Interactions of Potential Anti-COVID-19 Compounds with Multispecific ABC and OATP Drug Transporters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ABC Transporter Assays
2.3. OATP Transporter Assays
3. Results
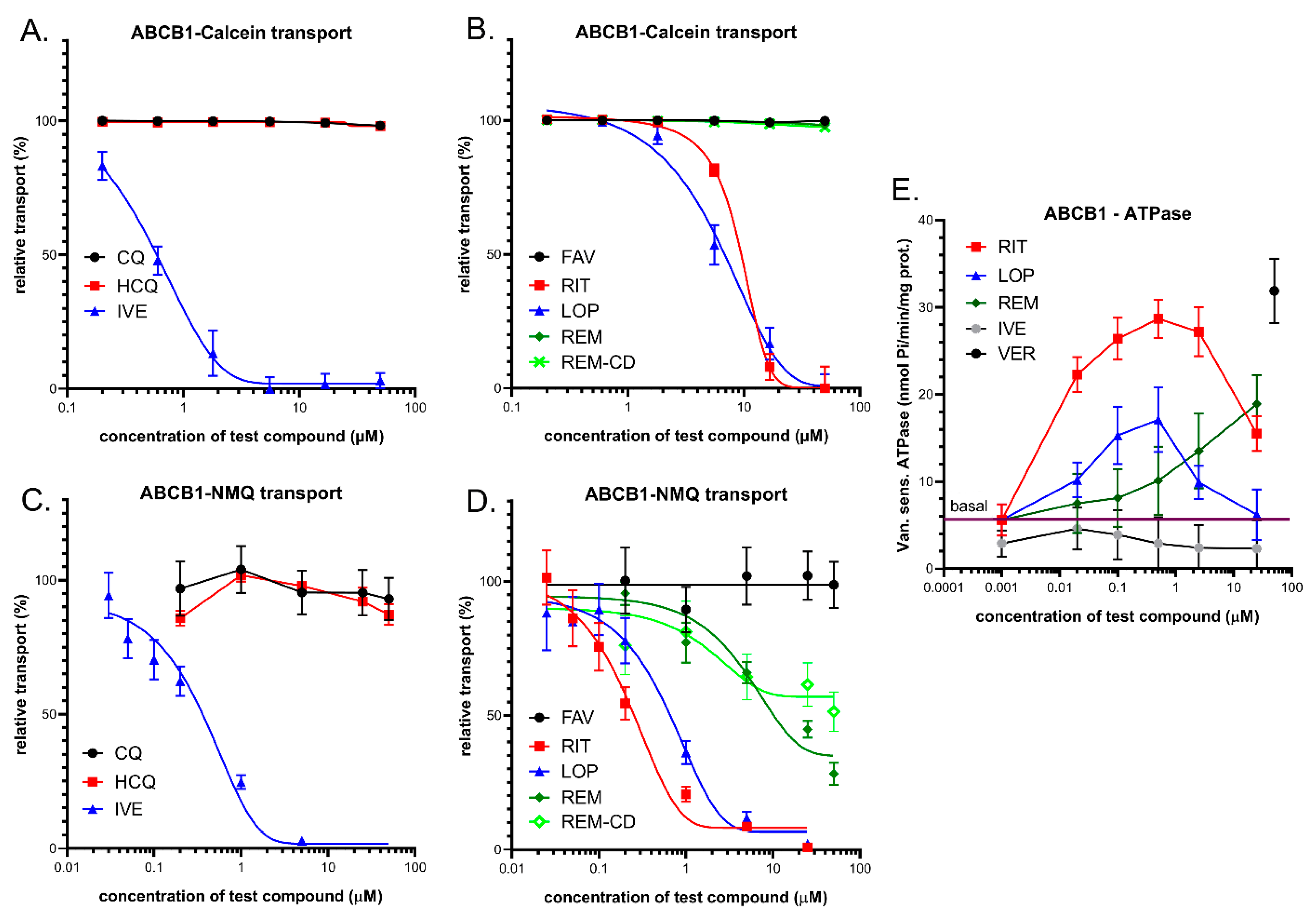
3.1. Interaction of Anti-COVID-19 Drug Candidates with ABCB1/MDR1/Pgp
3.1.1. Transport Assays in Intact Human PLB-985/ABCB1 Cells
3.1.2. Vesicular Transport Studies in HEK/ABCB1 Membrane Vesicles
3.1.3. ABCB1-ATPase Activity Measurements in Sf9 Membranes
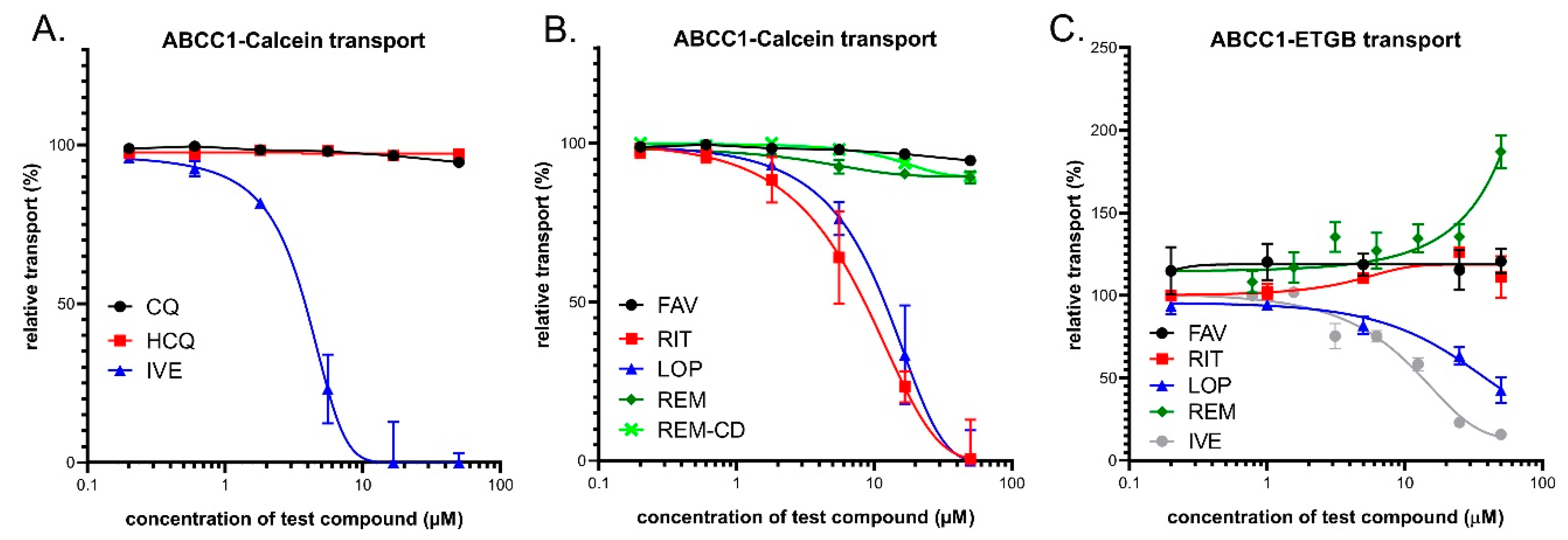
3.2. Interaction of Anti-COVID-19 Drug Candidates with ABCC1/MRP1
3.2.1. Transport Assay in Intact Human Cells—HL60/ABCC1 Cells
3.2.2. Vesicular Transport Studies in Sf9/ABCC1 Membrane Vesicles
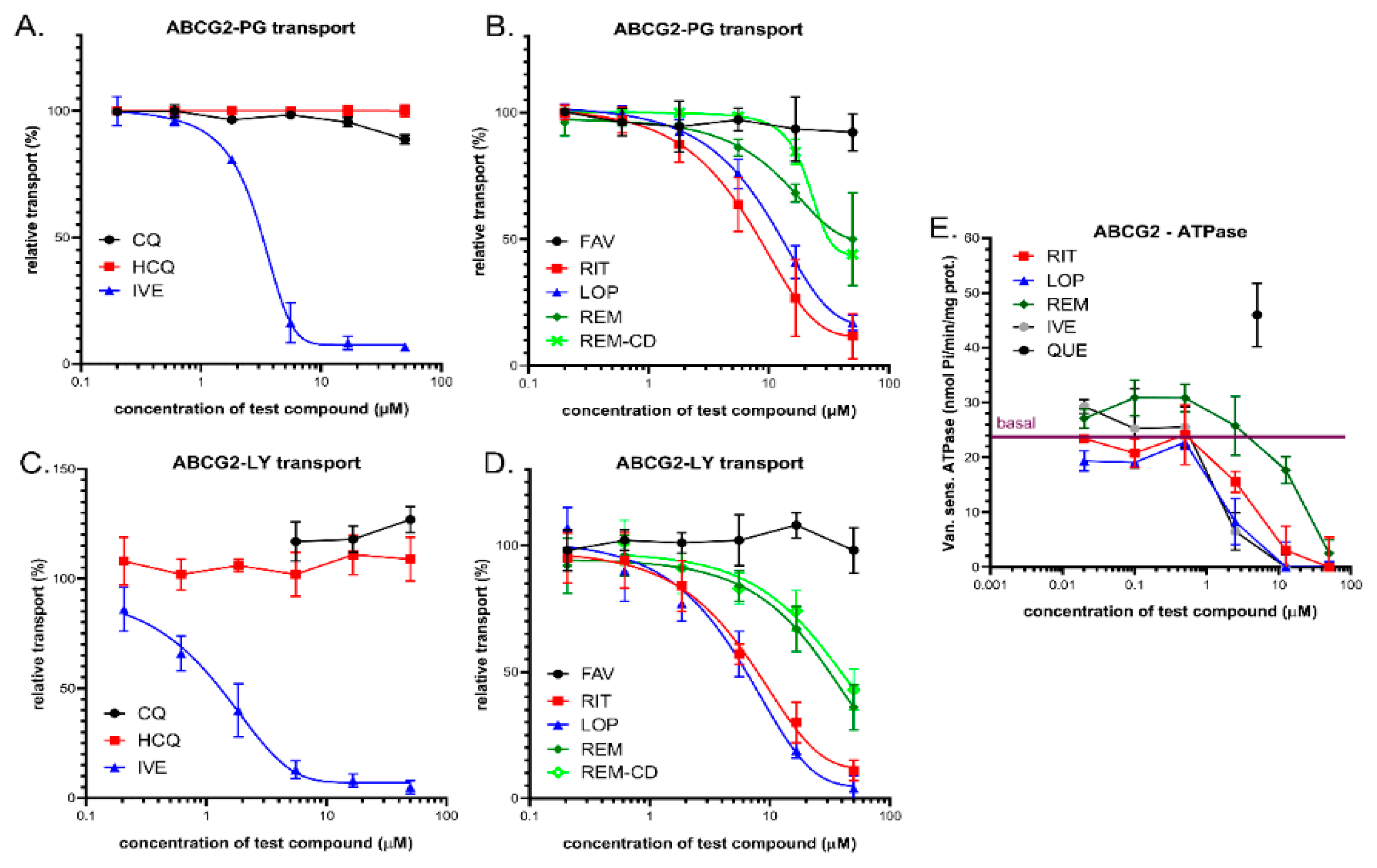
3.3. Interaction of Anti-COVID-19 Drug Candidates with ABCG2
3.3.1. Transport Measurements in Intact Human Cells—PLB/ABCG2 and HeLa/ABCG2
3.3.2. Vesicular Transport Studies in HEK/ABCG2 Membrane Vesicles
3.3.3. ABCG2-ATPase Activity Measurements in Sf9 Membranes
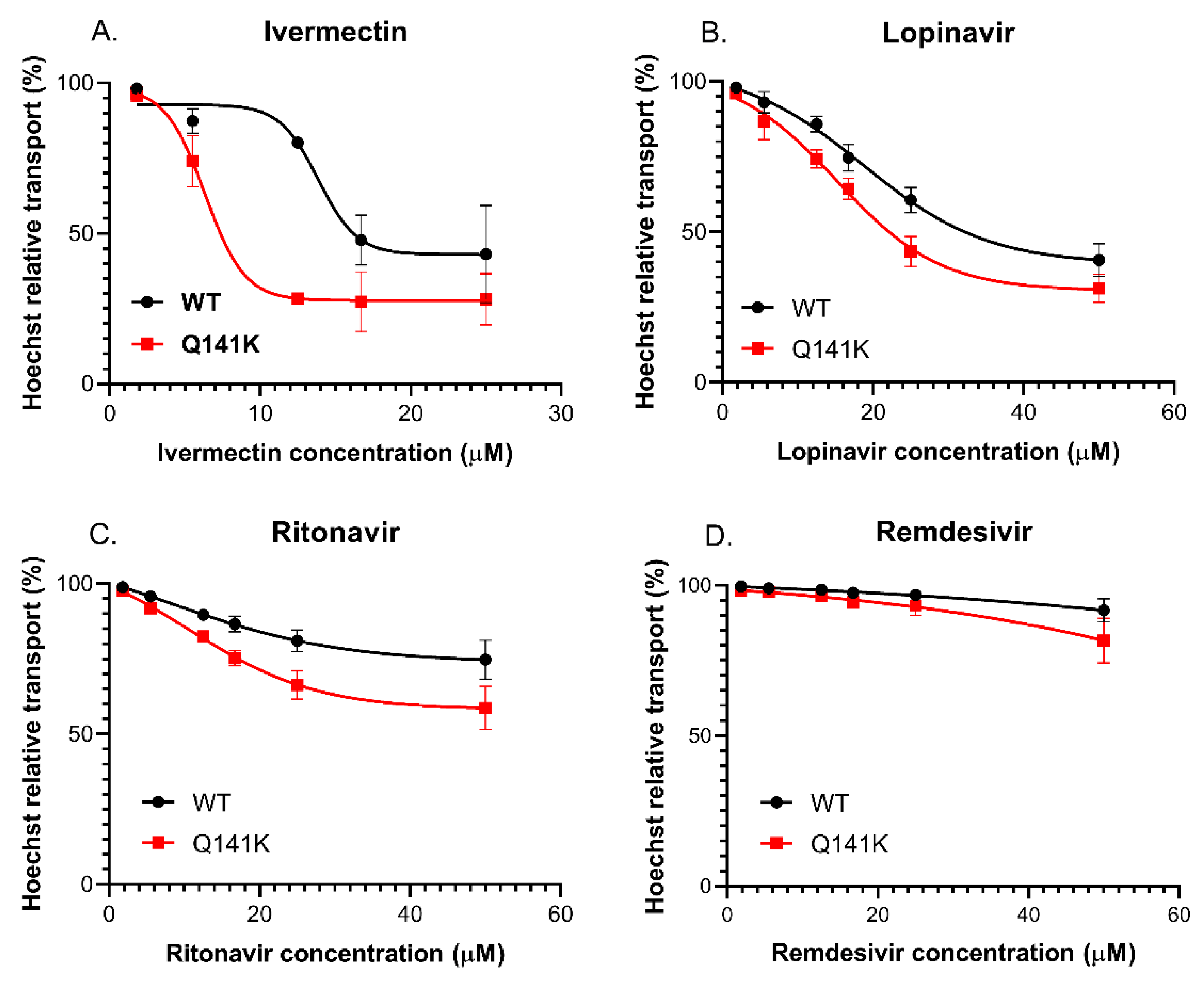
3.3.4. Effect of the Q141K-ABCG2 Polymorphism on the Inhibitory Potential of the Test Drugs
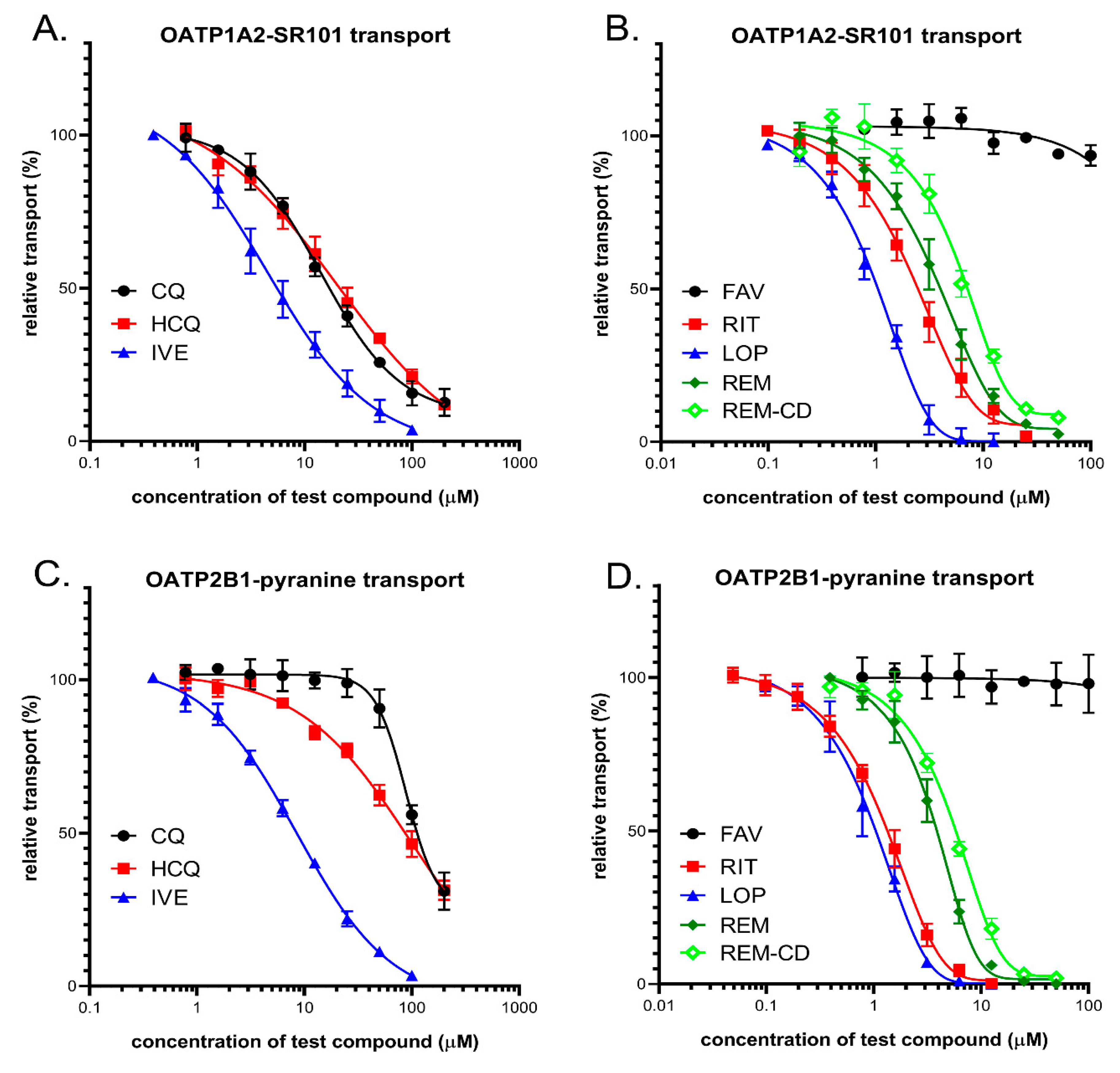
3.4. Interaction of Anti-COVID-19 Candidates with OATP1A2 and OATP2B1 Transporters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Singh, H.; Chauhan, P.; Kakkar, A.K. Hydroxychloroquine for the treatment and prophylaxis of COVID-19: The journey so far and the road ahead. Eur. J. Pharmacol. 2020, 173717. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.F.; Kovacs, R.J.; Stecker, E.C. Cardiology Magazine. Available online: https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19 (accessed on 20 November 2020).
- Roden, D.M.; Harrington, R.A.; Poppas, A.; Russo, A.M. Considerations for Drug Interactions on QTc in Exploratory COVID-19 Treatment. Circulation 2020, 141, e906–e907. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H.; Smit, J.J.; van Tellingen, O.; Beijnen, J.H.; Wagenaar, E.; van Deemter, L.; Mol, C.A.; van der Valk, M.A.; Robanus-Maandag, E.C.; te Riele, H.P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 1994, 77, 491–502. [Google Scholar] [CrossRef]
- Mealey, K.L.; Bentjen, S.A.; Gay, J.M.; Cantor, G.H. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics 2001, 11, 727–733. [Google Scholar] [CrossRef]
- Lespine, A.; Dupuy, J.; Orlowski, S.; Nagy, T.; Glavinas, H.; Krajcsi, P.; Alvinerie, M. Interaction of ivermectin with multidrug resistance proteins (MRP1, 2 and 3). Chem. Biol. Interact. 2006, 159, 169–179. [Google Scholar] [CrossRef]
- Pouliot, J.F.; L’Heureux, F.; Liu, Z.; Prichard, R.K.; Georges, E. Reversal of P-glycoprotein-associated multidrug resistance by ivermectin. Biochem. Pharmacol. 1997, 53, 17–25. [Google Scholar] [CrossRef]
- Didier, A.; Loor, F. The abamectin derivative ivermectin is a potent P-glycoprotein inhibitor. Anticancer. Drugs 1996, 7, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, K.; Sivakumaran, H.; Heaton, S.; Harrich, D.; Jans, D. Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and Dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, L.; Pinkham, C.; Baer, A.; Amaya, M.; Narayanan, A.; Wagstaff, K.M.; Jans, D.A.; Kehn-Hall, K. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antivir. Res. 2013, 100, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020, 177, 104760. [Google Scholar] [CrossRef]
- Bray, M.; Rayner, C.; Noël, F.; Jans, D.; Wagstaff, K. Ivermectin and COVID-19: A report in Antiviral Research, widespread interest, an FDA warning, two letters to the editor and the authors’ responses. Antivir. Res. 2020, 178, 104805. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Chaccour, C.; Hammann, F.; Ramón-García, S.; Rabinovich, N.R. Ivermectin and COVID-19: Keeping Rigor in Times of Urgency. Am. J. Trop. Med. Hyg. 2020, 102, 1156–1157. [Google Scholar] [CrossRef]
- Uzunova, K.; Filipova, E.; Pavlova, V.; Vekov, T. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed. Pharmacother. 2020, 131, 110668. [Google Scholar] [CrossRef]
- Arshad, U.; Pertinez, H.; Box, H.; Tatham, L.; Rajoli, R.K.R.; Curley, P.; Neary, M.; Sharp, J.; Liptrott, N.J.; Valentijn, A.; et al. Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics. Clin. Pharmacol. Ther. 2020, 108, 775–790. [Google Scholar] [CrossRef]
- Weiss, J.; Rose, J.; Storch, C.H.; Ketabi-Kiyanvash, N.; Sauer, A.; Haefeli, W.E.; Efferth, T. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J. Antimicrob. Chemother. 2007, 59, 238–245. [Google Scholar] [CrossRef]
- Martinec, O.; Huliciak, M.; Staud, F.; Cecka, F.; Vokral, I.; Cerveny, L. Anti-HIV and Anti-Hepatitis C Virus Drugs Inhibit P-Glycoprotein Efflux Activity in Caco-2 Cells and Precision-Cut Rat and Human Intestinal Slices. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Vaccher, E.; Sandron, S.; Sartor, I.; Tirelli, U.; Innocenti, F.; Toffoli, G. Lopinavir-ritonavir dramatically affects the pharmacokinetics of irinotecan in HIV patients with Kaposi’s sarcoma. Clin. Pharmacol. Ther. 2008, 83, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Pal, D.; Mitra, A.K. Both P-gp and MRP2 mediate transport of Lopinavir, a protease inhibitor. Int. J. Pharm. 2007, 339, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Janneh, O.; Jones, E.; Chandler, B.; Owen, A.; Khoo, S.H. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J. Antimicrob. Chemother. 2007, 60, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Zhang, Y.; Unadkat, J.D.; Mao, Q. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2). J. Pharmacol. Exp. Ther. 2004, 310, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Bierman, W.F.W.; Scheffer, G.L.; Schoonderwoerd, A.; Jansen, G.; van Agtmael, M.A.; Danner, S.A.; Scheper, R.J. Protease inhibitors atazanavir, lopinavir and ritonavir are potent blockers, but poor substrates, of ABC transporters in a broad panel of ABC transporter-overexpressing cell lines. J. Antimicrob. Chemother. 2010, 65, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-J.; Toots, M.; Lee, S.; Lee, M.-E.; Ludeke, B.; Luczo, J.M.; Ganti, K.; Cox, R.M.; Sticher, Z.M.; Edpuganti, V.; et al. Orally Efficacious Broad-Spectrum Ribonucleoside Analog Inhibitor of Influenza and Respiratory Syncytial Viruses. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef]
- Yang, K. What Do We Know About Remdesivir Drug Interactions? Clin. Transl. Sci. 2020, 13, 842–844. [Google Scholar] [CrossRef]
- Szakács, G.; Váradi, A.; Ozvegy-Laczka, C.; Sarkadi, B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov. Today 2008, 13, 379–393. [Google Scholar] [CrossRef]
- Takada, T.; Ichida, K.; Matsuo, H.; Nakayama, A.; Murakami, K.; Yamanashi, Y.; Kasuga, H.; Shinomiya, N.; Suzuki, H. ABCG2 dysfunction increases serum uric acid by decreased intestinal urate excretion. Nucleosides Nucleotides Nucleic Acids 2014, 33, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sarkadi, B.; Homolya, L.; Szakács, G.; Váradi, A. Human Multidrug Resistance ABCB and ABCG Transporters: Participation in a Chemoimmunity Defense System. Physiol. Rev. 2006, 1179–1236. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, S.; Dutheil, F.; Weaver, R.J.; Chassoux, F.; Daumas-Duport, C.; Couraud, P.-O.; Scherrmann, J.-M.; De Waziers, I.; Declèves, X. ABC transporters, cytochromes P450 and their main transcription factors: Expression at the human blood-brain barrier. J. Neurochem. 2008, 107, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Kamiie, J.; Ohtsuki, S.; Iwase, R.; Ohmine, K.; Katsukura, Y.; Yanai, K.; Sekine, Y.; Uchida, Y.; Ito, S.; Terasaki, T. Quantitative atlas of membrane transporter proteins: Development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm. Res. 2008, 25, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Daood, M.; Tsai, C.; Ahdab-Barmada, M.; Watchko, J.F. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics 2008, 39, 211–218. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Gui, C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 2008, 38, 778–801. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Stieger, B. The SLCO (former SLC21) superfamily of transporters. Mol. Asp. Med. 2013, 34, 396–412. [Google Scholar] [CrossRef]
- Shitara, Y.; Maeda, K.; Ikejiri, K.; Yoshida, K.; Horie, T.; Sugiyama, Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: Their roles in hepatic clearance and intestinal absorption. Biopharm. Drug Dispos. 2013, 34, 45–78. [Google Scholar] [CrossRef]
- Urquhart, B.L.; Kim, R.B. Blood-brain barrier transporters and response to CNS-active drugs. Eur. J. Clin. Pharmacol. 2009, 65, 1063–1070. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, Z.; Tay-Sontheimer, J.; Levy, R.H.; Ragueneau-Majlessi, I. Intestinal Drug Interactions Mediated by OATPs: A Systematic Review of Preclinical and Clinical Findings. J. Pharm. Sci. 2017, 106, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Kovacsics, D.; Patik, I.; Özvegy-Laczka, C. The role of organic anion transporting polypeptides in drug absorption, distribution, excretion and drug-drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.; Türk, D.; Telbisz, Á.; Kucsma, N.; Horváth, T.; Szakács, G.; Homolya, L.; Sarkadi, B.; Várady, G. A new fluorescent dye accumulation assay for parallel measurements of the ABCG2, ABCB1 and ABCC1 multidrug transporter functions. PLoS ONE 2018, 13, e0190629. [Google Scholar] [CrossRef] [PubMed]
- Zámbó, B.; Mózner, O.; Bartos, Z.; Török, G.; Várady, G.; Telbisz, Á.; Homolya, L.; Orbán, T.I.; Sarkadi, B. Cellular expression and function of naturally occurring variants of the human ABCG2 multidrug transporter. Cell. Mol. Life Sci. 2020, 77, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Sarkadi, B.; Bauzon, D.; Huckle, W.R.; Earp, H.S.; Berry, A.; Suchindran, H.; Price, E.M.; Olson, J.C.; Boucher, R.C.; Scarborough, G.A. Biochemical characterization of the cystic fibrosis transmembrane conductance regulator in normal and cystic fibrosis epithelial cells. J. Biol. Chem. 1992, 267, 2087–2095. [Google Scholar] [CrossRef]
- Patik, I.; Székely, V.; Német, O.; Szepesi, Á.; Kucsma, N.; Várady, G.; Szakács, G.; Bakos, É.; Özvegy-Laczka, C. Identification of novel cell-impermeant fluorescent substrates for testing the function and drug interaction of Organic Anion-Transporting Polypeptides, OATP1B1/1B3 and 2B1. Sci. Rep. 2018, 8, 2630. [Google Scholar] [CrossRef] [PubMed]
- Ozvegy, C.; Litman, T.; Szakács, G.; Nagy, Z.; Bates, S.; Váradi, A.; Sarkadi, B. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem. Biophys. Res. Commun. 2001, 285, 111–117. [Google Scholar] [CrossRef]
- Telbisz, A.; Müller, M.; Ozvegy-Laczka, C.; Homolya, L.; Szente, L.; Váradi, A.; Sarkadi, B. Membrane cholesterol selectively modulates the activity of the human ABCG2 multidrug transporter. Biochim. Biophys. Acta 2007, 1768, 2698–2713. [Google Scholar] [CrossRef]
- Ozvegy, C.; Váradi, A.; Sarkadi, B. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation. J. Biol. Chem. 2002, 277, 47980–47990. [Google Scholar] [CrossRef]
- Bakos, É.; Német, O.; Patik, I.; Kucsma, N.; Várady, G.; Szakács, G.; Özvegy-Laczka, C. A novel fluorescence-based functional assay for human OATP1A2 and OATP1C1 identifies interaction between third-generation P-gp inhibitors and OATP1A2. FEBS J. 2020, 287, 2468–2485. [Google Scholar] [CrossRef]
- Székely, V.; Patik, I.; Ungvári, O.; Telbisz, Á.; Szakács, G.; Bakos, É.; Özvegy-Laczka, C. Fluorescent probes for the dual investigation of MRP2 and OATP1B1 function and drug interactions. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 151, 105395. [Google Scholar] [CrossRef] [PubMed]
- Homolya, L.; Holló, Z.; Müller, M.; Mechetner, E.B.; Sarkadi, B. A new method for quantitative assessment of P-glycoprotein-related multidrug resistance in tumour cells. Br. J. Cancer 1996, 73, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Hooiveld, G.J.E.J.; Heegsma, J.; van Montfoort, J.E.; Jansen, P.L.M.; Meijer, D.K.F.; Müller, M. Stereoselective transport of hydrophilic quaternary drugs by human MDR1 and rat Mdr1b P-glycoproteins. Br. J. Pharmacol. 2002, 135, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Herédi-Szabó, K.; Palm, J.E.; Andersson, T.B.; Pál, Á.; Méhn, D.; Fekete, Z.; Beéry, E.; Jakab, K.T.; Jani, M.; Krajcsi, P. A P-gp vesicular transport inhibition assay—optimization and validation for drug-drug interaction testing. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 49, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Holló, Z.; Homolya, L.; Hegedûs, T.; Müller, M.; Szakács, G.; Jakab, K.; Antal, F.; Sarkadi, B. Parallel functional and immunological detection of human multidrug resistance proteins, P-glycoprotein and MRP1. Anticancer Res. 1998, 18, 2981–2987. [Google Scholar] [PubMed]
- Slot, A.J.; Wise, D.D.; Deeley, R.G.; Monks, T.J.; Cole, S.P.C. Modulation of human multidrug resistance protein (MRP) 1 (ABCC1) and MRP2 (ABCC2) transport activities by endogenous and exogenous glutathione-conjugated catechol metabolites. Drug Metab. Dispos. 2008, 36, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Strouse, J.J.; Ivnitski-Steele, I.; Waller, A.; Young, S.M.; Perez, D.; Evangelisti, A.M.; Ursu, O.; Bologa, C.G.; Carter, M.B.; Salas, V.M.; et al. Fluorescent substrates for flow cytometric evaluation of efflux inhibition in ABCB1, ABCC1, and ABCG2 transporters. Anal. Biochem. 2013, 437, 77–87. [Google Scholar] [CrossRef]
- Telford, W.G.; Bradford, J.; Godfrey, W.; Robey, R.W.; Bates, S.E. Side population analysis using a violet-excited cell-permeable DNA binding dye. Stem Cells 2007, 25, 1029–1036. [Google Scholar] [CrossRef]
- Boesch, M.; Reimer, D.; Rumpold, H.; Zeimet, A.G.; Sopper, S.; Wolf, D. DyeCycle Violet used for side population detection is a substrate of P-glycoprotein. Cytom. A 2012, 81, 517–522. [Google Scholar] [CrossRef]
- Nerada, Z.; Hegyi, Z.; Szepesi, Á.; Tóth, S.; Hegedüs, C.; Várady, G.; Matula, Z.; Homolya, L.; Sarkadi, B.; Telbisz, Á. Application of fluorescent dye substrates for functional characterization of ABC multidrug transporters at a single cell level. Cytom. A 2016, 89, 826–834. [Google Scholar] [CrossRef]
- Zong, Y.; Zhou, S.; Fatima, S.; Sorrentino, B.P. Expression of mouse Abcg2 mRNA during hematopoiesis is regulated by alternative use of multiple leader exons and promoters. J. Biol. Chem. 2006, 281, 29625–29632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, N.; van den Heuvel, J.J.M.W.; Koenderink, J.B.; Kidron, H. Transmembrane Domain Single-Nucleotide Polymorphisms Impair Expression and Transport Activity of ABC Transporter ABCG2. Pharm. Res. 2017, 34, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Mózner, O.; Bartos, Z.; Zámbó, B.; Homolya, L.; Hegedűs, T.; Sarkadi, B. Cellular Processing of the ABCG2 Transporter-Potential Effects on Gout and Drug Metabolism. Cells 2019, 8, 1215. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, K.M.; Balimane, P.V.; Cho, S.K.; Eadon, M.; Edeki, T.; Hillgren, K.M.; Huang, S.-M.; Sugiyama, Y.; Weitz, D.; Wen, Y.; et al. International Transporter Consortium commentary on clinically important transporter polymorphisms. Clin. Pharmacol. Ther. 2013, 94, 23–26. [Google Scholar] [CrossRef]
- Huang, S.-M.; Zhang, L.; Giacomini, K.M. The International Transporter Consortium: A collaborative group of scientists from academia, industry, and the FDA. Clin. Pharmacol. Ther. 2010, 87, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Geyer, J.; Gavrilova, O.; Petzinger, E. Brain penetration of ivermectin and selamectin in mdr1a,b P-glycoprotein- and bcrp- deficient knockout mice. J. Vet. Pharmacol. Ther. 2009, 32, 87–96. [Google Scholar] [CrossRef]
- Gao, B.; Hagenbuch, B.; Kullak-Ublick, G.A.; Benke, D.; Aguzzi, A.; Meier, P.J. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J. Pharmacol. Exp. Ther. 2000, 294, 73–79. [Google Scholar]
- Billington, S.; Salphati, L.; Hop, C.E.C.A.; Chu, X.; Evers, R.; Burdette, D.; Rowbottom, C.; Lai, Y.; Xiao, G.; Humphreys, W.G.; et al. Interindividual and Regional Variability in Drug Transporter Abundance at the Human Blood-Brain Barrier Measured by Quantitative Targeted Proteomics. Clin. Pharmacol. Ther. 2019, 106, 228–237. [Google Scholar] [CrossRef]
- Annaert, P.; Ye, Z.W.; Stieger, B.; Augustijns, P. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica 2010, 40, 163–176. [Google Scholar] [CrossRef]
- Tupova, L.; Hirschmugl, B.; Sucha, S.; Pilarova, V.; Székely, V.; Bakos, É.; Novakova, L.; Özvegy-Laczka, C.; Wadsack, C.; Ceckova, M. Interplay of drug transporters P-glycoprotein (MDR1), MRP1, OATP1A2 and OATP1B3 in passage of maraviroc across human placenta. Biomed. Pharmacother. 2020, 129, 110506. [Google Scholar] [CrossRef] [PubMed]
- Kis, O.; Zastre, J.A.; Ramaswamy, M.; Bendayan, R. pH dependence of organic anion-transporting polypeptide 2B1 in Caco-2 cells: Potential role in antiretroviral drug oral bioavailability and drug-drug interactions. J. Pharmacol. Exp. Ther. 2010, 334, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Hartkoorn, R.C.; Kwan, W.S.; Shallcross, V.; Chaikan, A.; Liptrott, N.; Egan, D.; Sora, E.S.; James, C.E.; Gibbons, S.; Bray, P.G.; et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet. Genom. 2010, 20, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Karlgren, M.; Vildhede, A.; Norinder, U.; Wisniewski, J.R.; Kimoto, E.; Lai, Y.; Haglund, U.; Artursson, P. Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): Influence of protein expression on drug-drug interactions. J. Med. Chem. 2012, 55, 4740–4763. [Google Scholar] [CrossRef] [PubMed]
- Hubeny, A.; Keiser, M.; Oswald, S.; Jedlitschky, G.; Kroemer, H.K.; Siegmund, W.; Grube, M. Expression of Organic Anion Transporting Polypeptide 1A2 in Red Blood Cells and Its Potential Impact on Antimalarial Therapy. Drug Metab. Dispos. 2016, 44, 1562–1568. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, L.; Chan, T.; Lu, X.; Shen, W.; Madigan, M.C.; Gillies, M.C.; Zhou, F. Chloroquine and Hydroxychloroquine Are Novel Inhibitors of Human Organic Anion Transporting Polypeptide 1A2. J. Pharm. Sci. 2016, 105, 884–890. [Google Scholar] [CrossRef]
- Cao, Y.-C.; Deng, Q.-X.; Dai, S.-X. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Med. Infect. Dis. 2020, 35, 101647. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Kebriaei, R.; Dresser, L.D. Remdesivir: Review of Pharmacology, Pre-clinical Data, and Emerging Clinical Experience for COVID-19. Pharmacotherapy 2020, 40, 659–671. [Google Scholar] [CrossRef]
| Potential Anti-COVID-19 Compounds | Mechanism of Action |
|---|---|
| chloroquine | Antimalarial—endosomal pH increase |
| hydroxychloroquine | Antimalarial—endosomal pH increase |
| ivermectin | Antiparasitic—glutamate-gated chloride channel and a GABA receptor inhibitor |
| lopinavir | (HIV) protease inhibitor |
| ritonavir | (HIV) protease inhibitor |
| remdesivir | Viral RNA-polymerase inhibitor |
| favipiravir | Viral RNA-polymerase inhibitor |
| Estimated Transporter Inhibition—IC50 (µM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ABCB1 | ABCC1 | ABCG2 | OATP Cellular Assays | |||||
| Potential anti-COVID-19 compounds | cellular assay | vesicular assay | cellular assay | vesicular assay | cellular assay | vesicular assay | OATP1A2 | OATP2B1 |
| chloroquine | - | - | - | - | - | - | 17.0 | 119 |
| hydroxychloroquine | - | - | - | - | - | - | 18.9 | 84 |
| ivermectin | 0.6 | 0.3 | 3.3 | 13.3 | 3.1 | 1.1 | 5.2 | 8.6 |
| lopinavir | 6.3 | 0.6 | 10.7 | 10 | 13.1 | 4.2 | 1.5 | 1.0 |
| ritonavir | 8.4 | 0.3 | 7.7 | - | 8.3 | 7.5 | 2.3 | 1.4 |
| remdesivir | - | >20 | - | -* | >50 | >50 | 3.8 | 3.8 |
| remdesivir-SBECD | - | >20 | - | NA | >50 | >50 | 6.1 | 5.6 |
| favipiravir | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telbisz, Á.; Ambrus, C.; Mózner, O.; Szabó, E.; Várady, G.; Bakos, É.; Sarkadi, B.; Özvegy-Laczka, C. Interactions of Potential Anti-COVID-19 Compounds with Multispecific ABC and OATP Drug Transporters. Pharmaceutics 2021, 13, 81. https://doi.org/10.3390/pharmaceutics13010081
Telbisz Á, Ambrus C, Mózner O, Szabó E, Várady G, Bakos É, Sarkadi B, Özvegy-Laczka C. Interactions of Potential Anti-COVID-19 Compounds with Multispecific ABC and OATP Drug Transporters. Pharmaceutics. 2021; 13(1):81. https://doi.org/10.3390/pharmaceutics13010081
Chicago/Turabian StyleTelbisz, Ágnes, Csilla Ambrus, Orsolya Mózner, Edit Szabó, György Várady, Éva Bakos, Balázs Sarkadi, and Csilla Özvegy-Laczka. 2021. "Interactions of Potential Anti-COVID-19 Compounds with Multispecific ABC and OATP Drug Transporters" Pharmaceutics 13, no. 1: 81. https://doi.org/10.3390/pharmaceutics13010081
APA StyleTelbisz, Á., Ambrus, C., Mózner, O., Szabó, E., Várady, G., Bakos, É., Sarkadi, B., & Özvegy-Laczka, C. (2021). Interactions of Potential Anti-COVID-19 Compounds with Multispecific ABC and OATP Drug Transporters. Pharmaceutics, 13(1), 81. https://doi.org/10.3390/pharmaceutics13010081







