Preparation and Evaluation of a Powdered Rebamipide Mouthwash as In-Hospital Formulation: Considering Dispersion before Use in Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Co-Grinding Mixtures
2.3. X-ray Powder Diffraction Analysis
2.4. Fourier Transform Infrared Spectroscopy
2.5. Preparation of Dispersion for Test Samples
2.6. Measurement of Mean Particle Size
2.7. Measurement of RB Solubility
2.8. Evaluation of the Dispersibility of the Suspension
3. Results and Discussion
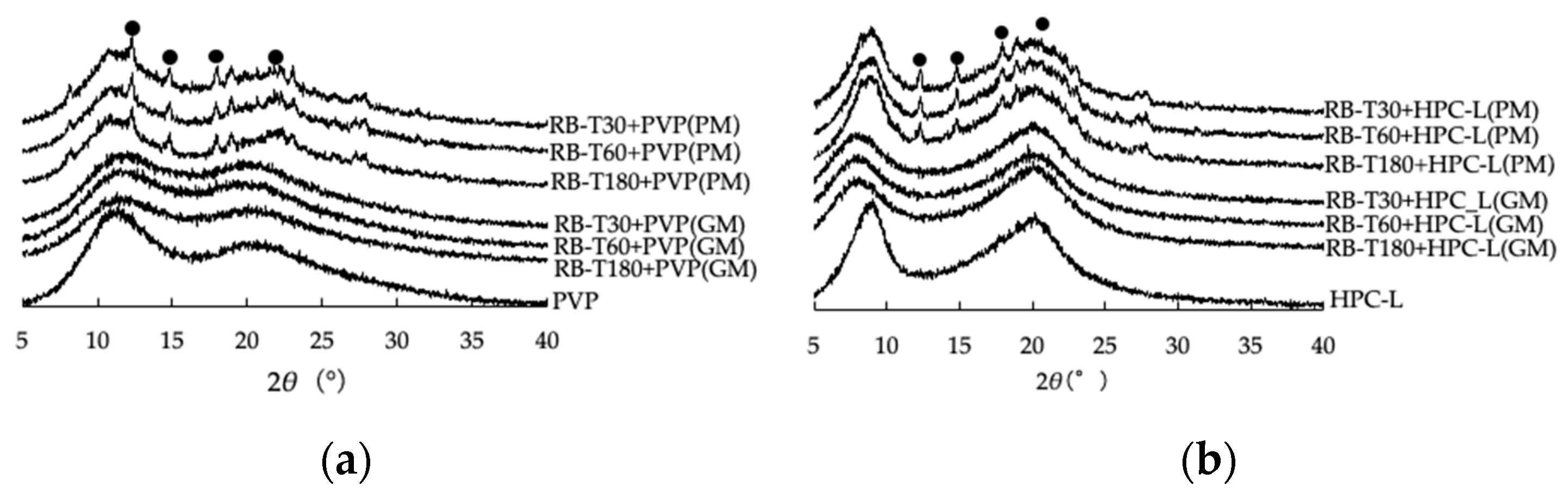
3.1. Effect of Grinding on the Crystallinity of Ground Mixtures
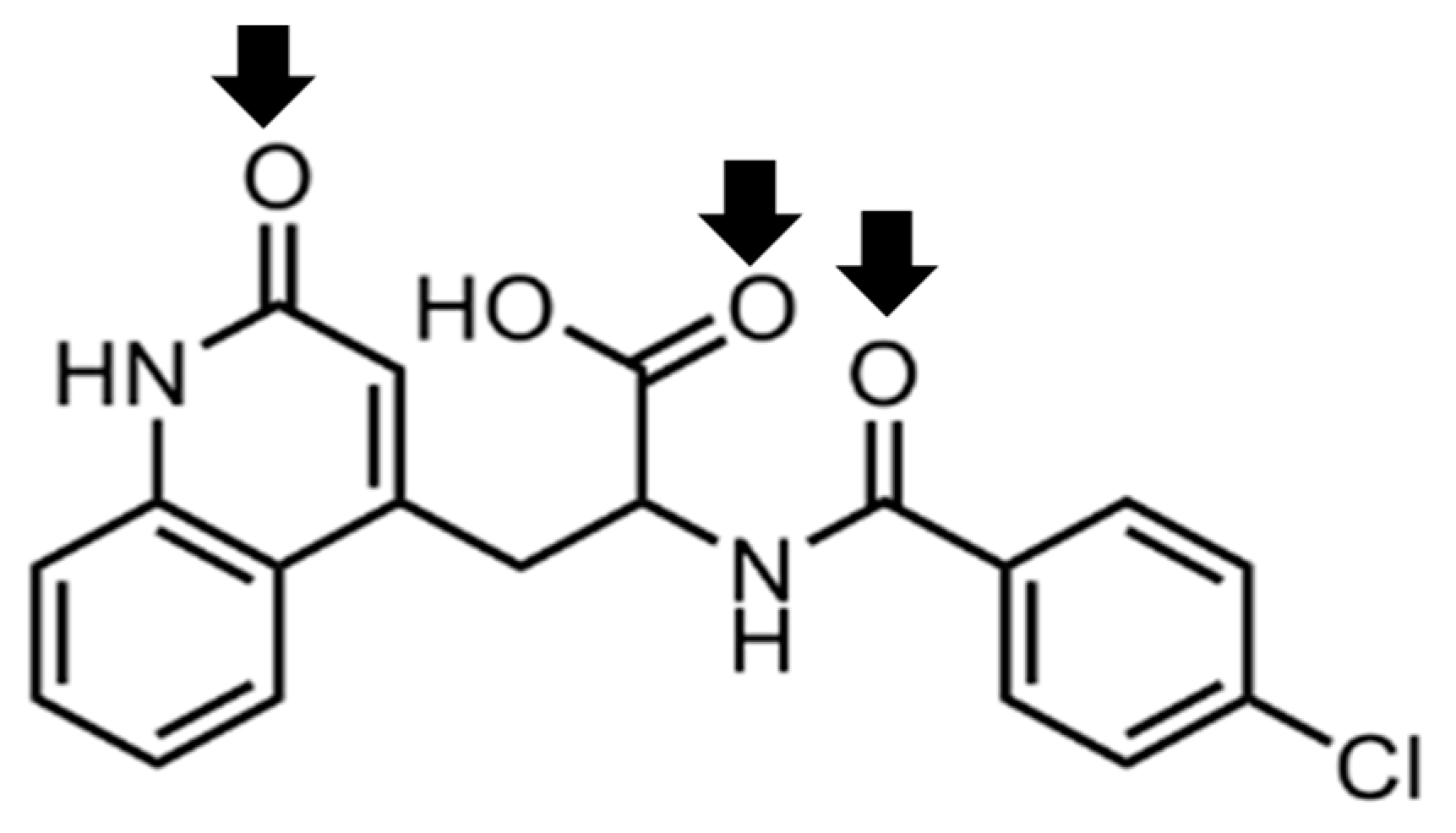
3.2. Evaluation of the Intermolecular Interaction by FTIR
3.3. Pilot Study for Evaluation of the Preservation Stability
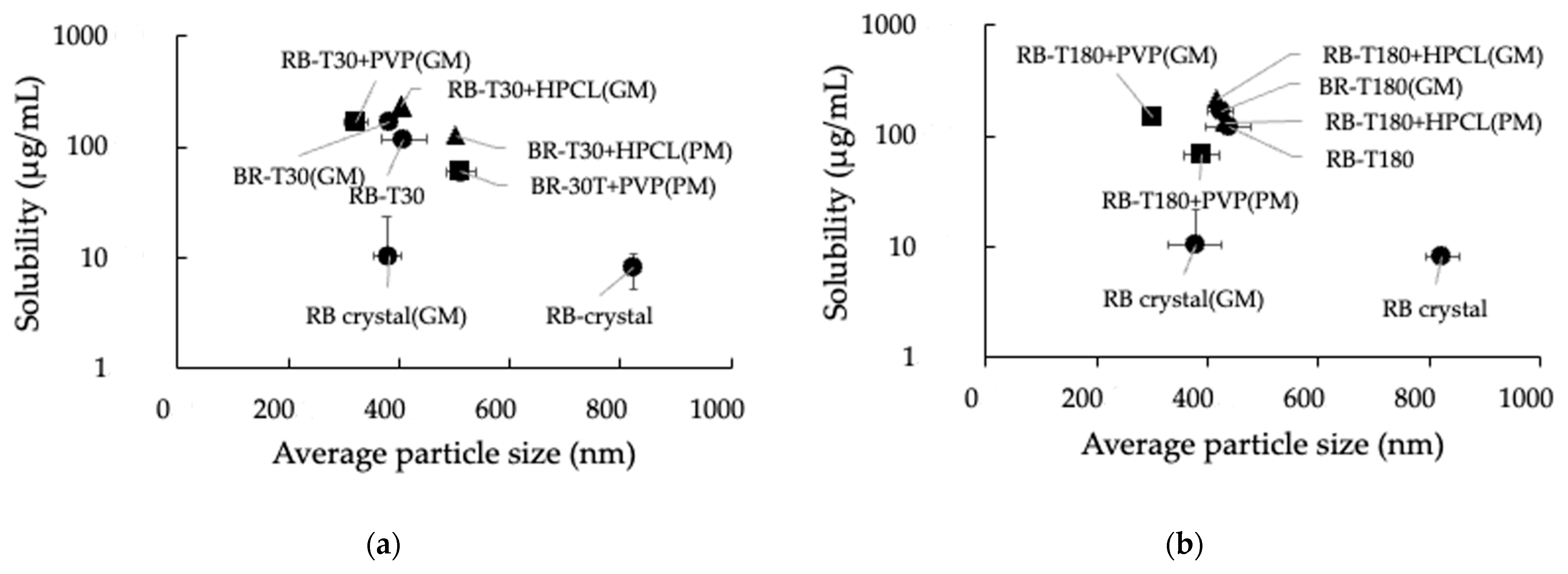
3.4. Effect of Grinding on the Particle Size of GMs
3.5. Effect of Grinding on the Solubility of RB
3.6. Evaluation of Dispersibility of GMs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement.
Informed Consent Statement.
Acknowledgments
Conflicts of Interest
References
- Naidu, M.U.R.; Ramana, G.V.; Rani, P.U.; Mohan, L.K.; Suman, A.; Roy, P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis-complicating the treatment of cancer. Neoplasia 2004, 6, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Sarumathy, S.; Ismail, A.M.; Anisamy, A.P. Efficacy and safety of oral glutamine in radiation induced oral mucositis in patients with head and neck cancer. Asian J. Pharm. Clin. Res. 2012, 5, 138–140. [Google Scholar]
- Parkhill, A.L. Oral mucositis and stomatitis associated with conventional and targeted anticancer therapy. J. Pharmacovigil. 2013, 1, 1–4. [Google Scholar] [CrossRef]
- Mahood, D.J.; Dose, A.M.; Loprinzi, C.L.; Veeder, M.H.; Athmann, L.M.; Therneau, T.M.; Sorensen, J.M.; Gainey, D.K.; Mailliard, J.A.; Gusa, N.L. Inhibition of fluorouracil-induced stomatitis by oral cryotherapy. J. Clin. Oncol. 1991, 9, 449–452. [Google Scholar] [CrossRef]
- Beaven, A.W.; Shea, T.C. The effect of palifermin on chemotherapyand radiation therapy–induced mucositis: A review of the current literature. Support. Cancer Ther. 2007, 4, 188–197. [Google Scholar] [CrossRef]
- Elad, S.; Rn, K.K.F.C.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Dds, D.P.S.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Keefe, D.M.; Schubert, M.M.; Elting, L.S.; Sonis, S.T.; Epstein, J.B.; Raber-Durlacher, J.E.; Migliorati, C.A.; McGuire, D.B.; Hutchins, R.D.; Peterson, D.E.; et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007, 109, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Cascinu, S.; Fedeli, A.; Fedeli, S.L.; Catalano, G. Oral cooling (cryotherapy), an effective treatment for the prevention of 5-fluorouracil-induced stomatitis. Oral Oncol. 1994, 30, 234–236. [Google Scholar] [CrossRef]
- Coda, B.A.; O’sullivan, B.; Donaldson, G.; Bohl, S.; Chapman, R.C.; Shen, D.D. Comparative efficacy of patient-controlled administration of morphine, hydromorphone, or sufentanil for the treatment of oral mucositis pain following bone marrow transplantation. Pain 1997, 72, 333–346. [Google Scholar] [CrossRef]
- Yasuno, N.; Watanabe, S.; Kanda, S.; Kizu, J.; Tsuchiya, M.; Nishitani, A.; Ono, H.; Imai, K.; Ishio, K.; Iinuma, T. Stability and clinical application of AzunolElaseXylocaineGargle (AEXG) in hospital preparation. Jpn. J. Hosp. Pharm. 1995, 21, 327–334. [Google Scholar] [CrossRef]
- Momo, K. Indomethacin spray preparation for the control of pain associated with stomatitis caused by chemotherapy and radiotherapy in cancer patients. Yakugaku Zasshi 2015, 135, 931–935. [Google Scholar] [CrossRef][Green Version]
- Tsavaris, N.; Komitsopoulou, P.; Tzannou, I.; Loucatou, P.; Tsaroucha-Noutsou, A.; Kilafis, G.; Kosmidis, P. Decreased oral toxicity with the local use of allopurinol in patients who received high dose 5-fluorouracil. Sel. Cancer Ther. 1991, 7, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Elzawawy, A. Treatment of 5-fluorouracil-induced stomatitis by allopurinol mouthwashes. Oncol. 1991, 48, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Kohri, N.; Ozawa, T.; Takasaki, M.; Eguchi, H.; Miwa, K.; Numa, J.; Abe, M. Development of camostat mesilate troche for the prevention of induced mucositis in the mouth at cancer chemotherapy. J. Pharm. Sci. Technol. Jpn. 2001, 61, 34–35. [Google Scholar] [CrossRef]
- Yosano, A.; Nomura, T.; Shibahara, T.; Hayashi, K.; Morituka, M.; Noma, H. Therapeutic experience by mesylic acid camostat component gargarism for ts-1 elicited stomatitis of a tongue cancer patient. J. Jpn. Soc. Oral Mucous Membr. 2005, 11, 16–20. [Google Scholar] [CrossRef][Green Version]
- Ishii, N.; Kawano, Y.; Suzuki, M.; Komoda, M.; Makino, K.; Hanawa, T. Effects of a camostat mesilate gargle on stomatitis caused by molecular target medicine. J. Pharm. Palliat. Care Sci. 2016, 9, 78–91. [Google Scholar]
- Shinohara, A.; Nakamura, M.; Onikubo, T.; Nakamura, K. Efficacy of rebamipide gargle against chemotherapy-induced oral mucositis. Yakugaku Zasshi 2015, 135, 937–941. [Google Scholar] [CrossRef][Green Version]
- Yoshida, M.; Kitahora, T.; Wakabayashi, G.; Tashiro, H.; Ono, H.; Otani, Y.; Shimazu, M.; Kubota, T.; Kumal, K.; Kitajima, M. Active oxygen species in formation of acute gastric mucosal lesions induced by thermal injury in rats. Dig. Dis. Sci. 1995, 40, 1306–1310. [Google Scholar] [CrossRef]
- Sonis, S. Mucositis as a biological process: A new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998, 34, 39–43. [Google Scholar] [CrossRef]
- Pico, J.; Avila-Garavito, A.; Naccache, P. Mucositis: Its occurrence, consequences, and treatment in the oncology setting. Oncologist 1998, 3, 446–451. [Google Scholar] [CrossRef]
- Kawano, Y.; Ishii, N.; Shimizu, Y.; Hanawa, T. Development and characterization of a suspension containing nanoparticulated rebamipide for a mouthwash for stomatitis. J. Pharm. Sci. Technol. Jpn. 2017, 77, 104–115. [Google Scholar] [CrossRef]
- Nagavarma, B.V.N.; Yadav, H.K.; Ayaz, A.V.L.S.; Vasudha, L.S.; Shivakumar, H.G. Different techniques for preoaration of polymeric nanoparticles. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Kamiya, S.; Yamada, M.; Washino, M.; Nakashima, K. Preparation of nanoparticles including antisolvent drugs by the combination of roll milling and high-pressure homogenization. Curr. Nanosci. 2018, 14, 143–147. [Google Scholar] [CrossRef]
- Moribe, K.; Higashi, K. Nanocrystal formulation of poorly water-soluble drug. Drug Deliv. Syst. 2015, 30, 92–99. [Google Scholar] [CrossRef]
- Kawano, Y.; Utsunomiya, Y.; Yokoyama, F.; Ishii, N.; Hanawa, T. Preparation and evaluation of rebamipide colloidal nanoparticles obtained by cogrinding in ternary ground mixtures. Colloids Interfaces. 2020, 4, 43. [Google Scholar] [CrossRef]
- Celia, C.; Locatelli, M.; Cilurzo, F.; Cosco, D.; Gentile, E.; Scalise, D.; Carafa, M.; Ventura, C.A.; Fleury, M.; Tisserand, C.; et al. Long term stability evaluation of prostacyclin released from biomedical device through turbiscan lab expert. Med. Chem. 2015, 11, 391–399. [Google Scholar] [CrossRef]
- Homayouni, A.; Sadeghi, F.; Nokhodchi, A.; Varshosaz, J.; Garekani, A.H. Preparation and characterization of celecoxib solid dispersions; Comparison of poloxamer-188 and PVP-K30 as carriers. Iran J. Basic Med. Sci. 2014, 17, 322–331. [Google Scholar] [PubMed]
- Vasconcelos, T.; Sarmento, B.; da Costa, P.J.C. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Liu, C.; Ren, T.; Wang, X.; Yang, Q.; Yang, Z.; Yang, Y.; Yang, S.; Gu, J.; Hu, C. Sodium salts and solvate of rebamipide: Synthesis, structure, and pharmacokinetic study. Cryst. Growth Des. 2016, 12, 3180–3189. [Google Scholar] [CrossRef]
- Gurunath, S.; Kumar, S.P.; Basavaraj, N.K.; Patil, P.A. Amorphous solid dispersion method for improving oral bioavailability of poorly water-soluble drugs. J. Pharm. Res. 2013, 6, 476–480. [Google Scholar] [CrossRef]
- Yoshioka, M.; Hancock, B.C.; Zografi, G. Crystallization of indomethacin from the amorphous state below and above its glass transition temperature. J. Pharm. Sci. 1994, 83, 1700–1705. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric amorphous solid dispersions: A review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Surikutchi, B.T.; Patil, S.P.; Shete, G.; Patel, S.; Bansal, K.A. Drug-excipient behavior in polymeric amorphous solid dispersions. J. Excip. Food Chem. 2013, 4, 70–94. [Google Scholar]
- Radhi, Z.A.; Ghareeb, M.M. Preparation and evaluation of extended release ocular inserts of rebamipide for local effect using casting technique. Iraqi J. Pharm. Sci. 2018, 28, 24–36. [Google Scholar]
- Mir, M.; Hayat, K.; Hussain, T.; Waoas, M.K.; Bukhari, N.I. Ball mill based co-milling: A promising way to enhance aqueous solubility of poorly soluble drugs employing norfloxacin as model drug. Acta Pol. Pharm. Drug Res. 2018, 75, 155–168. [Google Scholar]
- Ponchel, G.; Montisci, M.-J.; Dembri, A.; Durrer, C.; Duchêne, D. Mucoadhesion of colloidal particulate systems in the gastro-intestinal tract. Eur. J. Pharm. Biopharm. 1997, 44, 25–31. [Google Scholar] [CrossRef]






| Sample | Mixing Weight Ratio | Pre-Milling Time (Second) | Ground Time (Min) | ||
|---|---|---|---|---|---|
| RB-T *1 | PVPK-30 | HPC-L | |||
| RB-T30 | 1 | − | − | 30 | − |
| RB-T30 (GM) *2 | 1 | − | − | 30 | |
| RB-T30 + PVPK-30 (PM) *3 | 1 | 5 | − | − | |
| RB-T30 + PVP K-30 (GM) | 1 | 5 | − | 30 | |
| RB-T30 + HPC-L (PM) | 1 | − | 5 | − | |
| RB-T30 + HPC-L (GM) | 1 | − | 5 | 30 | |
| RB-T60 | 1 | − | − | 60 | − |
| RB-T60 (GM) | 1 | − | − | 30 | |
| RB-T60 + PVPK-30 (PM) | 1 | 5 | − | − | |
| RB-T60 + PVPK-30 (GM) | 1 | 5 | − | 30 | |
| RB-T60 + HPC-L (PM) | 1 | − | 5 | − | |
| RB-T60 + HPC-L (GM) | 1 | − | 5 | 30 | |
| RB-T180 | 1 | − | − | 180 | − |
| RB-T180 (GM) | 1 | − | − | 30 | |
| RB-T180 + PVPK-30 (PM) | 1 | 5 | − | − | |
| RB-T180 + PVPK-30 (GM) | 1 | 5 | − | 30 | |
| RB-T180 + HPC-L (PM) | 1 | − | 5 | − | |
| RB-T180 + HPC-L (GM) | 1 | − | 5 | 30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, N.; Mizobuchi, S.; Kawano, Y.; Hanawa, T. Preparation and Evaluation of a Powdered Rebamipide Mouthwash as In-Hospital Formulation: Considering Dispersion before Use in Patients. Pharmaceutics 2021, 13, 1848. https://doi.org/10.3390/pharmaceutics13111848
Ishii N, Mizobuchi S, Kawano Y, Hanawa T. Preparation and Evaluation of a Powdered Rebamipide Mouthwash as In-Hospital Formulation: Considering Dispersion before Use in Patients. Pharmaceutics. 2021; 13(11):1848. https://doi.org/10.3390/pharmaceutics13111848
Chicago/Turabian StyleIshii, Naoko, Senri Mizobuchi, Yayoi Kawano, and Takehisa Hanawa. 2021. "Preparation and Evaluation of a Powdered Rebamipide Mouthwash as In-Hospital Formulation: Considering Dispersion before Use in Patients" Pharmaceutics 13, no. 11: 1848. https://doi.org/10.3390/pharmaceutics13111848
APA StyleIshii, N., Mizobuchi, S., Kawano, Y., & Hanawa, T. (2021). Preparation and Evaluation of a Powdered Rebamipide Mouthwash as In-Hospital Formulation: Considering Dispersion before Use in Patients. Pharmaceutics, 13(11), 1848. https://doi.org/10.3390/pharmaceutics13111848








