Optimizing Solvent Selection and Processing Conditions to Generate High Bulk-Density, Co-Precipitated Amorphous Dispersions of Posaconazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.2.1. Spray-Dried Intermediate
2.2.2. Melt Quenched Dispersion
2.2.3. Co-Precipitated Amorphous Dispersion
2.2.4. cPAD Stability in Solvent Mixtures
2.2.5. Posaconazole Form III
2.2.6. Posaconazole Form γ
2.3. Characterization
2.3.1. X-ray Powder Diffraction
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Solid-State NMR Spectroscopy
2.3.4. Bulk Density
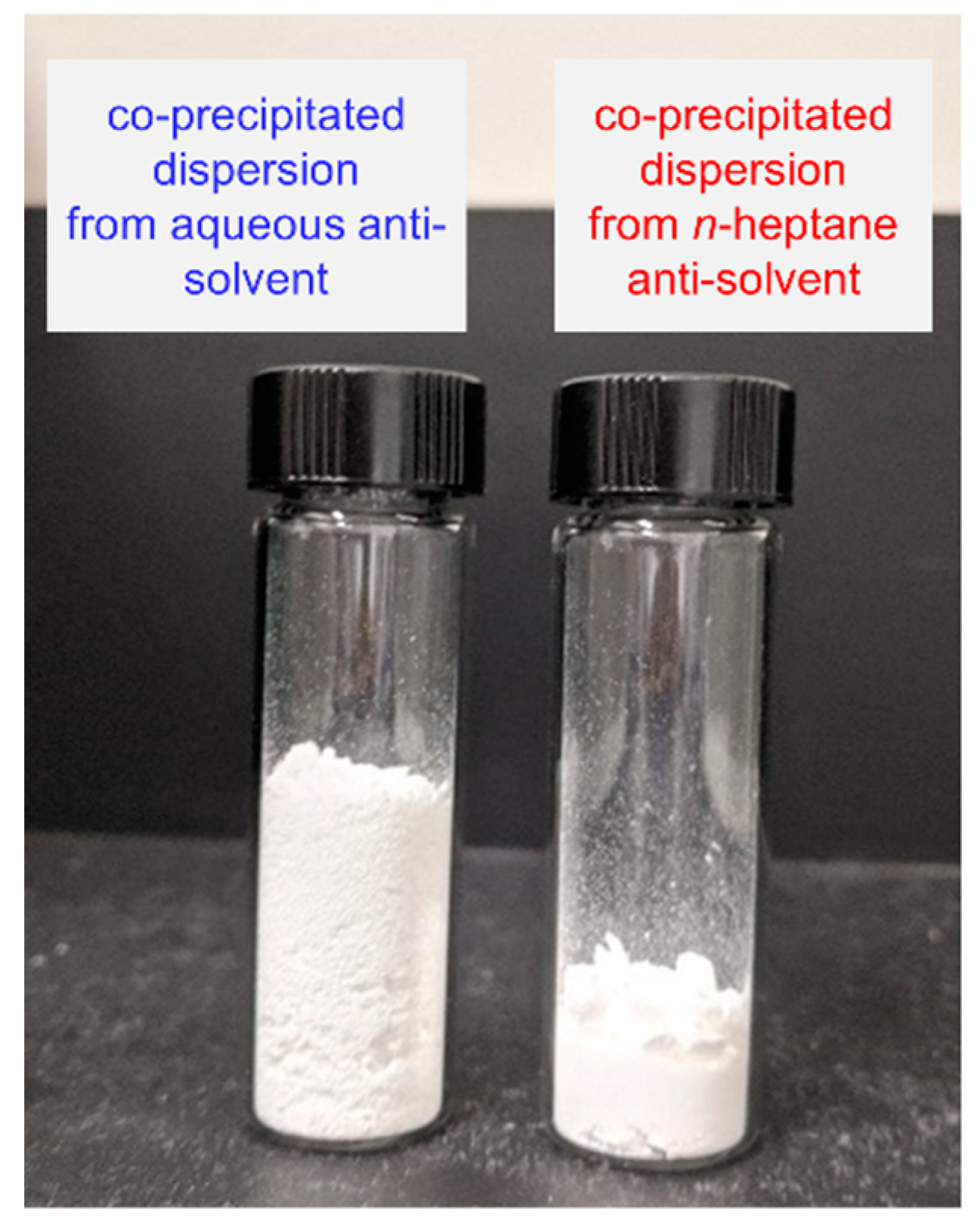
3. Results/Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Sandhu, H.; Choi, D.S.; Chokshi, H.; Malick, A.W. Amorphous solid dispersions. In Theory and Practice; Springer: Berlin, Germany, 2014. [Google Scholar]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric amorphous solid dispersions: A review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48, 27–42. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, Y.-C.; Shabani, Z.; Frankenfeld Lamm, C.; Zhu, W.; Li, Y.; Templeton, A. Processing impact on performance of solid dispersions. Pharmaceutics 2018, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Purohit, H.S.; Trasi, N.S.; Sun, D.D.; Chow, E.C.; Wen, H.; Zhang, X.; Gao, Y.; Taylor, L.S. Investigating the impact of drug crystallinity in amorphous tacrolimus capsules on pharmacokinetics and bioequivalence using discriminatory in vitro dissolution testing and physiologically based pharmacokinetic modeling and simulation. J. Pharm. Sci. 2018, 107, 1330–1341. [Google Scholar] [CrossRef]
- Purohit, H.S.; Trasi, N.S.; Osterling, D.J.; Stolarik, D.F.; Jenkins, G.J.; Gao, W.; Zhang, G.G.; Taylor, L.S. Assessing the impact of endogenously derived crystalline drug on the in vivo performance of amorphous formulations. Mol. Pharm. 2019, 16, 3617–3625. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Marques, S.; das Neves, J.; Sarmento, B. Amorphous solid dispersions: Rational selection of a manufacturing process. Adv. Drug Deliv. Rev. 2016, 100, 85–101. [Google Scholar] [CrossRef]
- Huang, S.; Williams, R.O. Effects of the preparation process on the properties of amorphous solid dispersions. AAPS PharmSciTech 2018, 19, 1971–1984. [Google Scholar] [CrossRef]
- Poozesh, S.; Setiawan, N.; Arce, F.; Sundararajan, P.; Della Rocca, J.; Rumondor, A.; Wei, D.; Wenslow, R.; Xi, H.; Zhang, S. Understanding the process-product-performance interplay of spray dried drug-polymer systems through complete structural and chemical characterization of single spray dried particles. Powder Technol. 2017, 320, 685–695. [Google Scholar] [CrossRef]
- Defrese, M.K.; Farmer, M.A.; Long, Y.; Timmerman, L.R.; Bae, Y.; Marsac, P.J. Approaches to Understanding the Solution-State Organization of Spray-Dried Dispersion Feed Solutions and Its Translation to the Solid State. Mol. Pharm. 2020, 17, 4548–4563. [Google Scholar] [CrossRef]
- Davis, M.T.; Potter, C.B.; Walker, G.M. Downstream processing of a ternary amorphous solid dispersion: The impacts of spray drying and hot melt extrusion on powder flow, compression and dissolution. Int. J. Pharm. 2018, 544, 242–253. [Google Scholar] [CrossRef]
- Rahimi, S.K.; O’Donnell, K.; Haight, B.; Machado, A.; Martin, C.; Meng, F.; Listro, T.; Zhang, F. Supercritical-CO2 Foam Extrusion of Hydroxypropyl Methyl Cellulose Acetate Succinate/Itraconazole Amorphous Solid Dispersions: Processing-Structure-Property Relations. J. Pharm. Sci. 2021, 110, 1444–1456. [Google Scholar] [CrossRef]
- Démuth, B.; Farkas, A.; Szabó, B.; Balogh, A.; Nagy, B.; Vágó, E.; Vigh, T.; Tinke, A.; Kazsu, Z.; Demeter, Á. Development and tableting of directly compressible powder from electrospun nanofibrous amorphous solid dispersion. Adv. Powder Technol. 2017, 28, 1554–1563. [Google Scholar] [CrossRef]
- Démuth, B.; Nagy, Z.K.; Balogh, A.; Vigh, T.; Marosi, G.; Verreck, G.; Van Assche, I.; Brewster, M. Downstream processing of polymer-based amorphous solid dispersions to generate tablet formulations. Int. J. Pharm. 2015, 486, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Jermain, S.V.; Brough, C.; Williams, R.O., III. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ho, C. Amorphous solid dispersions: Utilization and challenges in drug discovery and development. J. Pharm. Sci. 2015, 104, 3237–3258. [Google Scholar] [CrossRef]
- Mudie, D.M.; Buchanan, S.; Stewart, A.M.; Smith, A.; Shepard, K.B.; Biswas, N.; Marshall, D.; Ekdahl, A.; Pluntze, A.; Craig, C.D. A novel architecture for achieving high drug loading in amorphous spray dried dispersion tablets. Int. J. Pharm. X 2020, 2, 100042. [Google Scholar] [CrossRef]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Lakshman, J.P.; Cao, Y.; Kowalski, J.; Serajuddin, A.T. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Mol. Pharm. 2008, 5, 994–1002. [Google Scholar] [CrossRef]
- Lipinski, C. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- DiNunzio, J.C.; Brough, C.; Miller, D.A.; Williams, R.O., III; McGinity, J.W. Fusion processing of itraconazole solid dispersions by KinetiSol® dispersing: A comparative study to hot melt extrusion. J. Pharm. Sci. 2010, 99, 1239–1253. [Google Scholar] [CrossRef]
- DiNunzio, J.C.; Brough, C.; Hughey, J.R.; Miller, D.A.; Williams, R.O., III; McGinity, J.W. Fusion production of solid dispersions containing a heat-sensitive active ingredient by hot melt extrusion and Kinetisol® dispersing. Eur. J. Pharm. Biopharm. 2010, 74, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Sandhu, H.; Phuapradit, W.; Pinal, R.; Iyer, R.; Albano, A.; Chatterji, A.; Anand, S.; Choi, D.S.; Tang, K. Development of novel microprecipitated bulk powder (MBP) technology for manufacturing stable amorphous formulations of poorly soluble drugs. Int. J. Pharm. 2012, 438, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, J.M.; McConville, J.T.; Crisp, M.T.; Johnston, K.P.; Williams, R.O., III. Supersaturation produces high bioavailability of amorphous danazol particles formed by evaporative precipitation into aqueous solution and spray freezing into liquid technologies. Drug Dev. Ind. Pharm. 2006, 32, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, H.; Lang, B.; O’Donnell, K.; Zhang, H.; Wang, Z.; Dong, Y.; Wu, C.; Williams, R.O., III. Formulation and delivery of improved amorphous fenofibrate solid dispersions prepared by thin film freezing. Eur. J. Pharm. Biopharm. 2012, 82, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Badens, E.; Majerik, V.; Horváth, G.; Szokonya, L.; Bosc, N.; Teillaud, E.; Charbit, G. Comparison of solid dispersions produced by supercritical antisolvent and spray-freezing technologies. Int. J. Pharm. 2009, 377, 25–34. [Google Scholar] [CrossRef]
- Guo, Z.; Boyce, C.; Rhodes, T.; Liu, L.; Salituro, G.M.; Lee, K.-J.; Bak, A.; Leung, D.H. A novel method for preparing stabilized amorphous solid dispersion drug formulations using acoustic fusion. Int. J. Pharm. 2021, 592, 120026. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.K.; Schenck, L.; Koynov, A.; Rumondor, A.C.; Jin, X.; Marota, M.; Dalton, C. Producing amorphous solid dispersions via co-precipitation and spray drying: Impact to physicochemical and biopharmaceutical properties. J. Pharm. Sci. 2018, 107, 183–191. [Google Scholar] [CrossRef]
- Dong, Z.; Chatterji, A.; Sandhu, H.; Choi, D.S.; Chokshi, H.; Shah, N. Evaluation of solid state properties of solid dispersions prepared by hot-melt extrusion and solvent co-precipitation. Int. J. Pharm. 2008, 355, 141–149. [Google Scholar] [CrossRef]
- Schenck, L.; Boyce, C.; Frank, D.; Koranne, S.; Ferguson, H.M.; Strotman, N. Hierarchical Particle Approach for Co-Precipitated Amorphous Solid Dispersions for Use in Preclinical In Vivo Studies. Pharmaceutics 2021, 13, 1034. [Google Scholar] [CrossRef] [PubMed]
- Schenck, L.; Mann, A.K.P.; Liu, Z.; Milewski, M.; Zhang, S.; Ren, J.; Dewitt, K.; Hermans, A.; Cote, A. Building a better particle: Leveraging physicochemical understanding of amorphous solid dispersions and a hierarchical particle approach for improved delivery at high drug loadings. Int. J. Pharm. 2019, 559, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Bhujbal, S.V.; Pathak, V.; Zemlyanov, D.Y.; Taylor, L.S.; Zhou, Q.T. Physical stability and dissolution of lumefantrine amorphous solid dispersions produced by spray anti-solvent precipitation. J. Pharm. Sci. 2021, 110, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.H.; Rajesh, A.; Pandya, K.M.; Lubach, J.W.; Muliadi, A.; Yost, E.; Jia, W.; Nagapudi, K. Impact of method of preparation of amorphous solid dispersions on mechanical properties: Comparison of coprecipitation and spray drying. J. Pharm. Sci. 2019, 108, 870–879. [Google Scholar] [CrossRef]
- Harter, A.; Schenck, L.; Lee, I.; Cote, A. High-shear rotor–stator wet milling for drug substances: Expanding capability with improved scalability. Org. Process Res. Dev. 2013, 17, 1335–1344. [Google Scholar] [CrossRef]
- Cote, A.K.A.; O’Connor, R.; Schenck, L. Interrogation of Mixing in Custom High-Speed Rotor/Stator Wetmill. In North American Mixing Forum Mixing XXVI; North American Mixing Forum: San Juan, Puerto Rico, 2018. [Google Scholar]
- Paudel, A.; Van den Mooter, G. Influence of solvent composition on the miscibility and physical stability of naproxen/PVP K 25 solid dispersions prepared by cosolvent spray-drying. Pharm. Res. 2012, 29, 251–270. [Google Scholar] [CrossRef]
- Broman, E.; Khoo, C.; Taylor, L.S. A comparison of alternative polymer excipients and processing methods for making solid dispersions of a poorly water soluble drug. Int. J. Pharm. 2001, 222, 139–151. [Google Scholar] [CrossRef]
- Yang, Z.; Nollenberger, K.; Albers, J.; Moffat, J.; Craig, D.; Qi, S. The effect of processing on the surface physical stability of amorphous solid dispersions. Eur. J. Pharm. Biopharm. 2014, 88, 897–908. [Google Scholar] [CrossRef]
- Dohrn, S.; Luebbert, C.; Lehmkemper, K.; Kyeremateng, S.O.; Degenhardt, M.; Sadowski, G. Solvent influence on the phase behavior and glass transition of Amorphous Solid Dispersions. Eur. J. Pharm. Biopharm. 2021, 158, 132–142. [Google Scholar] [CrossRef]
- Dedroog, S.; Boel, E.; Kindts, C.; Appeltans, B.; Van den Mooter, G. The underestimated contribution of the solvent to the phase behavior of highly drug loaded amorphous solid dispersions. Int. J. Pharm. 2021, 609, 121201. [Google Scholar] [CrossRef]
- Wieser, J.; Pichler, A.; Hotter, A.; Griesser, U.; Langes, C. Crystalline Form of Posaconazole. Google Patents US8435998B2, 7 May 2013. [Google Scholar]
- Wieser, J.; Pichler, A.; Hotter, A.; Griesser, U.; Langes, C.; Laschober, C. Pharmaceutical Compositions Containing a Crystalline Form of Posaconazole. Google Patents US8563555B2, 22 October 2013. [Google Scholar]
- Huang, C.; Klinzing, G.; Procopio, A.; Yang, F.; Ren, J.; Burlage, R.; Zhu, L.; Su, Y. Understanding compression-induced amorphization of crystalline posaconazole. Mol. Pharm. 2018, 16, 825–833. [Google Scholar] [CrossRef]
- Lu, X.; Huang, C.; Lowinger, M.B.; Yang, F.; Xu, W.; Brown, C.D.; Hesk, D.; Koynov, A.; Schenck, L.; Su, Y. Molecular Interactions in Posaconazole Amorphous Solid Dispersions from Two-Dimensional Solid-State NMR Spectroscopy. Mol. Pharm. 2019, 16, 2579–2589. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, M.; Huang, C.; Lowinger, M.B.; Xu, W.; Yu, L.; Byrn, S.R.; Templeton, A.C.; Su, Y. Atomic-level Drug Substance and Polymer Interaction in Posaconazole Amorphous Solid Dispersion from Solid-State NMR. Mol. Pharm. 2020, 17, 2585–2598. [Google Scholar] [CrossRef] [PubMed]
- Mudie, D.M.; Stewart, A.M.; Biswas, N.; Brodeur, T.J.; Shepard, K.B.; Smith, A.; Morgen, M.M.; Baumann, J.M.; Vodak, D.T. Novel High-Drug-Loaded Amorphous Dispersion Tablets of Posaconazole; In Vivo and In Vitro Assessment. Mol. Pharm. 2020, 17, 4463–4472. [Google Scholar] [CrossRef] [PubMed]
- De Alencar Danda, L.J.; de Medeiros Batista, L.; Melo, V.C.S.; Sobrinho, J.L.S.; Soares, M.F.D.L.R. Combining amorphous solid dispersions for improved kinetic solubility of posaconazole simultaneously released from soluble PVP/VA64 and an insoluble ammonio methacrylate copolymer. Eur. J. Pharm. Sci. 2019, 133, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Posaconazole. Drugs 2005, 65, 1553–1567. [Google Scholar] [CrossRef]
- Cristofoletti, R.; Patel, N.; Dressman, J.B. Differences in food effects for 2 weak bases with similar BCS drug-related properties: What is happening in the intestinal lumen? J. Pharm. Sci. 2016, 105, 2712–2722. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Mann, A.K.; Zhang, D.; Yang, Z. Processing Impact on In Vitro and In Vivo Performance of Solid Dispersions—A Comparison between Hot-Melt Extrusion and Spray Drying. Pharmaceutics 2021, 13, 1307. [Google Scholar] [CrossRef]
- Elkhabaz, A.; Sarkar, S.; Simpson, G.J.; Taylor, L.S. Characterization of phase transformations for amorphous solid dispersions of a weakly basic drug upon dissolution in biorelevant media. Pharm. Res. 2019, 36, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Din, S.U.; Hughes, H.; O’Reilly, N.J.; Cathcart, H.; O’Ceallaigh, T.; Ndzie, E.; McLoughlin, P. Investigation into the stability, crystallization kinetics, and heating rate dependent crystallization of amorphous posaconazole. Cryst. Growth Des. 2020, 20, 5129–5142. [Google Scholar] [CrossRef]
- Andrews, D.R.; Leong, W.; Sudhakar, A. Crystalline Antifungal Polymorph. Google Patents US6958337B2, 25 October 2005. [Google Scholar]
- Yang, F.; Su, Y.; Small, J.; Huang, C.; Martin, G.E.; Farrington, A.M.; DiNunzio, J.; Brown, C.D. Probing the molecular-level interactions in an active pharmaceutical ingredient (API)-polymer dispersion and the resulting impact on drug product formulation. Pharm. Res. 2020, 37, 1–16. [Google Scholar] [CrossRef]
- Harmon, P.; Galipeau, K.; Xu, W.; Brown, C.; Wuelfing, W.P. Mechanism of dissolution-induced nanoparticle formation from a copovidone-based amorphous solid dispersion. Mol. Pharm. 2016, 13, 1467–1481. [Google Scholar] [CrossRef]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef]
- Zhu, Z. Flash nanoprecipitation: Prediction and enhancement of particle stability via drug structure. Mol. Pharm. 2014, 11, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Dohrn, S.; Reimer, P.; Luebbert, C.; Lehmkemper, K.; Kyeremateng, S.O.; Degenhardt, M.; Sadowski, G. Thermodynamic modeling of solvent-impact on phase separation in amorphous solid dispersions during drying. Mol. Pharm. 2020, 17, 2721–2733. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.S.; Matzger, A.J. Effect of polymer hydrophobicity on the stability of amorphous solid dispersions and supersaturated solutions of a hydrophobic pharmaceutical. Mol. Pharm. 2019, 16, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cape, J.L.; Mankani, B.R.; Zemlyanov, D.Y.; Shepard, K.B.; Morgen, M.M.; Taylor, L.S. Water-induced phase separation of spray-dried amorphous solid dispersions. Mol. Pharm. 2020, 17, 4004–4017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, C.; Chen, Z.; Su, C.; Hageman, M.; Hussain, M.; Haskell, R.; Stefanski, K.; Qian, F. Drug–polymer–water interaction and its implication for the dissolution performance of amorphous solid dispersions. Mol. Pharm. 2015, 12, 576–589. [Google Scholar] [CrossRef]
- Ekdahl, A.; Mudie, D.; Malewski, D.; Amidon, G.; Goodwin, A. Effect of spray-dried particle morphology on mechanical and flow properties of felodipine in PVP VA amorphous solid dispersions. J. Pharm. Sci. 2019, 108, 3657–3666. [Google Scholar] [CrossRef]
- Browne, E.; Charifou, R.; Worku, Z.A.; Babu, R.P.; Healy, A.M. Amorphous solid dispersions of ketoprofen and poly-vinyl polymers prepared via electrospraying and spray drying: A comparison of particle characteristics and performance. Int. J. Pharm. 2019, 566, 173–184. [Google Scholar] [CrossRef]
- Schenck, L.R.; Lamberto, D.J.; Kukura, I.J.L.; Guzman, F.J.; Cote, A.; Koynov, A. Process for Preparing Pharmaceutical Compositions. Google Patents WO2017106130A1, 22 June 2017. [Google Scholar]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Dobry, D.E.; Settell, D.M.; Baumann, J.M.; Ray, R.J.; Graham, L.J.; Beyerinck, R.A. A model-based methodology for spray-drying process development. J. Pharm. Innov. 2009, 4, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.; Moreira, J.; Doktorovová, S. QbD approach to downstream processing of spray-dried amorphous solid dispersions—A case study. Pharm. Dev. Technol. 2021, 26, 269–277. [Google Scholar] [CrossRef]
- Friesen, D.T.; Shanker, R.; Crew, M.; Smithey, D.T.; Curatolo, W.; Nightingale, J. Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: An overview. Mol. Pharm. 2008, 5, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Schmitt, E.A.; Law, D.; Brackemeyer, P.J.; Zhang, G.G. Assessing Physical Stability Risk Using the Amorphous Classification System (ACS) Based on Simple Thermal Analysis. Mol. Pharm. 2019, 16, 2742–2754. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]

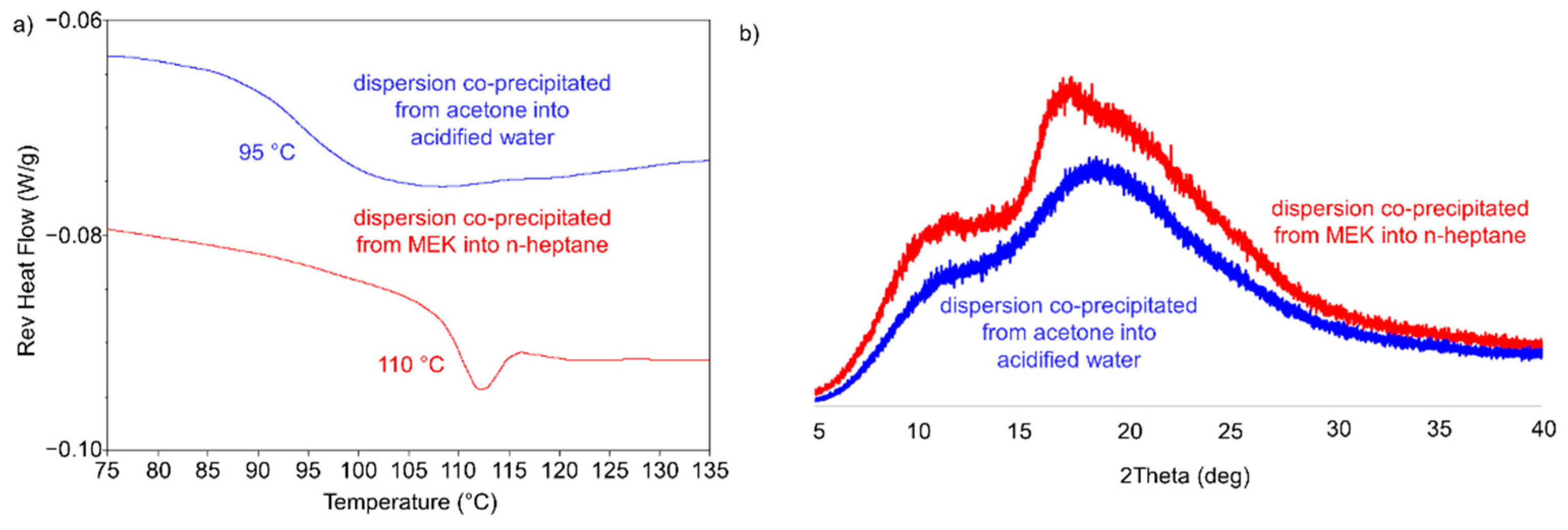
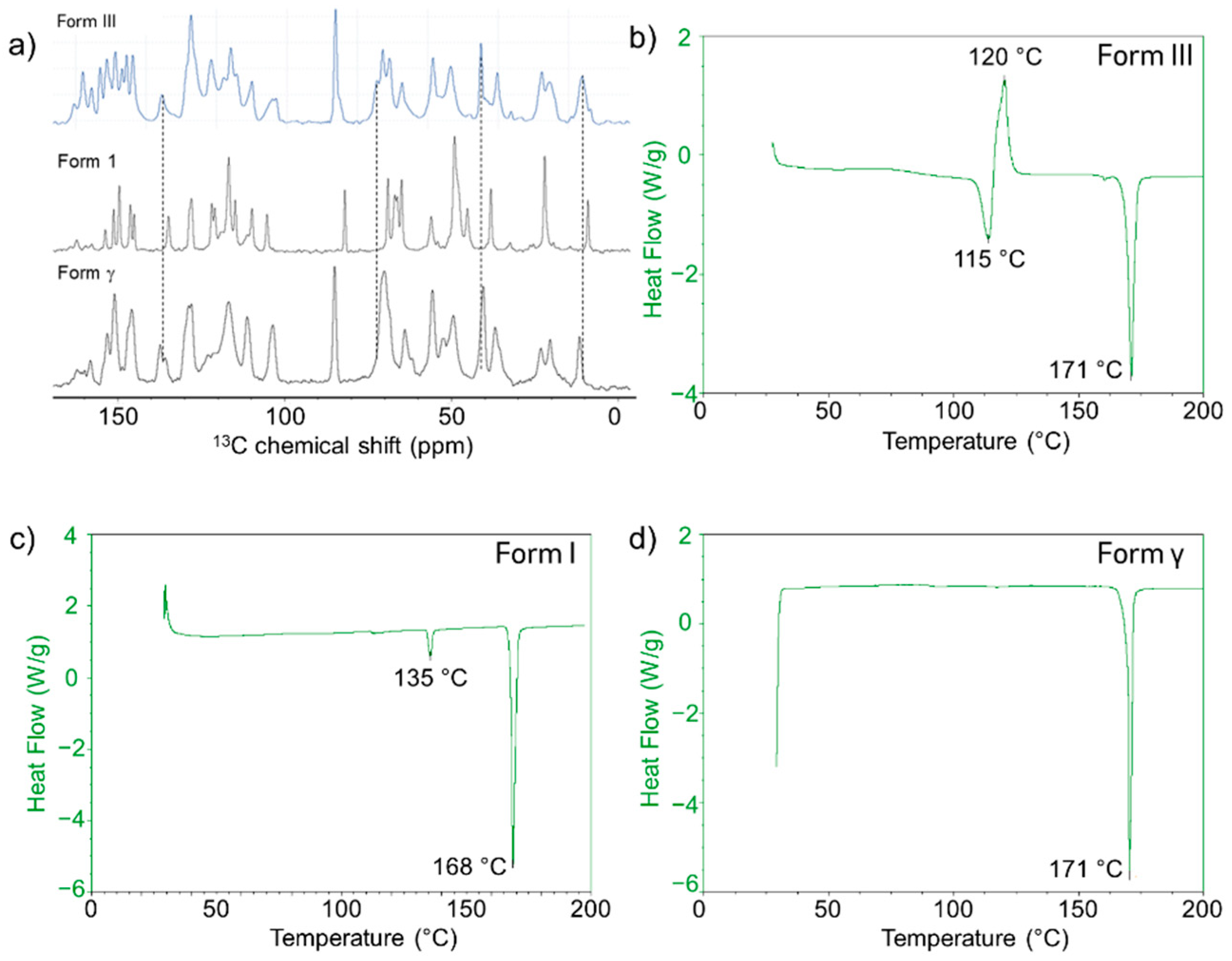
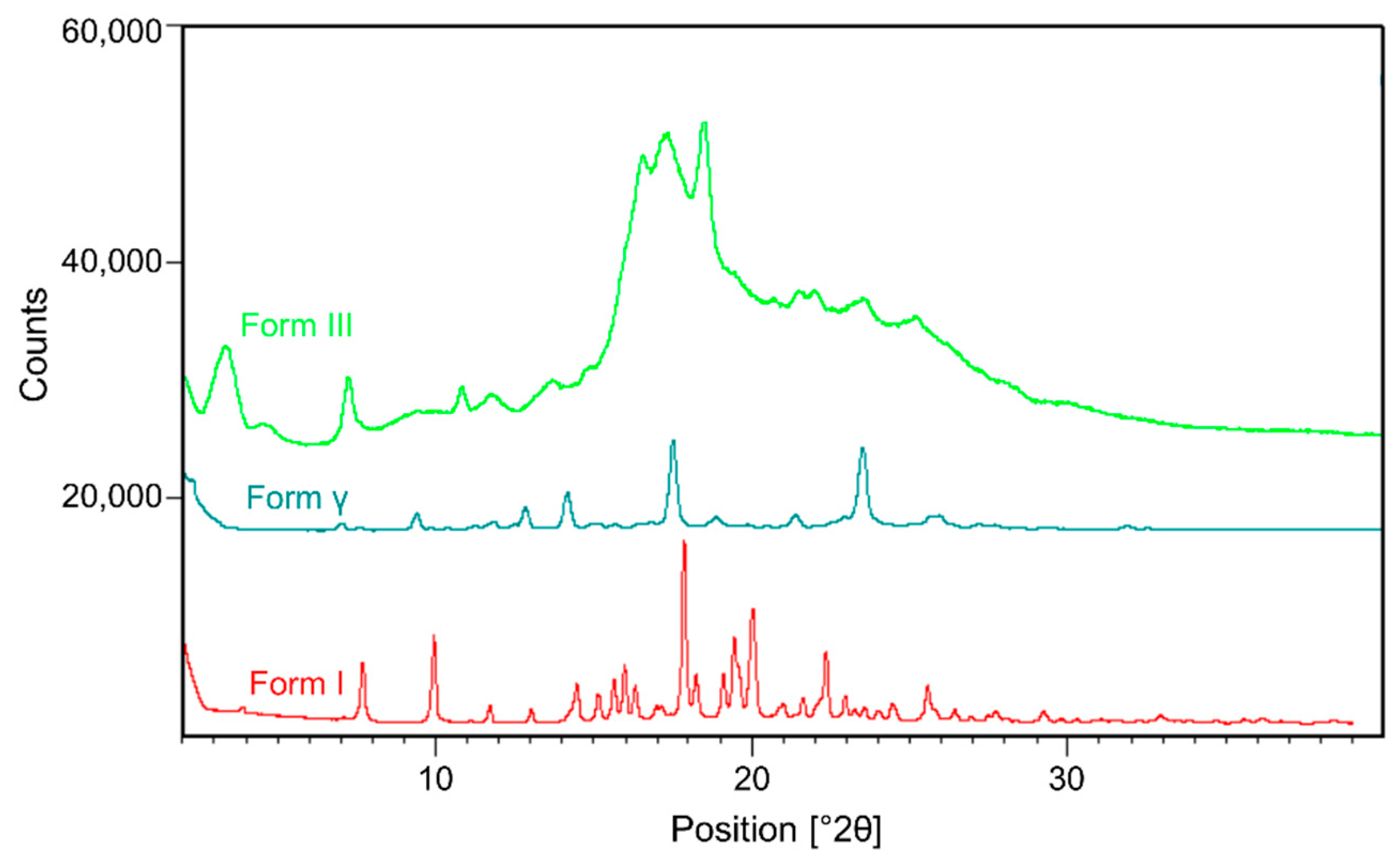
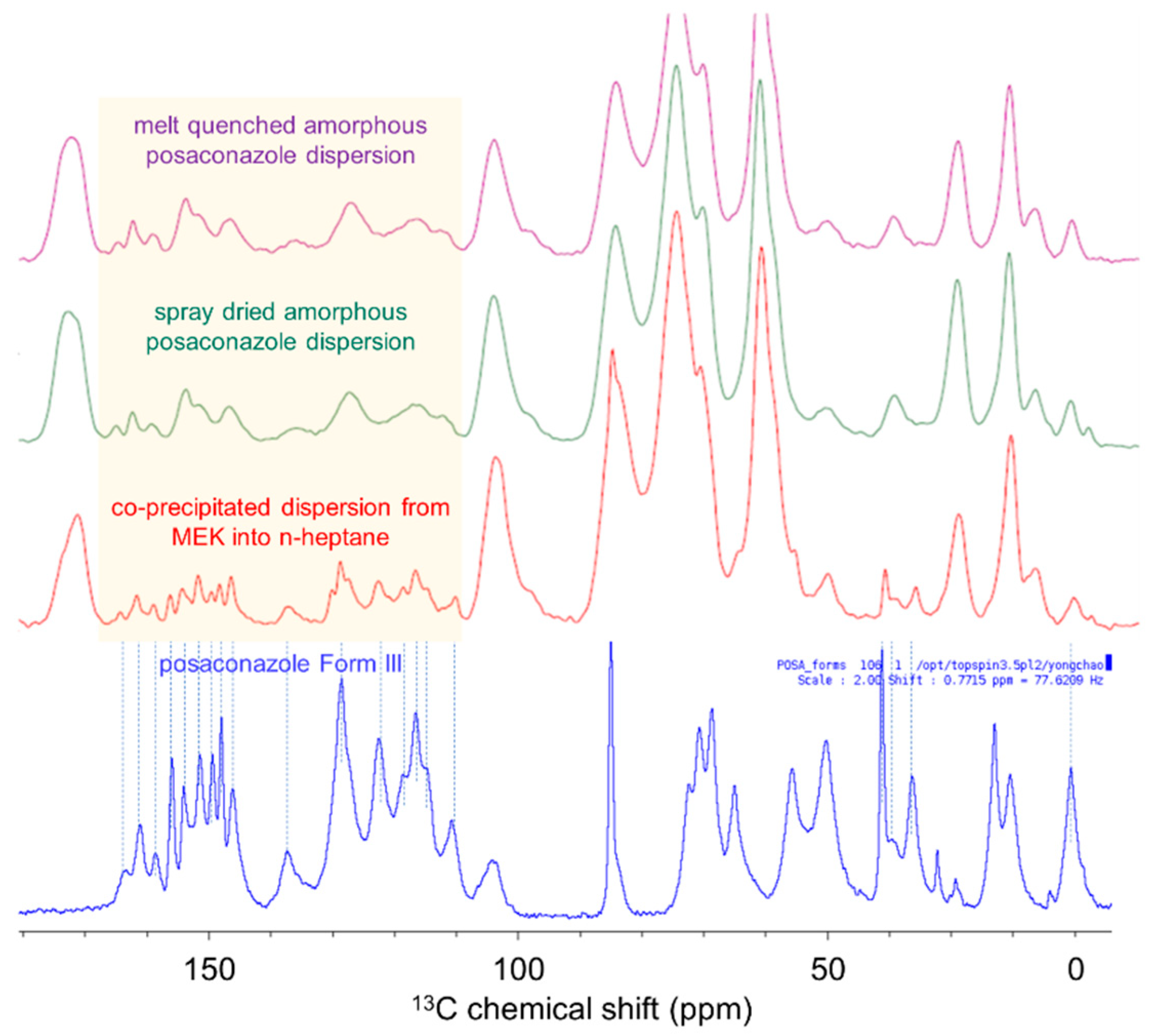

| Anti-Solvent | Solvent | Posaconazole Phase |
|---|---|---|
| n-heptane | Acetone | Form III |
| Acetone/water | Form III | |
| Methyl ethyl ketone | Form III | |
| Tetrahydrofuran | Form III | |
| Cyclohexane | Methyl ethyl ketone | Form III |
| 0.001 N HCl | Acetone | Amorphous |
| Ethanol/water | Amorphous | |
| Tetrahydrofuran | Amorphous/amorphous phase separation |
| Anti-Solvent | Solvent | Posaconazole Phase | Bulk Density |
|---|---|---|---|
| N-heptane (5 °C) | None | Amorphous | Retained |
| N-heptane (5 °C) | Acetone | Form III | Densified |
| N-heptane (5 °C) | Methyl ethyl ketone | Form III | Densified |
| N-heptane (5 °C) | Isopropanol | Form III | Densified |
| N-heptane (100 °C) | None | Form I | Densified |
| Methyl tert butyl ether | None | Form III | Densified |
| 0.001 N HCl (5 °C) | Acetone | Amorphous | Retained |
| 0.001 N HCl (5 °C) | MEK | Form III | Densified |
| 0.001 N HCl (60 °C) | None | Amorphous | Densified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frank, D.; Schenck, L.; Koynov, A.; Su, Y.; Li, Y.; Variankaval, N. Optimizing Solvent Selection and Processing Conditions to Generate High Bulk-Density, Co-Precipitated Amorphous Dispersions of Posaconazole. Pharmaceutics 2021, 13, 2017. https://doi.org/10.3390/pharmaceutics13122017
Frank D, Schenck L, Koynov A, Su Y, Li Y, Variankaval N. Optimizing Solvent Selection and Processing Conditions to Generate High Bulk-Density, Co-Precipitated Amorphous Dispersions of Posaconazole. Pharmaceutics. 2021; 13(12):2017. https://doi.org/10.3390/pharmaceutics13122017
Chicago/Turabian StyleFrank, Derek, Luke Schenck, Athanas Koynov, Yongchao Su, Yongjun Li, and Narayan Variankaval. 2021. "Optimizing Solvent Selection and Processing Conditions to Generate High Bulk-Density, Co-Precipitated Amorphous Dispersions of Posaconazole" Pharmaceutics 13, no. 12: 2017. https://doi.org/10.3390/pharmaceutics13122017
APA StyleFrank, D., Schenck, L., Koynov, A., Su, Y., Li, Y., & Variankaval, N. (2021). Optimizing Solvent Selection and Processing Conditions to Generate High Bulk-Density, Co-Precipitated Amorphous Dispersions of Posaconazole. Pharmaceutics, 13(12), 2017. https://doi.org/10.3390/pharmaceutics13122017






