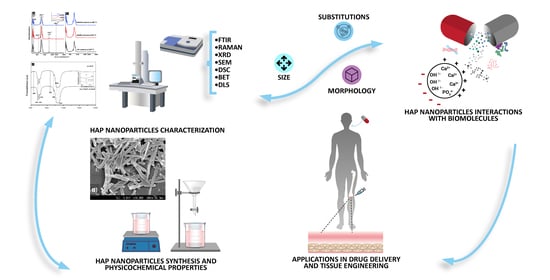
Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications
Abstract
:1. Introduction
- (1)
- Drugs conjugated or loaded with implanted HAP scaffolds;
- (2)
- Porous HAP or nano-HAP granular particles;
- (3)
- Polymer-coated HAP or nano-HAP particles.
2. Physicochemical Properties and Characterization
2.1. Chemical Structure and Properties of HAP Nanoparticles
2.2. Infrared Spectroscopy
2.3. Raman Spectroscopy
2.4. X-ray Diffraction
2.5. Scanning Electron Microscopy
2.6. Differential Scanning Calorimetry
2.7. Brunauer–Emmett–Teller Method
2.8. Dynamic Light Scattering
3. Interactions in Drug Delivery with HAP Nanoparticles
3.1. Proteins
3.2. Peptides
3.3. Drugs
3.4. Genetic Material
| Cargo | Heat Treatment | Size | Potential | SSA | Porosity | Pore Volume | Morphology | Amount Adsorbed | Application | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (nm) | (mV ) | (m2g−1) | (%) | (cm3g−1) | (-) | (mg) | |||||
| Fibrinogen | - | 2.53 | 2.39 | - | Spheres | 2.93 mg/m2 | ||||||
| Insulin | 80 overnight | 60 | 2.24 mg/m2 | Diabetes | [74] | |||||||
| Col-I | 1.12 mg/m2 | |||||||||||
| BSA | 1250 | 4 h | 1000 | −37 | 0.9 | micropores | - | Granules | 65.7 µg/mL | - | [7] | |
| BSA | 1000 | 15 h | 100 | 0 | 25.4 | micropores | - | Granules | 78.3 µg/mL | |||
| BSA | 600 | 3 h | 39 | −0.55 | 40 | - | - | - | 4.0 mg/m2 | |||
| Proteins | MG | 600 | 3 h | 39 | −0.55 | 40 | - | - | - | 1.0 µg/m2 | Blood compatibility | [85] |
| BSA | 700 | 3 h | 43 | −0.9 | 20 | - | - | - | 9.8 mg/m2 | |||
| MG | 700 | 3 h | 43 | −0.9 | 20 | - | - | - | 1.5 µg/m2 | |||
| BSA | 600 | 4 h | 32 | - | 73 | - | - | 89 µg/mg | Delivery | [19] | ||
| BSA | 700 | 4 h | 36 | - | 66 | - | - | 85 µg/mg | ||||
| Cyt c | 60 | 3 h | 60 × 30 | −24 | 96 | 0.79 | Rod | 60 µg/mg | ||||
| MGB | 43 µg/mg | Delivery | [32] | |||||||||
| BSA | 78 µg/mg | |||||||||||
| Peptides | APWHLSSQYSRT | 1350 | 1 h | - | - | 0.05 | - | - | Granules | 1 nmol | ||
| STLPIPHEFSRE | - | - | 2.4 nmol | Delivery | [28] | |||||||
| VTKHLNQISQSY | - | - | 2.5 nmol | |||||||||
| Drugs | Doxorubicin | 100 | 24 h | 400 × 600 | - | 163.2 | mesopores | 0.53 | Oval | 3 × 10 5 mol/g | Breast cancer | [8] |
| Ibuprofen | 1000 | 2 h | 79 | - | - | - | - | Plates | - | Arthritis | [54] | |
| Cisplatin | 80 | 93 × 29 | - | 96.8 | - | - | Plates | 2.4 mg/g | Cancer | [102] | ||
| Ampicilin | 1200 | 1 h | 8–9 ×103 | - | - | Mesopores | Spheres | 6.5 mg/g | Bacterial infection | [103] | ||
| DNA | Fish sperm DNA | 80 | 1.5 h | 20 | - | - | - | - | Spheres | 11 µg/mg | Gene therapy | [105] |
| EGFP-N1 pDNA | 170 | 2 h | 40–60 | - | - | - | - | Rod | 0.02 µg/ug | Gene therapy | [106] | |
| CDglyTK | 35 | 72 h | 23–34 | +16.8 | - | - | - | Feather | - | Antitumor | [107] | |
4. Intracellular Activity
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omidi, M.; Fatehinya, A.; Farahani, M.; Akbari, Z.; Shahmoradi, S.; Yazdian, F.; Tahriri, M.; Moharamzadeh, K.; Tayebi, L.; Vashaee, D. Characterization of biomaterials. In Biomaterials for Oral and Dental Tissue Engineering; Elsevier: San Diego, CA, USA, 2017; pp. 97–115. [Google Scholar]
- Agrawal, C.M.; Ong, J.L.; Appleford, M.R.; Mani, G. Introduction to Biomaterials. In Introduction to Biomaterials; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Galindo, T.G.P.; Chai, Y.; Tagaya, M. Hydroxyapatite nanoparticle coating on polymer for constructing effective biointeractive interfaces. J. Nanomater. 2019, 2019, 6495239. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.; Guerrero-Beltrán, C.E.; Ortega-Lara, W. Design and characterization of Gelatin/PVA hydrogels reinforced with ceramics for 3D printed prosthesis. Mater. Today Proc. 2019, 13, 324–331. [Google Scholar] [CrossRef]
- Fathi, M.H.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Rouahi, M.; Champion, E.; Gallet, O.; Jada, A.; Anselme, K. Physico-chemical characteristics and protein adsorption potential of hydroxyapatite particles: Influence on in vitro biocompatibility of ceramics after sintering. Colloids Surf. B Biointerfaces 2006, 47, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Liu, C.H.; Liang, Y.H.; Lin, F.H.; Wu, K.C.W. Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery. J. Mater. Chem. B 2013, 1, 2447–2450. [Google Scholar] [CrossRef]
- Kester, M.; Heakal, Y.; Fox, T.; Sharma, A.; Robertson, G.P.; Morgan, T.T.; Altinoğlu, E.İ.; Tabaković, A.; Parette, M.R.; Rouse, S.M.; et al. Calcium Phosphate Nanocomposite Particles for In Vitro Imaging and Encapsulated Chemotherapeutic Drug Delivery to Cancer Cells. Nano Lett. 2008, 8, 4116–4121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uskokovic, V.; Uskokovic, D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 152–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Beltrán, C.E.; Bernal-Ramírez, J.; Lozano, O.; Oropeza-Almazán, Y.; Castillo, E.C.; Garza, J.R.; García, N.; Vela, J.; García-García, A.; Ortega, E.; et al. Silica nanoparticles induce cardiotoxicity interfering with energetic status and Ca2+ handling in adult rat cardiomyocytes. Am. J. Physiol. Circ. Physiol. 2017, 312, H645–H661. [Google Scholar] [CrossRef] [Green Version]
- Ornelas-Soto, N.; Rubio-Govea, R.; Guerrero-Beltrán, C.E.; Vázquez-Garza, E.; Bernal-Ramírez, J.; García-García, A.; Oropeza-Almazán, Y.; García-Rivas, G.; Contreras-Torres, F.F. Enhancing internalization of silica particles in myocardial cells through surface modification. Mater. Sci. Eng. C 2017, 79, 831–840. [Google Scholar] [CrossRef]
- Contreras-Torres, F.F.; Rodríguez-Galván, A.; Guerrero-Beltrán, C.E.; Martínez-Lorán, E.; Vázquez-Garza, E.; Ornelas-Soto, N.; García-Rivas, G. Differential cytotoxicity and internalization of graphene family nanomaterials in myocardial cells. Mater. Sci. Eng. C 2017, 73, 633–642. [Google Scholar] [CrossRef]
- Eslami, H.; Solati-Hashjin, M.; Tahriri, M. The comparison of powder characteristics and physicochemical, mechanical and biological properties between nanostructure ceramics of hydroxyapatite and fluoridated hydroxyapatite. Mater. Sci. Eng. C 2009, 29, 1387–1398. [Google Scholar] [CrossRef]
- Uskokovic, V.; Desai, T.A. Phase composition control of calcium phosphate nanoparticles for tunable drug delivery kinetics and treatment of osteomyelitis. I. Preparation and drug release. J. Biomed. Mater. Res. Part A 2013, 101, 1416–1426. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Ghosh, S.S.; Atta, A.K.; Pramanik, N. A succinct overview of hydroxyapatite based nanocomposite biomaterials: Fabrications, physicochemical properties and some relevant biomedical applications. J. Bionanosci. 2018, 12, 143–158. [Google Scholar] [CrossRef]
- Mondal, S.; Dorozhkin, S.V.; Pal, U. Recent progress on fabrication and drug delivery applications of nanostructured hydroxyapatite. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1504. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Bandyopadhyay, A.; Bose, S. Reverse micelle-mediated synthesis of calcium phosphate nanocarriers for controlled release of bovine serum albumin. Acta Biomater. 2009, 5, 3112–3121. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Liu, F.; Liu, Y.; Li, C.; Wang, S.; Zhou, H.; Wang, W.; Zhu, H.; Winkler, D.A.; Yan, B. Toward a systematic exploration of nano-bio interactions. Toxicol. Appl. Pharmacol. 2017, 323, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Singh, R.P.; Jolly, S.S. Customized hydroxyapatites for bone-tissue engineering and drug delivery applications: A review. J. Sol-Gel Sci. Technol. 2020, 94, 505–530. [Google Scholar] [CrossRef]
- Porter, A.; Patel, N.; Brooks, R.; Best, S.; Rushton, N.; Bonfield, W. Effect of carbonate substitution on the ultrastructural characteristics of hydroxyapatite implants. J. Mater. Sci. Mater. Med. 2005, 16, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Bang, L.T.; Long, B.D.; Othman, R. Carbonate hydroxyapatite and silicon-substituted carbonate hydroxyapatite: Synthesis, mechanical properties, and solubility evaluations. Sci. World J. 2014, 2014, 969876. [Google Scholar] [CrossRef]
- Rekha, M.R.; Sharma, C.P. Nanoparticle Mediated Oral Delivery of Peptides and Proteins: Challenges and Perspectives; Elsevier: New York, NY, USA, 2011. [Google Scholar]
- Afroz, S.; Medhi, H.; Maity, S.; Minhas, G.; Battu, S.; Giddaluru, J.; Kumar, K.; Paik, P.; Khan, N. Mesoporous ZnO nanocapsules for the induction of enhanced antigen-specific immunological responses. Nanoscale 2017, 9, 14641–14653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilie, N.; Durner, J. Polymerization kinetic calculations in dental composites: A method comparison analysis. Clin. Oral Investig. 2014, 18, 1587–1596. [Google Scholar] [CrossRef]
- Moraes, L.G.P.; Rocha, R.S.F.; Menegazzo, L.M.; de Araújo, E.B.; Yukimito, K.; Moraes, J.C.S. Infrared spectroscopy: A tool for determination of the degree of conversion in dental composites. J. Appl. Oral Sci. 2008, 16, 145–149. [Google Scholar] [CrossRef]
- Segvich, S.J.; Smith, H.C.; Kohn, D.H. The adsorption of preferential binding peptides to apatite-based materials. Biomaterials 2009, 30, 1287–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Sofronia, A.M.; Baies, R.; Anghel, E.M.; Marinescu, C.A.; Tanasescu, S. Thermal and structural characterization of synthetic and natural nanocrystalline hydroxyapatite. Mater. Sci. Eng. C 2014, 43, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, A.; Rebelo, A.H.S.; Lemos, A.F.; Rocha, J.H.G.; Ventura, J.M.G.; Ferreira, J.M.F. Suitability evaluation of sol-gel derived Si-substituted hydroxyapatite for dental and maxillofacial applications through in vitro osteoblasts response. Dent. Mater. 2008, 24, 1374–1380. [Google Scholar] [CrossRef]
- Kojima, S.; Nagata, F.; Kugimiya, S.; Kato, K. Synthesis of peptide-containing calcium phosphate nanoparticles exhibiting highly selective adsorption of various proteins. Appl. Surf. Sci. 2018, 458, 438–445. [Google Scholar] [CrossRef]
- Wang, Y.; Tsuru, K.; Ishikawa, K.; Yokoi, T.; Kawashita, M. Fibronectin adsorption on carbonate-containing hydroxyapatite. Ceram. Int. 2021, 47, 11769–11776. [Google Scholar] [CrossRef]
- Daculsi, G. History of development and use of the bioceramics and biocomposites. In Handbook of Bioceramics and Biocomposites; Springer: Bucharest, Romania, 2016. [Google Scholar]
- Barralet, J.; Best, S.; Bonfield, W. Carbonate substitution in precipitated hydroxyapatite: An investigation into the effects of reaction temperature and bicarbonate ion concentration. J. Biomed. Mater. Res. 1998, 41, 79–86. [Google Scholar] [CrossRef]
- Spence, G.; Patel, N.; Brooks, R.; Rushton, N. Carbonate substituted hydroxyapatite: Resorption by osteoclasts modifies the osteoblastic response. J. Biomed. Mater. Res. Part A 2009, 90, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, E.; Bigi, A.; Cojazzi, G.; Gandolfi, M.; Panzavolta, S.; Roveri, N. Nanocrystals of magnesium and fluoride substituted hydroxyapatite. J. Inorg. Biochem. 1998, 72, 29–35. [Google Scholar] [CrossRef]
- Kim, S.-J.; Bang, H.-G.; Song, J.-H.; Park, S.-Y. Effect of fluoride additive on the mechanical properties of hydroxyapatite/alumina composites. Ceram. Int. 2009, 35, 1647–1650. [Google Scholar] [CrossRef]
- Cheng, K.; Weng, W.; Wang, H.; Zhang, S. In vitro behavior of osteoblast-like cells on fluoridated hydroxyapatite coatings. Biomaterials 2005, 26, 6288–6295. [Google Scholar] [CrossRef] [PubMed]
- Rintoul, L.; Wentrup-Byrne, E.; Suzuki, S.; Grøndahl, L. FT-IR spectroscopy of fluoro-substituted hydroxyapatite: Strengths and limitations. J. Mater. Sci. Mater. Med. 2007, 18, 1701–1709. [Google Scholar] [CrossRef] [Green Version]
- Botelho, C.M.; Lopes, M.A.; Gibson, I.R.; Best, S.M.; Santos, J.D.; De Engenharia, F.; Frias, R.R. Structural analysis of Si-substituted hydroxyapatite: Zeta potential and X-ray photoelectron spectroscopy. J. Mater. Sci. Mater. Med. 2002, 3, 1123–1127. [Google Scholar] [CrossRef]
- Porter, A.E.; Botelho, C.M.; Lopes, M.A.; Santos, J.D.; Best, S.M.; Bonfield, W. Ultrastructural comparison of dissolution and apatite precipitation on hydroxyapatite and silicon-substituted hydroxyapatitein vitro andin vivo. J. Biomed. Mater. Res. 2004, 69, 670–679. [Google Scholar] [CrossRef]
- Botelho, C.M.; Brooks, R.A.; Best, S.M.; Lopes, M.A.; Santos, J.D.; Rushton, N.; Bonfield, W. Human osteoblast response to silicon-substituted hydroxyapatite. J. Biomed. Mater. Res. Part A 2006, 79, 723–730. [Google Scholar] [CrossRef]
- Botelho, C.M.; Brooks, R.A.; Spence, G.; McFarlane, I.; Lopes, M.A.; Best, S.M.; Santos, J.D.; Rushton, N.; Bonfield, W. Differentiation of mononuclear precursors into osteoclasts on the surface of Si-substituted hydroxyapatite. J. Biomed. Mater. Res. Part A 2006, 78, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Silicon-substituted calcium phosphates—A critical view. Biomaterials 2009, 30, 6403–6406. [Google Scholar] [CrossRef]
- Lu, H.B.; Campbell, C.T.; Graham, D.J.; Ratner, B.D. Surface characterization of hydroxyapatite and related calcium phosphates by XPS and TOF-SIMS. Anal. Chem. 2000, 72, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Arends, J. Raman spectra of human dental calculus. J. Dent. Res. 1993, 72, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Arends, J. Raman spectroscopy in dental research: A short review of recent studies. Adv. Dent. Res. 1997, 11, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, Z.; Veselinović, L.; Marković, S.; Ignjatović, N.; Uskoković, D. Hydrothermal Synthesis of Nanosized Pure and Cobalt-Exchanged Hydroxyapatite. Mater. Manuf. Process. 2009, 24, 1096–1103. [Google Scholar] [CrossRef]
- Khan, A.F.; Awais, M.; Khan, A.S.; Tabassum, S.; Chaudhry, A.A.; Rehman, I.U. Raman spectroscopy of natural bone and synthetic apatites. Appl. Spectrosc. Rev. 2013, 48, 329–355. [Google Scholar] [CrossRef]
- Amer, W.; Abdelouahdi, K.; Ramananarivo, H.R.; Zahouily, M.; Fihri, A.; Djessas, K.; Zahouily, K.; Varma, R.S.; Solhy, A. Microwave-assisted synthesis of mesoporous nano-hydroxyapatite using surfactant templates. CrystEngComm 2014, 16, 543–549. [Google Scholar] [CrossRef]
- Mukherjee, B.; Santra, K.; Pattnaik, G.; Ghosh, S. Preparation, characterization and in-vitro evaluation of sustained release protein-loaded nanoparticles based on biodegradable polymers. Int. J. Nanomed. 2008, 3, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Palard, M.; Combes, J.; Champion, E.; Foucaud, S.; Rattner, A.; Bernache-Assollant, D. Effect of silicon content on the sintering and biological behaviour of Ca10(PO4)6−x(SiO4)x(OH)2−x ceramics. Acta Biomater. 2009, 5, 1223–1232. [Google Scholar] [CrossRef]
- Ryabenkova, Y.; Jadav, N.; Conte, M.; Hippler, M.F.A.; Reeves-Mclaren, N.; Coates, P.D.; Twigg, P.; Paradkar, A. Mechanism of Hydrogen-Bonded Complex Formation between Ibuprofen and Nanocrystalline Hydroxyapatite. Langmuir 2017, 33, 2965–2976. [Google Scholar] [CrossRef]
- Almora-Barrios, N.; De Leeuw, N.H. Modelling the interaction of a Hyp-Pro-Gly peptide with hydroxyapatite surfaces in aqueous environment. CrystEngComm 2010, 12, 960–967. [Google Scholar] [CrossRef]
- Natesan, K.; Shah, W.; Le, H.R.; Tredwin, C. A critical comparison on biocompatibility of different phases of sol–gel derived calcium phosphates as bone graft materials. J. Biomater. Tissue Eng. 2015, 5, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Sassa, K.; Yokogawa, Y.; Sakka, Y.; Okido, M.; Asai, S. Control of crystal orientation of hydroxyapatite by imposition of a high magnetic field. Mater. Trans. 2003, 44, 1133–1137. [Google Scholar] [CrossRef] [Green Version]
- Zandi, M.; Mirzadeh, H.; Mayer, C.; Urch, H.; Eslaminejad, M.B.; Bagheri, F.; Mivehchi, H. Biocompatibility evaluation of nano-rod hydroxyapatite/gelatin coated with nano-HAp as a novel scaffold using mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2010, 92, 1244–1255. [Google Scholar] [CrossRef]
- Yao, J.; Tjandra, W.; Chen, Y.Z.; Tam, K.C.; Ma, J.; Soh, B. Hydroxyapatite nanostructure material derived using cationic surfactant as a template. J. Mater. Chem. 2003, 13, 3053–3057. [Google Scholar] [CrossRef]
- Mohammad, N.F.; Othman, R.; Yee-Yeoh, F. Nanoporous hydroxyapatite preparation methods for drug delivery applications. Rev. Adv. Mater. Sci. 2014, 38, 138–147. [Google Scholar]
- Vallet-Regí, M. Bioceramics: From bone substitutes to nanoparticles for drug delivery. Pure Appl. Chem. 2019, 91, 687–706. [Google Scholar] [CrossRef]
- Motskin, M.; Wright, D.M.; Muller, K.; Kyle, N.; Gard, T.G.; Porter, A.E.; Skepper, J.N. Hydroxyapatite nano and microparticles: Correlation of particle properties with cytotoxicity and biostability. Biomaterials. 2009, 30, 3307–3317. [Google Scholar] [CrossRef]
- Barralet, J.; Knowles, J.C.; Best, S.; Bonfield, W. Thermal decomposition of synthesised carbonate hydroxyapatite. J. Mater. Sci. Mater. Med. 2002, 13, 529–533. [Google Scholar] [CrossRef]
- Nasir Vadia, S.R. Applications of mesoporous material for drug delivery. In Importance & Applications of Nanotechnology; MedDocs Publishers LLC: Reno, NV, USA, 2019. [Google Scholar]
- Yamamoto, T.; Endo, A.; Inagi, Y.; Ohmori, T.; Nakaiwa, M. Evaluation of thermoporometry for characterization of mesoporous materials. J. Colloid Interface Sci. 2005, 284, 614–620. [Google Scholar] [CrossRef]
- Jackson, C.L.; McKenna, G.B. The melting behavior of organic materials confined in porous solids. J. Chem. Phys. 1990, 93, 9002–9011. [Google Scholar] [CrossRef]
- Abbasi Aval, N.; Pirayesh Islamian, J.; Hatamian, M.; Arabfirouzjaei, M.; Javadpour, J.; Rashidi, M.R. Doxorubicin loaded large-pore mesoporous hydroxyapatite coated superparamagnetic Fe3O4 nanoparticles for cancer treatment. Int. J. Pharm. 2016, 509, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lett, J.A.; Sagadevan, S.; Prabhakar, J.J.; Hamizi, N.A.; Badruddin, I.A.; Johan, M.R.; Marlinda, A.R.; Wahab, Y.A.; Khan, T.M.Y.; Kamangar, S. Drug leaching properties of Vancomycin loaded mesoporous hydroxyapatite as bone substitutes. Processes 2019, 7, 826. [Google Scholar] [CrossRef] [Green Version]
- Cross, M.; Spycher, J. Cementless fixation techniques in joint replacement. In Joint Replacement Technology; Elsevier: New York, NY, USA, 2008; pp. 190–211. [Google Scholar]
- Bose, S.; Saha, S.K. Synthesis and Characterization of Hydroxyapatite Nanopowders by Emulsion Technique. Chem. Mater. 2003, 15, 4464–4469. [Google Scholar] [CrossRef]
- Uota, M.; Arakawa, H.; Kitamura, N.; Yoshimura, T.; Tanaka, J.; Kijima, T. Synthesis of high surface area hydroxyapatite nanoparticles by mixed surfactant-mediated approach. Langmuir 2005, 21, 4724–4728. [Google Scholar] [CrossRef]
- Boukha, Z.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Comparative Study of the Efficiency of Different Noble Metals Supported on Hydroxyapatite in the Catalytic Lean Methane Oxidation under Realistic Conditions. Materials 2021, 14, 3612. [Google Scholar] [CrossRef]
- Nga, N.K.; Thuy Chau, N.T.; Viet, P.H. Facile synthesis of hydroxyapatite nanoparticles mimicking biological apatite from eggshells for bone-tissue engineering. Colloids Surf. B Biointerfaces 2018, 172, 769–778. [Google Scholar] [CrossRef]
- Zhu, X.D.; Zhang, H.J.; Fan, H.S.; Li, W.; Zhang, X.D. Effect of phase composition and microstructure of calcium phosphate ceramic particles on protein adsorption. Acta Biomater. 2010, 6, 1536–1541. [Google Scholar] [CrossRef]
- Dhas, N.L.; Raval, N.J.; Kudarha, R.R.; Acharya, N.S.; Acharya, S.R. Core–shell nanoparticles as a drug delivery platform for tumor targeting. In Inorganic Frameworks as Smart Nanomedicines; Elsevier: Cambridge, MA, USA, 2018; pp. 387–448. [Google Scholar]
- Zhang, Y.; Zhang, L.; Ban, Q.; Li, J.; Li, C.H.; Guan, Y.Q. Preparation and characterization of hydroxyapatite nanoparticles carrying insulin and gallic acid for insulin oral delivery. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 353–364. [Google Scholar] [CrossRef]
- Han, Y.; Wang, X.; Li, S. A simple route to prepare stable hydroxyapatite nanoparticles suspension. J. Nanopart. Res. 2009, 11, 1235–1240. [Google Scholar] [CrossRef]
- Chen, L.; Mccrate, J.M.; Lee, J.C.-M.; Li, H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011, 22, 105708. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.H.; Stephen Inbaraj, B. Various physicochemical and surface properties controlling the bioactivity of cerium oxide nanoparticles. Crit. Rev. Biotechnol. 2018, 38, 1003–1024. [Google Scholar] [CrossRef] [PubMed]
- Loca, D.; Locs, J.; Dubnika, A.; Zalite, V.; Berzina-Cimdina, L. Porous hydroxyapatite for drug delivery. In Hydroxyapatite (Hap) for Biomedical Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Sandri, G.; Bonferoni, M.C.; Ferrari, F.; Rossi, S.; Caramella, C.M. The Role of Particle Size in Drug Release and Absorption. Part. Prod. 2014, 19, 323–341. [Google Scholar]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA Nanoparticles Improve the Oral Bioavailability of Curcumin in Rats: Characterizations and Mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef] [PubMed]
- Charoo, N.A.; Rahman, Z.; Khan, M.A. Nanoparticles for improvement in oral bioavailability. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 371–410. [Google Scholar]
- Wang, K.; Zhou, C.; Hong, Y.; Zhang, X. A review of protein adsorption on bioceramics. Interface Focus 2012, 2, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Kusudo, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Takashima, S. Selective protein adsorption and blood compatibility of hydroxy-carbonate apatites. J. Biomed. Mater. Res. Part A 2004, 69, 544–551. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide vaccine: Progress and challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Beltrán, C.E.; Mijares-Rojas, I.A.; Salgado-Garza, G.; Garay-Gutiérrez, N.F.; Carrión-Chavarría, B. Peptidic vaccines: The new cure for heart diseases? Pharmacol. Res. 2021, 164, 105372. [Google Scholar] [CrossRef]
- Krishnan-Sivadoss, I.; Mijares-Rojas, I.A.; Villarreal-Leal, R.A.; Torre-Amione, G.; Knowlton, A.A.; Guerrero-Beltrán, C.E. Heat shock protein 60 and cardiovascular diseases: An intricate love-hate story. Med. Res. Rev. 2021, 41, 29–71. [Google Scholar] [CrossRef]
- Garay-Gutiérrez, N.F.; Hernandez-Fuentes, C.P.; García-Rivas, G.; Lavandero, S.; Guerrero-Beltrán, C.E. Vaccines against components of the renin–angiotensin system. Heart Fail. Rev. 2021, 26, 711–726. [Google Scholar] [CrossRef]
- Tamerler, C.; Sarikaya, M. Molecular biomimetics: Utilizing nature’s molecular ways in practical engineering. Acta Biomater. 2007, 3, 289–299. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Nikolenko, N.V.; Esajenko, E.E. Surface Properties of Synthetic Calcium Hydroxyapatite. Adsorpt. Sci. Technol. 2005, 23, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Almora-Barrios, N.; De Leeuw, N.H. A density functional theory study of the interaction of collagen peptides with hydroxyapatite surfaces. Langmuir 2010, 26, 14535–14542. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. Hydroxyapatite crystallization in the presence of amino acids with uncharged polar side groups: Glycine, cysteine, cystine, and glutamine. Langmuir 2001, 17, 1074–1079. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. Inhibition of hydroxyapatite formation in aqueous solutions by amino acids with hydrophobic side groups. Langmuir 2000, 16, 6739–6744. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Dalas, E. Hydroxyapatite crystallization in the presence of serine, tyrosine and hydroxyproline amino acids with polar side groups. J. Cryst. Growth 2000, 216, 443–449. [Google Scholar] [CrossRef]
- Kawasaki, A.; Kawano, K.; Terada, Y.; Hirayasu, R. Adsorption of amino acids onto Hydroxyapatite. Nihon Hotetsu Shika Gakkai Zasshi 1989, 33, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A. Delivery. In Nanocarriers for Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Storm, G.; van Bloois, L.; Steerenberg, P.A.; van Etten, E.; de Groot, G.; Crommelin, D.J.A. Liposome encapsulation of doxorubicin: Pharmaceutical and therapeutic aspects. J. Control. Release 1989, 9, 215–229. [Google Scholar] [CrossRef]
- Moreno, E.C.; Kresak, M.; Hay, D.I. Adsorption of molecules of biological interest onto hydroxyapatite. Calcif. Tissue Int. 1984, 36, 48–59. [Google Scholar] [CrossRef]
- Lemaitre, A.B.A.J.; Rouxhet, P.G. Lysozyme on apatites: A model of protein adsorption controlled by electrostatic interactions. Colloids Surf. 1989, 37, 339–355. [Google Scholar] [CrossRef]
- Barroug, A.; Glimcher, M.J. Hydroxyapatite crystals as a local delivery system for cisplatin: Adsorption and release of cisplatin in vitro. J. Orthop. Res. 2002, 20, 274–280. [Google Scholar] [CrossRef]
- Queiroz, A.C.; Santos, J.D.; Monteiro, F.J.; Gibson, I.R.; Knowles, J.C. Adsorption and release studies of sodium ampicillin from hydroxyapatite and glass-reinforced hydroxyapatite composites. Biomaterials 2001, 22, 1393–1400. [Google Scholar] [CrossRef]
- Tram Do, T.N.; Lee, W.H.; Loo, C.Y.; Zavgorodniy, A.V.; Rohanizadeh, R. Hydroxyapatite nanoparticles as vectors for gene delivery. Ther. Deliv. 2012, 3, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Bertran, O.; Del Valle, L.J.; Revilla-López, G.; Chaves, G.; Cardús, L.; Casas, M.T.; Casanovas, J.; Turon, P.; Puiggalí, J.; Alemán, C. Mineralization of DNA into nanoparticles of hydroxyapatite. Dalt. Trans. 2014, 43, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.H.; Huang, B.Y.; Zhou, K.C.; Huang, S.P.; Liu, F.; Li, Y.M.; Xue, Z.G.; Long, Z.G. Hydroxyapatite nanoparticles as a novel gene carrier. J. Nanopart. Res. 2004, 6, 307–311. [Google Scholar] [CrossRef]
- Liu, T.; Tang, A.; Zhang, G.; Chen, Y.; Zhang, J.; Peng, S.; Cai, Z. Calcium phosphate nanoparticles as a novel nonviral vector for efficient transfection of DNA in cancer gene therapy. Cancer Biother. Radiopharm. 2005, 20, 141–149. [Google Scholar] [CrossRef]
- Wu, C.-W.K.; Yang, Y.-H.; Liang, Y.-H.; Chen, H.-Y.; Sung, E.; Yamauchi, Y.; Lin, F.-H. Facile Synthesis of Hollow Mesoporous Hydroxyapatite Nanoparticles for Intracellular Bio-imaging. Curr. Nanosci. 2011, 7, 926–931. [Google Scholar] [CrossRef]
- Fadeel, B.; Garcia-Bennett, A.E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. [Google Scholar] [CrossRef]
- Gentile, F.; Curcio, A.; Indolfi, C.; Ferrari, M.; Decuzzi, P. The margination propensity of spherical particles for vascular targeting in the microcirculation. J. Nanobiotechnol. 2008, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen Gupta, A. Role of particle size, shape, and stiffness in design of intravascular drug delivery systems: Insights from computations, experiments, and nature. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 255–270. [Google Scholar] [CrossRef]
- Li, X.; Zou, Q.; Li, W.; Chen, H. Intracellular Interaction of Hydroxyapatite-Based Nanocrystals with Uniform Shape and Traceable Fluorescence. Inorg. Chem. 2018, 57, 13739–13748. [Google Scholar] [CrossRef]
- Fulgione, A.; Ianniello, F.; Papaianni, M.; Contaldi, F.; Sgamma, T.; Giannini, C.; Pastore, S.; Velotta, R.; Della Ventura, B.; Roveri, N.; et al. Biomimetic hydroxyapatite nanocrystals are an active carrier for salmonella bacteriophages. Int. J. Nanomed. 2019, 14, 2219–2232. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Wang, M.; Wang, J.; Chen, X.; Li, X.; Xiao, Y.; Zhang, X. Effects of hydroxyapatite surface nano/micro-structure on osteoclast formation and activity. J. Mater. Chem. B 2019, 7, 7574–7587. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, Y.; Yan, W.; Hu, Q.; Tao, J.; Zhang, M.; Shi, Z.; Tang, R. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem. 2007, 17, 3780–3787. [Google Scholar] [CrossRef]
- Richards, J.M.; Kunitake, J.A.M.R.; Hunt, H.B.; Wnorowski, A.N.; Lin, D.W.; Boskey, A.L.; Donnelly, E.; Estroff, L.A.; Butcher, J.T. Crystallinity of hydroxyapatite drives myofibroblastic activation and calcification in aortic valves. Acta Biomater. 2018, 71, 24–36. [Google Scholar] [CrossRef] [Green Version]
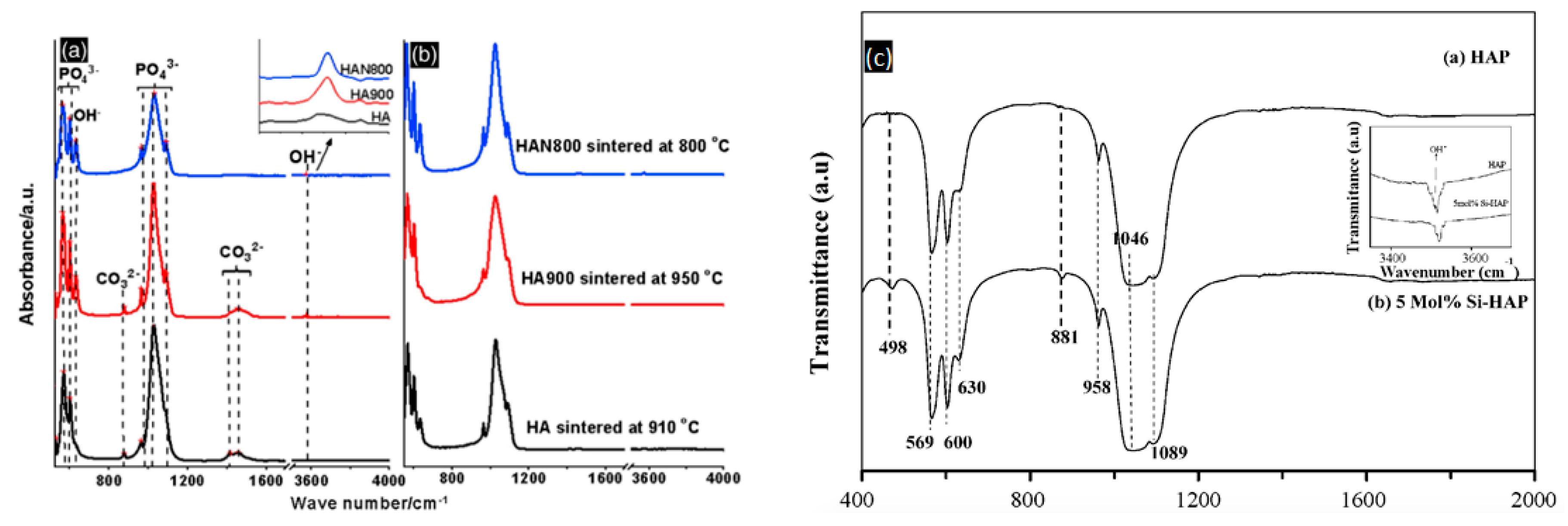

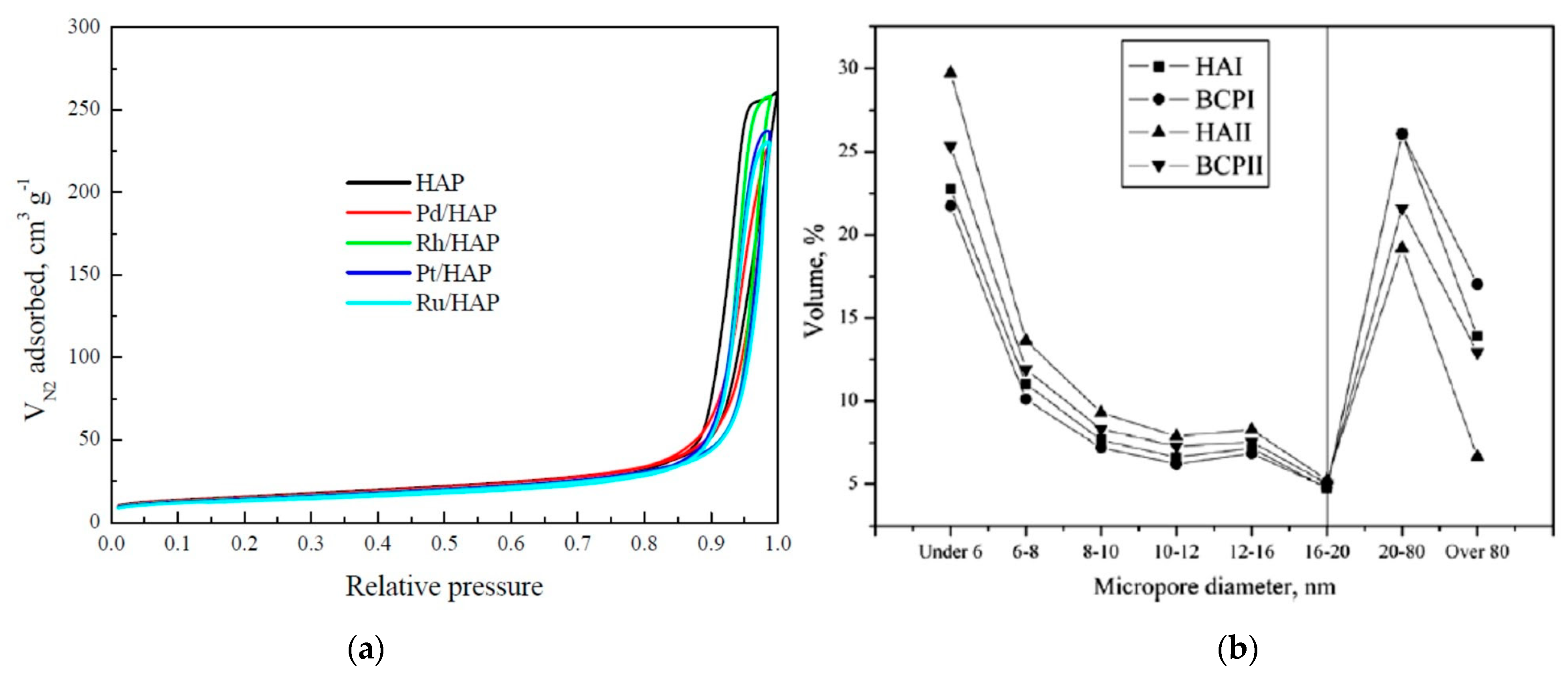
| Sintering Temperature | - | - | 60 °C | 600 °C | 950 °C | 1250 °C | 1350 °C |
|---|---|---|---|---|---|---|---|
| Sample | Natural HAP (cm−1) | Si-HAP (cm−1) | HAP-p (cm−1) | HAP (cm−1) | HAP (cm−1) | HAP (cm−1) | HAP (cm−1) |
| 1540 | - | 1540 | 1462.48 | 1456 | - | 1540 | |
| C-O | 1548 | - | - | - | - | - | - |
| 1418 | - | - | 1418.2 | 876 | - | - | |
| C=O | 1653 | - | 1650 | 1621.94 | - | - | - |
| C-N | 1560 | - | 1560 | - | - | - | - |
| N-H | 1560 | - | 1560 | - | - | - | - |
| Peptide | - | - | 1400 | - | - | - | - |
| P-O | 1087 | 1089 | 1020 | 1100 | 1091 | 1100 | 1087 |
| O-H | 3569 | 3570 | - | 3571 | 3572 | 3575 | - |
| SiO44− | - | 881 | - | - | - | - | - |
| - | 498 | - | - | - | - | - | |
| Reference | [30] | [31] | [32] | [6] | [30] | [7] | [28] |
| Synthetic HAP | Carbonated HAP | Mesoporous HAP NPs 80 °C | HAP 1400 °C | ||
|---|---|---|---|---|---|
| (cm−1) | (cm−1) | (cm−1) | (cm−1) | ||
| PO43− | v2 | 432 | 431 | 432 | N/A |
| 447 | 449 | 445 | |||
| PO43− | v4 | 585 | 581 | 577 | N/A |
| 597 | 592 | 590 | |||
| 614 | 608 | 608 | |||
| 622 | - | - | |||
| A CO32− | v4 | - | 664 | - | N/A |
| B CO32− | v4 | - | 730 | - | |
| TCP | 939 | ||||
| 946 | |||||
| 968 | |||||
| 1008 | |||||
| PO43− | v1 | 962 | 963 | 959 | 955 |
| - | - | - | - | ||
| - | - | - | - | ||
| PO43− | v3 | 1030 | - | - | 1026 |
| 1046 | 1048 | 1046 | 1047 | ||
| 1077 | 1078 | 1072 | 1075 | ||
| - | - | - | 1091 | ||
| B CO32− | v1 | - | 1069 | 1050 | - |
| A CO32− | v1 | - | 1114 | 1100 | - |
| Reference | [47] | [50] | [51] | [30] | |
| 2θ | d | Miller Index |
|---|---|---|
| (°) | (Å) | |
| 16.84 | 5.250 | (101) |
| 18.78 | 4.720 | (110) |
| 21.60 | 4.070 | (200) |
| 22.97 | 3.880 | (111) |
| 3.510 | (201) | |
| 25.90 | 3.440 | (002) |
| 28.22 | 3.170 | (102) |
| 29.14 | 3.080 | (210) |
| 31.86 | 2.814 | (211) |
| 32.20 | 2.778 | (112) |
| 32.90 | 2.720 | (300) |
| 34.22 | 2.631 | (202) |
| 35.51 | 2.528 | (301) |
| 39.26 | 2.296 | (212) |
| 39.86 | 2.262 | (310) |
| 2.228 | (221) | |
| 42.10 | 2.148 | (311) |
| 2.134 | (302) | |
| 43.80 | 2.065 | (113) |
| 2.040 | (400) | |
| 45.32 | 2.000 | (203) |
| 46.69 | 1.943 | (222) |
| 48.16 | 1.890 | (312) |
| 48.59 | 1.871 | (320) |
| 49.51 | 1.841 | (213) |
| 50.53 | 1.806 | (321) |
| 51.33 | 1.780 | (410) |
| 52.24 | 1.754 | (402), (303) |
| 53.27 | 1.722 | (004), (411) |
| 54.45 | 1.684 | (104) |
| 55.87 | 1.644 | (322), (223) |
| 57.15 | 1.611 | (313) |
| 58.17 | 1.587 | (501), (204) |
| 60.01 | 1.542 | (420) |
| 60.45 | 1.530 | (331) |
| 61.66 | 1.503 | (214), (421) |
| 63.07 | 1.474 | (502) |
| 1.465 | (510) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Ochoa, S.; Ortega-Lara, W.; Guerrero-Beltrán, C.E. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics 2021, 13, 1642. https://doi.org/10.3390/pharmaceutics13101642
Lara-Ochoa S, Ortega-Lara W, Guerrero-Beltrán CE. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics. 2021; 13(10):1642. https://doi.org/10.3390/pharmaceutics13101642
Chicago/Turabian StyleLara-Ochoa, Sofía, Wendy Ortega-Lara, and Carlos Enrique Guerrero-Beltrán. 2021. "Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications" Pharmaceutics 13, no. 10: 1642. https://doi.org/10.3390/pharmaceutics13101642
APA StyleLara-Ochoa, S., Ortega-Lara, W., & Guerrero-Beltrán, C. E. (2021). Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics, 13(10), 1642. https://doi.org/10.3390/pharmaceutics13101642