In Vivo Biodistribution, Clearance, and Biocompatibility of Multiple Carbon Dots Containing Nanoparticles for Biomedical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Cell Lines, and Animals
2.2. Synthesis and Characterization of CDs@PEI and PEI-CD Nanoparticles
2.3. PEGylation of CDs@PEI Nanoparticles
2.4. Cell Imaging Using the Confocal Laser Scanning Microscopy
2.5. In Vivo Biodistribution and Clearance of CDs@PEI Nanoparticles
2.6. Systemic Toxicity of the CDs@PEI Nanoparticles
3. Results and Discussion
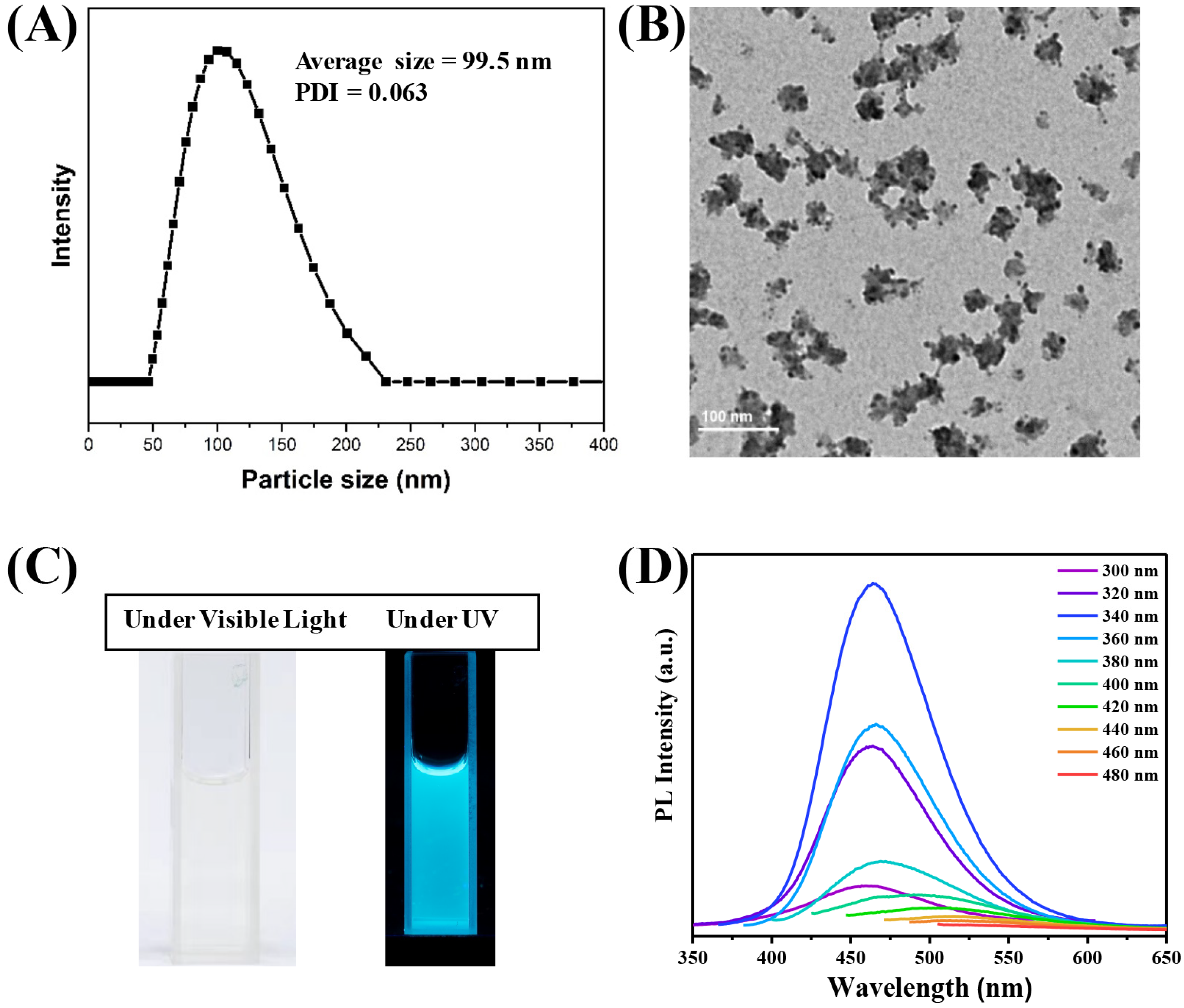
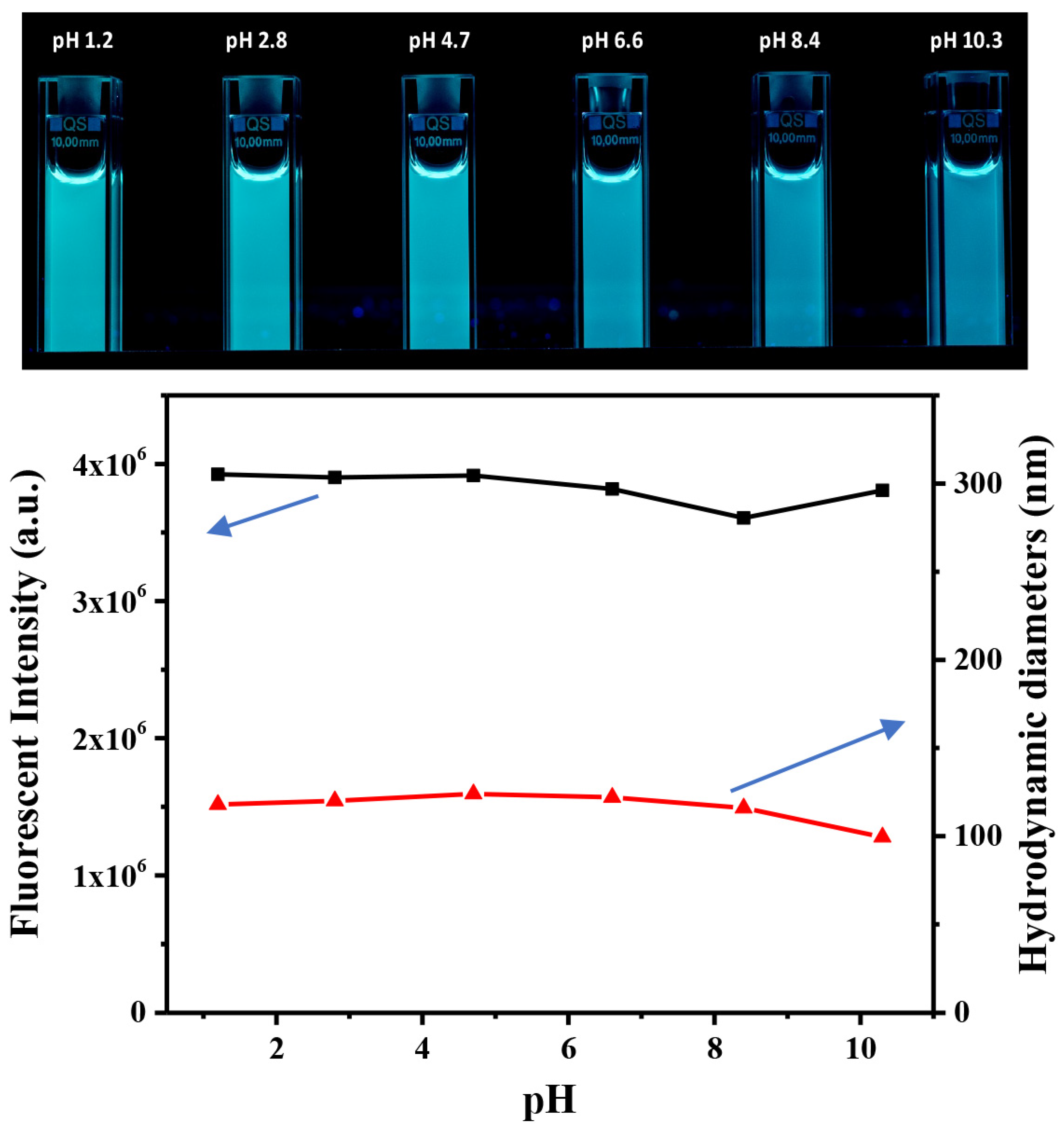
3.1. Preparation and Characterization of CDs@PEI Nanoparticles
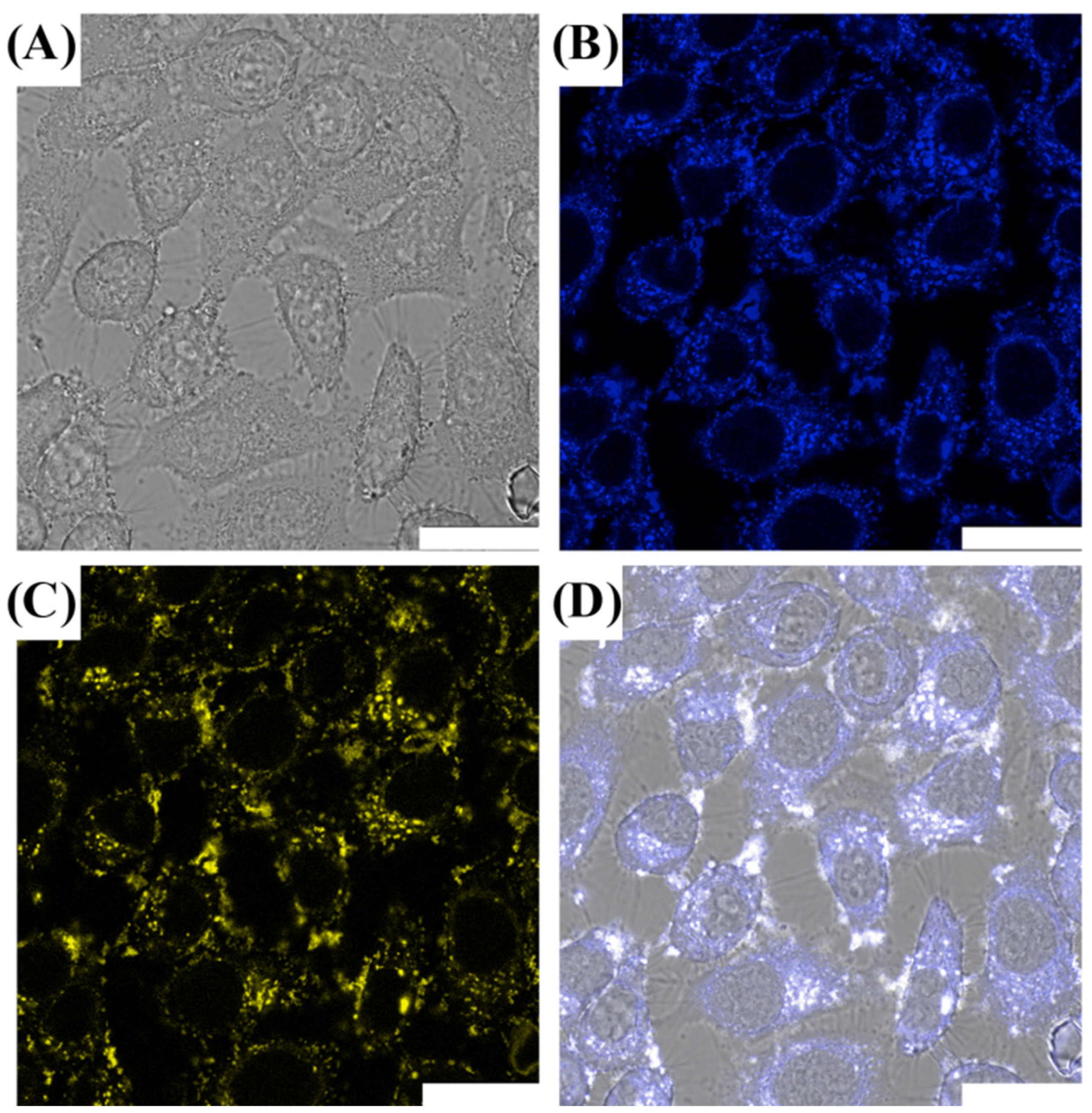
3.2. Cell Imaging of CDs@PEI Nanoparticles
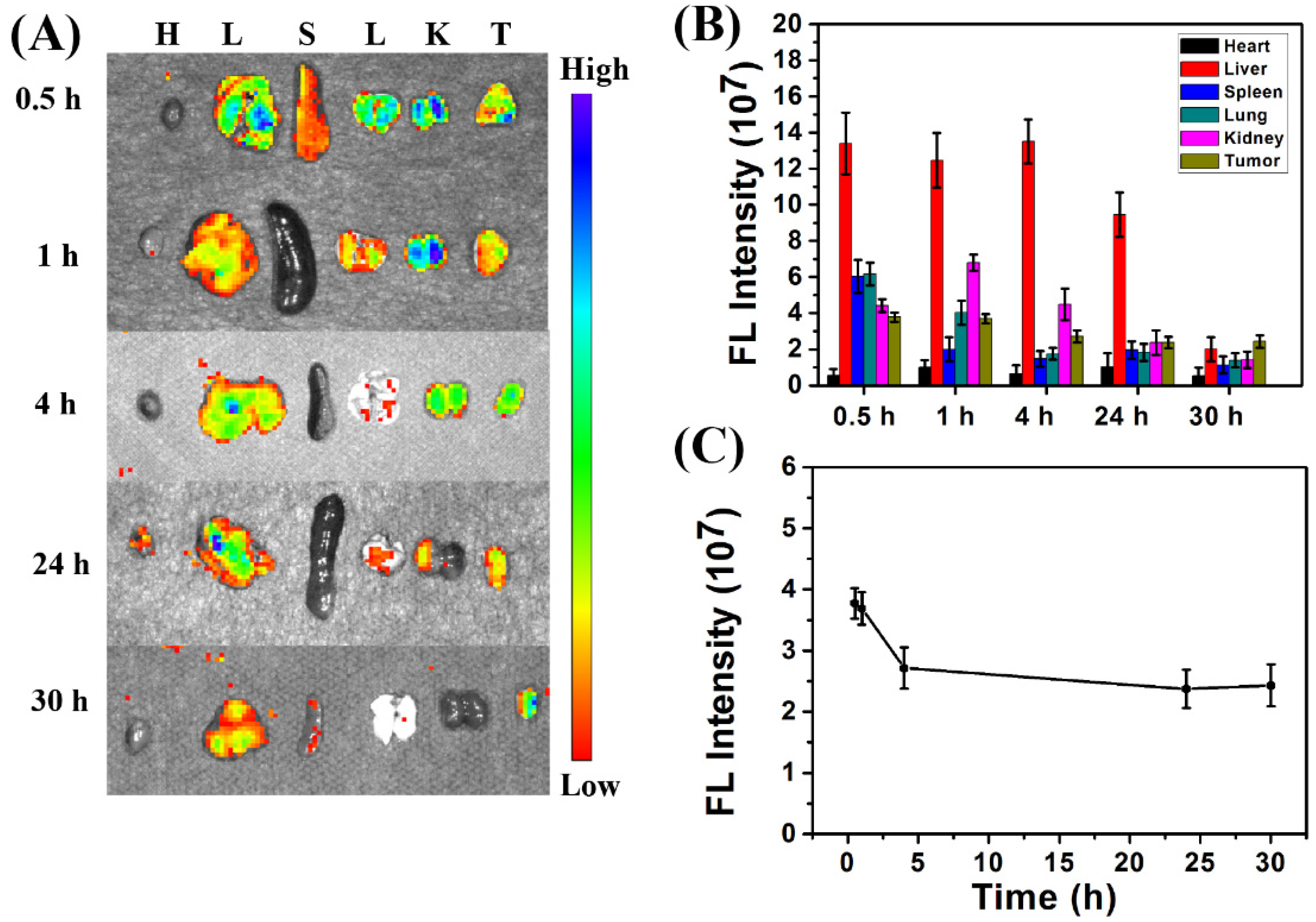
3.3. In Vivo Biodistribution of CDs@PEI Nanoparticles
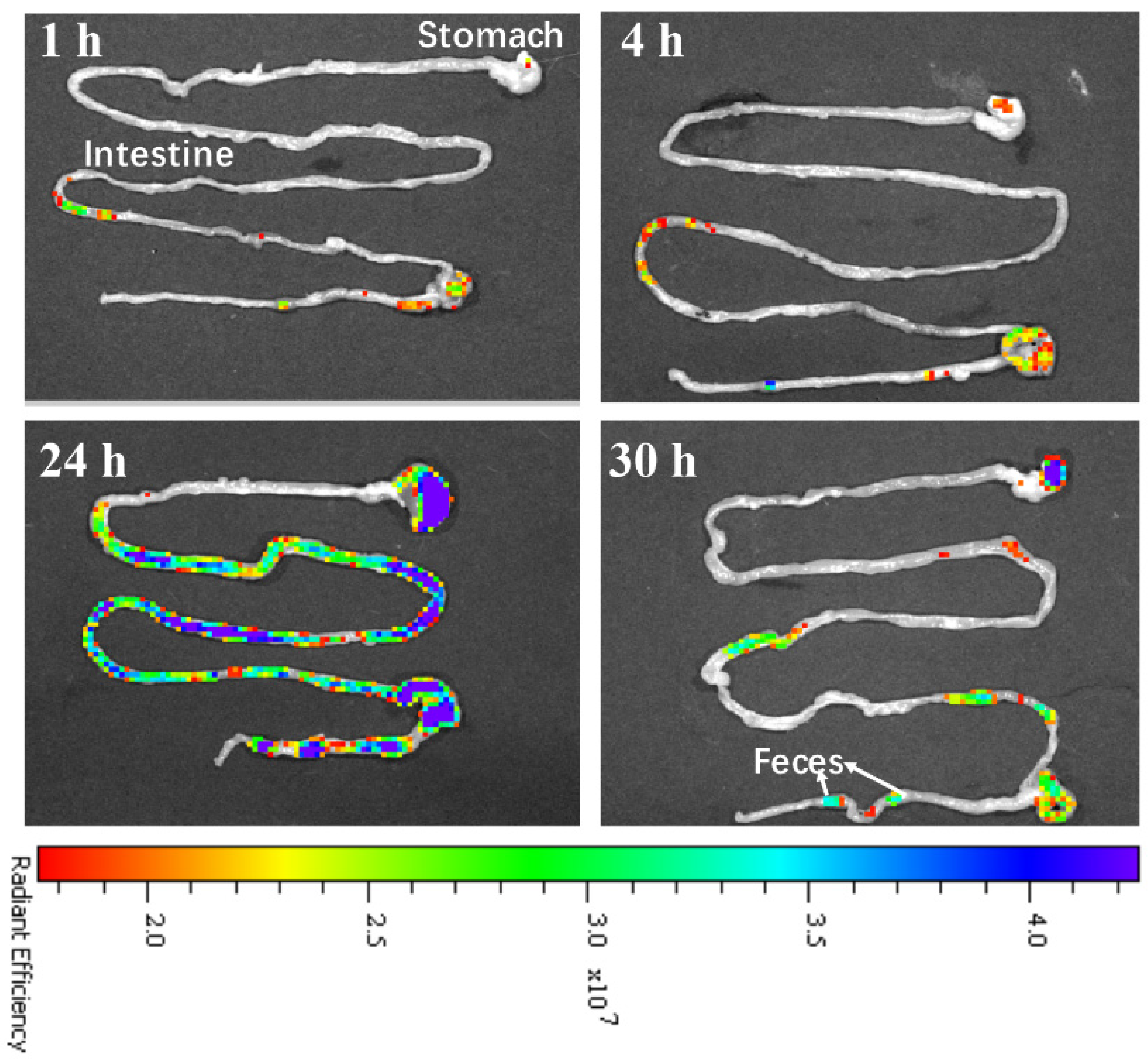
3.4. In Vivo Clearance of CDs@PEI Nanoparticles
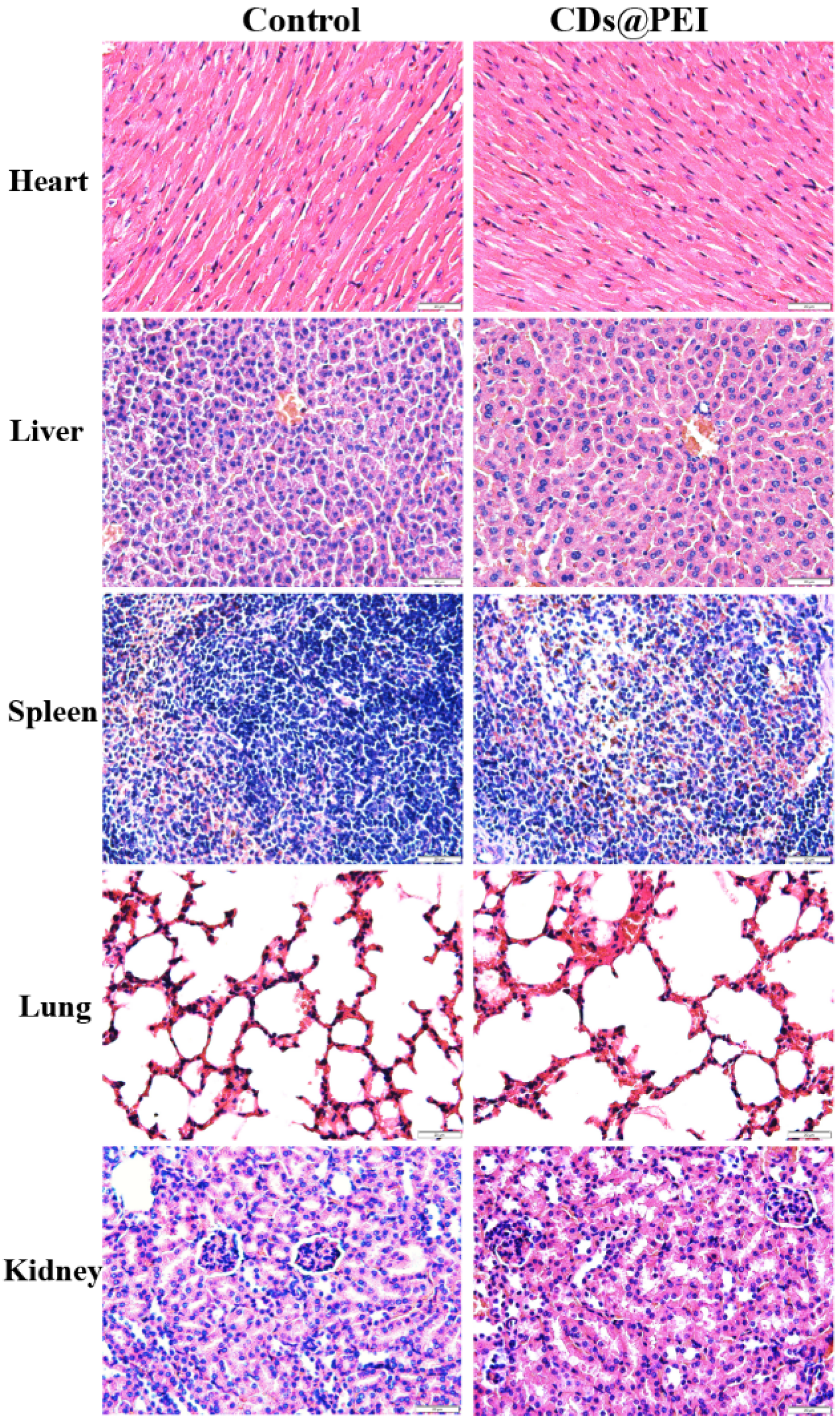
3.5. In Vivo Biocompatibility of the CDs@PEI Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaleel, J.A.; Pramod, K. Artful and multifaceted applications of carbon dot in biomedicine. J. Control. Release 2018, 269, 302–321. [Google Scholar] [CrossRef]
- Kang, Z.; Lee, S.T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef]
- Ghosal, K.; Ghosh, A. Carbon dots: The next generation platform for biomedical application. Mater. Sci. Eng. C 2019, 96, 887–903. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Lu, S.; Geng, T.; Xiao, G.; Zou, B.; Hong, B. Photoactivated fluorescence enhancement in F, N-doped carbon dots with piezochromic behavior. Angew. Chem. Int. Ed. Engl. 2020, 59, 9986–9991. [Google Scholar] [CrossRef]
- Yaghini, E.; Turner, H.; Pilling, A.; Naasani, I.; MacRobert, A.J. In vivo biodistribution and toxicology studies of cadmium-free indium-based quantum dot nanoparticles in a rat model. Nanomed. Nanotech. Biol. Med. 2018, 14, 2644–2655. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Wu, R.-S.; Wei, S.-C.; Huang, C.-C.; Chang, H.-T. Fluorescent carbon dots for selective labeling of subcellular organelles. ACS Omega 2020, 5, 11248–11261. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.; Xiao, L.; Liu, M.; Du, F.; Wang, Z. Recent advances in functional carbon quantum dots for antitumor. Int. J. Nanomed. 2021, 16, 7195–7229. [Google Scholar] [CrossRef]
- Su, Q.; Yang, X. Promoting room temperature phosphorescence through electron transfer from carbon dots to promethazine. ACS Appl. Mater. Interfaces 2021, 13, 41238–41248. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.-P.; Yang, L. Carbon dots as potent antimicrobial agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef]
- Fan, J.; Li, Q.; Chen, L.; Du, J.; Xue, W.; Yu, S.; Su, X.; Yang, Y. Research progress in the synthesis of targeting organelle carbon dots and their applications in cancer diagnosis and treatment. J. Biomed. Nanotechnol. 2021, 17, 1891–1916. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jo, G.; Chae, Y.; Subramani, S.; Lee, B.Y.; Kim, E.J.; Ji, M.-K.; Sim, U.; Hyun, H. Bioinspired Camellia japonica carbon dots with high near-infrared absorbance for efficient photothermal cancer therapy. Nanoscale 2021, 13, 14426–14434. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Q.; Yang, D.; Liao, J. Carbon dots: Highlight on their synthesis, properties and applications in tumor imaging and therapy. Nanosci. Nanotechnol. Lett. 2017, 9, 1827–1848. [Google Scholar] [CrossRef]
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-targeting fluorescent carbon dots for diagnosis of brain cancer cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef]
- Fu, X.; Fu, X.; Li, W.; Chen, Y.; Cai, Z. Ovalbumin as a precusor for green synthesis of highly fluorescent carbon dots for cell imaging. J. Biomed. Nanotechnol. 2019, 15, 1232–1240. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-convertible carbon dots for imaging guided drug delivery with enhanced in vivo cancer therapeutic efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.-S.; Zhong, N.; Gao, Q.-Y.; Xiong, H.-M. Highly efficient red-emitting carbon dots with gram-scale yield for bioimaging. Langmuir 2017, 33, 12635–12642. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Phatake, R.S.; Barnea, S.N.; Zerby, N.; Jelinek, R. Fluorescent self-healing carbon dot/polymer gels. ACS Nano 2019, 13, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zeng, A.; Liu, Z.; Zheng, C.; Wei, Y.; Yang, P.; Zhang, M.; Yang, F.; Xie, F. Carbon quantum dots: In vitro and in vivo studies on biocompatibility and biointeractions for optical imaging. Int. J. Nanomed. 2020, 15, 6519–6529. [Google Scholar] [CrossRef]
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 2, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Qi, J.; Ruan, J.; Shen, G. Highly sensitive detection of glucose by a “turn-off-on” fluorescent probe using gadolinium-doped carbon dots and carbon microparticles. J. Biomed. Nanotechnol. 2018, 14, 1117–1124. [Google Scholar] [CrossRef]
- Walia, S.; Shukla, A.K.; Sharma, C.; Acharya, A. Engineered bright blue- and red-emitting carbon dots facilitate synchronous imaging and inhibition of bacterial and cancer cell progression via 1O2-mediated DNA damage under photoirradiation. ACS Biomater. Sci. Eng. 2019, 5, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, R.; Wang, T.; Yang, W.; Wang, H.; Gu, W.; Ye, L. Carbon dots/prussian blue satellite/core nanocomposites for optical imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2018, 10, 1084–1092. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Zhu, L.; Choi, K.Y.; Guo, N.; Guo, J.; Tackett, K.; Anilkumar, P.; Liu, G.; Quan, Q. Effect of injection routes on the biodistribution, clearance, and tumor uptake of carbon dots. ACS Nano 2013, 7, 5684–5693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wang, G.D.; Tang, W.; Todd, T.; Xie, J. Gd-encapsulated carbonaceous dots with efficient renal clearance for magnetic resonance imaging. Adv. Mater. 2014, 26, 6761–6766. [Google Scholar] [CrossRef] [Green Version]
- Guan, C.; Zhao, Y.; Hou, Y.; Shan, G.; Yan, D.; Liu, Y. Glycosylated liposome loading carbon dots for targeted recognition to HepG2 cells. Talanta 2018, 182, 314–323. [Google Scholar] [CrossRef]
- Na, G.; Wen, Y.; Nie, H.; Gong, Y.; Zhang, X. Turn-on theranostic fluorescent nanoprobe by electrostatic self-assembly of carbon dots with doxorubicin for targeted cancer cell imaging, in vivo hyaluronidase, and targeted drug delivery. Biosens. Bioelectron. 2017, 96, 300–307. [Google Scholar]
- Yao, Y.; Niu, D.; Lee, C.H.; Li, Y.; Li, P. Aqueous synthesis of multi-carbon dot cross-linked polyethyleneimine particles with enhanced photoluminescent properties. Macromol. Rapid Commun. 2019, 40, 1800869. [Google Scholar] [CrossRef]
- Teng, C.; Chai, Z.; Yuan, Z.; Ren, L.; Lin, C.; Yan, Z. Desirable PEGylation for improving tumor selectivity of hyaluronic acid-based nanoparticles via low hepatic captured, long circulation times and CD44 receptor-mediated tumor targeting. Nanomed. Nanotech. Biol. Med. 2020, 24, 102105. [Google Scholar]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumor microenvironment. Chem. Soc. Rev. 2017, 46, 3830–3852. [Google Scholar] [CrossRef]
- Huang, L.; Chaurasiya, B.; Wu, D.; Wang, H.; Du, Y.; Tu, J.; Webster, T.J.; Sun, C. Versatile redox-sensitive pullulan nanoparticles for enhanced liver targeting and efficient cancer therapy. Nanomed. Nanotech. Biol. Med. 2018, 14, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.W.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumor. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngoune, R.; Peters, A.; Elverfeldt, D.; Winkler, K.; Pütz, G. Accumulating nanoparticles by EPR: A route of no return. J. Control. Release 2016, 238, 58–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Chio, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR effect: Combined strategies to improve the EPR effect in the tumor microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef]
- Ge, J.; Jia, Q.; Liu, W.; Lan, M.; Zhou, B.; Guo, L.; Zhou, H.; Zhang, H.; Wang, Y.; Gu, Y.; et al. Carbon dots with intrinsic theranostic properties for bioimaging, red-light-triggered photodynamic/photothermal simultaneous therapy in vitro and in vivo. Adv. Healthc. Mater. 2016, 5, 665–675. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Khatun, Z.; Huh, K.M.; Park, S.Y.; Lee, D.Y.; Cho, K.J.; Lee, Y.-K. In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano 2013, 7, 6858–6867. [Google Scholar] [CrossRef]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, D.; Zhang, J.; Zhang, W.; Yao, Y.; Li, P.; Gong, J. Amphiphilic core-shell nanoparticles containing polyethyleneimine shell for efficient delivery of microRNS to Kupffer cells. Int. J. Nanomed. 2016, 11, 2785–2797. [Google Scholar]
- Chung, E.J.; Mlinar, L.B.; Sugimoto, M.J.; Nord, K.; Roman, B.B.; Tirrell, M. In vivo biodistribution and clearance of peptide amphiphile micelles. Nanomed. Nanotech. Biol. Med. 2015, 11, 479–487. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, C.; Wang, S.; Li, Q.; Zhang, M.; Li, J.; Xu, K. Comparative study on in vivo behavior of PEGylated gadolinium oxide nanoparticles and Magnevist as MRI contrast agent. Nanomed. Nanotech. Biol. Med. 2018, 14, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, S.; Feng, L.; Liu, S.; Cai, S.; Dai, Y.; Xie, L.; Liu, B.; Yang, P.; Zhao, Y. Renal-clearable nickle-doped carbon dots with boosted photothermal conversion efficiency for multimodal imaging-guided cancer therapy in the second near-infrared biowindow. Adv. Funct. Mater. 2021, 31, 210059. [Google Scholar] [CrossRef]
- Pang, W.; Jiang, P.; Ding, S.; Bao, Z.; Wang, N.; Wang, H.; Qu, J.; Wang, D.; Gu, B.; Wei, X. Nucleolus-targeted photodynamic anticancer therapy using renal-clearable carbon dots. Adv. Healthc. Mater. 2020, 9, 2000607. [Google Scholar] [CrossRef]
- Hadad, C.; González-Domínguez, J.M.; Armelloni, S.; Mattinzoli, D.; Ikehata, M.; Istif, A.; Ostric, A.; Cellesi, F.; Alfieri, C.M.; Messa, P.; et al. Graphene quantum dots: From efficient preparation to safe renal excretion. Nano Res. 2021, 14, 674–683. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Yao, Y.; Lee, C.-H.; Wu, Y.; Li, P. In Vivo Biodistribution, Clearance, and Biocompatibility of Multiple Carbon Dots Containing Nanoparticles for Biomedical Application. Pharmaceutics 2021, 13, 1872. https://doi.org/10.3390/pharmaceutics13111872
Liao J, Yao Y, Lee C-H, Wu Y, Li P. In Vivo Biodistribution, Clearance, and Biocompatibility of Multiple Carbon Dots Containing Nanoparticles for Biomedical Application. Pharmaceutics. 2021; 13(11):1872. https://doi.org/10.3390/pharmaceutics13111872
Chicago/Turabian StyleLiao, Jinfeng, Yuan Yao, Cheng-Hao Lee, Yongzhi Wu, and Pei Li. 2021. "In Vivo Biodistribution, Clearance, and Biocompatibility of Multiple Carbon Dots Containing Nanoparticles for Biomedical Application" Pharmaceutics 13, no. 11: 1872. https://doi.org/10.3390/pharmaceutics13111872
APA StyleLiao, J., Yao, Y., Lee, C.-H., Wu, Y., & Li, P. (2021). In Vivo Biodistribution, Clearance, and Biocompatibility of Multiple Carbon Dots Containing Nanoparticles for Biomedical Application. Pharmaceutics, 13(11), 1872. https://doi.org/10.3390/pharmaceutics13111872





