Ultrasound and Microbubbles for the Treatment of Ocular Diseases: From Preclinical Research towards Clinical Application
Abstract
1. Introduction
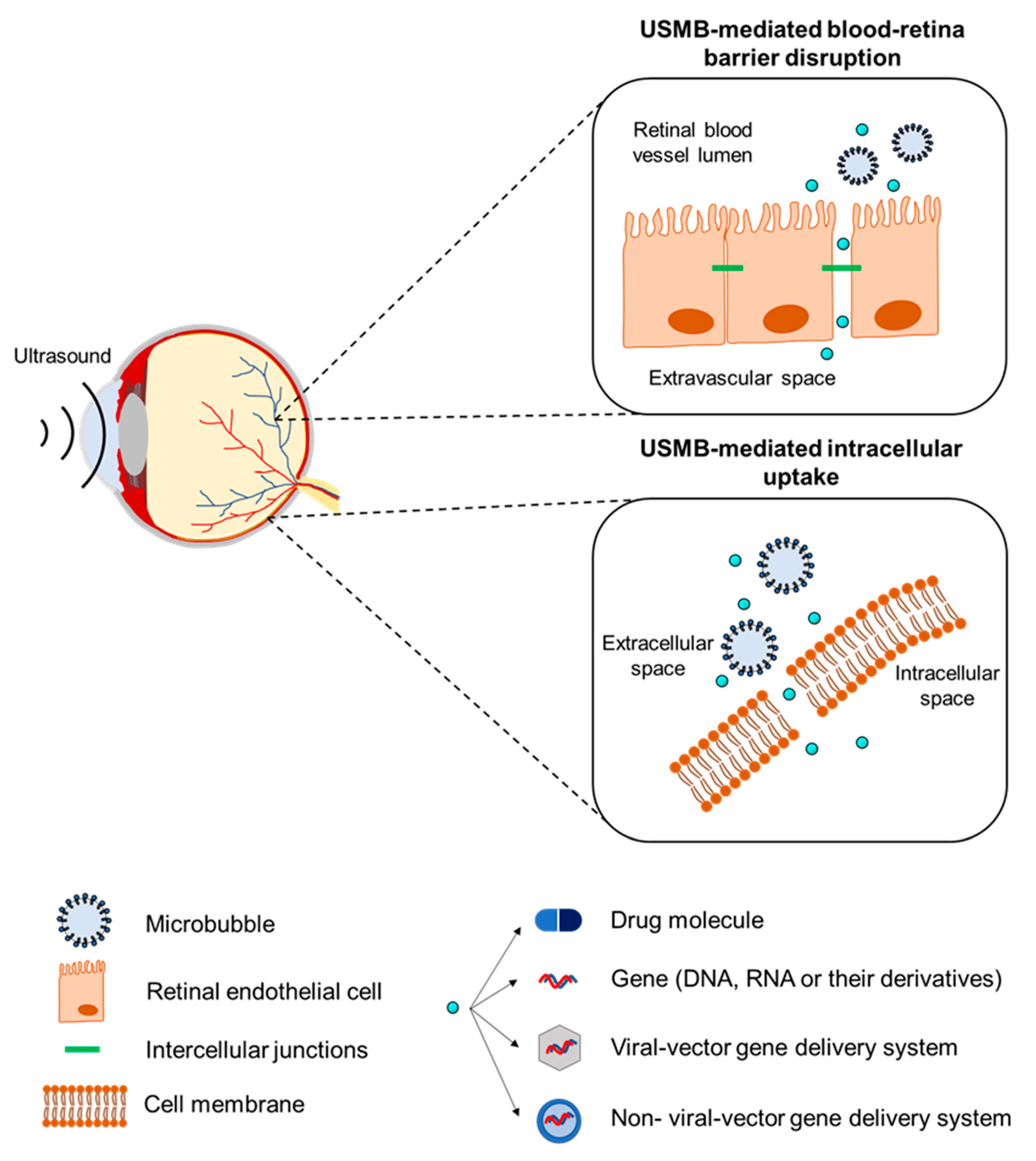
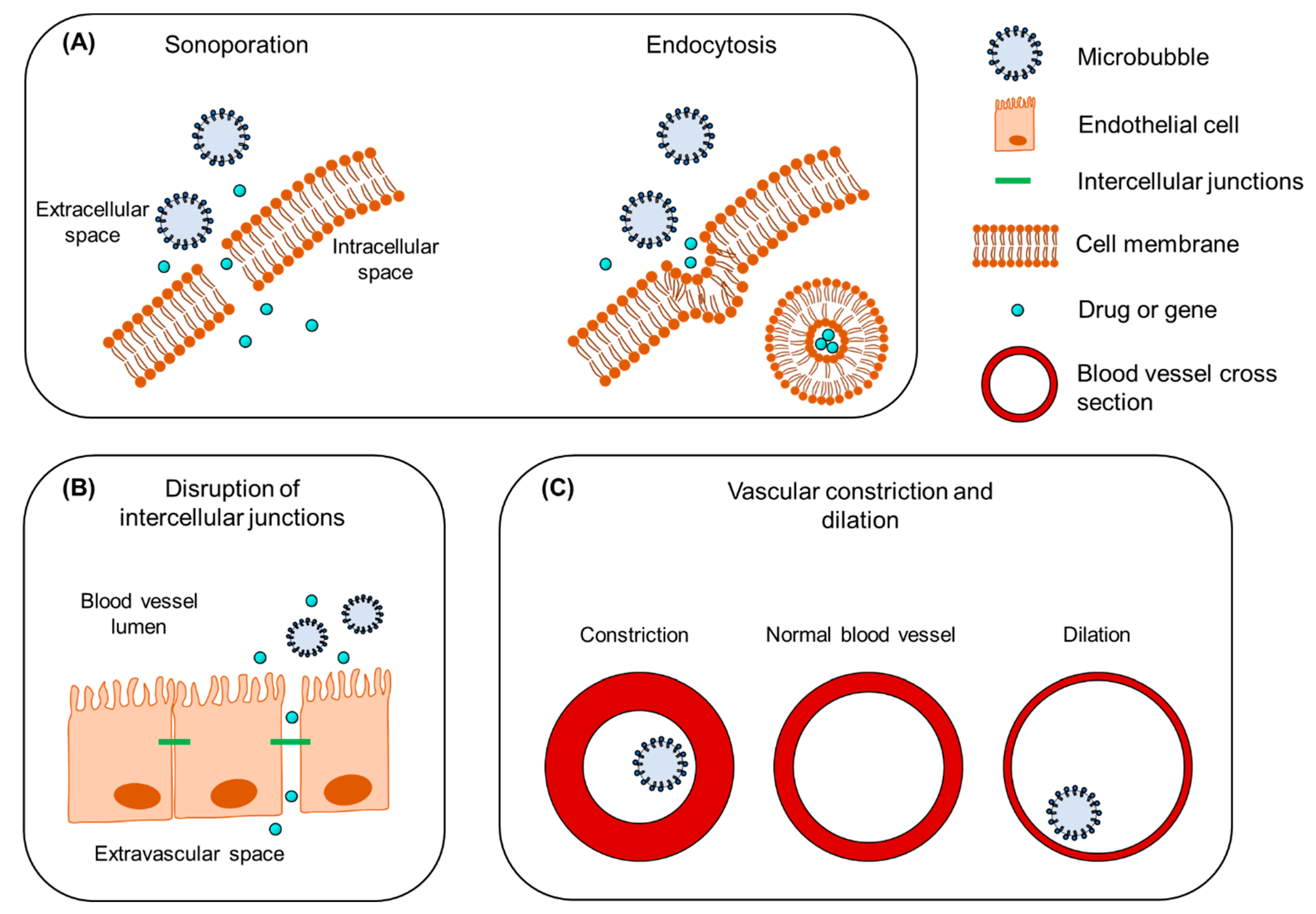
2. Mechanisms Underlying the Therapeutic Use of Ultrasound and Microbubbles
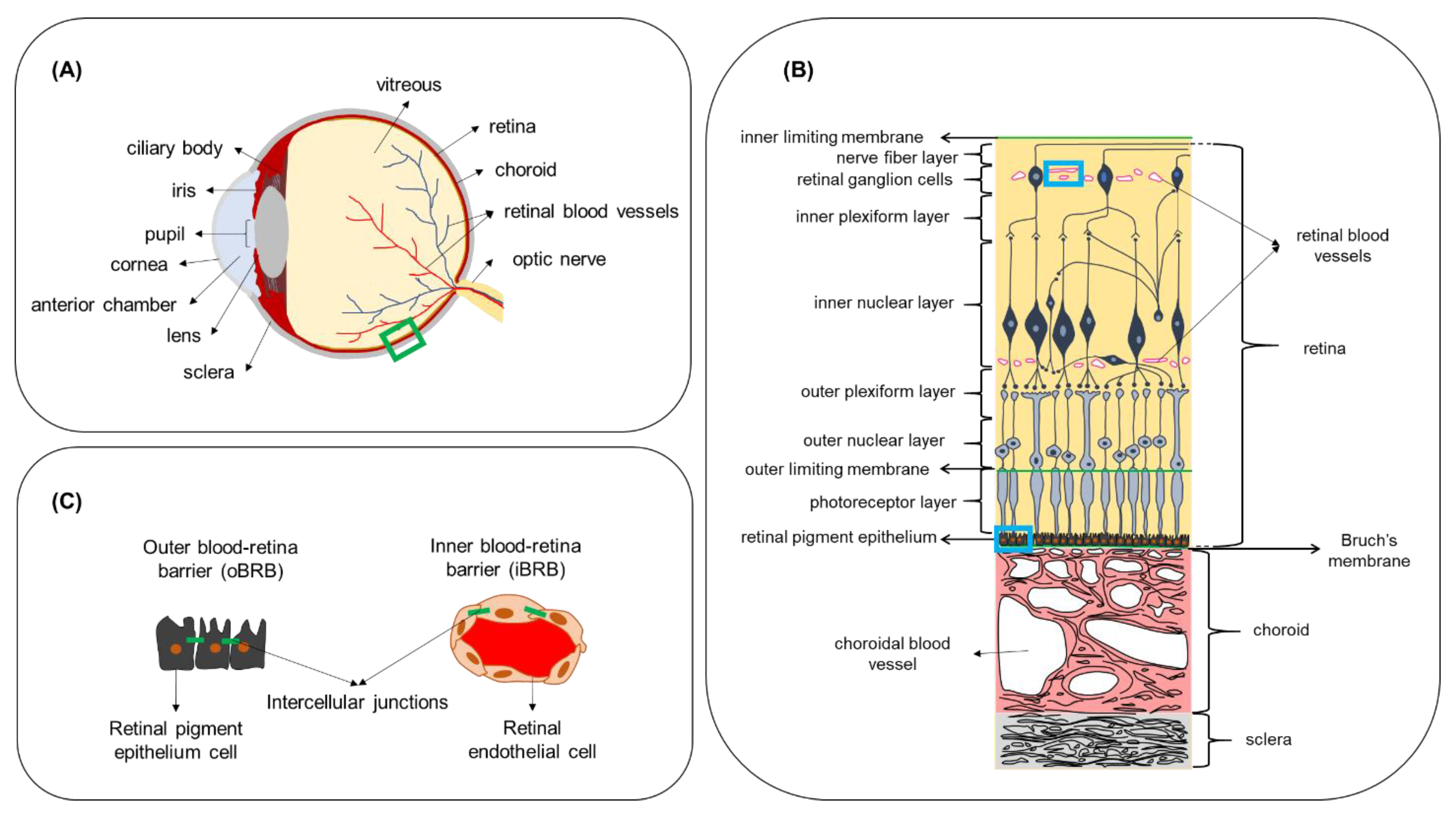
3. The Effect of Biological Barriers on the Pharmacokinetics of Ocular Drug Delivery
4. Ocular Pathologies That Could Benefit from Therapeutic Ultrasound and Microbubbles
4.1. Wet Age-Related Macular Degeneration
4.2. Glaucoma
4.3. Diabetic Retinopathy
4.4. Proliferative Vitreoretinopathy
4.5. Retinitis Pigmentosa
4.6. Retinoblastoma
4.7. Eyelid Malignant Melanoma
4.8. Corneal Opacity
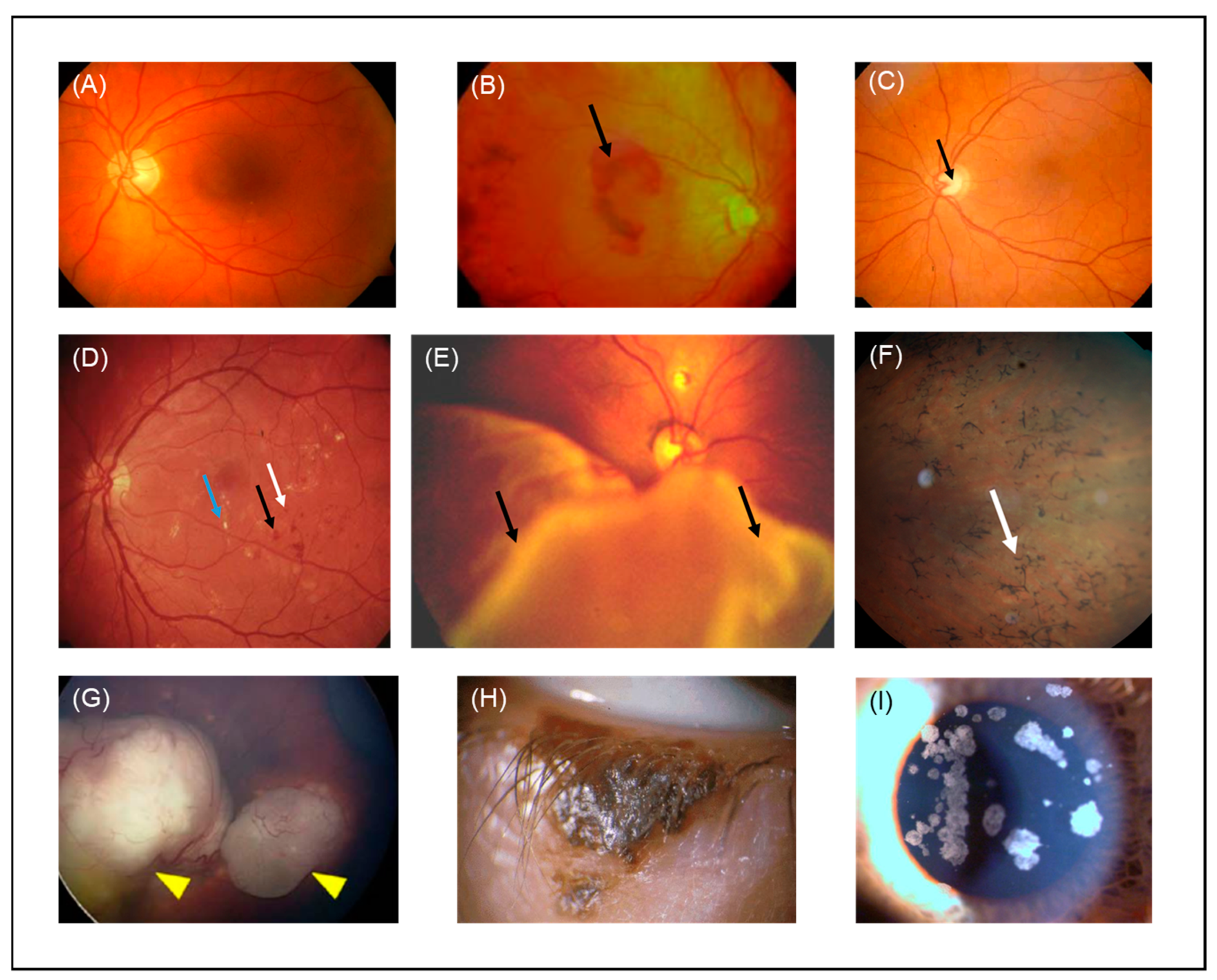
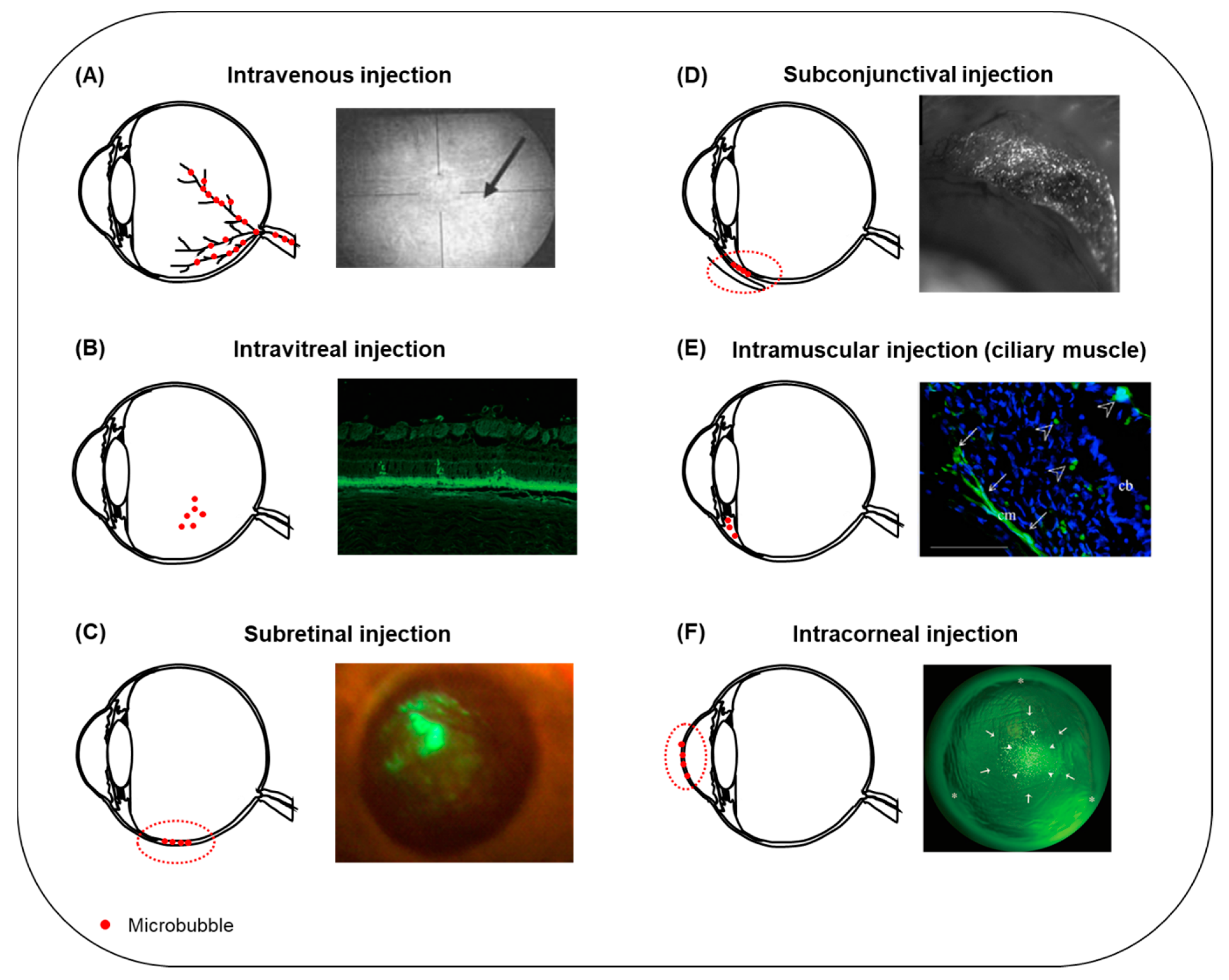
| Study Model | Delivered Compound | Microbubbles and In Vivo Administration Site | Ultrasound Parameters | Efficacy | Safety | Reference |
|---|---|---|---|---|---|---|
| In vivo, (rabbit) | Fluorescein | Definity®, intravenous | 2 MHz frequency, 0.2 and 1.7 MI, 5 min exposure time | Alteration in the diameter of uveal blood vessels observed in 20% and 80% of eyes treated al low and high MI, respectively. At high MI, vasoconstriction and extravasation of fluorescein were observed, and the mean number of altered segments in blood vessels was higher than at low MI. | No bleeding. | [79] |
| In vivo, (rat) | Gd | Definity®, intravenous | 0.69 MHz frequency, 0.81, 0.88 and 1.10 MPa PNP (MI 0.98, 1.06, and 1.32, respectively), 60 s exposure time. | Immediate increase in Gd signal after treatment indicating BRB disruption. For the two lower pressures, Gd signal was lowered 3.5 h post-treatment, revealing reversibility of BRB disruption, but not at 1.10 MPa PNP. | Extravasated erythrocytes in the nuclear layers of the retina with more severe damage at 1.10 MPa. | [80] |
| In vivo, (rat, mouse) | Evans blue, IgG, IgM | Definity®, intravenous | 1.1 MHz frequency, 0.36–0.84 MPa PNP (MI 0.34–0.80), 120 s exposure time. | Extravasation of Evans blue, IgG and IgM was observed in neural retina (INL and RGC) suggesting that the vascular plexi within these layers were permeabilized. No evidence for molecule transfer across the choroid and into the RPE. | Evidence for morphological damage, reactive gliosis, neuroinflammation and presence of erythroid cells. No megakaryocyte infiltration. | [81] |
| In vitro (RPE, Müller glia, photoreceptors) | IgG | Custom-made NBs with shells made of DPPC/DSPE-PEG(2k)-Ome and PFP inner gaseous phase | 1 MHz frequency, 0.5 W/cm2 intensity, 30 s exposure time | Increase in the intracellular uptake of IgG after treatment with USNB was cell line-dependent. USNB efficacy is highly dependent on ultrasound intensity and exposure time. | N/A | [82] |
| In vitro (RPE), in vivo (rat) | PEI/pEGFP | SonoVueTM, subretinal injection | 1 MHz frequency, 1–3 W/cm2 intensity, 1–5 min exposure time | In vitro: higher exposure time resulted in higher number of GFP-positive cells and decreased cell viability. In vivo: high density EGFP-positive cells were observed in animals treated with PEI/pEGFP + USMB, predominantly distributed in the retina. | No tissue damage. | [83] |
| In vivo (rat) | pEGFP-N1 | SonoVueTM, subretinal injection | 1 MHz frequency, 2 W/cm2 intensity, 5 min exposure time | The highest EGFP-positive signal was observed in the PEI/pDNA + USMB group, distributed in neural retina and RPE cells. The same trend was observed in the quantification of EGFP gene copy number and the EGFP mRNA expression level in the RPE and neural retina. | No evidence for corneal and retinal tissue damage, no morphological alterations and no inflammatory cell infiltration. | [84] |
| In vivo (rabbit) | pEGFP-N2 | Custom-made BL. Shells made by DSPC/DSPE-PEG (2k)-Ome, inner phase PFP gas. Intravitreal injection | 3 MHz frequency, 0.15 W/cm2 intensity, 60 s exposure time | Highest amount of GFP-score in the plasmid and USBL group. GFP-positive cells were colocalized with the areas exposed to ultrasound and were detected in the ONL. | No obvious tissue damage. | [85] |
| In vitro (human retinal pigment epithelium cells), in vivo (rat) | rAAV-EGFP | SonoVueTM, subretinal injection | 1 MHz frequency, 0.5–2 W/cm2 intensity, 1–5 min exposure time | In vitro, combined treatment with USMB resulted in the highest transduction efficiency than treatment with ultrasound only. In vivo, quantification of EGFP signal revealed significantly elevated values for the USMB group on the first 35 days post-treatment. EGFP-positive cells USMB group were found in neural retina and RPE cells. | No evidence for tissue damage. | [86] |
| In vitro (rat RPE cells), in vivo (rat) | rAAV2-EGFP | SonoVueTM, subretinal injection | 1 MHz frequency, 0.2–3 W/cm2 intensity, 15–300 s exposure time | Compared to the control group either ultrasound or microbubbles alone, but not their combination, increased rAAV-EGFP transduction of RPE-J cells in vitro. USMB-enhanced treatment resulted in a higher expression of EGFP in vivo. An increase in GFP-fluorescence was found until day 35 and reduced up to 120 days post-treatment. GFP signal was found in RPE and neural retina. | Adverse effects in cell viability in vitro observed at intensity of 3 W/cm2. In vivo, all retina cell layers were well preserved without photoreceptor loss or inflammation. | [87] |
| In vivo (rat) | Lipofectamine-formulated fluorescently labelled-siRNA | SonoVueTM, intravitreal injection | 1 MHz frequency, 2 W/cm2 intensity, 300 s exposure time | The greatest quantity of transduced cells was observed in the group treated with lipofectamine-formulated siRNA combined with USMB. No fluorescence was detected in either the untreated or treated with naked siRNA + ultrasound groups. | No significant cell viability reduction observed 12 h after transfection. Retina cell layers were well preserved without photoreceptor loss, nuclear layer vacuolation, or inflammation. | [88] |
| In vitro (human RPE cells) | rAAV-EGFP, PEI/pDNA and L/siRNA | SonoVueTM | 1 MHz frequency, 1–3 W/cm2 intensity, 60–120 s exposure time | Transfection efficiency of rAAV and PEI/pDNA vectors significantly improved when gene delivery was combined with USMB, in contrast to the L/siRNA efficiency that was benefited by ultrasound alone. Combined treatment with USMB did not cause structural alterations on the pDNA. | N/A | [89] |
| In vitro (rat RPE cells) | Fluorescently labelled siRNA encapsulated in mPEG-PLGA-PLL nanoparticles | SonoVueTM | 1 MHz frequency, 0.5–2 W/cm2 intensity, 30–60 s exposure time | Highest nanoparticle uptake observed in cells treated with ultrasound alone. Combination with USMB did not improve the nanoparticle uptake. | At the settings with the highest nanoparticle uptake, a temperature increase of 1.9 °C was reported with no influence on cell viability. | [90] |
| In vivo (rat) | Fluorescently labelled PDGF-BB siRNA encapsulated in mPEG-PLGA-PLL nanoparticles | SonoVueTM, intravitreal injection | 1 MHz frequency, 2 W/cm2 intensity, 5 min exposure time | The highest transfection efficiency in neural retina was achieved after combined treatment with USMB. | No evidence for tissue damage observed. All layers of the retina were well preserved without photoreceptor loss or inflammation. | [91] |
| In vivo (rabbit, intraocular hypertension animal model) | mNGF | SonoVueTM, intravitreal | 1 MHz frequency, 0.5 W/cm2 ultrasound intensity, 60 s exposure time | Function of optic nerve myelin and axons was improved in the group that received mNGF + USMB. Retinas treated with mNGF + USMB had clear and orderly arranged cell layers. The thickness of the inner and outer plexiform layers was nearly normal. Rod and cone cells were normally aligned without degeneration, and RGC were normal in structure. | N/A | [100] |
| In vitro (rat RGC) | pEGFP-N1 and bcl-xl | SonoVueTM | 0.3 MHz frequency, 0.25–1.25 W/cm2 intensity, 30–120 s exposure time | Improved transfection efficiency observed in pEGFP-N1 + USMB group. USMB-mediated bc1-xl transfection had a role in protection of RGCs from apoptosis, but not in complete apoptosis prevention. | N/A | [101] |
| In vivo (rat) | rAAV2-EGFP | Custom-made lipid microbubbles (shell: DSPC, 1,2-DSPE, DSPA, inner gas: PFP), intravitreal injection | 0.3 MHz frequency, 0.5–2.5 W/cm2 intensity, 60 s exposure time | Greatest EGFP expression was observed in retinas treated with rAAV2-EGFP + USMB. The majority of GFP-positive cells were RGC. | No structural, morphological alterations, no cellular infiltration in the vitreal cavity. | [102] |
| In vitro (rabbit cornea epithelial cells), in vivo (rat) | pEGFP-N2 | Custom-made BL. Shells made by DSPC/DSPE-PEG (2k)-Ome, inner gaseous phase PFP gas. Subconjunctival injection. | 1 MHz frequency, 0.8–1.2 W/cm2 intensity, 20–60 s exposure time | In vitro: The ratio of GFP-positive cells treated with USBL was about 2 times higher than the USMB group. No observed decrease in cell viability in any of the experimental groups. In vivo: GFP-positive cell density in eyes treated with USBL was significantly higher than the groups that received plasmid only, plasmid + ultrasound and plasmid + USMB. GFP-positive cells were mostly located beneath the conjunctival epithelium of the area exposed to ultrasound. No significant number of GFP-positive cells was observed in any other part of the eye. | The structure of conjunctiva was well preserved. No signs of hemorrhage, edema or inflammation. | [103] |
| In vitro (human retinal vascular endothelial cells) | ES-GFP | NMB: DPPC/DSPE-PEG2000 and cationic microbubbles CMB: DPPC/DSPE-PEG2000-Biotin/DC-Chol, containing PFP gas | 1 MHz frequency, 1 W/cm2 intensity, 1 min exposure time | CMBs had higher plasmid binding compared with NMBs. In cells treated with CMBs, the level of VEGF, Bcl-2, and Bcl-xl mRNA was decreased. | N/A | [111] |
| In vivo (rat) | pCMV-Gluc-1, pVAX1-LacZ, pEGFP-C1 | Artison, intra-muscle injection (ciliary muscle) | 1 MHz frequency, 0.7 MPa PNP, 120 s exposure time | One week after treatment, the group treated with pCMV-Gluc-1 plasmid + USMB had the greatest expression of luciferase. Enhanced expression of β-galactosidase in the ciliary muscle cells and sporadically around the ciliary body was observed microscopically. Similar enhancement and localization site of GFP protein observed in the pEGFP-C1 + USMB group. | No apoptotic cells detected in the conjunctiva, retina or cornea. A temperature increase of 3.7 °C in the lens and 7.3 °C in the ciliary muscle measured during ultrasound exposure. Normal temperature was immediately recovered. No alterations in the transparency of the lens for up to a month post-treatment. | [112] |
| In vivo (rat, proliferative vitreoretinopathy disease model) | rAAV2-TGF-β2-siRNA and rAAV2-PDGF-B-siRNA | SonoVueTM, intravitreal injection | 1 MHz frequency, 300 s exposure time | In the group treated with siRNAs + USMB, retinal morphologic alterations progressed slower than control groups. The numbers of effector cells, such as RPE cells, glial cells, fibroblasts and macrophages, and the incidence of retinal detachment, and proliferative membrane formation were significantly less than the eyes treated without USMB. | N/A | [119] |
| In vitro (human RB cells) | Doxorubicin | Artison | 1 MHz frequency, 0.3–10 W/cm2 intensity, 10 s exposure time | No significant differences in cell viability observed 24 h post-treatment between cells treated with doxorubicin alone and doxorubicin + USMB. Viability of cells exposed to doxorubicin + USMB was significantly lower compared with cells exposed to doxorubicin alone 48 and 72 h, but not 24 h post-treatment. | N/A | [133] |
| In vitro (mouse melanoma cells), in vivo (mouse) | Bleomycin | OptisonTM, intratumoral injection | 1 MHz frequency, 1–2 W/cm2 intensity, 60–240 s exposure time | Combination of bleomycin and USMB in vitro resulted in a significant decrease in cell viability at all concentrations tested. In vivo, in the bleomycin + USMB group, for drug concentrations of 0.06 mg/mL, 0.25 mg/mL and 0.5 mg/mL, tumors initially increased in weight but later had a continuous decrease until day 8. Tumors treated with 0.125 mg/mL bleomycin + USMB responded immediately after the 1st treatment with a continuous reduction in size. No reduction in size was observed in the group treated with bleomycin alone. | In vivo, temperature inside the tumor increased from 34 to 37 °C. The temperature of ultrasound probe changed in similar manner. No histological abnormalities were seen in the brain, lung, liver and heart. | [137] |
| In vitro, (rabbit corneal epithelial cells), in vivo (rabbit) | pEGFP-N2 | OptisonTM, intracorneal | 1 MHz frequency, 0.5–2 W/cm2 intensity, 15–120 s exposure time | In vitro: The greatest amount of GFP-positive cells ratio was significantly greater in samples treated with USMB. In vivo: The eyes that received plasmid + USMB showed the highest number of GFP-positive cells. GFP-positive cells appeared one day after treatment. Fluorescence intensity increased the first 8 days, significantly decreased on day 14, and was not measurable on day 30 after treatment. GFP was mainly located inside the corneal stroma. | Immediate corneal stroma haziness appeared at intensity >3 W/cm2, which spontaneously resolved immediately after treatment. No corneal damage, such as opacity or persistent epithelial defects, was observed. | [147] |
5. Safety and Tolerability of USMB in Ocular Therapeutic Applications
6. Future Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | age-related macular degeneration |
| BBB | blood–brain barrier |
| BLs | bubble liposomes |
| BRB | blood–retina barrier |
| CEUS | contrast-enhanced ultrasound |
| CMB | cationic microbubbles |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DSPA | 1,2-distearoyl-snglycero-phosphoacid |
| DSPC | 1,2-distearoyl-sn-glycero-phosphatidylcholine |
| DSPE-PEG(2k)-OMe | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] |
| DR | diabetic retinopathy |
| EGFP | enhanced green fluorescent protein |
| ES | endostatin |
| FDA | Food and Drug Administration |
| F-VEP | flash visual evoked potential |
| Gd | gadolinium |
| GFAP | glial fibrillary acidic protein |
| GFP | green fluorescence protein |
| H&E | hematoxylin and eosin |
| iBRB | inner blood–retina barrier |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| ILM | inner limiting membrane |
| INL | inner nuclear layer |
| IOP | intraocular pressure |
| L/siRNA | lipofectamine-formulated siRNA |
| MFI | mean fluorescence intensity |
| MI | mechanical index |
| mNGF | mouse neuron growth factor |
| mRNA | messenger RNA |
| mPEG-PLGA-PLL | monomethoxypoly(ethylene glycol)-poly(lactic-co-glycolic acid)-poly L-lysine |
| MW | molecular weight |
| NMB | neutral microbubbles |
| NBs | nanobubbles |
| oBRB | outer blood–retina barrier |
| OLM | outer limiting membrane |
| ONL | outer nuclear layer |
| PDGF | platelet-derived growth factor |
| pDNA | plasmid DNA |
| pEGFP | plasmid enhanced green fluorescence protein |
| PEI | polyethylenimine |
| PFP | perfluoropropane |
| PNP | peak-negative pressure |
| PVR | proliferative vitreoretinopathy |
| rAAV | recombinant adeno-associated virus |
| RB | retinoblastoma |
| RGC | retinal ganglion cells |
| RP | retinitis pigmentosa |
| RPE | retina pigment epithelium |
| siRNA | small interfering RNA |
| TGF-β2 | transforming growth factor-β2 |
| UBM | ultrasound biomicroscopy |
| USMB | ultrasound and microbubbles |
| USNB | ultrasound and nanobubbles |
| USBL | ultrasound and bubble liposomes |
| VEGF | vascular endothelial growth factor |
| WFUMB | World Federation of Ultrasound in Medicine and Biology |
References
- Henry Mundt, G.; Hughes, W.F. Ultrasonics in Ocular Diagnosis. Am. J. Ophthalmol. 1956, 41, 488–498. [Google Scholar] [CrossRef]
- Rosen, D.B.; Conway, M.D.; Ingram, C.P.; Ross, R.D.; Montilla, L.G.; Peyman, G.A. A Brief Overview of Ophthalmic Ultrasound Imaging. In Novel Diagnostic Methods in Ophthalmology; Nowinska, A., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-83880-311-7. [Google Scholar]
- Kendall, C.J.; Prager, T.C.; Cheng, H.; Gombos, D.; Tang, R.A.; Schiffman, J.S. Diagnostic Ophthalmic Ultrasound for Radiologists. Neuroimaging Clin. N. Am. 2015, 25, 327–365. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H. High-Resolution Ultrasound Imaging of the Eye—A Review. Clin. Experiment. Ophthalmol. 2009, 37, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Gramiak, R.; Shah, P.M. Echocardiography of the Aortic Root. Investig. Radiol. 1968, 3, 356–366. [Google Scholar] [CrossRef]
- Frinking, P.; Segers, T.; Luan, Y.; Tranquart, F. Three Decades of Ultrasound Contrast Agents: A Review of the Past, Present and Future Improvements. Ultrasound Med. Biol. 2020, 46, 892–908. [Google Scholar] [CrossRef]
- Klibanov, A.L. Ultrasound Contrast: Gas Microbubbles in the Vasculature. Investig. Radiol. 2021, 56, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble Agents: New Directions. Ultrasound Med. Biol. 2020, 46, 1326–1343. [Google Scholar] [CrossRef]
- Averkiou, M.A.; Bruce, M.F.; Powers, J.E.; Sheeran, P.S.; Burns, P.N. Imaging Methods for Ultrasound Contrast Agents. Ultrasound Med. Biol. 2020, 46, 498–517. [Google Scholar] [CrossRef]
- Cennamo, G.; Rosa, N.; Vallone, G.F.; Smaltino, F. First Experience with a New Echographic Contrast Agent. Br. J. Ophthalmol. 1994, 78, 823–826. [Google Scholar] [CrossRef][Green Version]
- Lemke, A.-J.; Hosten, N.; Richter, M.; Bechrakis, N.E.; Foerster, P.; Puls, R.; Gutberlet, M.; Felix, R. Contrast-Enhanced Color Doppler Sonography of Uveal Melanomas. J. Clin. Ultrasound 2001, 29, 205–211. [Google Scholar] [CrossRef]
- Forte, R.; Cennamo, G.; Staibano, S.; De Rosa, G. Echographic Examination with New Generation Contrast Agent of Choroidal Malignant Melanomas: Acta Ophthalmologica Scandinavica 2005. Acta Ophthalmol. Scand. 2005, 83, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; He, G.; Zhou, X.; Zheng, Y.; Zhu, Y.; Yang, J.; Zhang, M.; Zhou, Y. Contrast-Enhanced Ultrasound in the Diagnosis of Orbital Space-Occupying Lesions. Clin. Radiol. 2017, 72, 798.e1–798.e6. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, M.; Serafini, G.; Sconfienza, L.; Lacelli, F.; Cavallaro, M.; Coslovich, A.; Tognetto, D.; Cova, M. The Use of CEUS in the Diagnosis of Retinal/Choroidal Detachment and Associated Intraocular Masses—Preliminary Investigation in Patients with Equivocal Findings at Conventional Ultrasound. Ultraschall Med.—Eur. J. Ultrasound 2013, 35, 173–180. [Google Scholar] [CrossRef]
- Sng, W.J.; Kapur, J.; Sundar, G.; Lian, W.Q.D.; Tan, A.P. Utilization of Contrast-Enhanced Ultrasound in the Evaluation of Craniofacial Osseous Lesions: A Case Report. Clin. Imaging 2020, 60, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Williamson, T.H.; Harris, A. Color Doppler Ultrasound Imaging of Theeye and Orbit. Surv. Ophthalmol. 1996, 40, 255–267. [Google Scholar] [CrossRef]
- Skidmore, C.; Saurey, T.; Ferre, R.M.; Rodriguez-Brizuela, R.; Spaulding, J.; Lundgreen Mason, N. A Narrative Review of Common Uses of Ophthalmic Ultrasound in Emergency Medicine. J. Emerg. Med. 2021, 60, 80–89. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Lacelli, F.; Ardemagni, A.; Perrone, N.; Bertolotto, M.; Padolecchia, R.; Serafini, G. High-Resolution, Three-Dimensional, and Contrast-Enhanced Ultrasonographic Findings in Diseases of the Eye. J. Ultrasound 2010, 13, 143–149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Postema, M.; van Wamel, A.; Lancée, C.T.; de Jong, N. Ultrasound-Induced Encapsulated Microbubble Phenomena. Ultrasound Med. Biol. 2004, 30, 827–840. [Google Scholar] [CrossRef]
- Postema, M.; van Wamel, A.; ten Cate, F.J.; de Jong, N. High-Speed Photography during Ultrasound Illustrates Potential Therapeutic Applications of Microbubbles: Ultrasonic Microbubbles for Therapy. Med. Phys. 2005, 32, 3707–3711. [Google Scholar] [CrossRef]
- Qin, P.; Han, T.; Yu, A.C.H.; Xu, L. Mechanistic Understanding the Bioeffects of Ultrasound-Driven Microbubbles to Enhance Macromolecule Delivery. J. Control. Release 2018, 272, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, H.; Mayer, M.; Deng, C.X. Spatiotemporally Controlled Single Cell Sonoporation. Proc. Natl. Acad. Sci. USA 2012, 109, 16486–16491. [Google Scholar] [CrossRef]
- Lammertink, B.; Deckers, R.; Storm, G.; Moonen, C.; Bos, C. Duration of Ultrasound-Mediated Enhanced Plasma Membrane Permeability. Int. J. Pharm. 2015, 482, 92–98. [Google Scholar] [CrossRef]
- Meijering, B.D.M.; Juffermans, L.J.M.; van Wamel, A.; Henning, R.H.; Zuhorn, I.S.; Emmer, M.; Versteilen, A.M.G.; Paulus, W.J.; van Gilst, W.H.; Kooiman, K.; et al. Ultrasound and Microbubble-Targeted Delivery of Macromolecules Is Regulated by Induction of Endocytosis and Pore Formation. Circ. Res. 2009, 104, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Van Wamel, A.; Kooiman, K.; Harteveld, M.; Emmer, M.; ten Cate, F.J.; Versluis, M.; de Jong, N. Vibrating Microbubbles Poking Individual Cells: Drug Transfer into Cells via Sonoporation. J. Control. Release 2006, 112, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Schlicher, R.K.; Radhakrishna, H.; Tolentino, T.P.; Apkarian, R.P.; Zarnitsyn, V.; Prausnitz, M.R. Mechanism of Intracellular Delivery by Acoustic Cavitation. Ultrasound Med. Biol. 2006, 32, 915–924. [Google Scholar] [CrossRef]
- Afadzi, M.; Strand, S.P.; Nilssen, E.A.; Masoy, S.-E.; Johansen, T.F.; Hansen, R.; Angelsen, B.A.; de Davies, C.L. Mechanisms of the Ultrasound-Mediated Intracellular Delivery of Liposomes and Dextrans. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Paula, D.M.B.; Valero-Lapchik, V.B.; Paredes-Gamero, E.J.; Han, S.W. Therapeutic Ultrasound Promotes Plasmid DNA Uptake by Clathrin-Mediated Endocytosis: Plasmid DNA Endocytosis Induced by Ultrasound. J. Gene Med. 2011, 13, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Padilla, F. Ultrasound-Targeted Microbubble Destruction (UTMD) for Localized Drug Delivery into Tumor Tissue. IRBM 2019, 40, 10–15. [Google Scholar] [CrossRef]
- Endo-Takahashi, Y.; Negishi, Y. Microbubbles and Nanobubbles with Ultrasound for Systemic Gene Delivery. Pharmaceutics 2020, 12, 964. [Google Scholar] [CrossRef]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S.C.; Lentacker, I. Opening Doors with Ultrasound and Microbubbles: Beating Biological Barriers to Promote Drug Delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Abou-Elkacem, L.; Lee, T.; Dahl, J.; Lutz, A.M. Ultrasound and Microbubble Mediated Therapeutic Delivery: Underlying Mechanisms and Future Outlook. J. Controlled Release 2020, 326, 75–90. [Google Scholar] [CrossRef]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.C.; Moonen, C.T.W. Understanding Ultrasound Induced Sonoporation: Definitions and Underlying Mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Guo, X.; Tu, J.; Zhang, D. Mechanisms Underlying Sonoporation: Interaction between Microbubbles and Cells. Ultrason. Sonochem. 2020, 67, 105096. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of Focused Ultrasound Applied With an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.; O’Reilly, M.A.; Hynynen, K. Therapeutic Agent Delivery across the Blood–Brain Barrier Using Focused Ultrasound. Annu. Rev. Biomed. Eng. 2021, 23, 89–113. [Google Scholar] [CrossRef]
- Chen, K.-T.; Wei, K.-C.; Liu, H.-L. Focused Ultrasound Combined with Microbubbles in Central Nervous System Applications. Pharmaceutics 2021, 13, 1084. [Google Scholar] [CrossRef]
- Park, J.; Zhang, Y.; Vykhodtseva, N.; Jolesz, F.A.; McDannold, N.J. The Kinetics of Blood Brain Barrier Permeability and Targeted Doxorubicin Delivery into Brain Induced by Focused Ultrasound. J. Control. Release 2012, 162, 134–142. [Google Scholar] [CrossRef]
- Todd, N.; Angolano, C.; Ferran, C.; Devor, A.; Borsook, D.; McDannold, N. Secondary Effects on Brain Physiology Caused by Focused Ultrasound-Mediated Disruption of the Blood–brain Barrier. J. Control. Release 2020, 324, 450–459. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Sheikov, N.A.; Jolesz, F.A.; Vykhodtseva, N. Local and Reversible Blood–brain Barrier Disruption by Noninvasive Focused Ultrasound at Frequencies Suitable for Trans-Skull Sonications. NeuroImage 2005, 24, 12–20. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Effects of Acoustic Parameters and Ultrasound Contrast Agent Dose on Focused-Ultrasound Induced Blood-Brain Barrier Disruption. Ultrasound Med. Biol. 2008, 34, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Konofagou, E.E. The Size of Blood–Brain Barrier Opening Induced by Focused Ultrasound Is Dictated by the Acoustic Pressure. J. Cereb. Blood Flow Metab. 2014, 34, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Targeted Disruption of the Blood–brain Barrier with Focused Ultrasound: Association with Cavitation Activity. Phys. Med. Biol. 2006, 51, 793–807. [Google Scholar] [CrossRef]
- Chen, H.; Brayman, A.A.; Matula, T.J. Microbubble Dynamics in Microvessels: Observations of Microvessel Dilation, Invagination and Rupture. In Proceedings of the 2008 IEEE Ultrasonics Symposium, Beijing, China, 2–5 November 2008; pp. 1163–1166. [Google Scholar]
- Hwang, J.H.; Tu, J.; Brayman, A.A.; Matula, T.J.; Crum, L.A. Correlation between Inertial Cavitation Dose and Endothelial Cell Damage in Vivo. Ultrasound Med. Biol. 2006, 32, 1611–1619. [Google Scholar] [CrossRef]
- Keravnou, C.P.; De Cock, I.; Lentacker, I.; Izamis, M.-L.; Averkiou, M.A. Microvascular Injury and Perfusion Changes Induced by Ultrasound and Microbubbles in a Machine-Perfused Pig Liver. Ultrasound Med. Biol. 2016, 42, 2676–2686. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.B.; Suo, D.; Wang, Y.-N.; Kenerson, H.; Yeung, R.S.; Averkiou, M.A. Image-Guided Treatment of Primary Liver Cancer in Mice Leads to Vascular Disruption and Increased Drug Penetration. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Goertz, D.E.; Todorova, M.; Mortazavi, O.; Agache, V.; Chen, B.; Karshafian, R.; Hynynen, K. Antitumor Effects of Combining Docetaxel (Taxotere) with the Antivascular Action of Ultrasound Stimulated Microbubbles. PLoS ONE 2012, 7, e52307. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.K.W.; Ansaloni, S.; Ziemer, L.S.; Lee, W.M.-F.; Feldman, M.D.; Sehgal, C.M. The Antivascular Action of Physiotherapy Ultrasound on Murine Tumors. Ultrasound Med. Biol. 2005, 31, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.C.; Sultan, L.R.; Hunt, S.J.; Gade, T.P.; Karmacharya, M.B.; Schultz, S.M.; Brice, A.K.; Wood, A.K.W.; Sehgal, C.M. Microbubble Enhanced Ultrasound for the Antivascular Treatment and Monitoring of Hepatocellular Carcinoma. Nanotheranostics 2019, 3, 331–341. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, M.; Wang, J.; Xi, F.; Zhong, J.; Yang, Y.; Jin, H.; Liu, J. Improving the Therapeutic Effect of Ultrasound Combined With Microbubbles on Muscular Tumor Xenografts With Appropriate Acoustic Pressure. Front. Pharmacol. 2020, 11, 1057. [Google Scholar] [CrossRef]
- Bertuglia, S. Increase in Capillary Perfusion Following Low-Intensity Ultrasound and Microbubbles during Postischemic Reperfusion. Crit. Care Med. 2005, 33, 2061–2067. [Google Scholar] [CrossRef]
- Belcik, J.T.; Mott, B.H.; Xie, A.; Zhao, Y.; Kim, S.; Lindner, N.J.; Ammi, A.; Linden, J.M.; Lindner, J.R. Augmentation of Limb Perfusion and Reversal of Tissue Ischemia Produced by Ultrasound-Mediated Microbubble Cavitation. Circ. Cardiovasc. Imaging 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Remington, L.A. Clinical Anatomy and Physiology of the Visual System, 3rd ed.; Elsevier/Butterworth Heinemann: St. Louis, MO, USA, 2012; ISBN 9781437719260. [Google Scholar]
- Ansari, M.W. Atlas of Ocular Anatomy, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319427812. [Google Scholar]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.M.F.; Bergen, A.A.B. The Dynamic Nature of Bruch’s Membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef]
- Maurice, D.M.; Mishima, S. Ocular Pharmacokinetics. In Pharmacology of the Eye; Sears, M.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 69, pp. 19–116. ISBN1 978-3-642-69224-6. ISBN2 978-3-642-69222-2. [Google Scholar]
- Del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Kidron, H.; del Amo, E.M.; Vellonen, K.-S.; Urtti, A. Prediction of the Vitreal Half-Life of Small Molecular Drug-Like Compounds. Pharm. Res. 2012, 29, 3302–3311. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Vellonen, K.-S.; Kidron, H.; Urtti, A. Intravitreal Clearance and Volume of Distribution of Compounds in Rabbits: In Silico Prediction and Pharmacokinetic Simulations for Drug Development. Eur. J. Pharm. Biopharm. 2015, 95, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Robinson, M.R.; Lizak, M.J.; Tansey, G.; Lutz, R.J.; Yuan, P.; Wang, N.S.; Csaky, K.G. Controlled Drug Release from an Ocular Implant: An Evaluation Using Dynamic Three-Dimensional Magnetic Resonance Imaging. Investig. Opthalmol. Vis. Sci. 2004, 45, 2722. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.T.; Bell, R.A. Molecular Radii of Probes Used in Studies of Intestinal Permeability. Gut 1987, 28, 110–111. [Google Scholar] [CrossRef][Green Version]
- Ambati, J.; Canakis, C.S.; Miller, J.W.; Gragoudas, E.S.; Edwards, A.; Weissgold, D.J.; Kim, I.; Delori, F.C.; Adamis, A.P. Diffusion of High Molecular Weight Compounds through Sclera. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1181–1185. [Google Scholar]
- Pitkänen, L.; Ranta, V.-P.; Moilanen, H.; Urtti, A. Permeability of Retinal Pigment Epithelium: Effects of Permeant Molecular Weight and Lipophilicity. Investig. Opthalmol. Vis. Sci. 2005, 46, 641. [Google Scholar] [CrossRef]
- Smith, S.J.; Smith, B.D.; Mohney, B.G. Ocular Side Effects Following Intravitreal Injection Therapy for Retinoblastoma: A Systematic Review. Br. J. Ophthalmol. 2014, 98, 292–297. [Google Scholar] [CrossRef]
- Vo Kim, S.; Fajnkuchen, F.; Sarda, V.; Qu-Knafo, L.; Bodaghi, B.; Giocanti-Aurégan, A. Sustained Intraocular Pressure Elevation in Eyes Treated with Intravitreal Injections of Anti-Vascular Endothelial Growth Factor for Diabetic Macular Edema in a Real-Life Setting. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Sampat, K.M.; Garg, S.J. Complications of Intravitreal Injections. Curr. Opin. Ophthalmol. 2010, 21, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Urs, R.; Ketterling, J.A.; Yu, A.C.H.; Lloyd, H.O.; Yiu, B.Y.S.; Silverman, R.H. Ultrasound Imaging and Measurement of Choroidal Blood Flow. Transl. Vis. Sci. Technol. 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Gordiyenko, N.; Campos, M.; Lee, J.W.; Fariss, R.N.; Sztein, J.; Rodriguez, I.R. RPE Cells Internalize Low-Density Lipoprotein (LDL) and Oxidized LDL (oxLDL) in Large Quantities In Vitro and In Vivo. Investig. Opthalmol. Vis. Sci. 2004, 45, 2822. [Google Scholar] [CrossRef]
- Farkas, T.G.; Sylvester, V.; Archer, D.; Altona, M. The Histochemistry of Drusen. Am. J. Ophthalmol. 1971, 71, 1206–1215. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen Proteome Analysis: An Approach to the Etiology of Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-Related Macular Degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Dugel, P.U.; Jaffe, G.J.; Sallstig, P.; Warburton, J.; Weichselberger, A.; Wieland, M.; Singerman, L. Brolucizumab Versus Aflibercept in Participants with Neovascular Age-Related Macular Degeneration: A Randomized Trial. Ophthalmology 2017, 124, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lu, T.; Tuomi, L.; Jumbe, N.; Lu, J.; Eppler, S.; Kuebler, P.; Damico-Beyer, L.A.; Joshi, A. Pharmacokinetics of Ranibizumab in Patients with Neovascular Age-Related Macular Degeneration: A Population Approach. Investig. Opthalmol. Vis. Sci. 2013, 54, 1616. [Google Scholar] [CrossRef] [PubMed]
- Moisseiev, E.; Waisbourd, M.; Ben-Artsi, E.; Levinger, E.; Barak, A.; Daniels, T.; Csaky, K.; Loewenstein, A.; Barequet, I.S. Pharmacokinetics of Bevacizumab after Topical and Intravitreal Administration in Human Eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 331–337. [Google Scholar] [CrossRef]
- Semeraro, F.; Morescalchi, F.; Duse, S.; Parmeggiani, F.; Gambicorti, E.; Costagliola, C. Aflibercept in Wet AMD: Specific Role and Optimal Use. Drug Des. Devel. Ther. 2013, 711. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, T.; Karshafian, R.; Pavlin, C.J.; Burns, P.N. Insonation of the Eye in the Presence of Microbubbles: Preliminary Study of the Duration and Degree of Vascular Bioeffects-Work in Progress. J. Ultrasound Med. 2007, 26, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Zhang, Y.; Vykhodtseva, N.; Akula, J.D.; McDannold, N.J. Targeted and Reversible Blood-Retinal Barrier Disruption via Focused Ultrasound and Microbubbles. PLoS ONE 2012, 7, e42754. [Google Scholar] [CrossRef]
- Touahri, Y.; Dixit, R.; Kofoed, R.H.; Miloska, K.; Park, E.; Raeisossadati, R.; Markham-Coultes, K.; David, L.A.; Rijal, H.; Zhao, J.; et al. Focused Ultrasound as a Novel Strategy for Noninvasive Gene Delivery to Retinal Müller Glia. Theranostics 2020, 10, 2982–2999. [Google Scholar] [CrossRef]
- Thakur, S.S.; Ward, M.S.; Popat, A.; Flemming, N.B.; Parat, M.-O.; Barnett, N.L.; Parekh, H.S. Stably Engineered Nanobubbles and Ultrasound—An Effective Platform for Enhanced Macromolecular Delivery to Representative Cells of the Retina. PLoS ONE 2017, 12, e0178305. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Qian, J.; Li, F.; Li, H. Ultrasound-Targeted Microbubble Destruction Enhances Polyethylenimine-Mediated Gene Transfection in Vitro in Human Retinal Pigment Epithelial Cells and in Vivo in Rat Retina. Mol. Med. Rep. 2015, 12, 2835–2841. [Google Scholar] [CrossRef]
- Li, H.; Qian, J.; Yao, C.; Wan, C.; Li, F. Combined Ultrasound-Targeted Microbubble Destruction and Polyethylenimine-Mediated Plasmid DNA Delivery to the Rat Retina: Enhanced Efficiency and Accelerated Expression: US Assisted PEI/pDNA Delivery in Retina. J. Gene Med. 2016, 18, 47–56. [Google Scholar] [CrossRef]
- Sonoda, S.; Tachibana, K.; Yamashita, T.; Shirasawa, M.; Terasaki, H.; Uchino, E.; Suzuki, R.; Maruyama, K.; Sakamoto, T. Selective Gene Transfer to the Retina Using Intravitreal Ultrasound Irradiation. J. Ophthalmol. 2012, 2012, 412752. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zheng, X.Z.; Wang, H.P.; Li, F.; Wu, Y.; Du, L.F. Ultrasound-Targeted Microbubble Destruction Enhances AAV-Mediated Gene Transfection in Human RPE Cells in Vitro and Rat Retina in Vivo. Gene Ther. 2009, 16, 1146–1153. [Google Scholar] [CrossRef]
- Zheng, X.; Li, H.; Du, L.; Wang, H.; Gu, Q. In Vivo and in Vitro Effects of Ultrasound or/and Microbubbles on Recombinant Adeno-Associated Virus-Mediated Transgene Expression in the Retina. Asian Biomed. 2009, 3, 497–506. [Google Scholar]
- Zheng, X.; Ji, P.; Hu, J. Sonoporation Using Microbubbles Promotes Lipofectamine -Mediated siRNA Transduction to Rat Retina. Bosn. J. Basic Med. Sci. 2011, 11, 147. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Wan, C.; Li, F. Recombinant Adeno-Associated Virus-, Polyethylenimine/plasmid- and Lipofectamine/carboxyfluorescein-Labeled Small Interfering RNA-Based Transfection in Retinal Pigment Epithelial Cells with Ultrasound and/or SonoVue. Mol. Med. Rep. 2015, 11, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shi, Q.S.; Sun, Y.; Liu, P.F.; Zhu, M.J.; Du, L.F.; Duan, Y.R. Enhanced Delivery of Monomethoxypoly(ethylene Glycol)-Poly(lactic-Co-Glycolic Acid)-Poly L-Lysine Nanoparticles Loading Platelet-Derived Growth Factor BB Small Interfering RNA by Ultrasound And/or Microbubbles to Rat Retinal Pigment Epithelium Cells: Enhanced Delivery of NPs Loading siRNA by US And/or MBs. J. Gene Med. 2011, 13, 312–323. [Google Scholar] [CrossRef]
- Du, J.; Sun, Y.; Li, F.-H.; Du, L.-F.; Duan, Y.-R. Enhanced Delivery of Biodegradable mPEG-PLGA-PLL Nanoparticles Loading Cy3-Labelled PDGF-BB siRNA by UTMD to Rat Retina. J. Biosci. 2017, 42, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Harasymowycz, P.; Birt, C.; Gooi, P.; Heckler, L.; Hutnik, C.; Jinapriya, D.; Shuba, L.; Yan, D.; Day, R. Medical Management of Glaucoma in the 21st Century from a Canadian Perspective. J. Ophthalmol. 2016, 2016, 6509809. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef]
- Brooks, A.M.V.; Gillies, W.E. Ocular Beta-Blockers in Glaucoma Management: Clinical Pharmacological Aspects. Drugs Aging 1992, 2, 208–221. [Google Scholar] [CrossRef]
- Schwartz, K.; Budenz, D. Current Management of Glaucoma. Curr. Opin. Ophthalmol. 2004, 15, 119–126. [Google Scholar] [CrossRef]
- Apătăchioae, I.; Chiseliţă, D. Alpha-2 adrenergic agonists in the treatment of glaucoma. Oftalmol. Buchar. Rom. 1999, 47, 35–40. [Google Scholar]
- Tang, W.; Zhang, F.; Liu, K.; Duan, X. Efficacy and Safety of Prostaglandin Analogues in Primary Open-Angle Glaucoma or Ocular Hypertension Patients: A Meta-Analysis. Medicine 2019, 98, e16597. [Google Scholar] [CrossRef]
- Reardon, G.; Kotak, S.; Schwartz, G. Objective Assessment of Compliance and Persistence among Patients Treated for Glaucoma and Ocular Hypertension: A Systematic Review. Patient Prefer. Adherence 2011, 5, 441. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.K.; Erb, C.; Hoffmann, E.M.; Dietlein, T.; Pfeiffer, N. The Diagnosis and Treatment of Glaucoma. Dtsch. Aerzteblatt Online 2020, 117, 225. [Google Scholar] [CrossRef]
- Shen, X.; Huang, L.; Ma, D.; Zhao, J.; Xie, Y.; Li, Q.; Zeng, A.; Zeng, K.; Tian, R.; Wang, T.; et al. Ultrasound Microbubbles Enhance the Neuroprotective Effect of Mouse Nerve Growth Factor on Intraocular Hypertension-Induced Neuroretina Damage in Rabbits. J. Ophthalmol. 2016, 2016, 4235923. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, S.; Ren, J.; Xiong, H.; Yan, X.; Wang, Z. Gene Transfection to Retinal Ganglion Cells Mediated by Ultrasound Microbubbles in Vitro. Acad. Radiol. 2009, 16, 1086–1094. [Google Scholar] [CrossRef]
- Xie, W.; Liu, S.; Su, H.; Wang, Z.; Zheng, Y.; Fu, Y. Ultrasound Microbubbles Enhance Recombinant Adeno-Associated Virus Vector Delivery to Retinal Ganglion Cells In Vivo. Acad. Radiol. 2010, 17, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Sonoda, S.; Suzuki, R.; Arimura, N.; Tachibana, K.; Maruyama, K.; Sakamoto, T. A Novel Bubble Liposome and Ultrasound-Mediated Gene Transfer to Ocular Surface: RC-1 Cells in Vitro and Conjunctiva in Vivo. Exp. Eye Res. 2007, 85, 741–748. [Google Scholar] [CrossRef]
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L.; Klein, R. Retinopathy in Diabetes. Diabetes Care 2004, 27, S84–S87. [Google Scholar] [CrossRef]
- Mohamed, Q.; Gillies, M.C.; Wong, T.Y. Management of Diabetic Retinopathy: A Systematic Review. JAMA 2007, 298, 902. [Google Scholar] [CrossRef]
- Engerman, R.L. Pathogenesis of Diabetic Retinopathy. Diabetes 1989, 38, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Chawan-Saad, J.; Wu, M.; Wu, A.; Wu, L. Corticosteroids for Diabetic Macular Edema. Taiwan J. Ophthalmol. 2019, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simó, R.; et al. The Progress in Understanding and Treatment of Diabetic Retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Mansour, S.E.; Browning, D.J.; Wong, K.; Flynn Jr, H.W.; Bhavsar, A.R. The Evolving Treatment of Diabetic Retinopathy. Clin. Ophthalmol. 2020, 14, 653–678. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Yang, J.F.; Huang, Y.; Rosenblatt, M.I.; Chang, J.-H.; Azar, D.T. Endostatin’s Emerging Roles in Angiogenesis, Lymphangiogenesis, Disease, and Clinical Applications. Biochim. Biophys. Acta BBA—Gen. Subj. 2015, 1850, 2422–2438. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Z.; Zhou, Y.; Zhou, X.; Li, P.; Wang, Z.; Zhang, Q. Experimental Endostatin-GFP Gene Transfection into Human Retinal Vascular Endothelial Cells Using Ultrasound-Targeted Cationic Microbubble Destruction. Mol. Vis. 2015, 21, 930–938. [Google Scholar]
- Kowalczuk, L.; Boudinet, M.; El Sanharawi, M.; Touchard, E.; Naud, M.-C.; Saïed, A.; Jeanny, J.-C.; Behar-Cohen, F.; Laugier, P. In Vivo Gene Transfer into the Ocular Ciliary Muscle Mediated by Ultrasound and Microbubbles. Ultrasound Med. Biol. 2011, 37, 1814–1827. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Pathogenic Mechanisms in Proliferative Vitreoretinopathy. Arch. Ophthalmol. 1997, 115, 237. [Google Scholar] [CrossRef]
- Pastor, J.C.; de la Rúa, E.R.; Martín, F. Proliferative Vitreoretinopathy: Risk Factors and Pathobiology. Prog. Retin. Eye Res. 2002, 21, 127–144. [Google Scholar] [CrossRef]
- Ikuno, Y.; Leong, F.L.; Kazlauskas, A. Attenuation of Experimental Proliferative Vitreoretinopathy by Inhibiting the Platelet-Derived Growth Factor Receptor. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3107–3116. [Google Scholar]
- Zheng, X.-Z.; Du, L.-F.; Wang, H.-P. A Immunohistochemical Analysis of a Rat Model of Proliferative Vitreoretinopathy and a Comparison of the Expression of Tgf-β and PDGF among the Induction Methods. Bosn. J. Basic Med. Sci. 2010, 10, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Nagineni, C.N.; Kutty, V.; Detrick, B.; Hooks, J.J. Expression of PDGF and Their Receptors in Human Retinal Pigment Epithelial Cells and Fibroblasts: Regulation by TGF-β. J. Cell. Physiol. 2005, 203, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.C. Proliferative Vitreoretinopathy. Surv. Ophthalmol. 1998, 43, 3–18. [Google Scholar] [CrossRef]
- Zheng, X.; Du, L.; Wang, H.; Gu, Q. A Novel Approach to Attenuate Proliferative Vitreoretinopathy Using Ultrasound-Targeted Microbubble Destruction and Recombinant Adeno-Associated Virus-Mediated RNA Interference Targeting Transforming Growth Factor-β2 and Platelet-Derived Growth Factor-B: Attenuate PVR Using UTMD and RNAi Targeting TGF-β2 and PDGF-B. J. Gene Med. 2012, 14, 339–347. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis Pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Berson, E.L. Retinitis Pigmentosa. The Friedenwald Lecture. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1659–1676. [Google Scholar]
- Berson, E.L. A Randomized Trial of Vitamin A and Vitamin E Supplementation for Retinitis Pigmentosa. Arch. Ophthalmol. 1993, 111, 761. [Google Scholar] [CrossRef]
- Sibulesky, L.; Hayes, K.; Pronczuk, A.; Weigel-DiFranco, C.; Rosner, B.; Berson, E.L. Safety of <7500 RE (<25000 IU) Vitamin A Daily in Adults with Retinitis Pigmentosa. Am. J. Clin. Nutr. 1999, 69, 656–663. [Google Scholar] [CrossRef]
- Knudson, A.G. Mutation and Cancer: Statistical Study of Retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef]
- Friend, S.H.; Bernards, R.; Rogelj, S.; Weinberg, R.A.; Rapaport, J.M.; Albert, D.M.; Dryja, T.P. A Human DNA Segment with Properties of the Gene That Predisposes to Retinoblastoma and Osteosarcoma. Nature 1986, 323, 643–646. [Google Scholar] [CrossRef]
- Global Retinoblastoma Study Group; Fabian, I.D.; Abdallah, E.; Abdullahi, S.U.; Abdulqader, R.A.; Adamou Boubacar, S.; Ademola-Popoola, D.S.; Adio, A.; Afshar, A.R.; Aggarwal, P.; et al. Global Retinoblastoma Presentation and Analysis by National Income Level. JAMA Oncol. 2020, 6, 685. [Google Scholar] [CrossRef]
- MacCarthy, A.; Birch, J.M.; Draper, G.J.; Hungerford, J.L.; Kingston, J.E.; Kroll, M.E.; Stiller, C.A.; Vincent, T.J.; Murphy, M.F.G. Retinoblastoma: Treatment and Survival in Great Britain 1963 to 2002. Br. J. Ophthalmol. 2009, 93, 38–39. [Google Scholar] [CrossRef]
- Munier, F.L.; Mosimann, P.; Puccinelli, F.; Gaillard, M.-C.; Stathopoulos, C.; Houghton, S.; Bergin, C.; Beck-Popovic, M. First-Line Intra-Arterial versus Intravenous Chemotherapy in Unilateral Sporadic Group D Retinoblastoma: Evidence of Better Visual Outcomes, Ocular Survival and Shorter Time to Success with Intra-Arterial Delivery from Retrospective Review of 20 Years of Treatment. Br. J. Ophthalmol. 2017, 101, 1086–1093. [Google Scholar] [CrossRef]
- Fernandes, A.G.; Pollock, B.D.; Rabito, F.A. Retinoblastoma in the United States: A 40-Year Incidence and Survival Analysis. J. Pediatr. Ophthalmol. Strabismus 2018, 55, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ancona-Lezama, D.; Dalvin, L.; Shields, C. Modern Treatment of Retinoblastoma: A 2020 Review. Indian J. Ophthalmol. 2020, 68, 2356. [Google Scholar] [CrossRef]
- Manjandavida, F.; Stathopoulos, C.; Zhang, J.; Honavar, S.; Shields, C. Intra-Arterial Chemotherapy in Retinoblastoma—A Paradigm Change. Indian J. Ophthalmol. 2019, 67, 740. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Lally, S.E.; Leahey, A.M.; Jabbour, P.M.; Caywood, E.H.; Schwendeman, R.; Shields, J.A. Targeted Retinoblastoma Management: When to Use Intravenous, Intra-Arterial, Periocular, and Intravitreal Chemotherapy. Curr. Opin. Ophthalmol. 2014, 25, 374–385. [Google Scholar] [CrossRef]
- Lee, N.G.; Berry, J.L.; Lee, T.C.; Wang, A.T.; Honowitz, S.; Murphree, A.L.; Varshney, N.; Hinton, D.R.; Fawzi, A.A. Sonoporation Enhances Chemotherapeutic Efficacy in Retinoblastoma Cells In Vitro. Investig. Opthalmol. Vis. Sci. 2011, 52, 3868. [Google Scholar] [CrossRef]
- Giblin, M.E.; Shields, C.L.; Shields, J.A.; Eagle, R.C. Primary Eyelid Malignant Melanoma Associated With Primary Conjunctival Malignant Melanoma. Aust. N. Z. J. Ophthalmol. 1988, 16, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sains, R.S. Ocular Melanomas. Dermatol. Clin. 1985, 3, 297–307. [Google Scholar] [CrossRef]
- Esmaeli, B. Sentinel Lymph Node Mapping for Patients with Cutaneous and Conjunctival Malignant Melanoma. Ophthal. Plast. Reconstr. Surg. 2000, 16, 170–172. [Google Scholar] [CrossRef]
- Sonoda, S.; Tachibana, K.; Uchino, E.; Yamashita, T.; Sakoda, K.; Sonoda, K.-H.; Hisatomi, T.; Izumi, Y.; Sakamoto, T. Inhibition of Melanoma by Ultrasound-Microbubble-Aided Drug Delivery Suggests Membrane Permeabilization. Cancer Biol. Ther. 2007, 6, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, T.H.; Yin, J.; Dana, R. Methods for Assessing Corneal Opacity. Semin. Ophthalmol. 2019, 34, 205–210. [Google Scholar] [CrossRef]
- Memarzadeh, F.; Li, Y.; Francis, B.A.; Smith, R.E.; Gutmark, J.; Huang, D. Optical Coherence Tomography of the Anterior Segment in Secondary Glaucoma with Corneal Opacity after Penetrating Keratoplasty. Br. J. Ophthalmol. 2007, 91, 189–192. [Google Scholar] [CrossRef]
- Laibson, P.R. Corneal Infiltrates in Epidemic Keratoconjunctivitis: Response to Double-Blind Corticosteroid Therapy. Arch. Ophthalmol. 1970, 84, 36. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, E.C.A.; Cauchi, P.A.; Azuara-Blanco, A.; Foot, B. Surveillance of Severe Chemical Corneal Injuries in the UK. Br. J. Ophthalmol. 2009, 93, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.S.; Ferraz, C.A.; Hazarbassanov, R.M.; Allemann, N.; Campos, M. Phototherapeutic Keratectomy for the Treatment of Corneal Opacities After Epidemic Keratoconjunctivitis. Am. J. Ophthalmol. 2011, 151, 35–43.e1. [Google Scholar] [CrossRef]
- Gordon, Y.J. The Evolution of Antiviral Therapy for External Ocular Viral Infections Over Twenty-Five Years. Cornea 2000, 19, 673–680. [Google Scholar] [CrossRef]
- Tan, D.T.; Dart, J.K.; Holland, E.J.; Kinoshita, S. Corneal Transplantation. The Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef]
- Kupferman, A.; Pratt, M.; Suckewer, K. Topically Applied Steroids in Corneal Disease: III. The Role of Drug Derivative in Stromal Absorption of Dexamethasone. Arch. Ophthalmol. 1974, 91, 373. [Google Scholar] [CrossRef]
- Cox, W.; Allan, K.; Howard, L. Topically Applied Steroids in Corneal Disease: I. The Role of Inflammation in Stromal Absorption of Dexamethasone. Arch. Ophthalmol. 1972, 88, 308. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Tachibana, K.; Uchino, E.; Okubo, A.; Yamamoto, M.; Sakoda, K.; Hisatomi, T.; Sonoda, K.-H.; Negishi, Y.; Izumi, Y.; et al. Gene Transfer to Corneal Epithelium and Keratocytes Mediated by Ultrasound with Microbubbles. Investig. Opthalmol. Vis. Sci. 2006, 47, 558. [Google Scholar] [CrossRef] [PubMed]
- Dimaras, H.; Corson, T.W. Retinoblastoma, the Visible CNS Tumor: A Review: DIMARAS and CORSON. J. Neurosci. Res. 2019, 97, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Main, M.L.; Goldman, J.H.; Grayburn, P.A. Thinking outside the “Box”—The Ultrasound Contrast Controversy. J. Am. Coll. Cardiol. 2007, 50, 2434–2437. [Google Scholar] [CrossRef]
- Fisher, N.G.; Christiansen, J.P.; Klibanov, A.; Taylor, R.P.; Kaul, S.; Lindner, J.R. Influence of Microbubble Surface Charge on Capillary Transit and Myocardial Contrast Enhancement. J. Am. Coll. Cardiol. 2002, 40, 811–819. [Google Scholar] [CrossRef]
- Oyarzabal, N.A.; Longo Areso, N.; Bernedo Belar, N.; Popolizio, I.G.; Arregui, A.V.; Balza de Vallejo, O.V. Anaphylactic Shock Due to Allergy to Macrogol 4000 Contained in SonoVue®. Case Rep. Clin. Med. 2017, 6, 143–147. [Google Scholar] [CrossRef][Green Version]
- Krantz, M.S.; Liu, Y.; Phillips, E.J.; Stone, C.A. Anaphylaxis to PEGylated Liposomal Echocardiogram Contrast in a Patient with IgE-Mediated Macrogol Allergy. J. Allergy Clin. Immunol. Pract. 2020, 8, 1416–1419.e3. [Google Scholar] [CrossRef]
- Lindner, J.R.; Belcik, T.; Main, M.L.; Montanaro, A.; Mulvagh, S.L.; Olson, J.; Olyaei, A.; Porter, T.R.; Senior, R. Expert Consensus Statement from the American Society of Echocardiography on Hypersensitivity Reactions to Ultrasound Enhancing Agents in Patients with Allergy to Polyethylene Glycol. J. Am. Soc. Echocardiogr. 2021, 34, 707–708. [Google Scholar] [CrossRef]
- Williams, T.M.; Harvey, R.; Kratzert, W.B.; Fischer, M.A.; Neelankavil, J. Ultrasound-Enhancing Agent Safety: Understanding the New Food and Drug Administration Warning on Polyethylene Glycol. J. Cardiothorac. Vasc. Anesth. 2021. [Google Scholar] [CrossRef]
- Fix, S.M.; Nyankima, A.G.; McSweeney, M.D.; Tsuruta, J.K.; Lai, S.K.; Dayton, P.A. Accelerated Clearance of Ultrasound Contrast Agents Containing Polyethylene Glycol Is Associated with the Generation of Anti-Polyethylene Glycol Antibodies. Ultrasound Med. Biol. 2018, 44, 1266–1280. [Google Scholar] [CrossRef]
- Schneider, M.; Arditi, M.; Barrau, M.-B.; Brochot, J.; Broillet, A.; Ventrone, R.; Yan, F. BR1: A New Ultrasonographic Contrast Agent Based on Sulfur Hexafluoride-Filled Microbubbles. Investig. Radiol. 1995, 30, 451–457. [Google Scholar] [CrossRef]
- Abdelmoneim, S.S.; Mulvagh, S.L. Perflutren Lipid Microsphere Injectable Suspension for Cardiac Ultrasound. Imaging Med. 2012, 4, 171–191. [Google Scholar] [CrossRef]
- Park, J.; Park, D.; Shin, U.; Moon, S.; Kim, C.; Kim, H.; Park, H.; Choi, K.; Jung, B.; Oh, J.; et al. Synthesis of Laboratory Ultrasound Contrast Agents. Molecules 2013, 18, 13078–13095. [Google Scholar] [CrossRef]
- Wilson, S.R.; Burns, P.N. Microbubble-Enhanced US in Body Imaging: What Role? Radiology 2010, 257, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Sontum, P.C. Physicochemical Characteristics of SonazoidTM, A New Contrast Agent for Ultrasound Imaging. Ultrasound Med. Biol. 2008, 34, 824–833. [Google Scholar] [CrossRef]
- Al-Jawadi, S.; Thakur, S.S. Ultrasound-Responsive Lipid Microbubbles for Drug Delivery: A Review of Preparation Techniques to Optimise Formulation Size, Stability and Drug Loading. Int. J. Pharm. 2020, 585, 119559. [Google Scholar] [CrossRef] [PubMed]
- Van Elburg, B.; Collado-Lara, G.; Bruggert, G.-W.; Segers, T.; Versluis, M.; Lajoinie, G. Feedback-Controlled Microbubble Generator Producing One Million Monodisperse Bubbles per Second. Rev. Sci. Instrum. 2021, 92, 035110. [Google Scholar] [CrossRef]
- McMahon, D.; Poon, C.; Hynynen, K. Evaluating the Safety Profile of Focused Ultrasound and Microbubble-Mediated Treatments to Increase Blood-Brain Barrier Permeability. Expert Opin. Drug Deliv. 2019, 16, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-S.; Vlachos, F.; Choi, J.J.; Deffieux, T.; Selert, K.; Konofagou, E.E. In Vivo Transcranial Cavitation Threshold Detection during Ultrasound-Induced Blood–brain Barrier Opening in Mice. Phys. Med. Biol. 2010, 55, 6141–6155. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Hynynen, K. Blood-Brain Barrier: Real-Time Feedback-Controlled Focused Ultrasound Disruption by Using an Acoustic Emissions–based Controller. Radiology 2012, 263, 96–106. [Google Scholar] [CrossRef]
- FDA Guidance for Industry and FDA Staff. Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. 29 June 2019. Available online: https://www.fda.gov/media/71100/download (accessed on 22 July 2021).
- Kopechek, J.A.; Kim, H.; McPherson, D.D.; Holland, C.K. Calibration of the 1-MHz Sonitron Ultrasound System. Ultrasound Med. Biol. 2010, 36, 1762–1766. [Google Scholar] [CrossRef] [PubMed]
- Lafond, M.; Aptel, F.; Mestas, J.-L.; Lafon, C. Ultrasound-Mediated Ocular Delivery of Therapeutic Agents: A Review. Expert Opin. Drug Deliv. 2017, 14, 539–550. [Google Scholar] [CrossRef] [PubMed]
- WFUMB Symposium on Safety and Standardisation in Medical Ultrasound. Issues and Recommendations Regarding Thermal Mechanisms for Biological Effects of Ultrasound. Hornbaek, Denmark, 30 August–1 September 1991. Ultrasound Med. Biol. 1992, 18, 731–810.
- Aptel, F.; Tadjine, M.; Rouland, J.-F. Efficacy and Safety of Repeated Ultrasound Cycloplasty Procedures in Patients With Early or Delayed Failure After a First Procedure. J. Glaucoma 2020, 29, 24–30. [Google Scholar] [CrossRef]
- Torky, M.A.; Al Zafiri, Y.A.; Hagras, S.M.; Khattab, A.M.; Bassiouny, R.M.; Mokbel, T.H. Safety and Efficacy of Ultrasound Ciliary Plasty as a Primary Intervention in Glaucoma Patients. Int. J. Ophthalmol. 2019, 12, 597–602. [Google Scholar] [CrossRef]
- Kovacs, Z.I.; Kim, S.; Jikaria, N.; Qureshi, F.; Milo, B.; Lewis, B.K.; Bresler, M.; Burks, S.R.; Frank, J.A. Disrupting the Blood–brain Barrier by Focused Ultrasound Induces Sterile Inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E75–E84. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.; Hynynen, K. Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound Is Dependent on Microbubble Dose. Theranostics 2017, 7, 3989–4000. [Google Scholar] [CrossRef]
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular Signaling and Factors Involved in Müller Cell Gliosis: Neuroprotective and Detrimental Effects. Prog. Retin. Eye Res. 2009, 28, 423–451. [Google Scholar] [CrossRef]
- Carpentier, A. Transient Disruption of the Blood-Retinal Barrier of a Human and Uses Thereof for Treating a Retina Disorder. U.S. Patent Application No. 16/609,385, 13 February 2020. [Google Scholar]
- Nishioka, T.; Luo, H.; Fishbein, M.C.; Cercek, B.; Forrester, J.S.; Kim, C.-J.; Berglund, H.; Siegel, R.J. Dissolution of Thrombotic Arterial Occlusion by High Intensity, Low Frequency Ultrasound and Dodecafluoropentane Emulsion: An In Vitro and In Vivo Study. J. Am. Coll. Cardiol. 1997, 30, 561–568. [Google Scholar] [CrossRef]
- Mizushige, K.; Kondo, I.; Ohmori, K.; Hirao, K.; Matsuo, H. Enhancement of Ultrasound-Accelerated Thrombolysis by Echo Contrast Agents: Dependence on Microbubble Structure. Ultrasound Med. Biol. 1999, 25, 1431–1437. [Google Scholar] [CrossRef]
- Culp, W.C.; Porter, T.R.; Xie, F.; Goertzen, T.C.; McCowan, T.C.; Vonk, B.N.; Baxter, B.T. Microbubble Potentiated Ultrasound as a Method of Declotting Thrombosed Dialysis Grafts: Experimental Study in Dogs. Cardiovasc. Intervent. Radiol. 2001, 24, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Janjic, J.; Larsson, M.K.; Bjällmark, A. In-Vitro Sonothrombolysis Using Thick-Shelled Polymer Microbubbles—A Comparison with Thin-Shelled Microbubbles. Cardiovasc. Ultrasound 2020, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fawzi, A.; Ameri, H.; Humayun, M. Ultrasound and Microbubbles in Ocular Diagnostic and Therapies. U.S. Patent 8,764,658, 1 July 2014. [Google Scholar]
- Grubbs, R.; Stoller, M.; Han, Y.; Brodie, F. Method for Eye Lens Removal Using Cavitating Microbubbles. U.S. Patent Application 16/733,918, 9 July 2020. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousou, C.; Schuurmans, C.C.L.; Urtti, A.; Mastrobattista, E.; Storm, G.; Moonen, C.; Kaarniranta, K.; Deckers, R. Ultrasound and Microbubbles for the Treatment of Ocular Diseases: From Preclinical Research towards Clinical Application. Pharmaceutics 2021, 13, 1782. https://doi.org/10.3390/pharmaceutics13111782
Rousou C, Schuurmans CCL, Urtti A, Mastrobattista E, Storm G, Moonen C, Kaarniranta K, Deckers R. Ultrasound and Microbubbles for the Treatment of Ocular Diseases: From Preclinical Research towards Clinical Application. Pharmaceutics. 2021; 13(11):1782. https://doi.org/10.3390/pharmaceutics13111782
Chicago/Turabian StyleRousou, Charis, Carl C. L. Schuurmans, Arto Urtti, Enrico Mastrobattista, Gert Storm, Chrit Moonen, Kai Kaarniranta, and Roel Deckers. 2021. "Ultrasound and Microbubbles for the Treatment of Ocular Diseases: From Preclinical Research towards Clinical Application" Pharmaceutics 13, no. 11: 1782. https://doi.org/10.3390/pharmaceutics13111782
APA StyleRousou, C., Schuurmans, C. C. L., Urtti, A., Mastrobattista, E., Storm, G., Moonen, C., Kaarniranta, K., & Deckers, R. (2021). Ultrasound and Microbubbles for the Treatment of Ocular Diseases: From Preclinical Research towards Clinical Application. Pharmaceutics, 13(11), 1782. https://doi.org/10.3390/pharmaceutics13111782








