A Comparative Analysis of the Structure and Biological Properties of Films and Microfibrous Scaffolds Based on Silk Fibroin
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Silk Fibroin
2.2. Fabrication of Silk Fibroin Films
2.3. Fabrication of Silk Fibroin-Based Microfibrous Scaffolds
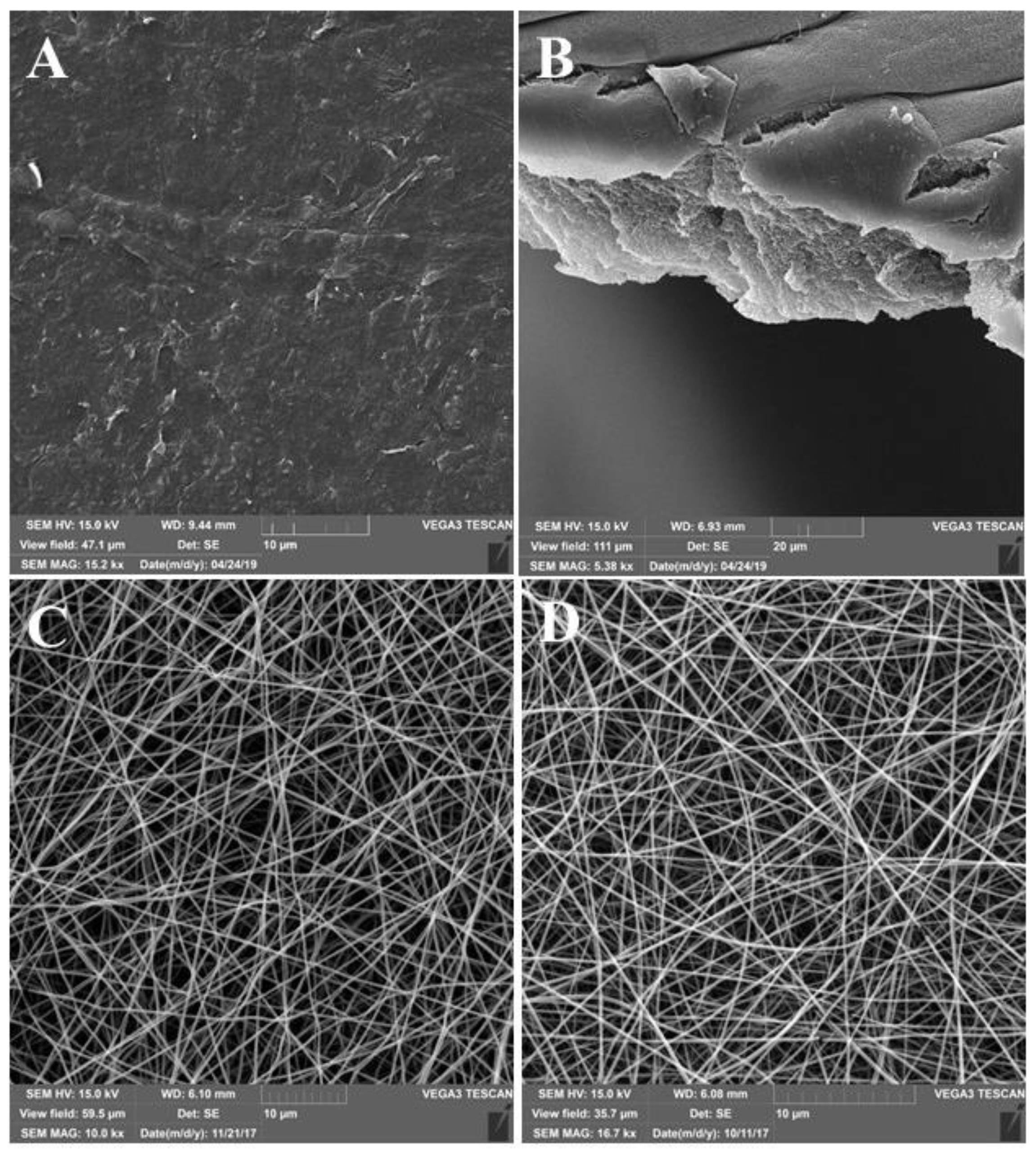
2.4. The Analysis of the Surface Structure Using Scanning Electron Microscopy (SEM)
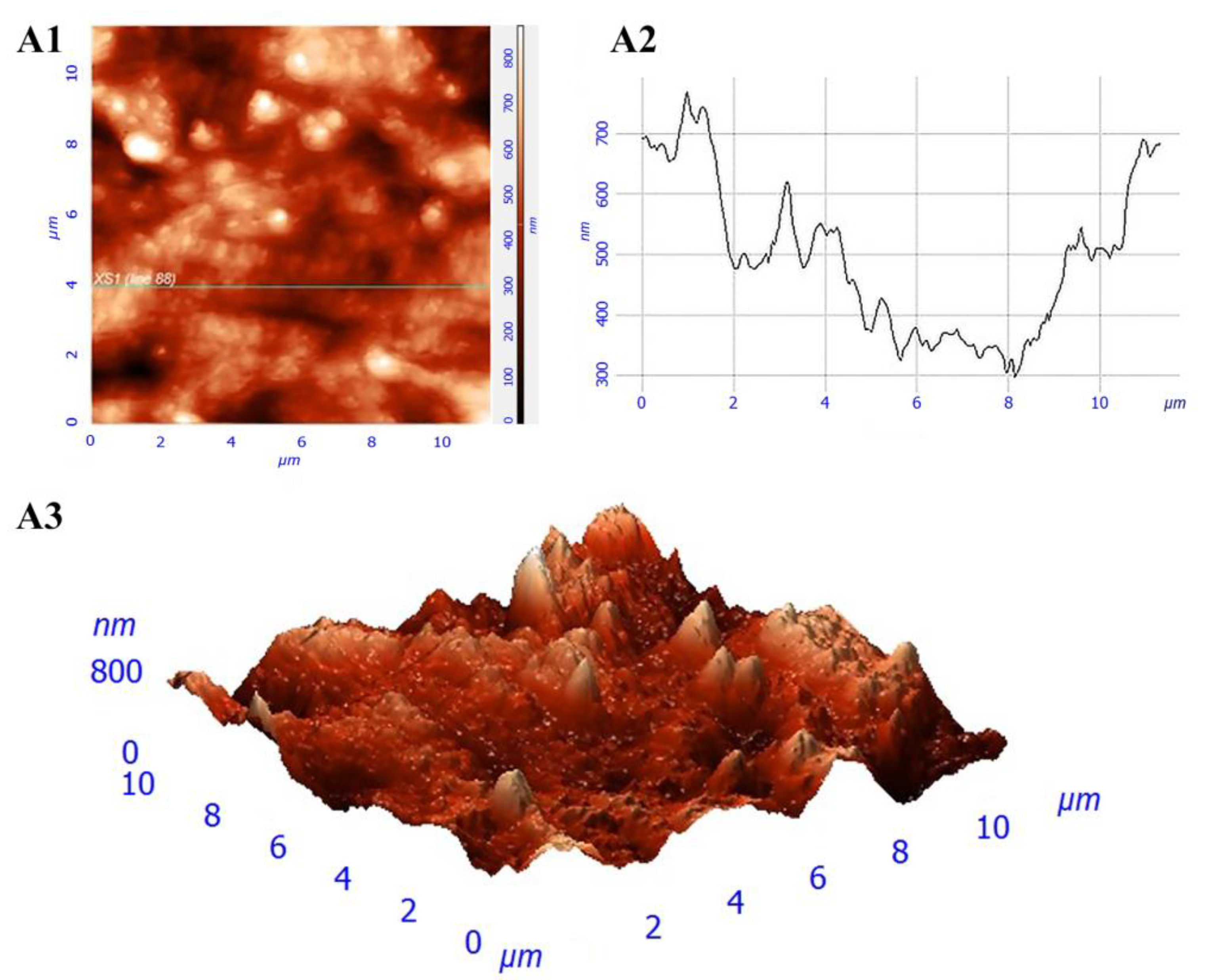
2.5. The Analysis of the Film Surface Structure Using Atomic Force Microscopy
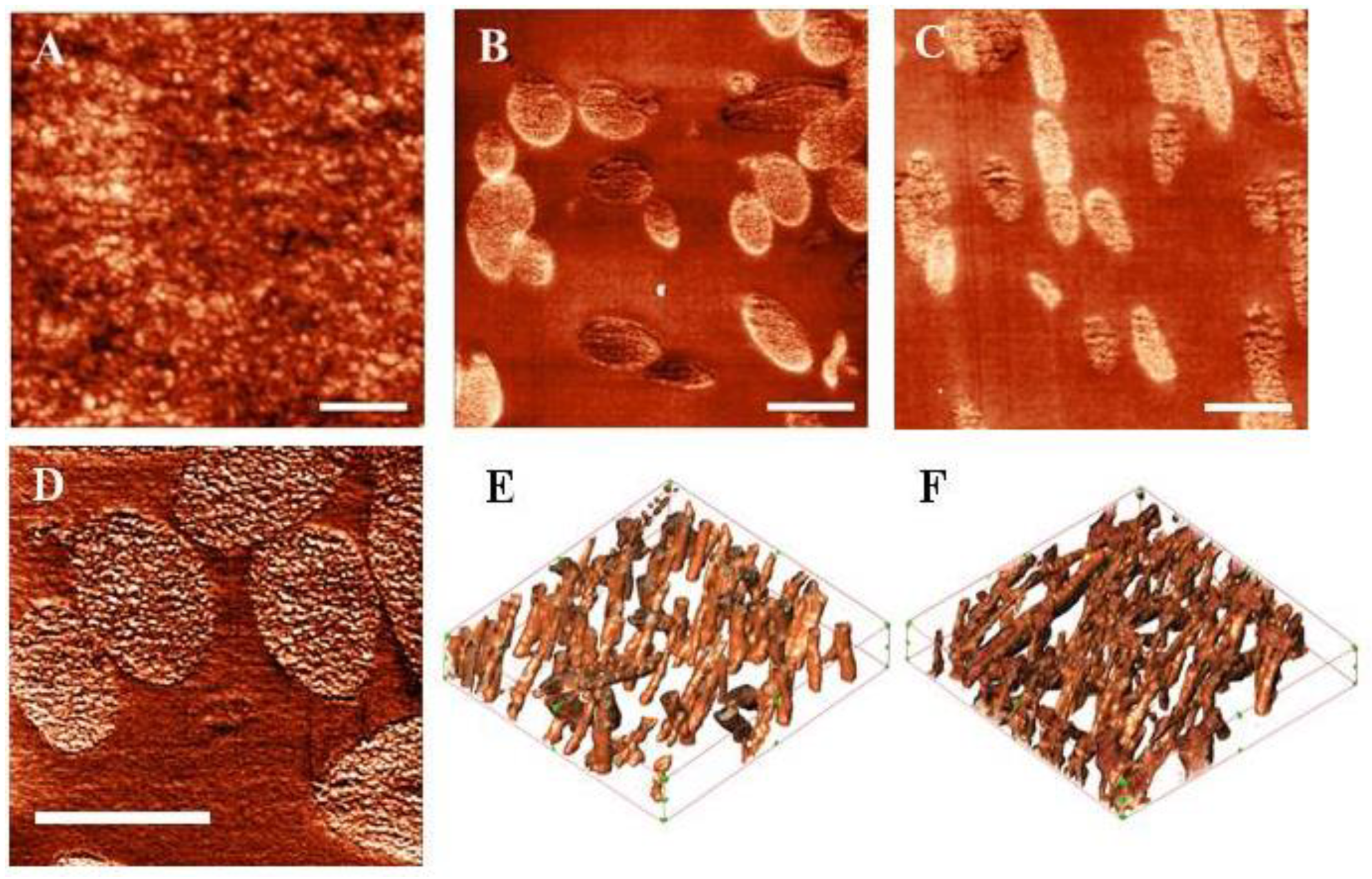
2.6. The Analysis of the Structure of Constructions by Scanning Probe Nanotomography (SPNT)
2.7. Experiments with Cells
2.8. Full-Thickness Skin Wound Healing of a Wistar Rat
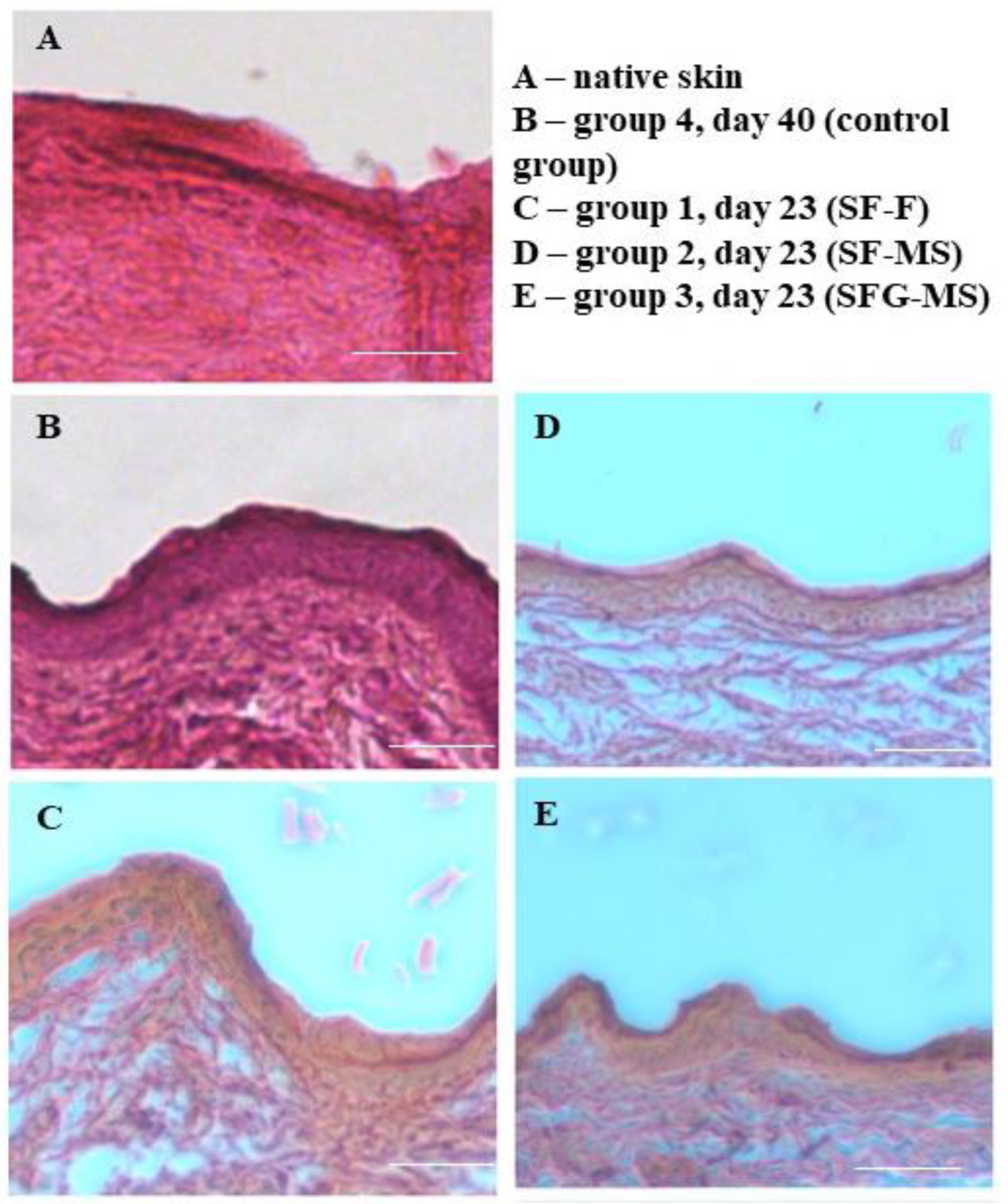
2.9. Histological Evaluation
2.10. Statistical Processing of Results
3. Results
3.1. Investigation of Construction Structure
3.2. Experiments with Cells
3.3. The Investigation of the Healing Process of the Full-Thickness Skin Wound of Wistar Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aramwit, P.; Motta, A.; Kundu, S.C. Tissue Engineering: From Basic Sciences to Clinical Perspectives. BioMed Res. Int. 2017, 2017, 1–2. [Google Scholar] [CrossRef]
- Shahriarpanah, S.; Nourmohammadi, J.; Amoabediny, G. Fabrication and characterization of carboxylated starch-chitosan bioactive scaffold for bone regeneration. Int. J. Biol. Macromol. 2016, 93, 1069–1078. [Google Scholar] [CrossRef]
- Venkatesan, J.; Singh, S.K.; Anil, S.; Kim, S.-K.; Shim, M.S. Preparation, Characterization and Biological Applications of Biosynthesized Silver Nanoparticles with Chitosan-Fucoidan Coating. Molecules 2018, 23, 1429. [Google Scholar] [CrossRef]
- Efimov, A.; Moisenovich, M.M.; Kuznetsov, A.G.; Safonova, L.A.; Bobrova, M.M.; Agapov, I.I. Investigation of micro- and nanostructure of biocompatible scaffolds from regenerated fibroin of Bombix mori by scanning probe nanotomography. Nanotechnol. Russ. 2014, 9, 688–692. [Google Scholar] [CrossRef]
- Bobrova, M.; Safonova, L.; Agapova, O.; Krasheninnikov, M.; Shagidulin, M.; Agapov, I.I. Liver Tissue Decellularization as a Promising Porous Scaffold Processing Technology for Tissue Engineering and Regenerative Medicine. Sovrem. Tehnol. V Med. 2015, 7, 6–13. [Google Scholar] [CrossRef][Green Version]
- Derakhti, S.; Safiabadi-Tali, S.H.; Amoabediny, G.; Sheikhpour, M. Attachment and detachment strategies in microcarrier-based cell culture technology: A comprehensive review. Mater. Sci. Eng. C 2019, 103, 109782. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Migliaresi, C.; Faccioni, F.; Torricelli, P.; Fini, M.; Giardino, R. Fibroin hydrogels for biomedical applications: Preparation, characterization and in vitro cell culture studies. J. Biomater. Sci. Polym. Ed. 2004, 15, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Venkatesan, J.; Vinodhini, P.; Sudha, P.N.; Kim, S.-K. Chitin and Chitosan Composites for Bone Tissue Regeneration. Adv. Food Nutr. Res. 2014, 73, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef]
- Ehrmann, A. Non-Toxic Crosslinking of Electrospun Gelatin Nanofibers for Tissue Engineering and Biomedicine—A Review. Polymers 2021, 13, 1973. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Van Le, Q. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef]
- Agapov, I.I.; Moisenovich, M.M.; Vasilyeva, T.V.; Pustovalova, O.L.; Kon’Kov, A.S.; Arkhipova, A.Y.; Sokolova, O.; Bogush, V.; Sevastianov, V.I.; Debabov, V.G.; et al. Biodegradable matrices from regenerated silk of Bombix mori. Dokl. Biochem. Biophys. 2010, 433, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Segnana, P.; Verin, L.; La Monica, S.; Fumarola, C.; Bucci, G.; Gussago, F.; Cantoni, A.M.; Ampollini, L.; Migliaresi, C. Physico-chemical characterization and biological evaluation of two fibroin materials. J. Tissue Eng. Regen. Med. 2012, 8, 874–885. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-X.; Zhang, N.-N.; Li, W.-F.; Jia, N.; Chen, B.-Y.; Zhou, K.; Zhang, J.; Chen, Y.; Zhou, C.-Z. N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease. J. Mol. Biol. 2012, 418, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Stoppato, M.; Stevens, H.Y.; Carletti, E.; Migliaresi, C.; Motta, A.; Guldberg, R.E. Effects of silk fibroin fiber incorporation on mechanical properties, endothelial cell colonization and vascularization of PDLLA scaffolds. Biomaterials 2013, 34, 4573–4581. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Hoyer, B.; Solati-Hashjin, M.; Lode, A.; Nosoudi, N.; Samadikuchaksaraei, A.; Gelinsky, M. Fabrication and characterization of regenerated silk scaffolds reinforced with natural silk fibers for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Edwards, S.; Hou, S.; Boudreau, R.; Yee, R.; Jeong, K.J. A multi-interpenetrating network (IPN) hydrogel with gelatin and silk fibroin. Biomater. Sci. 2019, 7, 1276–1280. [Google Scholar] [CrossRef]
- Panas-Perez, E.; Gatt, C.J.; Dunn, M.G. Development of a silk and collagen fiber scaffold for anterior cruciate ligament reconstruction. J. Mater. Sci. Mater. Electron. 2013, 24, 257–265. [Google Scholar] [CrossRef]
- Ghezzi, C.E.; Marelli, B.; Muja, N.; Hirota, N.; Martin, J.G.; Barralet, J.E.; Alessandrino, A.; Freddi, G.; Nazhat, S.N. Mesenchymal stem cell-seeded multilayered dense collagen-silk fibroin hybrid for tissue engineering applications. Biotechnol. J. 2011, 6, 1198–1207. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Gomes, A.; Cavaco-Paulo, A. Novel silk fibroin/elastin wound dressings. Acta Biomater. 2012, 8, 3049–3060. [Google Scholar] [CrossRef] [PubMed]
- Maghdouri-White, Y.; Bowlin, G.L.; Lemmon, C.; Dréau, D. Bioengineered silk scaffolds in 3D tissue modeling with focus on mammary tissues. Mater. Sci. Eng. C 2016, 59, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Soffer, L.; Wang, X.; Zhang, X.; Kluge, J.; Dorfmann, L.; Kaplan, D.L.; Leisk, G. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. J. Biomater. Sci. Polym. Ed. 2008, 19, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Kasoju, N.; Bora, U. Silk fibroin based biomimetic artificial extracellular matrix for hepatic tissue engineering applications. Biomed. Mater. 2012, 7, 045004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yan, S.; Li, M.; Wang, J. Growth of primary hippocampal neurons on multichannel silk fibroin scaffold. Fibers Polym. 2014, 15, 41–46. [Google Scholar] [CrossRef]
- Ajisawa, A. Dissolution of silk fibroin with calciumchloride/ethanol aqueous solution. J. Seric. Sci. Jpn. 1998, 67, 91–94. [Google Scholar] [CrossRef]
- Safonova, L.; Bobrova, M.; Agapova, O.; Kotliarova, M.; Arkhipova, A.; Moisenovich, M.; Agapov, I. Biological Properties of Regenerated Silk Fibroin Films. Sovrem. Tehnol. V Med. 2015, 7, 6–13. [Google Scholar] [CrossRef][Green Version]
- Efimov, A.E.; Agapova, O.I.; Safonova, L.A.; Bobrova, M.M.; Parfenov, V.; Koudan, E.V.; Pereira, F.D.A.S.; Bulanova, E.A.; Mironov, V.A.; Agapov, I.I. 3D scanning probe nanotomography of tissue spheroid fibroblasts interacting with electrospun polyurethane scaffold. Express Polym. Lett. 2019, 13, 632–641. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Safonova, L.A.; Bobrova, M.M.; Agapova, O.; Arkhipova, A.Y.; Goncharenko, A.V.; Agapov, I.I. Fibroin silk based films for rat’s full-thickness skin wound regeneration. Russ. J. Transpl. Artif. Organs 2016, 18, 74–84. [Google Scholar] [CrossRef]
- Sokolova, A.; Bobrova, M.; Safonova, L.; Agapova, O.; Moisenovich, M.; Agapov, I. The Relation of Biological Properties of the Silk Fibroin/Gelatin Scaffolds with the Composition and Fabrication Technology. Sovrem. Tehnol. V Med. 2016, 8, 6–15. [Google Scholar] [CrossRef][Green Version]
- Meng, Z.; Wang, Y.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Binan, L.; Tendey, C.; De Crescenzo, G.; El Ayoubi, R.; Ajji, A.; Jolicoeur, M. Differentiation of neuronal stem cells into motor neurons using electrospun poly-l-lactic acid/gelatin scaffold. Biomaterials 2014, 35, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, P.; Gioffrè, M.; Fiorani, A.; Panzavolta, S.; Gualandi, C.; Fini, M.; Focarete, M.L.; Bigi, A. Co-electrospun gelatin-poly(l-lactic acid) scaffolds: Modulation of mechanical properties and chondrocyte response as a function of composition. Mater. Sci. Eng. C 2014, 36, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Rodrigues, G.; Martins, G.; Roberto, M.; Mafra, M.; Henriques, C.; Silva, J. In vitro and in vivo evaluation of electrospun nanofibers of PCL, chitosan and gelatin: A comparative study. Mater. Sci. Eng. C 2015, 46, 348–358. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, X.; Jiang, X.; Brody, H.D.; Rowe, D.W.; Wei, M. Fabrication and characterization of biomimetic collagen–apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 2013, 9, 7308–7319. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, S.; Wang, Z. Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food Bioprod. Process. 2011, 89, 185–193. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Efimov, A.E.; Agapova, O.I.; Safonova, L.A.; Bobrova, M.M.; Volkov, A.D.; Khamkhash, L.; Agapov, I.I. Cryo scanning probe nanotomography study of the structure of alginate microcarriers. RSC Adv. 2017, 7, 8808–8815. [Google Scholar] [CrossRef]
- Surguchenko, V.A.; Ponomareva, A.S.; Efimov, A.E.; Nemets, E.A.; Agapov, I.I.; Sevastianov, V.I. Characteristics of adhesion and proliferation of mouse nih/3t3 fibroblasts on the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films with different surface roughness values. Russ. J. Transpl. Artif. Organs 2012, 14, 72–77. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Hernáez-Moya, R.; Iturriaga, L.; Pedraz, J.L.; Lakshminarayanan, R.; Dolatshahi-Pirouz, A.; Taebnia, N.; Orive, G. Recent advances in gelatin-based therapeutics. Expert Opin. Biol. Ther. 2019, 19, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Efimov, A.E.; Agapova, O.I.; Safonova, L.A.; Bobrova, M.M.; Agapov, I.I. Three-dimensional analysis of micro- and nanostructure of biomaterials and cells by method of scanning probe nanotomography. Russ. J. Transpl. Artif. Organs 2018, 19, 78–87. [Google Scholar] [CrossRef][Green Version]
- Efimov, A.E.; Moisenovich, M.M.; Bogush, V.; Agapov, I.I. 3D nanostructural analysis of silk fibroin and recombinant spidroin 1 scaffolds by scanning probe nanotomography. RSC Adv. 2014, 4, 60943–60947. [Google Scholar] [CrossRef]
- Servoli, E.; Maniglio, D.; Motta, A.; Predazzer, R.; Migliaresi, C. Surface Properties of Silk Fibroin Films and Their Interaction with Fibroblasts. Macromol. Biosci. 2005, 5, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Bobrova, M.M.; Safonova, L.A.; Agapova, O.I.; Efimov, A.E.; Agapov, I.I. The analysis of the proliferative activity of cells on microparticles obtained from decellularized liver and kidney tissue. Russ. J. Transpl. Artif. Organs 2019, 20, 69–75. [Google Scholar] [CrossRef]
- Schneider, A.; Wang, X.; Kaplan, D.; Garlick, J.; Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef]
- Gavrilova, N.; Borzenok, S.; Revishchin, A.; Tishchenko, O.; Ostrovkiy, D.; Bobrova, M.; Safonova, L.; Efimov, A.; Agapova, O.; Agammedov, M.; et al. The effect of biodegradable silk fibroin-based scaffolds containing glial cell line-derived neurotrophic factor (GDNF) on the corneal regeneration process. Int. J. Biol. Macromol. 2021, 185, 264–276. [Google Scholar] [CrossRef]
- Luangbudnark, W.; Viyoch, J.; Laupattarakasem, W.; Surakunprapha, P.; Laupattarakasem, P. Properties and Biocompatibility of Chitosan and Silk Fibroin Blend Films for Application in Skin Tissue Engineering. Sci. World J. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Pavankumar, K.N.; Jayakumar, K.; Chandrashekhara, N.; Narayanswamy, H.D.; Manjunatha, K.P.; Nirmala, G.C. The wound healing study of silk protein based biofilms in rats. J. Cell Tissue Res. 2013, 13, 3989–3996. [Google Scholar]
- Ni, S.; Sun, L.; Ercan, B.; Liu, L.; Ziemer, K.; Webster, T.J. A mechanism for the enhanced attachment and proliferation of fibroblasts on anodized 316L stainless steel with nano-pit arrays. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1297–1303. [Google Scholar] [CrossRef]
- Karahaliloğlu, Z.; Ercan, B.; Denkbaş, E.B.; Webster, T.J. Nanofeatured silk fibroin membranes for dermal wound healing applications. J. Biomed. Mater. Res. Part A 2015, 103, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Seidel, M. Rapid fabrication of laminated 3D hydrodynamic focusing microreactors and its application for synthesis of gold nanoparticles. Lab. Chip 2021. [CrossRef]
- Padol, A.; Jayakumar, K.; Shridhar, N.; Swamy, N.; Swamy, N.; Mohan, K. Safety evaluation of silk protein film (A novel wound healing agent) in terms of acute dermal toxicity, acute dermal irritation and skin sensitization. Toxicol. Int. 2011, 18, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.W.; Lee, O.J.; Lee, J.M.; Moon, B.M.; Park, H.J.; Park, Y.R.; Lee, M.C.; Kim, S.H.; Chao, J.R.; Ki, C.S.; et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int. J. Biol. Macromol. 2016, 85, 29–39. [Google Scholar] [CrossRef]




| Group Number | Wound Dressing Description |
|---|---|
| 1 | Silk fibroin aqueous solution with the protein concentration of 20 mg/mL, casting method (SF-F) |
| 2 | Silk fibroin HFIP-solution with the protein concentration of 50 mg/mL, electrospinning method (SF-MS) |
| 3 | Silk fibroin and gelatin HFIP-solution with the total protein concentration of 50 mg/mL which contains 70% of fibroin by total protein weight and 30% of gelatin by total protein weight, electrospinning method (SFG-MS) |
| 4 | (control) |
| Scaffold Description (Group Number) | Volume Porosity % | SA:V, μm−1 |
|---|---|---|
| SF-MS (group 2) | 81.3 ± 12.6 | 37.2 ± 9.7 |
| SFG-MS (group 3) | 86.4 ± 10.5 | 33.8 ± 7.4 |
| Group Number | 0 Day | 3 Day | 7 Day | 14 Day | 21 Day | 23 Day | 28 Day | 40 Day |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 2 ± 1 | 19 ± 0.6 * | 61 ± 1.5 * | 84 ± 1.5 * | 98 ± 1 * | 100 * | |
| 2 | 0 | 14 ± 1.5 * | 41 ± 1.2 * | 75 ± 0.6 * | 95 ± 0.6 * | 100 * | ||
| 3 | 0 | 2 ± 1.7 | 44 ± 1 * | 70 ± 1.5 * | 93 ± 0.6 * | 100 * | ||
| 4 | 0 | 0 | 12.5 ± 1 | 50 ± 1.5 | 68 ± 1.5 | 75 ± 1.5 | 87.5 ± 1.7 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safonova, L.; Bobrova, M.; Efimov, A.; Lyundup, A.; Agapova, O.; Agapov, I. A Comparative Analysis of the Structure and Biological Properties of Films and Microfibrous Scaffolds Based on Silk Fibroin. Pharmaceutics 2021, 13, 1561. https://doi.org/10.3390/pharmaceutics13101561
Safonova L, Bobrova M, Efimov A, Lyundup A, Agapova O, Agapov I. A Comparative Analysis of the Structure and Biological Properties of Films and Microfibrous Scaffolds Based on Silk Fibroin. Pharmaceutics. 2021; 13(10):1561. https://doi.org/10.3390/pharmaceutics13101561
Chicago/Turabian StyleSafonova, Liubov, Maria Bobrova, Anton Efimov, Alexey Lyundup, Olga Agapova, and Igor Agapov. 2021. "A Comparative Analysis of the Structure and Biological Properties of Films and Microfibrous Scaffolds Based on Silk Fibroin" Pharmaceutics 13, no. 10: 1561. https://doi.org/10.3390/pharmaceutics13101561
APA StyleSafonova, L., Bobrova, M., Efimov, A., Lyundup, A., Agapova, O., & Agapov, I. (2021). A Comparative Analysis of the Structure and Biological Properties of Films and Microfibrous Scaffolds Based on Silk Fibroin. Pharmaceutics, 13(10), 1561. https://doi.org/10.3390/pharmaceutics13101561






