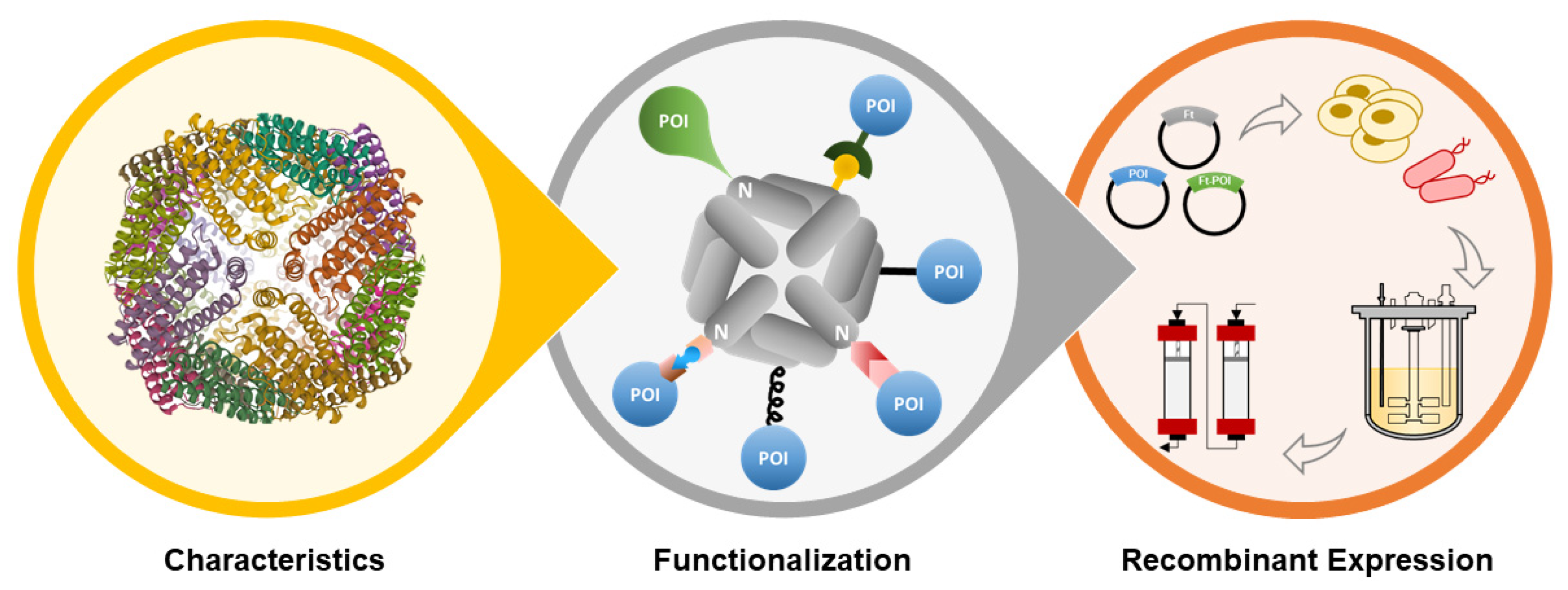
Functionalizing Ferritin Nanoparticles for Vaccine Development
Abstract
1. Introduction
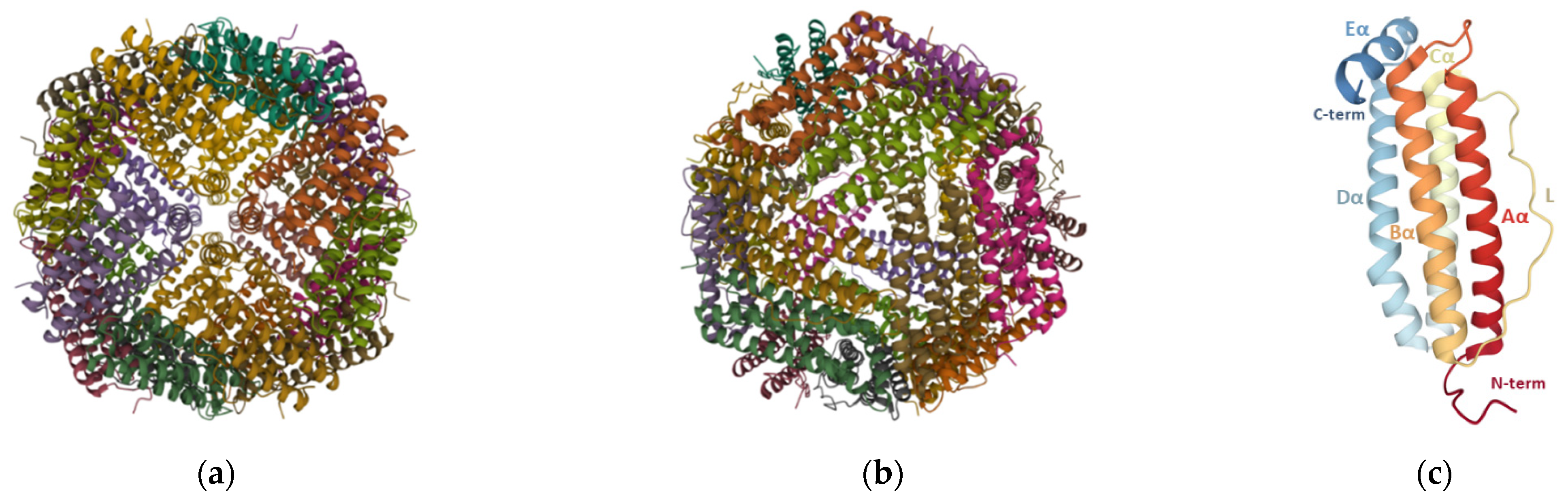
2. Ferritin Properties
2.1. Structure
2.2. Assembly Mechanism
2.3. Glycosylation Profiling
3. Functionalization of Ferritin Nanoparticles
3.1. Bioengineering Strategies
3.1.1. Genetic Fusion
| Expression System | Ferritin Source | Epitope/Antigen | No. Residues | Target | Architectonic Tags | Attachment Region on Ferritin | Yield (mg/L) | Application | Ref |
|---|---|---|---|---|---|---|---|---|---|
| E. coli | Human L-chain | Tat peptide | 9 | HIV-1 | Tat peptide and ferritin spaced by a GGG linker | N-terminal | 5–10 | Viral vaccination | [16] |
| E. coli BL21(DE3) | Human H-chain | RGD4C peptide | 9 | αvβ3 integrins upregulated on tumor vasculature | RGD4C and ferritin spaced by a GGGT linker | N-terminal | 10 | Tumor targeting | [68] |
| E. coli BL21(DE3) | P. furiosus | FcBP | 13 | Fc region of antibodies | FcBP and ferritin spaced by G-rich linkers | Loop connecting D and E helices | 10 | Antibody immobilization | [56] |
| E. coli BL21(DE3) | Rat H-chain | EV71 VP1 peptides | 4–15 | EV71 | - | Loop (res 146) orC- or N- terminal | - | Viral vaccination | [20] |
| E. coli BL21(DE3) | P. furiosus | OT-1 OT-2 | 8 17 | T-cell receptors of OT-1 and OT-2 | - | Loop (res 146) orC-terminal | - | DC-targeting and vaccination | [57] |
| E. coli BL21(DE3) | H. pylori | MtrE loop 1 MtrE loop 2 | 16 22 | N. gonorrhoeae | - | Loop connecting A and B helices (res 34) or N-terminal | - | Bacterial vaccination | [38] |
| E. coli BL21(DE3) | Human H-chain | RBM of S protein | 69 | SARS-CoV-2 | RBM and ferritin spaced by (GGGGS)3 linker | N-terminal | - | Viral vaccination | [66] |
| E. coli BL21(DE3) | Human H-chain | 3x M2e peptide | 72 | Influenza virus | M2e and ferritin spaced by a GGGGS linker | N-terminal | 0.5 | Viral vaccination | [72] |
| E. coli BL21(DE3) | Human H-chain | RFP | 225 | RFP-expressing melanoma tumor cells | RFP and ferritin spaced by a G3SG3TG3SG3 linker | C-terminal | - | Tumor targeting and vaccination | [71] |
| E. coli BL21(DE3) | H. pylori-bullfrog hybrid | OspA | 247 | B. burgdorferi | OspA and ferritin spaced by GS linker | N-terminal | - | Bacterial vaccination | [49] |
| E. coli Shuffle®T7 | E. coli K12 | hRID-RBD of S protein | 343 | MERS-CoV | TEV cleavage site between hRID and RBD, hRID-RBD, and ferritin spaced by an SSG linker | N-terminal | 1.6 | Viral vaccination | [37] |
| E. coli BL21(DE3) | H. pylori | VP6 | 410 | Rotavirus A | VP6 and ferritin spaced by an SGG linker | N-terminal | - | Viral vaccination | [39] |
| Sf-9 | H. pylori | GP5 | ~200 | PRRSV | GP5 and ferritin spaced by a GGGS linker | N-terminal | - | Viral vaccination | [41] |
| Sf-9 | H. pylori (N19Q) | FMDV VP1 G-H loop | 211 20 | FMDV | VP1 or G-H loop and ferritin spaced by a linker | N-terminal | - | Viral vaccination | [43] |
| Sf-9 | H. pylori (N19Q) | E2 from CSFV | 320 | CSFV | E2 and ferritin spaced by a GSG linker | N-terminal | - | Viral vaccination | [44] |
| HEK293F | H. pylori-bullfrog hybrid | RBD of HA | 208 | Several influenza virus strains | HA and ferritin spaced by an SG linker | N-terminal | - | Viral vaccination | [52] |
| HEK293T | H. pylori-bullfrog hybrid | RBD of S protein | 223 | SARS-CoV-2 | RBD and ferritin spaced by a SSGGASVLA linker | N-terminal | - | Viral vaccination | [48] |
| HEK293F | H. pylori-bullfrog hybrid | gp350 (D123) | 413 | EBV | gp350 and ferritin spaced by an (SG3)2 linker | N-terminal | - | Viral vaccination | [50] |
| HEK293F | H. pylori-bullfrog hybrid | HA | ~500 | Influenza virus | - | N-terminal | - | Viral vaccination | [51] |
| HEK293F | H. pylori (N19Q) | HA | 550 | Influenza virus | HA and ferritin spaced by an SGG linker | N-terminal | 2–10 | Viral vaccination | [45] |
| HEK293 | H. pylori (N19Q) | HA | 550 | Influenza virus | HA and ferritin spaced by an SGG linker | N-terminal | - | Viral vaccination | [47] |
| HEK293F | P. furiosus (R64K) | MD39 | 634 | HIV-1 | MD39 and ferritin spaced by a GSG linker | N-terminal | - | Viral vaccination | [9] |
| HEK293F | H. pylori | gp140 | 638 | HIV-1 | gp140 and ferritin spaced by a GSG linker | N-terminal | - | Viral vaccination | [78] |
| HEK293F | T. ni L- and H-chains | gp140 | ~638 | HIV-1 | gp140 and ferritin spaced by a (GS)5 linker | N-terminal | 1 | Viral vaccination | [63] |
| HEK293F | H. pylori | S protein | ~1148 | SARS-CoV-2 | S protein and ferritin spaced by a GSGGSG linker | N-terminal | 5 | Viral vaccination | [83] |
| HEK293F | H. pylori | S protein | 1213 | SARS-CoV-2 | S protein and ferritin spaced by an SGG linker | N-terminal | - | Viral vaccination | [81] |
| HEK293F CHO | H. pylori-bullfrog hybrid | F protein | 449 | RSV | F protein and ferritin spaced by an S(GS)2ES linker | N-terminal | 4.7 (HEK) 89 (CHO) | Viral vaccination | [53] |
| HEK293F CHO | - | E2 from HCV | ~500 | HCV | E2 and ferritin spaced by a (G4S)2 linker | N-terminal | 0.5 (HEK) 20 (CHO) | Viral vaccination | [84] |
| HEK293F CHO | - | gp120 gp140 | ~450 664 | HIV-1 | gp120 and ferritin spaced by an ASG linker; gp140 and ferritin spaced by a (GS)5ASG linker | N-terminal | - | Viral vaccination | [79] |
| CHO | - | gp140 | 664 | HIV-1 | - | N-terminal | - | Viral vaccination | [80] |
| CHO | - | S protein | 1149 | SARS-CoV-2 | S protein and ferritin spaced by a G4S linker | N-terminal | 0.8–1 | Viral vaccination | [82] |
3.1.2. Modular Assembly
Chemical Crosslinking
Chemically Inducible Dimerization (CID)
Click Chemistry
Enzyme-Catalyzed Conjugation
Tag/Catcher Technology
| Strategy | Expression System | Ferritin Source | Conjugate | No. Residues | Target | Architectonic Tags | Conjugation Mechanism | Attachment Region on Ferritin | Conjugation Efficiency | Application | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferritin | Conjugate | |||||||||||
| Chemical crosslinking | Equine spleen (out-sourced) | (out-sourced) | Equine spleen | KGDS peptide | 4 | Human activated platelets | - | Activation of KGDS with EDC and NHS; conjugation of KGDS-NHS to NH2 moieties on ferritin | Solvent-accessible NH2 moieties | - | Cell targeting | [86] |
| Chemical crosslinking | Equine spleen (out-sourced) | HEK293 (out-sourced) | Equine spleen | HA | 552 | Influenza virus | - | NHS-PEG-Mal crosslinker (NHS arm reacts with NH2 moieties on ferritin; Mal arm reacts with C residues on HA) | Solvent-accessible NH2 moieties | - | Viral vaccination | [90] |
| Chemical crosslinking | E. coli | Mice | Human | IgG | (~150 kDa) | Melanoma | - | NHS-PEG-Mal crosslinker (NHS arm reacts with NH2 moieties on IgG; Mal arm reacts with C residues on ferritin) | Solvent-accessible C residues | - | Tumor targeting | [85] |
| CID | E. coli | E. coli | P. furiosus | eGFP | 223 | - | FKBP fused to eGFP; FRB fused to ferritin | Heterodimerization of FKBP and FRB by addition of rapamycin | N-terminal | - | Multivalent protein-protein interaction | [58] |
| Click-chemistry | E. coli | Mice | Human L-chain | IgG | (~150 kDa) | Cell adhesion molecule ICAM-1 | 4-AzF incorporated in res 5 of ferritin; IgG functionalized with DBCO (via DBCO-NHS) | Conjugation of DBCO to 4-AzF by click chemistry | N-terminal | < 57% | Cell targeting | [65] |
| Enzyme-catalyzed | E. coli | E. coli | Human H-chain | 7D12 | (~15 kDa) | EGFR+ A431cancer cells | Q residue fused to C-terminal of 7D12; KK residues and ferritin spaced by a (GS)3 linker | Transglutaminase mediates stable isopeptide bond between Q and K residues | Fused KK residues atN-terminal | - | Tumor targeting | [73] |
| Tag/Catcher | E. coli BL21(DE3) | E. coli BL21(DE3) | P. furiosus | E7 Reps1 Adpgk Dpagt1 | 21 28 28 25 | E7-related or MC38 tumors | SpyTag fused to C-terminal of peptides; SpyCatcher and ferritin spaced by a (G4S)3 linker | Spontaneous isopeptide bond formation between SpyTag and SpyCatcher under nearly any common conditions | N-terminal | 90% | Tumor targeting | [59] |
| Tag/Catcher | E. coli BL21(DE3) | E. coli BL21(DE3) | P. furiosus or mouse H-chain | preS1 of HBV | 108 | HBV | SpyCatcher fused to C-terminal of preS1; SpyTag and ferritin spaced by a (G4S)3 linker | Spontaneous isopeptide bond formation between SpyTag and SpyCatcher under nearly any common conditions | N-terminal | 90% | Viral vaccination | [60] |
| Tag/Catcher | E. coli BL21(DE3) | HEK293F | P. furiosus | RBD of S protein | ~220 | SARS-CoV-2 | SpyTag fused to C-terminal of RBD; SpyCatcher and ferritin spaced by a (G4S)3 linker | Spontaneous isopeptide bond formation between SpyTag and SpyCatcher under nearly any common conditions | N-terminal | - | Viral vaccination | [55] |
| Tag/Catcher | E. coli BL21(DE3) | CHO | H. pylori | RBD and HR of S protein | 222 (RBD) 303 (HR) | SARS-CoV-2 | SpyTag fused to N-terminal of RBD or HR; SpyCatcher fused to ferritin | Spontaneous isopeptide bond formation between SpyTag and SpyCatcher under nearly any common conditions | N-terminal | - | Viral vaccination | [40] |
| Tag/Catcher | CHO | CHO | - | RBD of S protein | 202 | SARS-CoV-2 | SpyTag fused to C-terminal of RBD; SpyCatcher and ferritin; both spaced by a G4S linker | Spontaneous isopeptide bond formation between SpyTag and SpyCatcher under nearly any common conditions | N-terminal | - | Viral vaccination | [82] |
3.2. Attachment Site
3.3. Linkers
4. Production of Ferritin-Based Vaccine Candidates Using Recombinant Expression Systems
4.1. Expression Systems
4.2. Production of Ferritin Nanoparticles
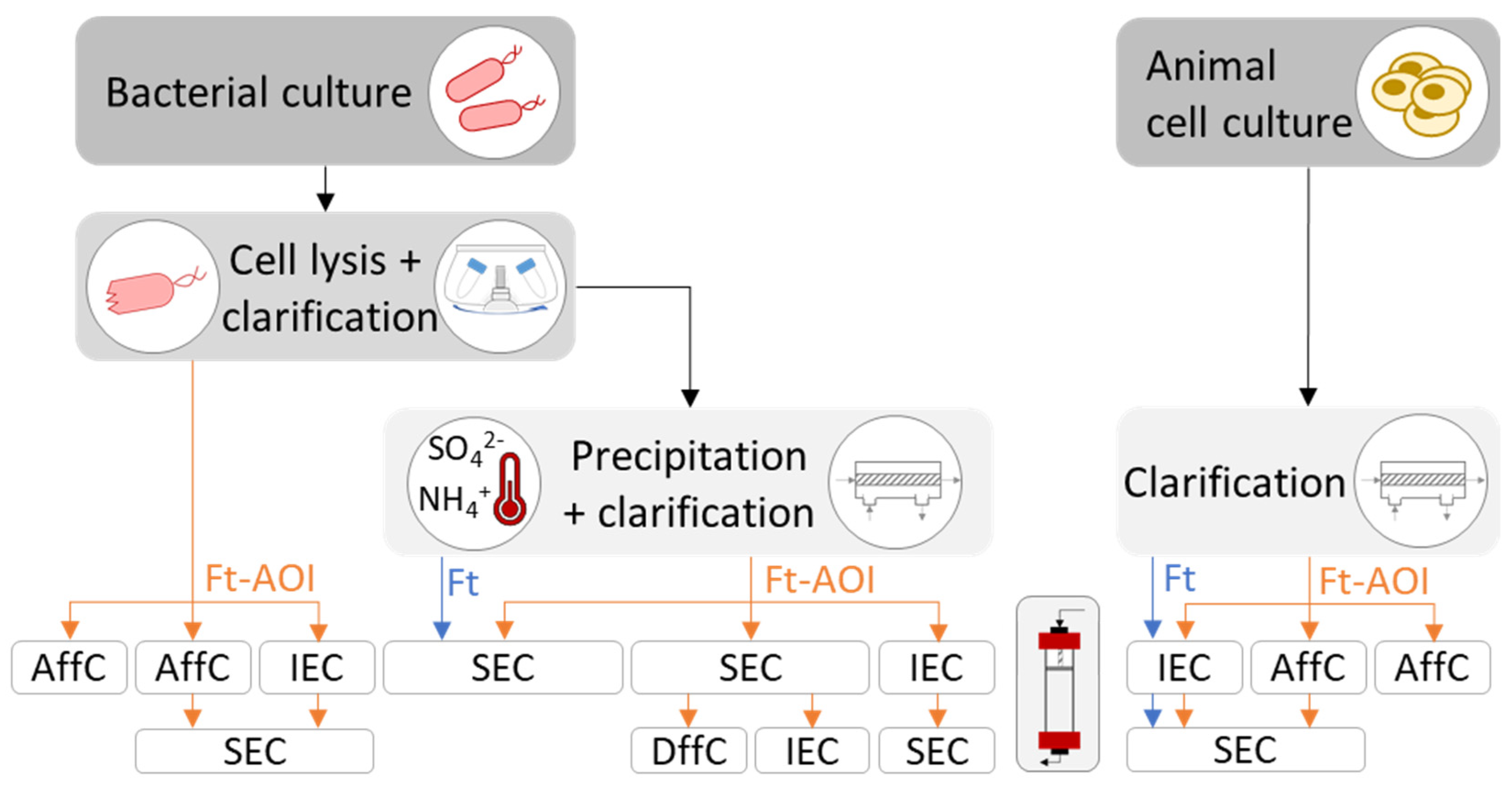
4.3. Purification of Ferritin Nanoparticles
4.3.1. Purification of Bacterial-Derived Ferritin Nanoparticles
4.3.2. Purification of Animal-Derived Ferritin Nanoparticles
4.4. Nanoparticle Characterization
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016, 120, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rehm, B.H. Bioengineering towards self-assembly of particulate vaccines. Curr. Opin. Biotechnol. 2017, 48, 42–53. [Google Scholar] [CrossRef]
- Parmiani, G.; Castelli, C.; Dalerba, P.; Mortarini, R.; Rivoltini, L.; Marincola, F.M.; Anichini, A. Cancer Immunotherapy With Peptide-Based Vaccines: What Have We Achieved? Where Are We Going? J. Natl. Cancer Inst. 2002, 94, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Yeste, A.; Nadeau, M.; Burns, E.J.; Weiner, H.L.; Quintana, F.J. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2012, 109, 11270–11275. [Google Scholar] [CrossRef]
- Hunter, Z.; McCarthy, D.P.; Yap, W.T.; Harp, C.T.; Getts, D.R.; Shea, L.D.; Miller, S.D. A Biodegradable Nanoparticle Platform for the Induction of Antigen-Specific Immune Tolerance for Treatment of Autoimmune Disease. ACS Nano 2014, 8, 2148–2160. [Google Scholar] [CrossRef]
- Smith, D.M.; Simon, J.K.; Jr, J.R.B. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef]
- Lung, P.; Yang, J.; Li, Q. Nanoparticle formulated vaccines: Opportunities and challenges. Nanoscale 2020, 12, 5746–5763. [Google Scholar] [CrossRef]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Tokatlian, T.; Read, B.J.; Jones, C.A.; Kulp, D.W.; Menis, S.; Chang, J.Y.H.; Steichen, J.M.; Kumari, S.; Allen, J.D.; Dane, E.L.; et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 2018, 363, 649–654. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, N.K.; Kim, I.-S. Bioengineered protein-based nanocage for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 157–171. [Google Scholar] [CrossRef]
- Irvine, D.J.; Swartz, M.A.; Szeto, G. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013, 12, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Munro, H.N.; Linder, M.C. Ferritin: Structure, biosynthesis, and role in iron metabolism. Physiol. Rev. 1978, 58, 317–396. [Google Scholar] [CrossRef]
- Bhaskar, S.; Lim, S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater. 2017, 9, e371. [Google Scholar] [CrossRef]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of Mammalian Iron Homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Soistman, E.; Carter, D.C. Ferritin nanoparticle technology...A new platform for antigen presentation and vaccine development. Ind. Biotechnol. 2006, 2, 143–147. [Google Scholar] [CrossRef]
- Khoshnejad, M.; Parhiz, H.; Shuvaev, V.V.; Dmochowski, I.J.; Muzykantov, V.R. Ferritin-based drug delivery systems: Hybrid nanocarriers for vascular immunotargeting. J. Control. Release 2018, 282, 13–24. [Google Scholar] [CrossRef]
- Uchida, M.; Kang, S.; Reichhardt, C.; Harlen, K.; Douglas, T. The ferritin superfamily: Supramolecular templates for materials synthesis. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 834–845. [Google Scholar] [CrossRef]
- He, D.; Marles-Wright, J. Ferritin family proteins and their use in bionanotechnology. New Biotechnol. 2015, 32, 651–657. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Yu, H.; Lv, P.; Lei, Z.; Zeng, Y.; Liu, G.; Cheng, T. Ferritin nanocage-based antigen delivery nanoplatforms: Epitope engineering for peptide vaccine design. Biomater. Sci. 2019, 7, 1794–1800. [Google Scholar] [CrossRef]
- Chiou, B.; Connor, J.R. Emerging and Dynamic Biomedical Uses of Ferritin. Pharmaceuticals 2018, 11, 124. [Google Scholar] [CrossRef]
- Chiancone, E.; Ceci, P.; Ilari, A.; Ribacchi, F.; Stefanini, S. Iron and proteins for iron storage and detoxification. BioMetals 2004, 17, 197–202. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin: Structure, Gene Regulation, and Cellular Function in Animals, Plants, and Microorganisms. Annu. Rev. Biochem. 1987, 56, 289–315. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. et Biophys. Acta (BBA) Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Watt, R.K.; Hilton, R.J.; Graff, D.M. Oxido-reduction is not the only mechanism allowing ions to traverse the ferritin protein shell. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 745–759. [Google Scholar] [CrossRef]
- Sehnal, D.; Rose, A.; Koca, J.; Burley, S.; Velankar, S. Mol*: Towards a Common Library and Tools for Web Molecular Graphics. In Workshop on Molecular Graphics and Visual Analysis of Molecular Data; Byska, J., Krone, M., Sommer, B., Eds.; The Eurographics Association: Geneve, Switzerland, 2018. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.D.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Banyard, S.H.; Stammers, D.K.; Harrison, P.M. Electron density map of apoferritin at 2.8-Å resolution. Nature 1978, 271, 282–284. [Google Scholar] [CrossRef]
- Gerl, M.; Jaenicke, R. Mechanism of the self-assembly of apoferritin from horse spleen. Eur. Biophys. J. 1987, 15, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Ohtomo, H.; Yamada, Y.; Hikima, T.; Kurobe, A.; Fujiwara, K.; Ikeguchi, M. Ferritin Assembly Revisited: A Time-Resolved Small-Angle X-ray Scattering Study. Biochemistry 2016, 55, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Treffry, A.; Lee, P.J.; Harrison, P.M. Iron-induced changes in rat liver isoferritins. Biochem. J. 1984, 220, 717–722. [Google Scholar] [CrossRef]
- Ihara, K.; Maeguchi, K.; Young, C.T.; Theil, E.C. Cell-specific properties of red cell and liver ferritin from bullfrog tadpoles probed by phosphorylation in vitro. J. Biol. Chem. 1984, 259, 278–283. [Google Scholar] [CrossRef]
- Cragg, S.J.; Wagstaff, M.; Worwood, M. Detection of a glycosylated subunit in human serum ferritin. Biochem. J. 1981, 199, 565–571. [Google Scholar] [CrossRef]
- Cragg, S.J.; Wagstaff, M.; Worwood, M. Sialic Acid and the Microheterogeneity of Human Serum Ferritin. Clin. Sci. 1980, 58, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Halliday, J.W.; Mack, U.; Powell, L.W. The Kinetics of Serum and Tissue Ferritins: Relation to Carbohydrate Content. Br. J. Haematol. 1979, 42, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Son, A.; Kim, J.; Bin Kwon, S.; Kim, M.H.; Kim, P.; Kim, J.; Byun, Y.H.; Sung, J.; Lee, J.; et al. Chaperna-Mediated Assembly of Ferritin-Based Middle East Respiratory Syndrome-Coronavirus Nanoparticles. Front. Immunol. 2018, 9, 1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xing, D.; Le Van, A.; Jerse, A.E.; Wang, S. Structure-based design of ferritin nanoparticle immunogens displaying antigenic loops of Neisseria gonorrhoeae. FEBS Open Bio 2017, 7, 1196–1207. [Google Scholar] [CrossRef]
- Li, Z.; Cui, K.; Wang, H.; Liu, F.; Huang, K.; Duan, Z.; Wang, F.; Shi, D.; Liu, Q. A milk-based self-assemble rotavirus VP6–ferritin nanoparticle vaccine elicited protection against the viral infection. J. Nanobiotechnology 2019, 17, 13. [Google Scholar] [CrossRef]
- Ma, X.; Zou, F.; Yu, F.; Li, R.; Yuan, Y.; Zhang, Y.; Zhang, X.; Deng, J.; Chen, T.; Song, Z.; et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity 2020, 53, 1315–1330. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Li, J.; Zhao, Z.; Zhang, H.; Hao, G.; Chen, H.; Qian, P. Immunization with a recombinant fusion of porcine reproductive and respiratory syndrome virus modified GP5 and ferritin elicits enhanced protective immunity in pigs. Virology 2020, 552, 112–120. [Google Scholar] [CrossRef]
- Qu, Z.; Li, M.; Guo, Y.; Liu, Y.; Wang, J.; Gao, M. Expression, purification, and characterisation of recombinant ferritin in insect cells using the baculovirus expression system. Biotechnol. Lett. 2019, 42, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Y.; Chen, H.; Li, X.; Qian, P. A ferritin nanoparticle vaccine for foot-and-mouth disease virus elicited partial protection in mice. Vaccine 2020, 38, 5647–5652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Chen, Y.; Li, H.; Fang, K.; Chen, H.; Li, X.; Qian, P. A Self-Assembling Ferritin Nanoplatform for Designing Classical Swine Fever Vaccine: Elicitation of Potent Neutralizing Antibody. Vaccines 2021, 9, 45. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Wei, C.-J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.R.; Rao, S.S.; Kong, W.-P.; Wang, L.; Nabel, G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.-J.; Kanekiyo, M.; Kong, W.-P.; Gallagher, J.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Kelly, H.G.; Tan, H.X.; Juno, J.A.; Esterbauer, R.; Ju, Y.; Jiang, W.; Wimmer, V.C.; Duckworth, B.C.; Groom, J.R.; Caruso, F.; et al. Self-assembling influenza nanoparticle vaccines drive extended germinal center activity and memory B cell maturation. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Kim, D.; Yu, K.-M.; Seo, H.D.; Lee, S.-A.; Casel, M.A.B.; Jang, S.-G.; Kim, S.; Jung, W.; Lai, C.-J.; et al. Development of Spike Receptor-Binding Domain Nanoparticles as a Vaccine Candidate against SARS-CoV-2 Infection in Ferrets. mBio 2021, 12. [Google Scholar] [CrossRef]
- Kamp, H.D.; Swanson, K.A.; Wei, R.R.; Dhal, P.K.; Dharanipragada, R.; Kern, A.; Sharma, B.; Sima, R.; Hajdusek, O.; Hu, L.T.; et al. Design of a broadly reactive Lyme disease vaccine. npj Vaccines 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Bu, W.; Joyce, M.G.; Meng, G.; Whittle, J.R.; Baxa, U.; Yamamoto, T.; Narpala, S.; Todd, J.-P.; Rao, S.S.; et al. Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell 2015, 162, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Darricarrère, N.; Pougatcheva, S.; Duan, X.; Rudicell, R.S.; Chou, T.-H.; DiNapoli, J.; Ross, T.M.; Alefantis, T.; Vogel, T.U.; Kleanthous, H.; et al. Development of a Pan-H1 Influenza Vaccine. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Joyce, M.G.; Gillespie, R.A.; Gallagher, J.R.; Andrews, S.F.; Yassine, H.M.; Wheatley, A.K.; Fisher, B.E.; Ambrozak, D.R.; Creanga, A.; et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol. 2019, 20, 362–372. [Google Scholar] [CrossRef]
- Swanson, K.A.; Rainho-Tomko, J.N.; Williams, Z.P.; Lanza, L.; Peredelchuk, M.; Kishko, M.; Pavot, V.; Alamares-Sapuay, J.; Adhikarla, H.; Gupta, S.; et al. A respiratory syncytial virus (RSV) F protein nanoparticle vaccine focuses antibody responses to a conserved neutralization domain. Sci. Immunol. 2020, 5, eaba6466. [Google Scholar] [CrossRef]
- Calisti, L.; Benni, I.; Trabuco, M.C.; Baiocco, P.; Ruzicka, B.; Boffi, A.; Falvo, E.; Malatesta, F.; Bonamore, A. Probing bulky ligand entry in engineered archaeal ferritins. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, B.; Zhu, Y.; Tan, W.; Zhu, M. Ferritin nanoparticle-based SARS-CoV-2 RBD vaccine induces a persistent antibody response and long-term memory in mice. Cell. Mol. Immunol. 2021, 18, 749–751. [Google Scholar] [CrossRef]
- Kang, H.J.; Kang, Y.J.; Lee, Y.-M.; Shin, H.-H.; Chung, S.J.; Kang, S. Developing an antibody-binding protein cage as a molecular recognition drug modular nanoplatform. Biomaterials 2012, 33, 5423–5430. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-A.; Kang, Y.J.; Shin, C.; Ra, J.-S.; Shin, H.-H.; Hong, S.Y.; Do, Y.; Kang, S. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (DC)-based vaccine development. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Ducasse, R.; Wang, W.-A.; Navarro, M.G.-J.; DeBons, N.; Colin, A.; Gautier, J.; Guigner, J.-M.; Guyot, F.; Gueroui, Z. Programmed Self-Assembly of a Biochemical and Magnetic Scaffold to Trigger and Manipulate Microtubule Structures. Sci. Rep. 2017, 7, 11344. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Zhou, X.; Guo, Z.; Zhang, J.; Zhu, P.; Yao, S.; Zhu, M. Ferritin nanoparticle-based SpyTag/SpyCatcher-enabled click vaccine for tumor immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2018, 16, 69–78. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Bian, Y.; Wang, S.; Chai, Q.; Guo, Z.; Wang, Z.; Zhu, P.; Peng, H.; Yan, X.; et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 2020, 15, 406–416. [Google Scholar] [CrossRef]
- de Turris, V.; Trabuco, M.C.; Peruzzi, G.; Boffi, A.; Testi, C.; Vallone, B.; Montemiglio, L.C.; Georges, A.D.; Calisti, L.; Benni, I.; et al. Humanized archaeal ferritin as a tool for cell targeted delivery. Nanoscale 2016, 9, 647–655. [Google Scholar] [CrossRef]
- Palombarini, F.; Ghirga, F.; Boffi, A.; Macone, A.; Bonamore, A. Application of crossflow ultrafiltration for scaling up the purification of a recombinant ferritin. Protein Expr. Purif. 2019, 163, 105451. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, I.S.; Joyce, M.G.; Chen, R.E.; Leung, K.; McKee, K.; Druz, A.; Van Galen, J.G.; Kanekiyo, M.; Tsybovsky, Y.; Yang, E.S.; et al. Two-Component Ferritin Nanoparticles for Multimerization of Diverse Trimeric Antigens. ACS Infect. Dis. 2018, 4, 788–796. [Google Scholar] [CrossRef]
- Lin, X.; Xie, J.; Niu, G.; Zhang, F.; Gao, H.; Yang, M.; Quan, Q.; Aronova, M.A.; Zhang, G.; Lee, S.; et al. Chimeric Ferritin Nanocages for Multiple Function Loading and Multimodal Imaging. Nano Lett. 2011, 11, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Khoshnejad, M.; Greineder, C.F.; Pulsipher, K.W.; Villa, C.H.; Altun, B.; Pan, D.C.; Tsourkas, A.; Dmochowski, I.J.; Muzykantov, V.R. Ferritin Nanocages with Biologically Orthogonal Conjugation for Vascular Targeting and Imaging. Bioconjugate Chem. 2018, 29, 1209–1218. [Google Scholar] [CrossRef]
- Yao, D.; Lao, F.; Zhang, Z.; Liu, Y.; Cheng, J.; Ding, F.; Wang, X.; Xi, L.; Wang, C.; Yan, X.; et al. Human H-ferritin presenting RBM of spike glycoprotein as potential vaccine of SARS-CoV-2. bioRxiv 2020, 115618. [Google Scholar] [CrossRef]
- Zou, W.; Liu, X.; Zhao, X.; Wang, J.; Chen, D.; Li, J.; Ji, L.; Hua, Z. Expression, purification, and characterization of recombinant human L-chain ferritin. Protein Expr. Purif. 2016, 119, 63–68. [Google Scholar] [CrossRef]
- Uchida, M.; Flenniken, .M.L.; Allen, .M.; Willits, .D.A.; Crowley, .B.E.; Brumfield, .S.; Willis, .A.F.; Jackiw, .L.; Jutila, .M.; Young, M.J.; et al. Targeting of Cancer Cells with Ferrimagnetic Ferritin Cage Nanoparticles. J. Am. Chem. Soc. 2006, 128, 16626–16633. [Google Scholar] [CrossRef]
- Ceci, P.; Vannucci, L.; Falvo, E.; Fornara, M.; Di Micco, P.; Benada, O.; Krizan, J.; Svoboda, J.; Hulikova-Capkova, K.; Morea, V.; et al. Selective targeting of melanoma by PEG-masked protein-based multifunctional nanoparticles. Int. J. Nanomed. 2012, 7, 1489–1509. [Google Scholar] [CrossRef][Green Version]
- Vannucci, L.; Falvo, E.; Failla, C.M.; Carbo, M.; Fornara, M.; Canese, R.; Cecchetti, S.; Rajsiglova, L.; Stakheev, D.; Krizan, J.; et al. In Vivo Targeting of Cutaneous Melanoma Using an Melanoma Stimulating Hormone-Engineered Human Protein Cage with Fluorophore and Magnetic Resonance Imaging Tracers. J. Biomed. Nanotechnol. 2015, 11, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-R.; Ko, H.K.; Ryu, J.H.; Ahn, K.Y.; Lee, Y.-H.; Oh, S.J.; Na, J.H.; Kim, T.W.; Byun, Y.; Kwon, I.C.; et al. Engineered Human Ferritin Nanoparticles for Direct Delivery of Tumor Antigens to Lymph Node and Cancer Immunotherapy. Sci. Rep. 2016, 6, 35182. [Google Scholar] [CrossRef]
- Qi, M.; Zhang, X.-E.; Sun, X.; Zhang, X.; Yao, Y.; Liu, S.; Chen, Z.; Li, W.; Zhang, Z.; Chen, J.; et al. Intranasal Nanovaccine Confers Homo- and Hetero-Subtypic Influenza Protection. Small 2018, 14, e1703207. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, Y.; Wu, T.; Cheng, J.; Liu, Y. Nanobody-Ferritin Conjugate for Targeted Photodynamic Therapy. Chem. A Eur. J. 2020, 26, 7442–7450. [Google Scholar] [CrossRef]
- Kowarik, M.; Young, N.M.; Numao, S.; Schulz, B.L.; Hug, I.; Callewaert, N.; Mills, D.C.; Watson, D.C.; Hernandez, M.; Kelly, J.F.; et al. Definition of the bacterial N-glycosylation site consensus sequence. EMBO J. 2006, 25, 1957–1966. [Google Scholar] [CrossRef]
- Harrison, P.M. The structure and function of ferritin. Biochem. Educ. 1986, 14, 154–162. [Google Scholar] [CrossRef]
- E Huard, D.J.; Kane, K.M.; Tezcan, F.A. Re-engineering protein interfaces yields copper-inducible ferritin cage assembly. Nat. Chem. Biol. 2013, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, S.; Xu, C.; Zhao, G. Engineering protein interfaces yields ferritin disassembly and reassembly under benign experimental conditions. Chem. Commun. 2016, 52, 7402–7405. [Google Scholar] [CrossRef] [PubMed]
- Sliepen, K.; Ozorowski, G.; Burger, J.A.; Van Montfort, T.; Stunnenberg, M.; Labranche, C.C.; Montefiori, D.C.; Moore, J.P.; Ward, A.B.; Sanders, R.W. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology 2015, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- He, L.; De Val, N.; Morris, C.D.; Vora, N.; Thinnes, T.C.; Kong, L.; Azadnia, P.; Sok, D.; Zhou, B.; Burton, D.R.; et al. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat. Commun. 2016, 7, 12041. [Google Scholar] [CrossRef] [PubMed]
- American Association for the Advancement of Science. An Erratum for the Research Article: “HIV-1 vaccine design through minimizing envelope metastability” by L. He, S. Kumar, J. D. Allen, D. Huang, X. Lin, C. J. Mann, K. L. Saye-Francisco, J. Copps, A. Sarkar, S. S. Blizard, G. Ozorowski, D. Sok, M. Crispin, A. B. Ward, D. Nemazee, D. R. Burton, I. A. Wilson and J. Zhu. Sci. Adv. 2020, 6, eabd8600. [Google Scholar] [CrossRef]
- Powell, A.E.; Zhang, K.; Sanyal, M.; Tang, S.; Weidenbacher, P.A.; Li, S.; Pham, T.D.; Pak, J.E.; Chiu, W.; Kim, P.S. A Single Immunization with Spike-Functionalized Ferritin Vaccines Elicits Neutralizing Antibody Responses against SARS-CoV-2 in Mice. ACS Central Sci. 2021, 7, 183–199. [Google Scholar] [CrossRef]
- He, L.; Lin, X.; Wang, Y.; Abraham, C.; Sou, C.; Ngo, T.; Zhang, Y.; Wilson, I.A.; Zhu, J. Single-component, self-assembling, protein nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci. Adv. 2021, 7, eabf1591. [Google Scholar] [CrossRef]
- Joyce, M.G.; Chen, W.-H.; Sankhala, R.S.; Hajduczki, A.; Thomas, P.V.; Choe, M.; Chang, W.; Peterson, C.E.; Martinez, E.; Morrison, E.B.; et al. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. bioRxiv Prepr. Serv. Biol. 2021, 150. [Google Scholar] [CrossRef]
- He, L.; Tzarum, N.; Lin, X.; Shapero, B.; Sou, C.; Mann, C.J.; Stano, A.; Zhang, L.; Nagy, K.; Giang, E.; et al. Proof of concept for rational design of hepatitis C virus E2 core nanoparticle vaccines. Sci. Adv. 2020, 6, eaaz6225. [Google Scholar] [CrossRef] [PubMed]
- Falvo, E.; Tremante, E.; Fraioli, R.; Leonetti, C.; Zamparelli, C.; Boffi, A.; Morea, V.; Ceci, P.; Giacomini, P. Antibody–drug conjugates: Targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale 2013, 5, 12278–12285. [Google Scholar] [CrossRef]
- Luo, W.; Guo, H.; Ye, Y.; Huang, C.; Lin, L.; Wu, Y.; Chen, H. Construction and in vitro studies of magnetic-apoferritin nanocages conjugated with KGDS peptide targeted at activated platelets for the MRI diagnosis of thrombus. J. Nanoparticle Res. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Derose, R.; Miyamoto, T.; Inoue, T. Manipulating signaling at will: Chemically-inducible dimerization (CID) techniques resolve problems in cell biology. Pflügers Arch. Eur. J. Physiol. 2013, 465, 409–417. [Google Scholar] [CrossRef]
- Lobba, M.J.; Fellmann, C.; Marmelstein, A.M.; Maza, J.C.; Kissman, E.N.; Robinson, S.A.; Staahl, B.T.; Urnes, C.; Lew, R.J.; Mogilevsky, C.S.; et al. Site-Specific Bioconjugation through Enzyme-Catalyzed Tyrosine–Cysteine Bond Formation. ACS Central Sci. 2020, 6, 1564–1571. [Google Scholar] [CrossRef]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.; Yang, Y.; Ma, G.; Su, Z.; Zhang, S. An Apoferritin–Hemagglutinin Conjugate Vaccine with Encapsulated Nucleoprotein Antigen Peptide from Influenza Virus Confers Enhanced Cross Protection. Bioconjugate Chem. 2020, 31, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yun, J.; Shang, Z.; Zhang, X.; Pan, B. Design and optimization of a linker for fusion protein construction. Prog. Nat. Sci. 2009, 19, 1197–1200. [Google Scholar] [CrossRef]
- Gustavsson, M.; Lehtiö, J.; Denman, S.; Teeri, T.T.; Hult, K.; Martinelle, M. Stable linker peptides for a cellulose-binding domain–lipase fusion protein expressed in Pichia pastoris. Protein Eng. Des. Sel. 2001, 14, 711–715. [Google Scholar] [CrossRef]
- Van Rosmalen, M.; Krom, M.; Merkx, M. Tuning the Flexibility of Glycine-Serine Linkers To Allow Rational Design of Multidomain Proteins. Biochemistry 2017, 56, 6565–6574. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2012, 65, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.; Novarra, S.; Zhu, L.; Mugabe, S.; Thisted, T.; Baca, M.; Depaz, R.; Barton, C. O-xylosylation in a Recombinant Protein is Directed at a Common Motif on Glycine–Serine Linkers. J. Pharm. Sci. 2013, 102, 3920–3924. [Google Scholar] [CrossRef]
- Johnson, Q.R.; Lindsay, R.J.; Raval, S.R.; Dobbs, J.S.; Nellas, R.B.; Shen, T. Effects of Branched O-Glycosylation on a Semiflexible Peptide Linker. J. Phys. Chem. B 2014, 118, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fan, K.; Yan, X. Ferritin drug carrier (FDC) for tumor targeting therapy. J. Control. Release 2019, 311-312, 288–300. [Google Scholar] [CrossRef]
- Jacob, S.I.; Khogeer, B.; Bampos, N.; Sheppard, T.; Schwartz, R.; Lowe, C.R. Development and Application of Synthetic Affinity Ligands for the Purification of Ferritin-Based Influenza Antigens. Bioconjugate Chem. 2017, 28, 1931–1943. [Google Scholar] [CrossRef]

| Ferritin Source | Uniprot | Expression System | Putative N-Glycosylation Site | Sequon 1 | References |
|---|---|---|---|---|---|
| E. coli K-12 | P0A998 | E. coli | - | - | [37] |
| H. pylori | P52093 | E. coli | Eukaryote and bacteria | E(17)-M(18)-N(19)-S(20)-S(21) | [38,39,40] |
| H. pylori (N19Q) 2 | - | IC-BEVS, HEK293 | - | - | [41,42,43,44,45,46,47] |
| H. pylori-bullfrog hybrid 3 | - | E. coli, HEK293, CHO | - | - | [48,49,50,51,52,53] |
| P. furiosus | I6V0I9 | E. coli | - | - | [54,55,56,57,58,59,60] |
| P. furiosus (R64K) 4 | - | HEK293 | - | - | [9] |
| A. fulgidus | O29424 | E. coli | Eukaryote and bacteria | E(96)-V(97)-N(98)-V(99)-T(100) | [54,61,62] |
| T. ni | L-chain: Q52SA8 H-chain: Q52SA9 | HEK293 | Eukaryote (L-chain only) | L-chain: R(101)-K(102)-N(103)-Y(104)-T(105) | [63] |
| Rat | L-chain: P02793 H-chain: P19132 | HEK293 | Eukaryote (both chains) 5 | L-chain: R(6)-Q(7)-N(8)-Y(9)-S(10) H-chain: S(110)-V(111)-N(112)-Q(113)-S(114) | [20,60] |
| Human | L-chain: P02792 H-chain: P02794 | E. coli | Eukaryote (both chains) 5 | L-chain: R(6)-Q(7)-N(8)-Y(9)-S(10) H-chain: N(110)-V(111)-N(112)-Q(113)-S(114) and S(179)-D(180)-N(181)-E(182)-S(183) | [16,64,65,66,67,68,69,70,71,72,73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.Q.; Alves, P.M.; Roldão, A. Functionalizing Ferritin Nanoparticles for Vaccine Development. Pharmaceutics 2021, 13, 1621. https://doi.org/10.3390/pharmaceutics13101621
Rodrigues MQ, Alves PM, Roldão A. Functionalizing Ferritin Nanoparticles for Vaccine Development. Pharmaceutics. 2021; 13(10):1621. https://doi.org/10.3390/pharmaceutics13101621
Chicago/Turabian StyleRodrigues, Margarida Q., Paula M. Alves, and António Roldão. 2021. "Functionalizing Ferritin Nanoparticles for Vaccine Development" Pharmaceutics 13, no. 10: 1621. https://doi.org/10.3390/pharmaceutics13101621
APA StyleRodrigues, M. Q., Alves, P. M., & Roldão, A. (2021). Functionalizing Ferritin Nanoparticles for Vaccine Development. Pharmaceutics, 13(10), 1621. https://doi.org/10.3390/pharmaceutics13101621







