Cinnamon (Cinnamomum verum) Essential Oil
5.3.3. Memecylon Edule Extract
5.3.4. Aspalathus Linearis Fermented Extract (AL Extract)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rani, M.; Schwacha, M.G. Aging and the pathogenic response to burn. Aging Dis. 2012, 3, 171. [Google Scholar] [PubMed]
- Kim, J.H.; Unnithan, A.R.; Kim, H.J.; Tiwari, A.P.; Park, C.H.; Kim, C.S. Electrospun badger (Meles meles) oil/Ag nanoparticle based anti-bacterial mats for biomedical applications. J. Ind. Eng. Chem. 2015, 30, 254–260. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Pichiah, P.T.; Gnanasekaran, G.; Seenivasan, K.; Barakat, N.A.; Cha, Y.S.; Jung, C.H.; Shanmugam, A.; Kim, H.Y. Emu oil-based electrospun nanofibrous scaffolds for wound skin tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 454–460. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Madamsetty, V.S.; Kumar, A.; Varzandeh, M.; Dehshahri, A.; Zarrabi, A.; Sharififar, F.; Mohammadi, M.; Fahimipour, A.; Ramakrishna, S. Electrospun nanocarriers for delivering natural products for cancer therapy. Trends Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Shishir, M.R.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Acevedo, F.; Hermosilla, J.; Sanhueza, C.; Mora-Lagos, B.; Fuentes, I.; Rubilar, M.; Concheiro, A.; Alvarez-Lorenzo, C. Gallic acid loaded PEO-core/zein-shell nanofibers for chemopreventive action on gallbladder cancer cells. Eur. J. Pharm. Sci. 2018, 119, 49–61. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity-A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef] [PubMed]
- Amina, M.; Amna, T.; Hassan, M.S.; Ibrahim, T.A.; Khil, M.S. Facile single mode electrospinning way for fabrication of natural product based silver decorated polyurethane nanofibrous membranes: Prospective medicated bandages. Colloids Surf. A: Physicochem. Eng. Asp. 2013, 425, 115–121. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Pérez-González, G.L.; Bogdanchikova, N.; Pestryakov, A.; Nimaev, V.; Soloveva, A.; Cornejo-Bravo, J.M.; Toledaño-Magaña, Y. Antimicrobial Effect of Electrospun Nanofibers Loaded with Silver Nanoparticles: Influence of Ag Incorporation Method. J. Nanomater. 2021, 2021, 9920755. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Mater. Sci. Eng. C 2019, 101, 306–322. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef]
- Zhmayev, E.; Cho, D.; Joo, Y.L. Nanofibers from gas-assisted polymer melt electrospinning. Polymer 2010, 51, 4140–4144. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Shan, Y.; Xiong, J.; Hu, Z.; Zhang, Y.; Gao, J. In vivo study of silk fibroin/gelatin electrospun nanofiber dressing loaded with astragaloside IV on the effect of promoting wound healing and relieving scar. J. Drug Deliv. Sci. Technol. 2019, 52, 272–281. [Google Scholar] [CrossRef]
- Wang, X.; Nakane, K. Preparation of polymeric nanofibers via immersion electrospinning. Eur. Polym. J. 2020, 134, 109837. [Google Scholar] [CrossRef]
- Antunes, B.P.; Moreira, A.F.; Gaspar, V.M.; Correia, I.J. Chitosan/arginine–chitosan polymer blends for assembly of nanofibrous membranes for wound regeneration. Carbohydr. Polym. 2015, 130, 104–112. [Google Scholar] [CrossRef]
- Ramalingam, R.; Dhand, C.; Mayandi, V.; Leung, C.M.; Ezhilarasu, H.; Karuppannan, S.K.; Prasannan, P.; Ong, S.T.; Sunderasan, N.; Kaliappan, I.; et al. Core–Shell Structured Antimicrobial Nanofiber Dressings Containing Herbal Extract and Antibiotics Combination for the Prevention of Biofilms and Promotion of Cutaneous Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 24356–24369. [Google Scholar] [CrossRef] [PubMed]
- Ilomuanya, M.O.; Adebona, A.C.; Wang, W.; Sowemimo, A.; Eziegbo, C.L.; Silva, B.O.; Adeosun, S.O.; Joubert, E.; De Beer, D. Development and characterization of collagen-based electrospun scaffolds containing silver sulphadiazine and Aspalathus linearis extract for potential wound healing applications. SN Appl. Sci. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Bayat, S.; Amiri, N.; Pishavar, E.; Kalalinia, F.; Movaffagh, J.; Hashemi, M. Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019, 229, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, Q.; Yao, H.; Zhu, Y.; Topham, P.D.; Yue, K.; Wang, L. Superhydrophobic hierarchical fiber/bead composite membranes for efficient treatment of burns. Acta Biomater. 2019, 92, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Mayandi, V.; Wen Choong, A.C.; Dhand, C.; Lim, F.P.; Aung, T.T.; Sriram, H.; Dwivedi, N.; Periayah, M.H.; Sridhar, S.; Fazil, M.H.U.T.; et al. Multifunctional antimicrobial nanofiber dressings containing ε-polylysine for the eradication of bacterial bioburden and promotion of wound healing in critically colonized wounds. ACS Appl. Mater. Interfaces 2020, 12, 15989–16005. [Google Scholar] [CrossRef]
- Elshishiny, F.; Mamdouh, W. Fabrication of Nanofibrous/Xerogel Layer-by-Layer Biocomposite Scaffolds for Skin Tissue Regeneration: In Vitro Study. ACS Omega 2020, 5, 2133–2147. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Epidermal differentiation of stem cells on poly (3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV) nanofibers. Ann. Biomed. Eng. 2014, 42, 2589–2599. [Google Scholar] [CrossRef]
- Rujitanaroj, P.O.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Amer, S.; Attia, N.; Nouh, S.; El-Kammar, M.; Korittum, A.; Abu-Ahmed, H. Fabrication of sliver nanoparticles/polyvinyl alcohol/gelatin ternary nanofiber mats for wound healing application. J. Biomater. Appl. 2020, 35, 287–298. [Google Scholar] [CrossRef]
- Dong, R.H.; Jia, Y.X.; Qin, C.C.; Zhan, L.; Yan, X.; Cui, L.; Long, Y.Z. In situ deposition of a personalized nanofibrous dressing via a handy electrospinning device for skin wound care. Nanoscale 2016, 8, 3482–3488. [Google Scholar] [CrossRef]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core–Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20, 1900328. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Bogdał, D.; Matýsek, D. Hybrid bilayer PLA/chitosan nanofibrous scaffolds doped with ZnO, Fe3O4, and Au nanoparticles with bioactive properties for skin tissue engineering. Polymers 2020, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Paaver, U.; Tamm, I.; Laidmäe, I.; Lust, A.; Kirsimäe, K.; Veski, P.; Heinämäki, J. Soluplus graft copolymer: Potential novel carrier polymer in electrospinning of nanofibrous drug delivery systems for wound therapy. Biomed Res. Int. 2014, 2014, 789765. [Google Scholar] [CrossRef]
- Rivero, G.; Meuter, M.; Pepe, A.; Guevara, M.G.; Boccaccini, A.R.; Abraham, G.A. Nanofibrous membranes as smart wound dressings that release antibiotics when an injury is infected. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124313. [Google Scholar] [CrossRef]
- Abd Elhaleem, M.B.; Farghali, A.A.; El-Shahawy, A.A.; El-Ela, F.I.; Eldine, Z.E.; Mahmoud, R.K. Chemisorption and sustained release of cefotaxime between a layered double hydroxide and polyvinyl alcohol nanofibers for enhanced efficacy against second degree burn wound infection. RSC Adv. 2020, 10, 13196–13214. [Google Scholar] [CrossRef]
- Ribeiro, N.; Sousa, A.; Cunha-Reis, C.; Oliveira, A.L.; Granja, P.L.; Monteiro, F.J.; Sousa, S.R. New prospects in skin regeneration and repair using nanophased hydroxyapatite embedded in collagen nanofibers. Nanomed. Nanotechnol. Biol. Med. 2021, 33, 102353. [Google Scholar] [CrossRef]
- Heo, D.N.; Yang, D.H.; Lee, J.B.; Bae, M.S.; Kim, J.H.; Moon, S.H.; Kwon, I.K. Burn-wound healing effect of gelatin/polyurethane nanofiber scaffold containing silver-sulfadiazine. J. Biomed. Nanotechnol. 2013, 9, 511–515. [Google Scholar] [CrossRef]
- Jeong, L.; Kim, M.H.; Jung, J.Y.; Min, B.M.; Park, W.H. Effect of silk fibroin nanofibers containing silver sulfadiazine on wound healing. Int. J. Nanomed. 2014, 9, 5277. [Google Scholar] [CrossRef][Green Version]
- Macri, L.K.; Sheihet, L.; Singer, A.J.; Kohn, J.; Clark, R.A. Ultrafast and fast bioerodible electrospun fiber mats for topical delivery of a hydrophilic peptide. J. Control. Release 2012, 161, 813–820. [Google Scholar] [CrossRef]
- Kim, G.; Yoon, H.; Lee, H.; Park, G.M.; Koh, Y. Polycarprolactone ultrafine fiber membrane fabricated using a charge-reduced electrohydrodynamic process. Macromol. Res. 2009, 17, 533–537. [Google Scholar] [CrossRef]
- Romano, I.; Summa, M.; Heredia-Guerrero, J.A.; Spanò, R.; Ceseracciu, L.; Pignatelli, C.; Athanassiou, A. Fumarate-loaded electrospun nanofibers with anti-inflammatory activity for fast recovery of mild skin burns. Biomed. Mater. 2016, 11, 041001. [Google Scholar] [CrossRef]
- Leung, C.M.; Dhand, C.; Mayandi, V.; Ramalingam, R.; Lim, F.P.; Barathi, V.A.; Dwivedi, N.; Orive, G.; Beuerman, R.W.; Ramakrishna, S.; et al. Wound healing properties of magnesium mineralized antimicrobial nanofiber dressings containing chondroitin sulphate–a comparison between blend and core–shell nanofibers. Biomater. Sci. 2020, 8, 3454–3471. [Google Scholar] [CrossRef]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Zheng, G.; Jiang, J.; Wang, X.; Li, W.; Yu, Z.; Lin, L. High-aspect-ratio three-dimensional electrospinning via a tip guiding electrode. Mater. Des. 2021, 198, 109304. [Google Scholar] [CrossRef]
- Vilchez, A.; Acevedo, F.; Cea, M.; Seeger, M.; Navia, R. Applications of electrospun nanofibers with antioxidant properties: A review. Nanomaterials 2020, 10, 175. [Google Scholar] [CrossRef]
- Robinson, A.J.; Pérez-Nava, A.; Ali, S.C.; González-Campos, J.B.; Holloway, J.L.; Cosgriff-Hernandez, E.M. Comparative analysis of fiber alignment methods in electrospinning. Matter 2021, 4, 821–844. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, P.U.; Growney Kalaf, E.A.; Dunn, A.J.; Shornick, L.P.; Sell, S.A. Comparison of silk fibroin electrospun scaffolds with poloxamer and honey additives for burn wound applications. J. Bioact. Compat. Polym. 2018, 33, 79–94. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Mele, E. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef]
- Buzgo, M.; Mickova, A.; Rampichova, M.; Doupnik, M. Blend electrospinning, coaxial electrospinning, and emulsion electrospinning techniques. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Woodhead Publishing: Cambridge, UK, 2018; pp. 325–347. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Wei, Y. One-dimensional composite nanomaterials: Synthesis by electrospinning and their applications. Small 2009, 5, 2349–2370. [Google Scholar] [CrossRef]
- Xu, Y.; Ndayikengurukiye, J.; Akono, A.T.; Guo, P. Fabrication of fiber-reinforced polymer ceramic composites by wet electrospinning. Manuf. Lett. 2021. [Google Scholar] [CrossRef]
- Chen, S.; Gao, J.; Yan, E.; Wang, Y.; Li, Y.; Lu, H.; Fan, L.; Wang, D.; An, Q. A novel porous composite membrane of PHA/PVA via coupling of electrospinning and spin coating for antibacterial applications. Mater. Lett. 2021, 301, 130279. [Google Scholar] [CrossRef]
- Qin, X. Coaxial electrospinning of nanofibers. In Electrospun Nanofibers; Woodhead Publishing: Cambridge, UK, 2017; pp. 41–71. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, L.; Ruan, H.; Zhang, L.; Wang, J.; Jiang, H.; Wu, Y.; Feng, S.; Chen, J. Electrospun chitosan oligosaccharide/polycaprolactone nanofibers loaded with wound-healing compounds of Rutin and Quercetin as antibacterial dressings. Int. J. Biol. Macromol. 2021, 183, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Ketabchi, N.D.; Adabi, M.; Gholami, M.; Firoozi, S.; Amanzadi, B.; Faridi-Majidi, R. Study of Third-Degree Burn Wounds Debridement and Treatment by Actinidin Enzyme Immobilized on Electrospun Chitosan/PEO Nanofibers in Rats. Biointerface Res. Appl. Chem. 2021, 11, 10358–10370. [Google Scholar] [CrossRef]
- Talukder, M.E.; Hasan, K.F.; Wang, J.; Yao, J.; Li, C.; Song, H. Novel fibrin functionalized multilayered electrospun nanofiber membrane for burn wound treatment. J. Mater. Sci. 2021, 56, 12814–12834. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Bera, H.; Zhang, H.; Chen, Y.; Cun, D.; Foderà, V.; Yang, M. α-Lactalbumin-based nanofiber dressings improve burn wound healing and reduce scarring. ACS Appl. Mater. Interfaces 2020, 12, 45702–45713. [Google Scholar] [CrossRef] [PubMed]
- Kossyvaki, D.; Suarato, G.; Summa, M.; Gennari, A.; Francini, N.; Gounaki, I.; Venieri, D.; Tirelli, N.; Bertorelli, R.; Athanassiou, A.; et al. Keratin–cinnamon essential oil biocomposite fibrous patches for skin burn care. Mater. Adv. 2020, 1, 1805–1816. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Annamalai, S.K.; Arunachalam, K.D.; Ramakrishna, S. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials 2013, 34, 724–734. [Google Scholar] [CrossRef]
- Steffens, D.; Leonardi, D.; da Luz Soster, P.R.; Lersch, M.; Rosa, A.; Crestani, T.; Pranke, P. Development of a new nanofiber scaffold for use with stem cells in a third degree burn animal model. Burns 2014, 40, 1650–1660. [Google Scholar] [CrossRef]
- Li, W.; Wu, D.; Zhu, S.; Liu, Z.; Luo, B.; Lu, L.; Zhou, C. Sustained release of plasmid DNA from PLLA/POSS nanofibers for angiogenic therapy. Chem. Eng. J. 2019, 365, 270–281. [Google Scholar] [CrossRef]
- Venugopal, J.R.; Zhang, Y.; Ramakrishna, S. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif. Organs 2006, 30, 440–446. [Google Scholar] [CrossRef]
- Sadeghi-Avalshahr, A.R.; Khorsand-Ghayeni, M.; Nokhasteh, S.; Molavi, A.M.; Naderi-Meshkin, H. Synthesis and characterization of PLGA/collagen composite scaffolds as skin substitute produced by electrospinning through two different approaches. J. Mater. Sci. Mater. Med. 2017, 28, 14. [Google Scholar] [CrossRef]
- Saeed, S.M.; Mirzadeh, H.; Zandi, M.; Barzin, J. Designing and fabrication of curcumin loaded PCL/PVA multi-layer nanofibrous electrospun structures as active wound dressing. Prog. Biomater. 2017, 6, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef]
- Zhang, Y.; Atala, A. Regenerative medicine of the bladder. In Principles of Regenerative Medicine, 3rd ed.; Academic Press: Waltham, MA, USA, 2019; pp. 1263–1279. [Google Scholar] [CrossRef]
- Souza, P.R.; de Oliveira, A.C.; Vilsinski, B.H.; Kipper, M.J.; Martins, A.F. Polysaccharide-Based Materials Created by Physical Processes: From Preparation to Biomedical Applications. Pharmaceutics 2021, 13, 621. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Singer, A.J.; Taira, B.R.; Anderson, R.; McClain, S.A.; Rosenberg, L. Reepithelialization of mid-dermal porcine burns after rapid enzymatic debridement with Debrase®. J. Burn Care Res. 2011, 32, 647–653. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Hongo, C.; Fukushima, R.; Wu, G.; Narita, H.; Noguchi, K.; Tanaka, Y.; Nishino, N. Crystal structures of collagen model peptides with Pro-Hyp-Gly repeating sequence at 1.26 Å resolution: Implications for proline ring puckering. Pept. Sci. Orig. Res. Biomol. 2004, 76, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.Z.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatin–formic acid solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Dhand, C.; Barathi, V.A.; Ong, S.T.; Venkatesh, M.; Harini, S.; Dwivedi, N.; Goh, E.T.L.; Nandhakumar, M.; Venugopal, J.R.; Diaz, S.M.; et al. Latent oxidative polymerization of catecholamines as potential cross-linkers for biocompatible and multifunctional biopolymer scaffolds. ACS Appl. Mater. Interfaces 2016, 8, 32266–32281. [Google Scholar] [CrossRef]
- Slater, S.; Mitsky, T.A.; Houmiel, K.L.; Hao, M.; Reiser, S.E.; Taylor, N.B.; Tran, M.; Valentin, H.E.; Rodriguez, D.J.; Stone, D.A.; et al. Metabolic engineering of Arabidopsis and Brassica for poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production. Nat. Biotechnol. 1999, 17, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Oliveira, J.M.; Sá-Lima, H.; Sousa, R.A.; Mano, J.F.; Reis, R.L. 2.211-Polymers of Biological Origin. Compr. Biomater. 2011, 2, 187–205. [Google Scholar] [CrossRef]
- Lu, J.W.; Zhu, Y.L.; Guo, Z.X.; Hu, P.; Yu, J. Electrospinning of sodium alginate with poly (ethylene oxide). Polymer 2006, 47, 8026–8031. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Bailey, F.E.; Koleske, J.V. Polyoxyalkylenes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly (ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, B.; Xia, G.; Choi, S.H. FDA’s poly (lactic-co-glycolic acid) research program and regulatory outcomes. AAPS J. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.A.; Flores-Sahagun, T.H.; Ribeiro, A.M. Biodegradable poly (L-lactic acid)(PLLA) and PLLA-3-arm blend membranes: The use of PLLA-3-arm as a plasticizer. Polym. Test. 2017, 60, 84–93. [Google Scholar] [CrossRef]
- Chaouat, M.; Le Visage, C.; Baille, W.E.; Escoubet, B.; Chaubet, F.; Mateescu, M.A.; Letourneur, D. A novel cross-linked poly (vinyl alcohol)(PVA) for vascular grafts. Adv. Funct. Mater. 2008, 18, 2855–2861. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly (vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Biopolym. PVA Hydrogels Anionic Polym. Nanocomposites 2000, 153, 37–65. [Google Scholar] [CrossRef]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-vinylpyrrolidone: Synthesis, characterization and uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef]
- Siparsky, G.L.; Voorhees, K.J.; Miao, F. Hydrolysis of polylactic acid (PLA) and polycaprolactone (PCL) in aqueous acetonitrile solutions: Autocatalysis. J. Environ. Polym. Degrad. 1989, 6, 31–41. [Google Scholar] [CrossRef]
- Yu, C.H.; Chan, V.; Li, C.; Hsieh, J.H.; Lin, P.H.; Tsai, Y.H.; Chen, Y. Fabrication of Polylactic Acid/Paclitaxel Nano Fibers by Electrospinning for Cancer Therapeutics. BMC Chem. 2020, 14, 1–12. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications–a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Kohli, N.; Sharma, V.; Brown, S.J.; García-Gareta, E. Synthetic polymers for skin biomaterials. In Biomaterials for Skin Repair and Regeneration; Woodhead Publishing: Cambridge, UK, 2019; pp. 125–149. [Google Scholar] [CrossRef]
- Frantz, A.C.; San, E.D.; Pope, L.C.; Burke, T. Using genetic methods to investigate dispersal in two badger (Meles meles) populations with different ecological characteristics. Heredity 2010, 104, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.F.; Fernández, I.D.; Maldonado, H.; Torres, R.; Zavaleta, A.; Vaisberg, A.J.; Hammond, G.B. Evaluation of the wound-healing activity of selected traditional medicinal plants from Peru. J. Ethnopharmacol. 1997, 55, 193–200. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Homayoni, H.; Ravandi, S.A.; Valizadeh, M. Electrospinning of chitosan nanofibers: Processing optimization. Carbohydr. Polym. 2009, 77, 656–661. [Google Scholar] [CrossRef]
- De Vrieze, S.; Westbroek, P.; Van Camp, T.; Van Langenhove, L. Electrospinning of chitosan nanofibrous structures: Feasibility study. J. Mater. Sci. 2007, 42, 8029–8034. [Google Scholar] [CrossRef]
- Zhou, H.; Beevers, C.S.; Huang, S. The targets of curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Chromek, M.; Slamová, Z.; Bergman, P.; Kovács, L.; Podracká, L.U.; Ehrén, I.; Hökfelt, T.; Gudmundsson, G.H.; Gallo, R.L.; Agerberth, B.; et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006, 12, 636–641. [Google Scholar] [CrossRef]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- San José-Rodríguez, J.C.; de León, M.S. La miel como antibiótico tópico en las úlceras por presión Actualización. Med. Natur. 2015, 9, 93–103. [Google Scholar]
- Gao, C.; Yan, T.; Du, J.; He, F.; Luo, H.; Wan, Y. Introduction of broad spectrum antibacterial properties to bacterial cellulose nanofibers via immobilising ε-polylysine nanocoatings. Food Hydrocoll. 2014, 36, 204–211. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Martín-Gil, J.; Pérez-Lebeña, E.; Ruano-Rosa, D.; Revuelta, J.L.; Casanova-Gascón, J.; Ramos-Sánchez, M.C.; Martín-Ramos, P. Antifungal agents based on chitosan oligomers, ε-polylysine and Streptomyces spp. secondary metabolites against three Botryosphaeriaceae species. Antibiotics 2019, 8, 99. [Google Scholar] [CrossRef]
- Khan, F.; Sarker, M.; Rahman, M.; Ming, L.C.; Mohamed, I.N.; Zhao, C.; Sheikh, B.Y.; Tsong, H.F.; Rashid, M.A. Comprehensive review on phytochemicals, pharmacological and clinical potentials of Gymnema sylvestre. Front. Pharmacol. 2019, 10, 1223. [Google Scholar] [CrossRef]
- Amid, A.; Ismail, N.A.; Yusof, F.; Salleh, H.M. Expression, purification, and characterization of a recombinant stem bromelain from Ananas comosus. Process Biochem. 2011, 46, 2232–2239. [Google Scholar] [CrossRef]
- Abdul Muhammad, Z.; Ahmad, T. Therapeutic uses of pineapple-extracted bromelain in surgical care—A review. JPMA J. Pak. Med. Assoc. 2017, 67, 121. [Google Scholar] [PubMed]
- Amini, A.; Masoumi-Moghaddam, S.; Morris, D.L. Utility of Bromelain and N-Acetylcysteine in Treatment of Peritoneal Dissemination of Gastrointestinal Mucin-Producing Malignancies; Springer: London, UK, 2016. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Starakis, I.K.; Papadomanolaki, M.G.; Mavroeidi, N.G.; Ganotakis, E.S. The hypolipidaemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population: A prospective study. J. Sci. Food Agric. 2014, 94, 432–437. [Google Scholar] [CrossRef]
- De Morais, M.G.; Stillings, C.; Dersch, R.; Rudisile, M.; Pranke, P.; Costa, J.A.; Wendorff, J. Preparation of nanofibers containing the microalga Spirulina (Arthrospira). Bioresour. Technol. 2010, 101, 2872–2876. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef]
- Jiang, Z.; Mao, Z. Astragaloside IV (AS-IV) alleviates the malignant biological behavior of hepatocellular carcinoma via Wnt/β-catenin signaling pathway. RSC Adv. 2019, 9, 35473–35482. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Sadiq, A.; Shah, A.; Jeschke, M.G.; Belo, C.; Qasim Hayat, M.; Murad, S.; Amini-Nik, S. The role of serotonin during skin healing in post-thermal injury. Int. J. Mol. Sci. 2018, 19, 1034. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Fibrin formation, structure and properties. Fibrous Proteins Struct. Mech. 2017, 82, 405–456. [Google Scholar] [CrossRef]
- Shirvani Farsani, Z.; Mansouri, K.; Bidmeshkipour, A.; Mostafaie, A. Isolation and Primary Culture of Rat Hepatocytes Using Kiwifruit Actinidin. Sci. J. Hamedan Univ. Med. Sci. Health Serv. 2007, 14, 16–22. [Google Scholar]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Sasannejad, P.; Saeedi, M.; Shoeibi, A.; Gorji, A.; Abbasi, M.; Foroughipour, M. Lavender essential oil in the treatment of migraine headache: A placebo-controlled clinical trial. Eur. Neurol. 2012, 67, 288–291. [Google Scholar] [CrossRef]
- Cavanagh, H.; Wilkinson, J. Biological activities of Lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid.-Based Complementary Altern. Med. eCAM 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Pringle, N.A.; Koekemoer, T.C.; Holzer, A.; Young, C.; Venables, L.; van de Venter, M. Potential therapeutic benefits of green and fermented Rooibos (Aspalathus linearis) in dermal wound healing. Planta Med. 2018, 84, 645–652. [Google Scholar] [CrossRef] [PubMed]
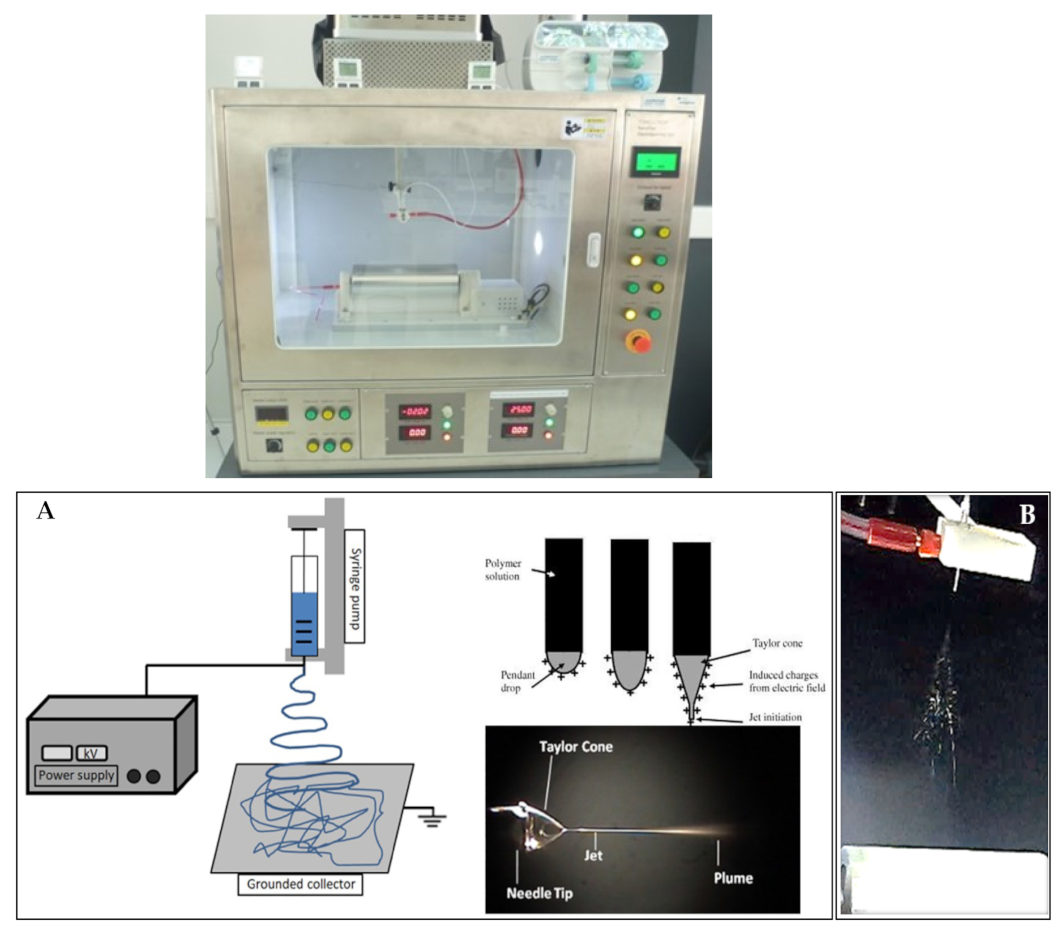
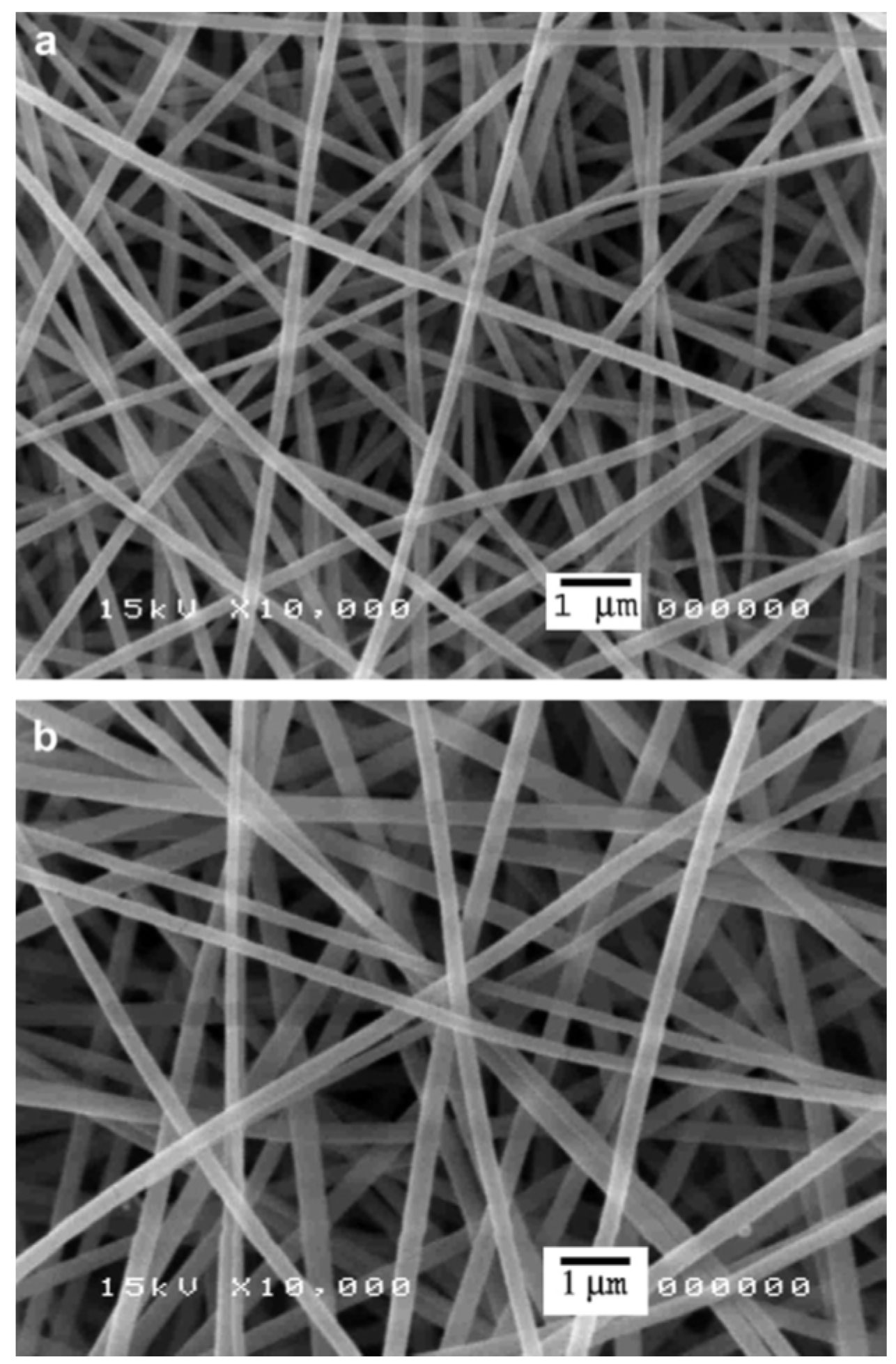
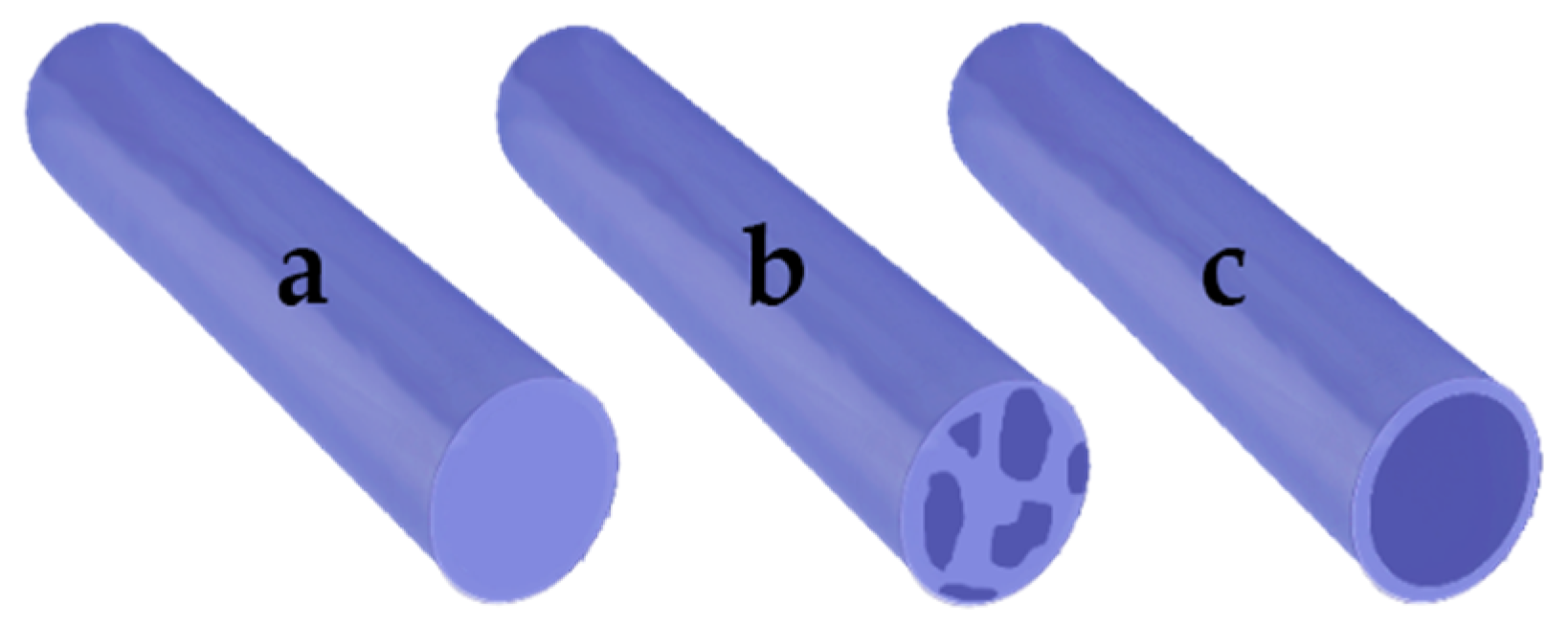
| Active Compound | Reference | |
|---|---|---|
| Metallic Particles | Silver Nitrate | [27] |
| Silver NanoparticlesSilver Nitrate | [28] | |
| Mesoporous Silica Nanoparticles with Silver Nanoparticles | [29] | |
| Zinc Oxide Nanoparticles | [30,31] | |
| Iron oxide Nanoparticles | [31] | |
| Gold Nanoparticles | [31] | |
| Piroxicam | [32] | |
| Synthetic compounds | Nitrofurazone | [33] |
| Cefotaxime | [34] | |
| Hydroxyapatite | [35] | |
| Silver Sulfadiazine | [20,36,37] | |
| Peptide P12 | [38] | |
| Polycaprolactone | [39] | |
| Polypropylene Fumarate | [40] | |
| Mineralized Magnesium | [41] | |
| Matrix | Encapsulated Bioactive Compound | Electrospinning Parameters | Diameter of Fibers | Fiber Type | Biological Effects of Electrospun Fibers | Reference |
|---|---|---|---|---|---|---|
| Polyurethane | Badger (Meles meles) oil | Voltage: 20 kV Flow Rate:—L/h Distance:15 cm | 375–518 nm | Blend-composite | Antibacterial | [2] |
| Polyurethane/Silver nanoparticles (10/3% w/w) | Olive Oil (Olea europaea L.) | Voltage: 15 kV Flow Rate:—mL/h Distance: 10 cm | 250–550 nm | Blend-composite | Antibacterial | [10] |
| Silk fibroin/Gelatin (1:3 w/w) | Astragaloside IV | Voltage: 15 kV Flow Rate: 0.1 mL/h Distance:—cm | _ | Blend-composite | Accelerate the process of wound healing | [16] |
| Chitosan-Deacetylated | Chitosan/L-arginine | Voltage: 28 kV Flow Rate: 1.2 mL/h Distance: 10 cm | 50–500 nm | Blend-composite | Antibacterial | [18] |
| Polycaprolactone/Gelatin (Core) (8/4% w/w) Gelatin (Shell) | Minocycline hydrochloride G. sylvestre extracts | Voltage: 13 kV Flow Rate: 1.2 & 1 mL/h Distance: 12 cm | 300–450 nm | Core/Shell | Antibacterial Nanofibers | [19] |
| Polylactide/Collagen (20/4% w/v) | Fermented rooibos A. linearis extracts | Voltage: 25 kV Flow Rate: 0.1 mL/min Distance: 22 cm | 13–23 µm | Blend-composite | Antibacterial Nanofibers; Accelerate the process of wound healing | [20] |
Chitosan | Bromelain | Voltage: 10 kV Flow Rate: 0.5 mL/h Distance: 20 cm. | 140–360 nm | Blend-composite | Accelerate the process of wound healing | [21] |
| Polylactide/Poly(ethylene glycol) (Core) (1:1 w/w) Polylactide/Poly(vinyl pyrrolidone) (Shell) (5:5, 7:3, 8:2, 9:1 w/w) | Peptides HHC36 Curcumin | Voltage: 20 kV Flow Rate:—mL/h Distance: 15 cm | 3.2–4.6 μm | Core/Shell | Antibacterial | [22] |
| Gelatin | ε-Polylysine | Voltage: 12 kV Flow Rate: 0.8 mL/h Distance: 12 cm | 425 ± 33 nm | Blend-composite | Antibacterial | [24] |
| Poly(vinyl alco-hol) | Chitosan | Voltage: 18 kV Flow Rate: 0.8 mL/h Distance: 12 cm | 130–170 nm | Blend-composite | Antibacterial | [25] |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) | _ | Voltage: 8 kV Flow Rate: 0.002 mL/min Distance: 12 cm | 510–670 nm | Simple Fibers (Mono-polymer) | Accelerate the process of wound healing | [26] |
| Silk fibroin/Poloxamer 407 (P407) (1:0, 3:1, 1:1 w/w) | Manuka Honey | Voltage: 25–23 kV Flow Rate: 3–4 mL/h Distance: 16.5–18 cm | 2.4–5.9 μm | Blend-composite | Antibacterial; Accelerate the process of wound healing | [47] |
| Sodium Alginate-Poly(ethylene glycol)/Pluronic F127 (surfactant) (8:2 w/w—1.5% w/v) | Lavender essential oil (Lavandula angustifolia) | Voltage: 25 kV Flow Rate: 0.5 mL/h Distance: 20 cm | 50–125 nm | Blend-Emulsion Electrospinning | Antibacterial Nanofibers; Accelerate the process of wound healing | [48] |
| Polycaprolactone/Chitosan (10, 15, 20/15% w/w) | Quercetin/Rutin | Voltage: 24–32 kV Flow Rate: 0.77 mL/h Distance: 15 cm | 90–120 nm | Blend-composite | Antibacterial Nanofibers; Accelerate the process of wound healing | [55] |
| Chitosan/Poly(ethylene oxide) (2/0.5% w/w) | Actinidin | Voltage:—kV Flow Rate: 0.5–1.5 mL/h Distance: 7–9 cm | 100–200 nm | Blend-composite + Actinidin enzyme immobilization | Antibacterial Nanofibers; Accelerate the process of wound healing | [56] |
| Gelatin (layer 1) Poly(vinyl alcohol)/Sodium Alginate (layer 2) (13/2.5% w/v) Chitosan/Poly(vinyl alcohol) (layer 3) (2/15% w/v) | Fibrin | Voltage: 25–30 kV Flow Rate: 0.8–1.1 mL/h Distance:—cm | 150–350 nm | Blend-composite | Antibacterial Nanofibers; Accelerate the process of wound healing | [57] |
| Polycaprolactone | α-Lactalbumin | Voltage: 9–18 kV Flow Rate: 0.3–0.6 mL/min Distance: 15 cm | 183–344 nm | Blend-composite | Accelerate the process of wound healing | [58] |
| Poly(vinyl pyrrolidone)/Keratin (3:1, 2:1, 1:1 w/w) | Cinnamon essential oil | Voltage: 24 kV Flow Rate: 350–850 μL/h Distance: 25 cm | 315–466 nm | Blend-composite | Antibacterial Nanofibers; Accelerate the process of wound healing | [59] |
| Polycaprolactone/Gelatin (6:4 w/w) | Plant extracts: I. aspalathoides A. indica M. edule M. andamanica | Voltage: 15 kV Flow Rate: 1 mL/h Distance: 12 cm | 266–601 nm | Blend-composite | Accelerate the process of wound healing | [60] |
| Poly-D,L-lactic acid | Microalga Spirulina (Arthrospira platensis) | Voltage: 15 kV Flow Rate: 2 mL/h Distance: 15 cm | 260–270 nm | Blend-composite | Accelerate the process of wound healing | [61] |
| Poly(L-lactic acid)/polyhedral oligomeric silsesquioxane nanoparticles (24:1 w/w) | Plasmid DNA Encoding Angiopoietin-1 (pAng) | Voltage: 13 kV Flow Rate: 0.8 mL/h Distance: 15 cm | 580–780 nm | Blend-composite | Accelerate the process of wound healing | [62] |
| Polycaprolactone/Collagen (55:25 w/v) | _ | Voltage: 13 kV Flow Rate: 3 mL/h Distance: 13 cm | 170–275 nm | Blend-composite | Accelerate the process of wound healing | [63] |
| Poly(lactic-co-glycolic acids)/Collagen (4:1 w/w) | _ | Voltage: 28 kV Flow Rate: 1 mL/h Distance: 17 cm | 100–300 nm | Blend-composite | Accelerate the process of wound healing | [64] |
| Polycaprolactone (12.5% w/v) Poly(vinyl al-co-hol) (8% w/v) | Curcumin | Voltage: 12, 18, 24 kV Flow Rate: 1, 2, 3 mL/h Distance: 16 cm | _ | Blend-composite | Antibacterial | [65] |
| Material Type | Material Name | Reference |
|---|---|---|
| Natural | Chitosan | [18,21,55,56,57] |
| Collagen | [20,63,64] | |
| Gelatin | [19,57,60,66,67] | |
| Keratin | [59] | |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) | [26] | |
| Silk Fibroin | [16] | |
| Sodium Alginate | [48,57] | |
| Synthetic | Poly(ethylene glycol)/Poly(ethylene oxide)/Polyoxyethylene | [22,56] |
| Poly(lactic-co-glycolic acids) | [64] | |
| Poly(L-lactic acid) | [62] | |
| Poly(vinyl alcohol) | [25,57,65] | |
| Poly(vinyl pyrrolidone) | [22,59] | |
| Polycaprolactone | [19,55,58,60,63,65] | |
| Poly-D,L-lactic acid | [61] | |
| Polylactide | [20,22] | |
| Polyurethane | [2] |
| Biological Effects | Bioactive Compounds | Reference |
|---|---|---|
| Antimicrobial | Badger (Meles meles) oil | [2] |
| Olive (Olea europaea L.) oil | [16] | |
| CH—CH/L-arginine | [18,25] | |
| Gymnema sylvestre extract | [19] | |
| ε-Polylysine | [24] | |
| Manuka Honey (*) | [47] | |
| Peptides HHC36 | [62] | |
| Curcumin | [62,65] | |
| Wound healing accelerator | Astragaloside IV | [16] |
| Bromelain | [21] | |
| Quercetin/Rutin | [55] | |
| Actinidin | [56] | |
| Fibrin | [57] | |
| α-Lactalbumin | [58] | |
| Microalga Spirulina (Arthrospira platensis) | [61] | |
| Plasmid DNA Encoding Angiopoietin-1 | [62] | |
| Antimicrobial and wound healing accelerator | Aspalathus linearis fermented extract | [20] |
| Lavender (Lavandula angustifolia) essential oil | [48] | |
| Cinnamon (Cinnamomum verum) essential oil | [59] | |
| Indigofera aspalathoides extract | [60] | |
| Azadirachta indica extract | [60] | |
| Memecylon edule extract | [60] | |
| Myristica andamanica extract | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermosilla, J.; Pastene-Navarrete, E.; Acevedo, F. Electrospun Fibers Loaded with Natural Bioactive Compounds as a Biomedical System for Skin Burn Treatment. A Review. Pharmaceutics 2021, 13, 2054. https://doi.org/10.3390/pharmaceutics13122054
Hermosilla J, Pastene-Navarrete E, Acevedo F. Electrospun Fibers Loaded with Natural Bioactive Compounds as a Biomedical System for Skin Burn Treatment. A Review. Pharmaceutics. 2021; 13(12):2054. https://doi.org/10.3390/pharmaceutics13122054
Chicago/Turabian StyleHermosilla, Jeyson, Edgar Pastene-Navarrete, and Francisca Acevedo. 2021. "Electrospun Fibers Loaded with Natural Bioactive Compounds as a Biomedical System for Skin Burn Treatment. A Review" Pharmaceutics 13, no. 12: 2054. https://doi.org/10.3390/pharmaceutics13122054
APA StyleHermosilla, J., Pastene-Navarrete, E., & Acevedo, F. (2021). Electrospun Fibers Loaded with Natural Bioactive Compounds as a Biomedical System for Skin Burn Treatment. A Review. Pharmaceutics, 13(12), 2054. https://doi.org/10.3390/pharmaceutics13122054








