The Neonatal and Juvenile Pig in Pediatric Drug Discovery and Development
Abstract
1. Introduction
2. Anatomical, Physiological and Developmental Similarities between Pigs and Humans
2.1. Characterization of the Pig As a Relevant Animal Model
2.2. The Pig in Pediatric Research
2.2.1. Pig and Human Postnatal Development
| Organ System | Feature | Similarity | References |
|---|---|---|---|
| Gastrointestinal | Physiology of digestion | Very similar | [55] |
| Ontogeny of digestive enzymes | Similar in most cases (more than the rat) | [56] | |
| Neonatal gastric pH | Different: pigs show adult-like values early in life (5 days of age) and humans reach that point at 2 years of age | [35,57,58] | |
| Gastric emptying | Maturation of gastric emptying with age has not been established in pigs. Prolonged emptying is expected in newborn pigs, as observed in humans | [56] | |
| Intestinal transit | Similar: longer in neonates than juvenile/adults | [56,59] | |
| Intestinal surface | Similar: smaller than juvenile/adults, leads to similar nutrient absorption | [40,60] | |
| Microbiome | Similar: mainly consists of Firmicutes and Bacteriodetes phyla | [61] | |
| Liver | Similar relation to body weight in adults (about 2%) | [35] | |
| Slightly higher ratio in human (around 5%) than minipig (3%) neonates | |||
| Cardiovascular | Drainage | Different | [40] |
| Main central vessels | Different relative importance | ||
| Cardiac output | Different | ||
| Cardiac myocyte maturation | Similar (compared to other species) | [62] | |
| Serum proteins (albumin and globulins) | Different in neonatal pigs and humans, but even at infant stages | [40,63] | |
| Central nervous | Anatomical complexity | Similar | [28,35] |
| Distribution of grey and white matter | Similar | ||
| Brain growth pattern | Similar | ||
| Renal | Nephrogenesis | Different: completes after weaning (3 weeks of age) in pig and 34–36 weeks gestational age in humans | [64] |
| Glomerular filtration rate | Similar maturation: adult levels at 8 weeks (pig) and 1 year (human) of age | [65] | |
| Effective renal plasma flow | Within the same range in growing pigs and children | [66] | |
| Urinary pH | [66] | ||
| Immune | Immune genes | High similarity (>80%) | [67,68] |
| Skeletal and neuromuscular | Development | Different: faster in pigs, in which locomotion patterns reach mature levels as early as 8 h after birth | [69] |
| Respiratory | Anatomy and histology | Similar | [70] |
| Maturation | Faster | [36] | |
| Alveoli multiplication | Earlier in pigs | [71] |
2.2.2. Hepatic Drug Metabolism in the Neonatal and Juvenile Pig
2.2.3. PBPK Models in the Neonatal and Juvenile Pig
3. Fields of Application
3.1. History of Pharmacological Studies in Pigs
3.2. Current Pharmacological Research in Pigs
3.2.1. Nonclinical Safety Models for Small and Large Molecules
3.2.2. Examples of Pig Models in Condition-Specific Drug Development Research
Neonatal Asphyxia and Therapeutic Hypothermia: Effects on Drug PK
The Special Case of the Neonatal Preterm Pig
- Respiratory Pathologies and Lung Development
- 2.
- Digestive Alterations
Other Relevant Models from a Clinical Perspective
4. Pitfalls, Limitations and Unexplored Areas of the Model
4.1. Imbalance between Disease Model and Drug Development
4.2. Differences in Organ Maturation
4.3. Limitations of the Current Pig Proteome
4.4. Practical/Logistical Disadvantages
5. Conclusions and Expert Opinion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anker, J.V.D.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58, S10–S25. [Google Scholar] [CrossRef]
- Germovsek, E.; Barker, C.I.S.; Sharland, M.; Standing, J.F. Scaling clearance in paediatric pharmacokinetics: All models are wrong, which are useful? Br. J. Clin. Pharmacol. 2017, 83, 777–790. [Google Scholar] [CrossRef]
- Stillhart, C.; Vučićević, K.; Augustijns, P.; Basit, A.W.; Batchelor, H.; Flanagan, T.R.; Gesquiere, I.; Greupink, R.; Keszthelyi, D.; Koskinen, M.; et al. Impact of gastrointestinal physiology on drug absorption in special populations—An UNGAP review. Eur. J. Pharm. Sci. 2020, 147, 105280. [Google Scholar] [CrossRef] [PubMed]
- Van Groen, B.D.; Krekels, E.H.J.; Mooij, M.G.; Van Duijn, E.; Vaes, W.H.J.; Windhorst, A.D.; Van Rosmalen, J.; Hartman, S.J.F.; Hendrikse, N.H.; Koch, B.C.P.; et al. The Oral Bioavailability and Metabolism of Midazolam in Stable Critically Ill Children: A Pharmacokinetic Microtracing Study. Clin. Pharmacol. Ther. 2021, 109, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.M.; the International Neonatal Consortium (INC); Benjamin, D.; Barrett, J.S.; Allegaert, K.; Portman, R.; Davis, J.M.; Turner, M.A. Safety, dosing, and pharmaceutical quality for studies that evaluate medicinal products (including biological products) in neonates. Pediatr. Res. 2016, 81, 692–711. [Google Scholar] [CrossRef] [PubMed]
- Oyaert, M.; Spriet, I.; Allegaert, K.; Smits, A.; Vanstraelen, K.; Peersman, N.; Wauters, J.; Verhaegen, J.; Vermeersch, P.; Pauwels, S. Factors Impacting Unbound Vancomycin Concentrations in Different Patient Populations. Antimicrob. Agents Chemother. 2015, 59, 7073–7079. [Google Scholar] [CrossRef]
- Allegaert, K.; Anker, J.V.D. Ontogeny of Phase I Metabolism of Drugs. J. Clin. Pharmacol. 2019, 59, S33–S41. [Google Scholar] [CrossRef]
- Cristea, S.; Krekels, E.H.J.; Rostami-Hodjegan, A.; Allegaert, K.; Knibbe, C.A.J. The Influence of Drug Properties and Ontogeny of Transporters on Pediatric Renal Clearance through Glomerular Filtration and Active Secretion: A Simulation-Based Study. AAPS J. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Cheung, K.W.K.; Van Groen, B.D.; Spaans, E.; Van Borselen, M.D.; De Bruijn, A.C.; Simons-Oosterhuis, Y.; Tibboel, D.; Samsom, J.N.; Verdijk, R.M.; Smeets, B.; et al. A Comprehensive Analysis of Ontogeny of Renal Drug Transporters: mRNA Analyses, Quantitative Proteomics, and Localization. Clin. Pharmacol. Ther. 2019, 106, 1083–1092. [Google Scholar] [CrossRef]
- De Cock, R.F.W.; Allegaert, K.; Brussee, J.M.; Sherwin, C.M.T.; Mulla, H.; De Hoog, M.; Anker, J.N.V.D.; Danhof, M.; Knibbe, C.A.J. Simultaneous Pharmacokinetic Modeling of Gentamicin, Tobramycin and Vancomycin Clearance from Neonates to Adults: Towards a Semi-physiological Function for Maturation in Glomerular Filtration. Pharm. Res. 2014, 31, 2643–2654. [Google Scholar] [CrossRef]
- Aladjem, M.; Kaplinsky, C.; Wolfish, N.; Laufer, Y.; Halkin, H. Maturation of Renal Tubular Transport of Digoxin. Pediatr. Res. 1981, 15, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Simons, S.; Tibboel, D.; Krekels, E.H.; Knibbe, C.A.; Anker, J.N.V.D. Non-maturational covariates for dynamic systems pharmacology models in neonates, infants, and children: Filling the gaps beyond developmental pharmacology. Eur. J. Pharm. Sci. 2017, 109, S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Mulla, H. Understanding Developmental Pharmacodynamics. Pediatr. Drugs 2010, 12, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Rakhade, S.N.; Jensen, F.E. Epileptogenesis in the immature brain: Emerging mechanisms. Nat. Rev. Neurol. 2009, 5, 380–391. [Google Scholar] [CrossRef]
- Marshall, J.A.; Kearns, G.L. Developmental pharmacodynamics of cyclosporine. Clin. Pharmacol. Ther. 1999, 66, 66–75. [Google Scholar] [CrossRef]
- Krasemann, T.; Bente, K.; Burkhardtsmaier, G. The corrected QT interval in 24 h ECGs in neonates. Clin. Res. Cardiol. 2010, 99, 309–314. [Google Scholar] [CrossRef]
- Barrow, P.; Schmitt, G. Juvenile Nonclinical Safety Studies in Support of Pediatric Drug Development. Adv. Struct. Saf. Stud. 2017, 1641, 25–67. [Google Scholar] [CrossRef]
- Anker, J.V.D.; McCune, S.; Annaert, P.; Baer, G.R.; Mulugeta, Y.; Abdelrahman, R.; Wu, K.; Krudys, K.M.; Fisher, J.; Slikker, W.; et al. Approaches to Dose Finding in Neonates, Illustrating the Variability between Neonatal Drug Development Programs. Pharmaceutics 2020, 12, 685. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/paediatric-medicines/paediatric-regulation (accessed on 23 October 2020).
- Food and Drug Administration. Draft Guidance: Demonstrating Substantial Evidence of Effectiveness for Human Drug and Biological Products. Guidance for Industry. Available online: https://www.fda.gov/media/133660/download (accessed on 23 October 2020).
- Tassinari, M.S.; Benson, K.; Elayan, I.; Espandiari, P.; Davis-Bruno, K. Juvenile animal studies and pediatric drug development retrospective review: Use in regulatory decisions and labeling. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2011, 92, 261–265. [Google Scholar] [CrossRef]
- Baldrick, P. Juvenile Animal Testing: Assessing Need and Use in the Drug Product Label. Ther. Innov. Regul. Sci. 2018, 52, 641–648. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/results-juvenile-animal-studies-jas-impact-anti-cancer-medicine-development-use-children_en.pdf (accessed on 23 October 2020).
- Burrin, D.; Sangild, P.T.; Stoll, B.; Thymann, T.; Buddington, R.; Marini, J.; Olutoye, O.; Shulman, R.J. Translational Advances in Pediatric Nutrition and Gastroenterology: New Insights from Pig Models. Annu. Rev. Anim. Biosci. 2020, 8, 321–354. [Google Scholar] [CrossRef] [PubMed]
- Barouxis, D.; Chalkias, A.; Syggelou, A.; Iacovidou, N.; Xanthos, T. Research in human resuscitation: What we learn from animals. J. Matern. Neonatal Med. 2012, 25, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.; Annaert, P.; Van Cruchten, S.; Allegaert, K. A Physiology-Based Pharmacokinetic Framework to Support Drug Development and Dose Precision During Therapeutic Hypothermia in Neonates. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Sciascia, Q.; Das, G.; Metges, C.C. Review: The pig as a model for humans: Effects of nutritional factors on intestinal function and health1. J. Anim. Sci. 2016, 94, 441–452. [Google Scholar] [CrossRef]
- Mudd, A.T.; Dilger, R. Early-Life Nutrition and Neurodevelopment: Use of the Piglet as a Translational Model. Adv. Nutr. 2017, 8, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.; Makin, A.; Herron, A.; Clubb, F., Jr.; Frazier, K. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Swindle, M.M.; Smith, A.C. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Butler, J.E.; Sun, J.; Wertz, N.; Sinkora, M. Antibody repertoire development in swine. Dev. Comp. Immunol. 2006, 30, 199–221. [Google Scholar] [CrossRef]
- Forster, R.; Bode, G.; Ellegaard, L.; Van Der Laan, J.W. The RETHINK project on minipigs in the toxicity testing of new medicines and chemicals: Conclusions and recommendations. J. Pharmacol. Toxicol. Methods 2010, 62, 236–242. [Google Scholar] [CrossRef]
- Ferenc, K.; Pietrzak, P.; Godlewski, M.M.; Piwowarski, J.; Kilianczyk, R.; Guilloteau, P.; Zabielski, R. Intrauterine growth retarded piglet as a model for humans-Studies on the perinatal development of the gut structure and function. Reprod. Biol. 2014, 14, 51–60. [Google Scholar] [CrossRef]
- Stricker-Krongrad, A.; Shoemake, C.R.; Bouchard, G.F. The Miniature Swine as a Model in Experimental and Translational Medicine. Toxicol. Pathol. 2016, 44, 612–623. [Google Scholar] [CrossRef]
- Van Peer, E.; Downes, N.; Casteleyn, C.; Van Ginneken, C.; Weeren, A.; Van Cruchten, S. Organ data from the developing Göttingen minipig: First steps towards a juvenile PBPK model. J. Pharmacokinet. Pharmacodyn. 2016, 43, 179–190. [Google Scholar] [CrossRef] [PubMed]
- ICH. Ich Guideline s11 on Nonclinical Safety Testing in Support of Development of Paediatric Pharmaceuticals; Committee for Medicinal Products for Human Use, 2020; Available online: https://www.ema.europa.eu/en/ich-guideline-s11-nonclinical-safety-testing-support-development-paediatric-pharmaceuticals-step-5vv (accessed on 18 October 2020).
- Summerfield, A.; Rziha, H.-J.; Saalmüller, A. Functional characterization of porcine CD4+ CD8+ extrathymic T lymphocytes. Cell. Immunol. 1996, 168, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, O. (Ed.) The minipig in toxicology. In Proceedings of the Satellite Symposium to Eurotox’97, Aarhus, Denmark; 1997. [Google Scholar]
- Suenderhauf, C.; Parrott, N. A Physiologically Based Pharmacokinetic Model of the Minipig: Data Compilation and Model Implementation. Pharm. Res. 2012, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gasthuys, E.; Vandecasteele, T.; De Bruyne, P.; Walle, J.V.; De Backer, P.; Cornillie, P.; Devreese, M.; Croubels, S. The potential use of piglets as human pediatric surrogate for preclinical pharmacokinetic and pharmacodynamic drug testing. Curr. Pharm. Des. 2016, 22, 4069–4085. [Google Scholar] [CrossRef]
- Baber, N. Guide to Paediatric Clinical Research. Br. J. Clin. Pharmacol. 2008, 65, 282. [Google Scholar] [CrossRef]
- Heining, P.; Ruysschaert, T. The Use of Minipig in Drug Discovery and Development. Toxicol. Pathol. 2016, 44, 467–473. [Google Scholar] [CrossRef]
- Van Peer, E.M.; Verbueken, E.; Saad, M.; Casteleyn, C.; Van Ginneken, C.J.; Van Cruchten, S. Ontogeny of CYP3A and P-Glycoprotein in the Liver and the Small Intestine of the Göttingen Minipig: An Immunohistochemical Evaluation. Basic Clin. Pharmacol. Toxicol. 2013, 114, 387–394. [Google Scholar] [CrossRef]
- Van Peer, E.M.; De Bock, L.; Boussery, K.; Van Bocxlaer, J.; Casteleyn, C.; Van Ginneken, C.; Van Cruchten, S. Age-related Differences in CYP3A Abundance and Activity in the Liver of the Göttingen Minipig. Basic Clin. Pharmacol. Toxicol. 2015, 117, 350–357. [Google Scholar] [CrossRef]
- Van Peer, E.; Jacobs, F.; Snoeys, J.; Van Houdt, J.; Pijpers, I.; Casteleyn, C.; Van Ginneken, C.; Van Cruchten, S. In vitro Phase I-and Phase II-Drug Metabolism in The Liver of Juvenile and Adult Göttingen Minipigs. Pharm. Res. 2017, 34, 750–764. [Google Scholar] [CrossRef]
- Crick, S.J.; Sheppard, M.N.; Ho, S.Y.; Gebstein, L.; Anderson, R.H. Anatomy of the pig heart: Comparisons with normal human cardiac structure. J. Anat. 1998, 193, 105–119. [Google Scholar] [CrossRef]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83. [Google Scholar] [CrossRef]
- Butler, J.E.; Lager, K.; Splichal, I.; Francis, D.; Kacskovics, I.; Sinkora, M.; Wertz, N.; Sun, J.; Zhao, Y.; Brown, W.; et al. The piglet as a model for B cell and immune system development. Veter-Immunol. Immunopathol. 2009, 128, 147–170. [Google Scholar] [CrossRef]
- Comstock, S.S.; Reznikov, E.A.; Contractor, N.; Donovan, S.M. Dietary Bovine Lactoferrin Alters Mucosal and Systemic Immune Cell Responses in Neonatal Piglets. J. Nutr. 2014, 144, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Valent, D.; Yeste, N.; Hernández-Castellano, L.E.; Arroyo, L.; Wu, W.; García-Contreras, C.; Vazquez-Gomez, M.; González-Bulnes, A.; Bendixen, E.; Bassols, A. SWATH-MS quantitative proteomic investigation of intrauterine growth restriction in a porcine model reveals sex differences in hippocampus development. J. Proteom. 2019, 204, 103391. [Google Scholar] [CrossRef] [PubMed]
- Gortner, L.; Shen, J.; Tutdibi, E. Sexual Dimorphism of Neonatal Lung Development. Klin. Pädiatrie 2013, 225, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Óvilo, C.; Gonzalez-Bulnes, A.; Benítez, R.; Ayuso, M.; Barbero, A.; Pérez-Solana, M.L.; Barragán, C.; Astiz, S.; Fernández, A.; López-Bote, C. Prenatal programming in an obese swine model: Sex-related effects of maternal energy restriction on morphology, metabolism and hypothalamic gene expression. Br. J. Nutr. 2014, 111, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Anadkat, J.S.; Kuzniewicz, M.W.; Chaudhari, B.P.; Cole, F.S.; Hamvas, A. Increased risk for respiratory distress among white, male, late preterm and term infants. J. Perinatol. 2012, 32, 780–785. [Google Scholar] [CrossRef]
- Spengler, D.; Rintz, N.; Krause, M.F. An Unsettled Promise: The Newborn Piglet Model of Neonatal Acute Respiratory Distress Syndrome (NARDS). Physiologic Data and Systematic Review. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Ziegler, A.L.; Gonzalez, L.; Blikslager, A.T. Large Animal Models: The Key to Translational Discovery in Digestive Disease Research. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 716–724. [Google Scholar] [CrossRef]
- Neal-Kluever, A.; Fisher, J.; Grylack, L.; Kakiuchi-Kiyota, S.; Halpern, W.G. Physiology of the Neonatal Gastrointestinal System Relevant to the Disposition of Orally Administered Medications. Drug Metab. Dispos. 2018, 47, 296–313. [Google Scholar] [CrossRef]
- Fallingborg, J.; Christensen, L.A.; Ingeman-Nielsen, M.; Jacobsen, B.A.; Abildgaard, K.; Rasmussen, H.H.; Rasmussen, S.N. Measurement of Gastrointestinal pH and Regional Transit Times in Normal Children. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, I.H.; Rademaker, C.M.A.; Schobben, A.F.A.M.; Anker, J.N.V.D. Guidelines on Paediatric Dosing on the Basis of Developmental Physiology and Pharmacokinetic Considerations. Clin. Pharmacokinet. 2006, 45, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.Y.; Sourial, M.; Hutson, J.M.; Southwell, B.R. Short-Term Interferential Transabdominal Electrical Stimulation Did Not Change Oral-Rectal Transit Time in Piglets. Neuromodul. Technol. Neural Interface 2018, 21, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, T.; Piedra, J.V.; Skrzypek, H.; Kazimierczak, W.; Szymanczyk, S.; Zabielski, R. Changes in pig small intestinal absorptive area during the first 14days of life. Livest. Sci. 2010, 133, 53–56. [Google Scholar] [CrossRef]
- Heinritz, S.N.; Mosenthin, R.; Weiss, E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef]
- Kim, M.Y.; Eiby, Y.A.; Lumbers, E.R.; Wright, L.L.; Gibson, K.J.; Barnett, A.C.; Lingwood, B.E. Effects of Glucocorticoid Exposure on Growth and Structural Maturation of the Heart of the Preterm Piglet. PLoS ONE 2014, 9, e93407. [Google Scholar] [CrossRef]
- Christoffersen, B.Ø.; Jensen, S.J.; Ludvigsen, T.P.; Nilsson, S.K.; Grossi, A.B.; Heegaard, P.M.H. Age- and Sex-Associated Effects on Acute-Phase Proteins in Göttingen Minipigs. Comp. Med. 2015, 65, 333–341. [Google Scholar]
- Friis, C. Postnatal development of the pig kidney: Ultrastucure of the glomerulus and the proximal tubule. J. Anat. 1980, 130, 513–526. [Google Scholar]
- De Cock, R.F.; Allegaert, K.; Schreuder, M.F.; Sherwin, C.M.; de Hoog, M.; van den Anker, J.N.; Danhof, M.; Knibbe, C.A.J. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin. Pharmacokinet. 2012, 51, 105–117. [Google Scholar] [CrossRef]
- Dhondt, L.; Croubels, S.; De Paepe, P.; Wallis, S.C.; Pandey, S.; Roberts, J.A.; Lipman, J.; De Cock, P.; Devreese, M. Conventional Pig as Animal Model for Human Renal Drug Excretion Processes: Unravelling the Porcine Renal Function by Use of a Cocktail of Exogenous Markers. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Dawson, H. A Comparative Assessment of the Pig, Mouse and Human Genomes; CRC Press: Boca Raton, FL, USA, 2011; pp. 323–342. [Google Scholar]
- Palermo, S.; Capra, E.; Torremorell, M.; Dolzan, M.; Davoli, R.; Haley, C.; Giuffra, E. Toll-like receptor 4genetic diversity among pig populations. Anim. Genet. 2009, 40, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Hole, C.V.; Goyens, J.; Prims, S.; Fransen, E.; Hernando, M.A.; Van Cruchten, S.; Aerts, P.; Van Ginneken, C. How innate is locomotion in precocial animals? A study on the early development of spatio-temporal gait variables and gait symmetry in piglets. J. Exp. Biol. 2017, 220, 2706–2716. [Google Scholar] [CrossRef] [PubMed]
- Judge, E.P.; Hughes, J.M.L.; Egan, J.J.; Maguire, M.; Molloy, E.L.; O’Dea, S. Anatomy and Bronchoscopy of the Porcine Lung. A Model for Translational Respiratory Medicine. Am. J. Respir. Cell Mol. Biol. 2014, 51, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Haworth, S.G.; Hislop, A.A. Adaptation of the pulmonary circulation to extra-uterine life in the pig and its relevance to the human infant. Cardiovasc. Res. 1981, 15, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Achour, B.; Barber, J.; Rostami-Hodjegan, A. Cytochrome P450 Pig Liver Pie: Determination of Individual Cytochrome P450 Isoform Contents in Microsomes from Two Pig Livers Using Liquid Chromatography in Conjunction with Mass Spectrometry. Drug Metab. Dispos. 2011, 39, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Anzenbacherová, E.; Baranová, J.; Zuber, R.; Pěchová, A.; Anzenbacher, P.; Souček, P.; Martínková, J. Model Systems Based on Experimental Animals for Studies on Drug Metabolism in Man: (Mini)Pig Cytochromes P450 3A29 and 2E1. Basic Clin. Pharmacol. Toxicol. 2005, 96, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Brunius, C.; Rasmussen, M.K.; Lacoutière, H.; Andersson, K.; Ekstrand, B.; Zamaratskaia, G. Expression and activities of hepatic cytochrome P450 (CYP1A, CYP2A and CYP2E1) in entire and castrated male pigs. Animal 2012, 6, 271–277. [Google Scholar] [CrossRef]
- Burkina, V.; Rasmussen, M.K.; Oliinychenko, Y.; Zamaratskaia, G. Porcine cytochrome 2A19 and 2E1. Basic Clin. Pharmacol. Toxicol. 2018, 124, 32–39. [Google Scholar] [CrossRef]
- Kojima, M.; Degawa, M. Sex differences in constitutive mRNA levels of CYP2B22, CYP2C33, CYP2C49, CYP3A22, CYP3A29 and CYP3A46 in the pig liver: Comparison between Meishan and Landrace pigs. Drug Metab. Pharmacokinet. 2016, 31, 185–192. [Google Scholar] [CrossRef]
- Schelstraete, W.; De Clerck, L.; Govaert, E.; Millecam, J.; Devreese, M.; Deforce, D.; Van Bocxlaer, J.; Croubels, S. Characterization of Porcine Hepatic and Intestinal Drug Metabolizing CYP450: Comparison with Human Orthologues from A Quantitative, Activity and Selectivity Perspective. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Skaanild, M.T. Porcine cytochrome P450 and metabolism. Curr. Pharm. Des. 2006, 12, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Skaanild, M.T.; Friis, C. Cytochrome P450 Sex Differences in Minipigs and Conventional Pigs. Pharmacol. Toxicol. 1999, 85, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Skaanild, M.T.; Friis, C. Is Cytochrome P450 CYP2D Activity Present in Pig Liver? Pharmacol. Toxicol. 2002, 91, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Skaanild, M.T.; Friis, C. Analyses of CYP2C in Porcine Microsomes. Basic Clin. Pharmacol. Toxicol. 2008, 103, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Baranová, J.; Anzenbacherová, E.; Anzenbacher, P.; Souček, P. Minipig Cytochrome P450 2e1: Comparison with Human Enzyme. Drug Metab. Dispos. 2005, 33, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Heckel, T.; Schmucki, R.; Berrera, M.; Ringshandl, S.; Badi, L.; Steiner, G.; Ravon, M.; Küng, E.; Kuhn, B.; Kratochwil, N.A.; et al. Functional analysis and transcriptional output of the Göttingen minipig genome. BMC Genom. 2015, 16, 932. [Google Scholar] [CrossRef]
- Lignet, F.; Sherbetjian, E.; Kratochwil, N.; Jones, R.; Suenderhauf, C.; Otteneder, M.B.; Singer, T.; Parrott, N. Characterization of Pharmacokinetics in the Göttingen Minipig with Reference Human Drugs: An In Vitro and In Vivo Approach. Pharm. Res. 2016, 33, 2565–2579. [Google Scholar] [CrossRef]
- Shang, H.; Guo, K.; Liu, Y.; Yang, J.; Wei, H. Constitutive expression of CYP3A mRNA in Bama miniature pig tissues. Gene 2013, 524, 261–267. [Google Scholar] [CrossRef]
- Skaanild, M.T.; Friis, C. Characterization of the P450 System in Göttingen Minipigs. Pharmacol. Toxicol. 1997, 80, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Souček, P.; Zuber, R.; Anzenbacherová, E.; Anzenbacher, P.; Guengerich, F.P. Minipig cytochrome P450 3A, 2A and 2C enzymes have similar properties to human analogs. BMC Pharmacol. 2001, 1, 11. [Google Scholar] [CrossRef]
- Millecam, J.; De Clerck, L.; Govaert, E.; Devreese, M.; Gasthuys, E.; Schelstraete, W.; Deforce, D.; De Bock, L.; Van Bocxlaer, J.; Sys, S.; et al. The Ontogeny of Cytochrome P450 Enzyme Activity and Protein Abundance in Conventional Pigs in Support of Preclinical Pediatric Drug Research. Front. Pharmacol. 2018, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Cazeneuve, C.; Pons, G.; Rey, E.; Treluyer, J.M.; Cresteil, T.; Thiroux, G.; D’Athis, P.; Olive, G. Biotransformation of caffeine in human liver microsomes from foetuses, neonates, infants and adults. Br. J. Clin. Pharmacol. 1994, 37, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, D.; Sonnier, M.; Moncion, A.; Cheron, G.; Cresteil, T. Expression of CYP3A in the Human Liver-Evidence that the Shift between CYP3A7 and CYP3A4 Occurs Immediately After Birth. JBIC J. Biol. Inorg. Chem. 1997, 247, 625–634. [Google Scholar] [CrossRef]
- Sonnier, M.; Cresteil, T. Delayed ontogenesis of CYP1A2 in the human liver. JBIC J. Biol. Inorg. Chem. 1998, 251, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Treluyer, J.M.; Gueret, G.; Cheron, G.; Sonnier, M.; Cresteil, T. Developmental expression of CYP2C and CYP2C-dependent activities in the human liver: In-vivo/in-vitro correlation and inducibility. Pharmacogenetics 1997, 7, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Treluyer, J.-M.; Jacqz-Aigrain, E.; Alvarez, F.; Cresteil, T. Expression of CYP2D6 in developing human liver. JBIC J. Biol. Inorg. Chem. 1991, 202, 583–588. [Google Scholar] [CrossRef]
- Hu, S.X. Age-related change of hepatic uridine diphosphate glucuronosyltransferase and sulfotransferase activities in male chickens and pigs. J. Veter. Pharmacol. Ther. 2016, 40, 270–278. [Google Scholar] [CrossRef]
- Millecam, J.; De Baere, S.; Croubels, S.; Devreese, M. In Vivo Metabolism of Ibuprofen in Growing Conventional Pigs: A Pharmacokinetic Approach. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Upreti, V.V.; Wahlstrom, J.L. Meta-analysis of hepatic cytochrome P450 ontogeny to underwrite the prediction of pediatric pharmacokinetics using physiologically based pharmacokinetic modeling. J. Clin. Pharmacol. 2015, 56, 266–283. [Google Scholar] [CrossRef]
- Lu, H.; Rosenbaum, S. Developmental Pharmacokinetics in Pediatric Populations. J. Pediatr. Pharmacol. Ther. 2014, 19, 262–276. [Google Scholar] [CrossRef]
- Brouwer, K.L.R.; Aleksunes, L.M.; Brandys, B.; Giacoia, G.P.; Knipp, G.T.; Lukacova, V.; Meibohm, B.; Nigam, S.K.; Rieder, M.; De Wildt, S.N.; et al. Human Ontogeny of Drug Transporters: Review and Recommendations of the Pediatric Transporter Working Group. Clin. Pharmacol. Ther. 2015, 98, 266–287. [Google Scholar] [CrossRef] [PubMed]
- Schneckener, S.; Preuss, T.G.; Kuepfer, L.; Witt, J. A workflow to build PBTK models for novel species. Arch. Toxicol. 2020, 94, 3847–3860. [Google Scholar] [CrossRef]
- Suenderhauf, C.; Tuffin, G.; Lorentsen, H.; Grimm, H.-P.; Flament, C.; Parrott, N. Pharmacokinetics of Paracetamol in Göttingen Minipigs: In Vivo Studies and Modeling to Elucidate Physiological Determinants of Absorption. Pharm. Res. 2014, 31, 2696–2707. [Google Scholar] [CrossRef] [PubMed]
- Kesisoglou, F.; Balakrishnan, A.; Manser, K. Utility of PBPK Absorption Modeling to Guide Modified Release Formulation Development of Gaboxadol, a Highly Soluble Compound With Region-Dependent Absorption. J. Pharm. Sci. 2016, 105, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Shida, S.; Yamazaki, H. Human plasma concentrations of five cytochrome P450 probes extrapolated from pharmacokinetics in dogs and minipigs using physiologically based pharmacokinetic modeling. Xenobiotica 2015, 46, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P.; Collet, S.H.; Atrux-Tallau, N.; Linget, J.-M.; Hennequin, L.; Wilson, C.E. Application of the Tissue Composition–Based Model to Minipig for Predicting the Volume of Distribution at Steady State and Dermis-to-Plasma Partition Coefficients of Drugs Used in the Physiologically Based Pharmacokinetics Model in Dermatology. J. Pharm. Sci. 2019, 108, 603–619. [Google Scholar] [CrossRef]
- Oliver, W.T.; Funnell, H.S.; Oki, Y.; Oliver, H.S.F.W.T. Some Effects of Chlorpromazine on the Activity of Pig Serum Cholinesterase. Nat. Cell Biol. 2006, 200, 361–362. [Google Scholar] [CrossRef]
- Walkenstein, S.S.; Wiser, R.; Gudmundsen, C.H.; Kimmel, H.B.; Corradino, R.A. Absorption, Metabolism, and Excretion of Oxazepam and Its Succinate Half-Ester. J. Pharm. Sci. 2006, 53, 1181–1186. [Google Scholar] [CrossRef]
- Barr, W. The Use of Physical and Animal Models to Assess Bioavailability. Pharmacology 1972, 8, 55–101. [Google Scholar] [CrossRef]
- Bay, W.; Gleiser, C.; Dukes, T.; Brown, R. The experimental production and evaluation of drug-induced phototoxicity in swine. Toxicol. Appl. Pharmacol. 1970, 17, 538–547. [Google Scholar] [CrossRef]
- Joiner, P.D.; Kadowitz, P.J.; Hughes, J.P.; Hyman, A.L. NE and ACh responses of intrapulmonary vessels from dog, swine, sheep, and man. Am. J. Physiol. Content 1975, 228, 1821–1827. [Google Scholar] [CrossRef]
- Kadowitz, P.J.; Joiner, P.D.; Hyman, A.L. Effect of prostaglandin E2 on pulmonary vascular resistance in intact dog, swine and lamb. Eur. J. Pharmacol. 1975, 31, 72–80. [Google Scholar] [CrossRef]
- Jacob, S.W.; Wood, D.C. Dimethyl sulfoxide (DMSO) toxicology, pharmacology, and clinical experience. Am. J. Surg. 1967, 114, 414–426. [Google Scholar] [CrossRef]
- Farber, T.M.; Smith, E.J.; Earl, F.L.; Van Loon, E. The effect of lindane and phenobarbital on microsomal enzyme induction in dogs and miniature swine. Toxicol. Appl. Pharmacol. 1976, 37, 319–330. [Google Scholar] [CrossRef]
- Noordewier, B.; Bailie, M.D.; Hood, J.B. Pharmacological analysis of the action of diuretics in the newborn pig. J. Pharmacol. Exp. Ther. 1978, 207, 236–242. [Google Scholar] [PubMed]
- de Roth, L.; Chand, N. Pharmacological study of tracheal smooth muscle in neonatal swine. Zent. Veterinärmed. Reihe A 1979, 26, 393–401. [Google Scholar] [CrossRef]
- Paap, C.M.; Nahata, M.C. Clinical Pharmacokinetics of Antibacterial Drugs in Neonates. Clin. Pharmacokinet. 1990, 19, 280–318. [Google Scholar] [CrossRef]
- Nau, H.; Kuhnz, W.; Egger, H.-J.; Helge, H. Anticonvulsants during Pregnancy and Lactation Transplacental, Maternal and Neonatal Pharmacokinetics. Clin. Pharmacokinet. 1982, 7, 508–543. [Google Scholar] [CrossRef]
- Anderson, B.J.; Woollard, G.A.; Holford, N.H.G. A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br. J. Clin. Pharmacol. 2000, 50, 125–134. [Google Scholar] [CrossRef]
- De Backer, P. Comparative Veterinary Pharmacology, Toxicology and Therapy; Springer: Berlin/Heidelberg, Germany, 1986; pp. 161–171. [Google Scholar]
- Nouws, J. Pharmacokinetics in immature animals: A review. J. Anim. Sci. 1992, 70, 3627–3634. [Google Scholar] [CrossRef]
- Šatas, S.; Hoem, N.O.; Melby, K.; Porter, H.; Lindgren, C.G.; Whitelaw, A.; Thoresen, M. Influence of mild hypothermia after hypoxia-ischemia on the pharmacokinetics of gentamicin in newborn pigs. Biol. Neonate 2000, 77, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Short, C.R.; Davis, L.E. Perinatal development of drug-metabolizing enzyme activity in swine. J. Pharmacol. Exp. Ther. 1970, 174, 185–196. [Google Scholar] [PubMed]
- Swinney, D.C.; Anthony, J. How were new medicines discovered? Nat. Rev. Drug Discov. 2011, 10, 507–519. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/presentation/presentation-pre-clinical-requirements-support-development-paediatric-medicines-janina-karres_en.pdf (accessed on 2 November 2020).
- Roth, W.J.; Kissinger, C.B.; McCain, R.R.; Cooper, B.R.; Marchant-Forde, J.N.; Vreeman, R.C.; Hannou, S.; Knipp, G.T. Assessment of Juvenile Pigs to Serve as Human Pediatric Surrogates for Preclinical Formulation Pharmacokinetic Testing. AAPS J. 2013, 15, 763–774. [Google Scholar] [CrossRef]
- Rai, A.; Bhalla, S.; Rebello, S.S.; Kastrissios, H.; Gulati, A. Disposition of morphine in plasma and cerebrospinal fluid varies during neonatal development in pigs. J. Pharm. Pharmacol. 2005, 57, 981–985. [Google Scholar] [CrossRef]
- Boudreaux, J.P.; Schieber, R.A.; Cook, D.R. Hemodynamic effects of halothane in the newborn piglet. Anesth. Analg. 1984, 63, 731–737. [Google Scholar] [CrossRef]
- Kajimoto, M.; Atkinson, D.B.; Ledee, D.R.; Kayser, E.-B.; Morgan, P.G.; Sedensky, M.M.; Isern, N.G.; Rosiers, C.D.; Portman, M. Propofol Compared with Isoflurane Inhibits Mitochondrial Metabolism in Immature Swine Cerebral Cortex. Br. J. Pharmacol. 2014, 34, 514–521. [Google Scholar] [CrossRef]
- Whitaker, E.E.; Zheng, C.Z.; Bissonnette, B.; Miller, A.D.; Koppert, T.L.; Tobias, J.D.; Pierson, C.R.; Christofi, F.L. Use of a Piglet Model for the Study of Anesthetic-induced Developmental Neurotoxicity (AIDN): A Translational Neuroscience Approach. J. Vis. Exp. 2017, 124, e55193. [Google Scholar] [CrossRef]
- Millecam, J.; Van Bergen, T.; Schauvliege, S.; Antonissen, G.; Martens, A.; Chiers, K.; Gehring, R.; Gasthuys, E.; Walle, J.V.; Croubels, S.; et al. Developmental Pharmacokinetics and Safety of Ibuprofen and Its Enantiomers in the Conventional Pig as Potential Pediatric Animal Model. Front. Pharmacol. 2019, 10, 505. [Google Scholar] [CrossRef]
- Gasthuys, E.; Vermeulen, A.; Croubels, S.; Millecam, J.; Schauvliege, S.; Van Bergen, T.; De Bruyne, P.; Walle, J.V.; Devreese, M. Population Pharmacokinetic Modeling of a Desmopressin Oral Lyophilisate in Growing Piglets as a Model for the Pediatric Population. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Ulrich, P.; Blaich, G.; Baumann, A.; Fagg, R.; Hey, A.; Kiessling, A.; Kronenberg, S.; Lindecrona, R.H.; Mohl, S.; Richter, W.F.; et al. Biotherapeutics in non-clinical development: Strengthening the interface between safety, pharmacokinetics-pharmacodynamics and manufacturing. Regul. Toxicol. Pharmacol. 2018, 94, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.E.; Atkinson, S.A. Growth Hormone and Insulin-like Growth Factor-I Therapy Promote Protein Deposition and Growth in Dexamethasone-treated Piglets. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.E.; Donovan, S.M.; Atkinson, S.A. Dexamethasone-Induced Abnormalities in Growth and Bone Metabolism in Piglets Are Partially Attenuated by Growth Hormone with No Synergistic Effect of Insulin-Like Growth Factor-I. Pediatr. Res. 1998, 44, 215–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thygesen, P.; Andersen, H.S.; Behrens, C.; Fels, J.J.; Nørskov-Lauritsen, L.; Rischel, C.; Johansen, N.L. Nonclinical pharmacokinetic and pharmacodynamic characterisation of somapacitan: A reversible non-covalent albumin-binding growth hormone. Growth Horm. IGF Res. 2017, 35, 8–16. [Google Scholar] [CrossRef]
- Ramos, L.; Obregon-Henao, A.; Henao-Tamayo, M.; Bowen, R.; Izzo, A.; Lunney, J.K.; Gonzalez-Juarrero, M. Minipigs as a neonatal animal model for tuberculosis vaccine efficacy testing. Veter. Immunol. Immunopathol. 2019, 215, 109884. [Google Scholar] [CrossRef]
- Busignies, V.; Simon, G.; Mollereau, G.; Bourry, O.; Mazel, V.; Rosa-Calatrava, M.; Tchoreloff, P. Development and pre-clinical evaluation in the swine model of a mucosal vaccine tablet for human influenza viruses: A proof-of-concept study. Int. J. Pharm. 2018, 538, 87–96. [Google Scholar] [CrossRef]
- Rajao, D.S.; Vincent, A.L. Swine as a Model for Influenza A Virus Infection and Immunity. ILAR J. 2015, 56, 44–52. [Google Scholar] [CrossRef]
- Starbæk, S.M.R.; Brogaard, L.; Dawson, H.D.; Smith, A.D.; Heegaard, P.M.H.; Larsen, L.E.; Jungersen, G.; Skovgaard, K. Animal Models for Influenza A Virus Infection Incorporating the Involvement of Innate Host Defenses: Enhanced Translational Value of the Porcine Model. ILAR J. 2018, 59, 323–337. [Google Scholar] [CrossRef]
- Gala, R.P.; Popescu, C.; Knipp, G.T.; McCain, R.R.; Ubale, R.V.; Addo, R.; Bhowmik, T.; Kulczar, C.D.; D’Souza, M.J. Physicochemical and Preclinical Evaluation of a Novel Buccal Measles Vaccine. AAPS PharmSciTech 2016, 18, 283–292. [Google Scholar] [CrossRef]
- Hinderer, C.; Katz, N.; Buza, E.L.; Dyer, C.; Goode, T.; Bell, P.; Richman, L.K.; Wilson, J.M. Severe Toxicity in Nonhuman Primates and Piglets Following High-Dose Intravenous Administration of an Adeno-Associated Virus Vector Expressing Human SMN. Hum. Gene Ther. 2018, 29, 285–298. [Google Scholar] [CrossRef]
- Dias, N.; Stein, C. Antisense oligonucleotides: Basic concepts and mechanisms. Mol. Cancer Ther. 2002, 1, 347–355. [Google Scholar]
- M3(R2); ICH EIMR. Guidance on Non-Clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals. In Proceedings of the International Conference on Harmonisation, Japan; 2009. Available online: http://www.nifds.go.kr/apec/Guideline/19.ICH_M3_R2__Guideline.pdf (accessed on 28 September 2020).
- Vamathevan, J.; Hall, M.D.; Hasan, S.; Woollard, P.; Xu, M.; Yang, Y.; Li, X.; Wang, X.; Kenny, S.; Brown, J.R.; et al. Minipig and beagle animal model genomes aid species selection in pharmaceutical discovery and development. Toxicol. Appl. Pharmacol. 2013, 270, 149–157. [Google Scholar] [CrossRef]
- Braendli-Baiocco, A.; Festag, M.; Erichsen, K.D.; Persson, R.; Mihatsch, M.J.; Fisker, N.; Funk, J.; Mohr, S.; Constien, R.; Ploix, C.; et al. The Minipig is a Suitable Non-Rodent Model in the Safety Assessment of Single Stranded Oligonucleotides. Toxicol. Sci. 2017, 157, 112–128. [Google Scholar] [CrossRef]
- Srinivasan, S.K.; Tewary, H.K.; Iversen, P.L. Characterization of Binding Sites, Extent of Binding, and Drug Interactions of Oligonucleotides with Albumin. Antisense Res. Dev. 1995, 5, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.S.; Norris, D.; Yu, R.; Bennett, C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 46–51. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Anastasopoulos, F.; Pouton, C.W.; Boyd, B.J. Overcoming biological barriers to in vivo efficacy of antisense oligonucleotides. Expert Rev. Mol. Med. 2009, 11, e10. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.S. Antisense Oligonucleotide Therapies. Toxicol. Pathol. 2014, 43, 78–89. [Google Scholar] [CrossRef]
- Burel, S.A.; Hart, C.E.; Cauntay, P.; Hsiao, J.; Machemer, T.; Katz, M.; Watt, A.T.; Bui, H.-H.; Younis, H.; Sabripour, M.; et al. Hepatotoxicity of high affinity gapmer antisense oligonucleotides is mediated by RNase H1 dependent promiscuous reduction of very long pre-mRNA transcripts. Nucleic Acids Res. 2016, 44, 2093–2109. [Google Scholar] [CrossRef]
- Sewing, S.; Roth, A.B.; Winter, M.; Dieckmann, A.; Bertinetti-Lapatki, C.; Tessier, Y.; McGinnis, C.; Huber, S.; Koller, E.; Ploix, C.; et al. Assessing single-stranded oligonucleotide drug-induced effects in vitro reveals key risk factors for thrombocytopenia. PLoS ONE 2017, 12, e0187574. [Google Scholar] [CrossRef]
- Pliszczak-Król, A.; Rząsa, A.; Gemra, M.; Król, J.; Łuczak, G.; Zyzak, A.; Zalewski, D.; Iwaszko-Simonik, A.; Graczyk, S. Age-related changes of platelet and plasma coagulation parameters in young pigs. J. Veter. Diagn. Investig. 2016, 28, 561–567. [Google Scholar] [CrossRef]
- Bollen, P.; Madsen, L.; Meyer, O.; Ritskes-Hoitinga, J. Growth differences of male and female Göttingen minipigs during ad libitum feeding: A pilot study. Lab. Anim. 2005, 39, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef]
- Schmitt, G.; Parrott, N.; Prinssen, E.; Barrow, P. The great barrier belief: The blood–brain barrier and considerations for juvenile toxicity studies. Reprod. Toxicol. 2017, 72, 129–135. [Google Scholar] [CrossRef]
- Weaver, M.L.; Grossi, A.B.; Schützsack, J.; Parish, J.; Løgsted, J.; Bøgh, I.B.; Cameron, D.; Harvey, W.; Festag, M.; Downes, N.; et al. Vehicle Systems and Excipients Used in Minipig Drug Development Studies. Toxicol. Pathol. 2015, 44, 367–372. [Google Scholar] [CrossRef]
- Kerenyi, A.; Kelen, D.; Faulkner, S.D.; Bainbridge, A.; Chandrasekaran, M.; Cady, E.B.; Golay, X.; Robertson, N.J. Systemic effects of whole-body cooling to 35 °C, 33.5 °C, and 30 °C in a piglet model of perinatal asphyxia: Implications for therapeutic hypothermia. Pediatr. Res. 2012, 71, 573–582. [Google Scholar] [CrossRef]
- Pang, R.; Avdic-Belltheus, A.; Meehan, C.; Martinello, K.; Mutshiya, T.; Yang, Q.; Sokolska, M.; Torrealdea, F.; Hristova, M.; Bainbridge, A.; et al. Melatonin and/or erythropoietin combined with hypothermia in a piglet model of perinatal asphyxia. Brain Commun. 2020. [Google Scholar] [CrossRef]
- Robertson, N.J.; Lingam, I.; Meehan, C.; Martinello, K.A.; Avdic-Belltheus, A.; Stein, L.; Tachrount, M.; Price, D.; Sokolska, M.; Bainbridge, A.; et al. High-Dose Melatonin and Ethanol Excipient Combined with Therapeutic Hypothermia in a Newborn Piglet Asphyxia Model. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu309701 (accessed on 2 November 2020).
- Solevåg, A.; Schmölzer, G.; Cheung, P.-Y. Novel interventions to reduce oxidative-stress related brain injury in neonatal asphyxia. Free. Radic. Biol. Med. 2019, 142, 113–122. [Google Scholar] [CrossRef]
- Interventional Procedures Guidance (26 May 2010). Therapeutic Hypothermia with Intracorporeal Temperature Monitoring for Hypoxic Perinatal Brain Injury. Available online: https://wwwniceorguk/guidance/ipg347 (accessed on 2 November 2020).
- Favié, L.M.A.; Groenendaal, F.; Broek, M.P.V.D.; Rademaker, C.M.; De Haan, T.R.; Van Straaten, H.L.; Dijk, P.H.; Van Heijst, A.; Simons, S.H.; Dijkman, K.P.; et al. Phenobarbital, Midazolam Pharmacokinetics, Effectiveness, and Drug-Drug Interaction in Asphyxiated Neonates Undergoing Therapeutic Hypothermia. Neonatology 2019, 116, 154–162. [Google Scholar] [CrossRef]
- Róka, A.; Vásárhelyi, B.; Bodrogi, E.; Machay, T.; Szabó, M. Changes in laboratory parameters indicating cell necrosis and organ dysfunction in asphyxiated neonates on moderate systemic hypothermia. Acta Paediatr. 2007, 96, 1118–1121. [Google Scholar] [CrossRef]
- Temesvári, P.; Hencz, P.; Joó, F.; Eck, E.; Szerdahelyi, P.; Boda, D. Modulation of the Blood-Brain Barrier Permeability in Neonatal Cytotoxic Brain Edema: Laboratory and Morphological Findings Obtained on Newborn Piglets with Experimental Pneumothorax. Neonatology 1984, 46, 198–208. [Google Scholar] [CrossRef]
- Domoki, F.; Zimmermann, A.; Cserni, G.; Bori, R.; Temesvári, P.; Bari, F. Reventilation with room air or 100% oxygen after asphyxia differentially affects cerebral neuropathology in newborn pigs. Acta Paediatr. 2006, 95, 1109–1115. [Google Scholar] [CrossRef]
- Imai, H.; Konno, K.; Nakamura, M.; Shimizu, T.; Kubota, C.; Seki, K.; Honda, F.; Tomizawa, S.; Tanaka, Y.; Hata, H.; et al. A new model of focal cerebral ischemia in the miniature pig. J. Neurosurg. Pediatr. 2006, 104, 123–132. [Google Scholar] [CrossRef]
- Cheung, P.-Y.; Gill, R.S.; Bigam, D.L. A Swine Model of Neonatal Asphyxia. J. Vis. Exp. 2011, 56, 3166. [Google Scholar] [CrossRef]
- Kattwinkel, J.; Perlman, J.M.; Aziz, K.; Colby, C.; Fairchild, K.; Gallagher, J.; Hazinski, M.F.; Halamek, L.P.; Kumar, P.; Little, G.; et al. Part 15: Neonatal Resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010, 122, S909–S919. [Google Scholar] [CrossRef]
- Broek, M.P.H.V.D.; Groenendaal, F.; Egberts, A.C.; Rademaker, C.M.A. Effects of Hypothermia on Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2010, 49, 277–294. [Google Scholar] [CrossRef]
- Ezzati, M.; Broad, K.; Kawano, G.; Faulkner, S.; Hassell, J.; Fleiss, B.; Gressens, P.; Fierens, I.; Rostami, J.; Maze, M.; et al. Pharmacokinetics of dexmedetomidine combined with therapeutic hypothermia in a piglet asphyxia model. Acta Anaesthesiol. Scand. 2014, 58, 733–742. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Kochanek, P.M.; Poloyac, S.M. Effects of hypothermia on drug disposition, metabolism, and response: A focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit. Care Med. 2007, 35, 2196–2204. [Google Scholar] [CrossRef]
- Zanelli, S.; Buck, M.; Fairchild, K.D. Physiologic and pharmacologic considerations for hypothermia therapy in neonates. J. Perinatol. 2010, 31, 377–386. [Google Scholar] [CrossRef]
- Pokorna, P.; Wildschut, E.; Vobruba, V.; Anker, J.V.D.; Tibboel, D. The Impact of Hypothermia on the Pharmacokinetics of Drugs Used in Neonates and Young Infants. Curr. Pharm. Des. 2015, 21, 5705–5724. [Google Scholar] [CrossRef]
- Pasquin, M.P.; Cheung, P.-Y.; Patel, S.; Lu, M.; Lee, T.-F.; Wagner, M.; O’Reilly, M.; Schmölzer, G.M. Comparison of Different Compression to Ventilation Ratios (2: 1, 3: 1, and 4: 1) during Cardiopulmonary Resuscitation in a Porcine Model of Neonatal Asphyxia. Neonatology 2018, 114, 37–45. [Google Scholar] [CrossRef]
- Solevåg, A.L.; Schmölzer, G.M.; O’Reilly, M.; Lu, M.; Lee, T.-F.; Hornberger, L.K.; Nakstad, B.; Cheung, P.-Y. Myocardial perfusion and oxidative stress after 21% vs. 100% oxygen ventilation and uninterrupted chest compressions in severely asphyxiated piglets. Resuscitation 2016, 106, 7–13. [Google Scholar] [CrossRef]
- Wagner, M.; Cheung, P.-Y.; Li, E.S.; Lee, T.-F.; Lu, M.; O’Reilly, M.; Olischar, M.; Schmölzer, G.M. Effects of epinephrine on hemodynamic changes during cardiopulmonary resuscitation in a neonatal piglet model. Pediatr. Res. 2018, 83, 897–903. [Google Scholar] [CrossRef]
- Niño, D.F.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 590–600. [Google Scholar] [CrossRef]
- Caminita, F.; Van Der Merwe, M.; Hance, B.; Krishnan, R.; Miller, S.; Buddington, K.; Buddington, R.K. A preterm pig model of lung immaturity and spontaneous infant respiratory distress syndrome. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L118–L129. [Google Scholar] [CrossRef][Green Version]
- Eiby, Y.A.; Wright, L.L.; Kalanjati, V.P.; Miller, S.M.; Bjorkman, S.T.; Keates, H.L.; Lumbers, E.R.; Colditz, P.B.; Lingwood, B.E. A Pig Model of the Preterm Neonate: Anthropometric and Physiological Characteristics. PLoS ONE 2013, 8, e68763. [Google Scholar] [CrossRef]
- Kollisch-Singule, M.; Jain, S.V.; Satalin, J.; Andrews, P.; Searles, Q.; Liu, Z.; Zhou, Y.; Wang, G.; Meier, A.H.; Gatto, L.A.; et al. Limiting ventilator-associated lung injury in a preterm porcine neonatal model. J. Pediatr. Surg. 2017, 52, 50–55. [Google Scholar] [CrossRef]
- Qian, L.; Liu, H.; Yu, W.; Wang, X.; Sun, Z.; Wang, W.; Zhu, L.; Sun, B. Effects of Positive End-Expiratory Pressure, Inhaled Nitric Oxide and Surfactant on Expression of Proinflammatory Cytokines and Growth Factors in Preterm Piglet Lungs. Pediatr. Res. 2008, 64, 17–23. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef]
- Degraeuwe, P.L.; Thunnissen, E.; Vos, G.D.; Blanco, C.E. High-Frequency Oscillatory Ventilation, Partial Liquid Ventilation, or Conventional Mechanical Ventilation in NewbornPiglets with Saline Lavage-Induced Acute Lung Injury. A Comparison of Gas-Exchange Efficacy and Lung Histomorphology. Biol. Neonate. 1999, 75, 118–129. [Google Scholar] [CrossRef]
- Merz, U. Effects of prolonged partial liquid ventilation, high frequency ventilation and convential ventilation on gas exchange and lung pathology in newborn surfactant-depleted piglets. Shock 2000, 13, 472–477. [Google Scholar] [CrossRef]
- Stoltz, D.A.; Meyerholz, D.K.; Pezzulo, A.A.; Ramachandran, S.; Rogan, M.P.; Davis, G.J.; Hanfland, R.A.; Wohlford-Lenane, C.; Dohrn, C.L.; Bartlett, J.A.; et al. Cystic Fibrosis Pigs Develop Lung Disease and Exhibit Defective Bacterial Eradication at Birth. Sci. Transl. Med. 2010, 2, 29ra31. [Google Scholar] [CrossRef]
- Vegge, A.; Siggers, R.H.; Schmidt, M.; Elnif, J.; Bjørnvad, C.R.; Thymann, T.; Grondahl, M.L.; Hansen, A.K.; Jensen, S.K.; Boye, M.; et al. Diet- and Colonization-Dependent Intestinal Dysfunction Predisposes to Necrotizing Enterocolitis in Preterm Pigs. Gastroenterology 2006, 130, 1776–1792. [Google Scholar] [CrossRef]
- Ghoneim, N.; Bauchart-Thevret, C.; Oosterloo, B.; Stoll, B.; Kulkarni, M.; De Pipaón, M.S.; Zamora, I.J.; Olutoye, O.O.; Berg, B.; Wittke, A.; et al. Delayed Initiation but Not Gradual Advancement of Enteral Formula Feeding Reduces the Incidence of Necrotizing Enterocolitis (NEC) in Preterm Pigs. PLoS ONE 2014, 9, e106888. [Google Scholar] [CrossRef]
- Jensen, M.L.; Vegge, A.; Lykke, M.; Schmidt, M.; Boye, M.; Jensen, B.B.; Thymann, T. Similar efficacy of human banked milk and bovine colostrum to decrease incidence of necrotizing enterocolitis in preterm piglets. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R4–R12. [Google Scholar] [CrossRef]
- Vegge, A.; Jensen, M.L.; Chatterton, D.E.W.; Jensen, B.B.; Thymann, T.; Kvistgaard, A.S.; Sangild, P.T. Raw bovine milk improves gut responses to feeding relative to infant formula in preterm piglets. Am. J. Physiol. Liver Physiol. 2014, 306, G81–G90. [Google Scholar] [CrossRef]
- Benight, N.M.; Stoll, B.; Olutoye, O.O.; Holst, J.J.; Burrin, D.G. GLP-2 Delays but Does Not Prevent the Onset of Necrotizing Enterocolitis in Preterm Pigs. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 623–630. [Google Scholar] [CrossRef]
- Brunse, A.; Deng, L.; Pan, X.; Hui, Y.; Kot, W.; Nguyen, D.N.; Bojsen-Møller Secher, J.; Sandris Nielsen, D.; Thymann, T. Fecal filtrate transfer protects against necrotizing enterocolitis in preterm pigs. bioRxiv 2020. [Google Scholar] [CrossRef]
- Grzywacz, K.; Butcher, J.; Romain, G.; Li, J.; Stintzi, A. The impact of probiotics and lactoferrin supplementation on piglet gastrointestinal microbial communities. BioMetals 2019, 32, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Vegge, A.; Thymann, T.; Cilieborg, M.S.; Lykke, M.; Mølbak, L.; Jensen, B.B.; Schmidt, M.; Kelly, D.; Mulder, I.; Burrin, D.G.; et al. Antibiotics modulate intestinal immunity and prevent necrotizing enterocolitis in preterm neonatal piglets. Am. J. Physiol. Liver Physiol. 2014, 306, G59–G71. [Google Scholar] [CrossRef]
- Jiang, P.; Trimigno, A.; Stanstrup, J.; Khakimov, B.; Viereck, N.; Engelsen, S.B.; Sangild, P.T.; Dragsted, L.O. Antibiotic Treatment Preventing Necrotising Enterocolitis Alters Urinary and Plasma Metabolomes in Preterm Pigs. J. Proteome Res. 2017, 16, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Birck, M.M.; Nguyen, D.N.; Cilieborg, M.S.; Kamal, S.S.; Nielsen, D.S.; Damborg, P.; Olsen, J.E.; Lauridsen, C.; Sangild, P.T.; Thymann, T. Enteral but not parenteral antibiotics enhance gut function and prevent necrotizing enterocolitis in formula-fed newborn preterm pigs. Am. J. Physiol. Liver Physiol. 2016, 310, G323–G333. [Google Scholar] [CrossRef]
- Wales, P.W.; De Silva, N.; Kim, J.; Lecce, L.; To, T.; Moore, A. Neonatal short bowel syndrome: Population-based estimates of incidence and mortality rates. J. Pediatr. Surg. 2004, 39, 690–695. [Google Scholar] [CrossRef]
- Bines, J.E.; Taylor, R.G.; Justice, F.; Paris, M.C.; Sourial, M.; Nagy, E.; Emselle, S.; Catto-Smith, A.G.; Fuller, P.J. Influence of diet complexity on intestinal adaptation following massive small bowel resection in a preclinical model. J. Gastroenterol. Hepatol. 2002, 17, 1170–1179. [Google Scholar] [CrossRef]
- Sacher, P.; Stauffer, U. An Animal Model for Short-Bowel Syndrome in Piglets to Assess the Efficiency of Bowel-Lengthening Procedures. Eur. J. Pediatr. Surg. 1997, 7, 207–211. [Google Scholar] [CrossRef]
- Benhamou, P.; Canarelli, J.; Cordonnier, C.; Postel, J.; Grenier, E.; Leke, A.; Dupont, C. Human recombinant growth hormone increases small bowel lengthening after massive small bowel resection in piglets. J. Pediatr. Surg. 1997, 32, 1332–1336. [Google Scholar] [CrossRef]
- Welters, C.F.; Deutz, N.E.P.; DeJong, C.H.; Soeters, P.B.; Heineman, E. Supplementation of enteral nutrition with butyrate leads to increased portal efflux of amino acids in growing pigs with short bowel syndrome. J. Pediatr. Surg. 1996, 31, 526–529. [Google Scholar] [CrossRef]
- Heemskerk, V.H.; Van Heurn, L.W.E.; Farla, P.; Buurman, W.A.; Piersma, F.; Ter Riet, G.; Heineman, E. Effect of IGF-Rich Colostrum on Bowel Adaptation in Neonatal Piglets With Short Bowel Syndrome. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 47–51. [Google Scholar] [CrossRef]
- Lim, D.W.; Levesque, C.L.; Vine, D.F.; Muto, M.; Koepke, J.R.; Nation, P.N.; Wizzard, P.R.; Li, J.; Bigam, D.L.; Brubaker, P.L.; et al. Synergy of glucagon-like peptide-2 and epidermal growth factor coadministration on intestinal adaptation in neonatal piglets with short bowel syndrome. Am. J. Physiol. Liver Physiol. 2017, 312, G390–G404. [Google Scholar] [CrossRef]
- Suri, M.; Turner, J.M.; Sigalet, D.L.; Wizzard, P.R.; Nation, P.N.; Ball, R.O.; Pencharz, P.B.; Brubaker, P.L.; Wales, P.W. Exogenous glucagon-like peptide-2 improves outcomes of intestinal adaptation in a distal-intestinal resection neonatal piglet model of short bowel syndrome. Pediatr. Res. 2014, 76, 370–377. [Google Scholar] [CrossRef]
- Jeppesen, P.; Pertkiewicz, M.; Messing, B.; Iyer, K.; Seidner, D.L.; O’Keefe, S.J.; Forbes, A.; Heinze, H.; Joelsson, B. Teduglutide Reduces Need for Parenteral Support Among Patients With Short Bowel Syndrome With Intestinal Failure. Gastroenterology 2012, 143, 1473–1481.e3. [Google Scholar] [CrossRef] [PubMed]
- Thymann, T.; Stoll, B.; Mecklenburg, L.; Burrin, D.G.; Vegge, A.; Qvist, N.; Eriksen, T.; Jeppesen, P.B.; Sangild, P.T. Acute Effects of the Glucagon-Like Peptide 2 Analogue, Teduglutide, On Intestinal Adaptation in Newborn Pigs with Short Bowel Syndrome. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Slim, G.M.; Lansing, M.; Wizzard, P.; Nation, P.N.; Wheeler, S.E.; Brubaker, P.L.; Jeppesen, P.B.; Wales, P.W.; Turner, J.M. Novel Long-Acting GLP-2 Analogue, FE 203799 (Apraglutide), Enhances Adaptation and Linear Intestinal Growth in a Neonatal Piglet Model of Short Bowel Syndrome with Total Resection of the Ileum. J. Parenter. Enter. Nutr. 2019, 43, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Porcine models of digestive disease: The future of large animal translational research. Transl. Res. 2015, 166, 12–27. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Irritable Bowel Syndrome and Stress-Related Psychiatric Co-morbidities: Focus on Early Life Stress; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; Volume 239, pp. 219–246. [Google Scholar]
- Vergauwen, H.; DeGroote, J.; Prims, S.; Wang, W.; Fransen, E.; De Smet, S.; Casteleyn, C.; Van Cruchten, S.; Michiels, J.; Van Ginneken, C. Artificial rearing influences the morphology, permeability and redox state of the gastrointestinal tract of low and normal birth weight piglets. J. Anim. Sci. Biotechnol. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Brown, D.R.; Timmermans, J.-P. Lessons from the porcine enteric nervous system. Neurogastroenterol. Motil. 2004, 16, 50–54. [Google Scholar] [CrossRef]
- Huygelen, V.; De Vos, M.; Willemen, S.; Fransen, E.; Casteleyn, C.; Van Cruchten, S.; Van Ginneken, C. Age-related differences in mucosal barrier function and morphology of the small intestine in low and normal birth weight piglets1. J. Anim. Sci. 2014, 92, 3398–3406. [Google Scholar] [CrossRef]
- Willemen, S.A.; Che, L.; Dewilde, S.; Van Hauwaert, M.L.; De Vos, M.; Huygelen, V.; Fransen, E.; Tambuyzer, B.R.; Casteleyn, C.; Van Cruchten, S.; et al. Enteric and serological distribution of serotonin and its precursor tryptophan in perinatal low and normal weight piglets. Animal 2014, 8, 792–799. [Google Scholar] [CrossRef]
- Huygelen, V.; De Vos, M.; Willemen, S.; Tambuyzer, B.; Casteleyn, C.; Knapen, D.; Van Cruchten, S.; Van Ginneken, C.J. Increased intestinal barrier function in the small intestine of formula-fed neonatal piglets. J. Anim. Sci. 2012, 90, 315–317. [Google Scholar] [CrossRef]
- Oste, M.; Van Ginneken, C.J.; Van Haver, E.R.; Bjornvad, C.R.; Thymann, T.; Sangild, P.T. The Intestinal Trophic Response to Enteral Food Is Reduced in Parenterally Fed Preterm Pigs and Is Associated with More Nitrergic Neurons1. J. Nutr. 2005, 135, 2657–2663. [Google Scholar] [CrossRef]
- Wang, W.; DeGroote, J.; Van Ginneken, C.; Van Poucke, M.; Vergauwen, H.; Dam, T.M.T.; Vanrompay, D.; Peelman, L.J.; De Smet, S.; Michiels, J. Intrauterine growth restriction in neonatal piglets affects small intestinal mucosal permeability and mRNA expression of redox-sensitive genes. FASEB J. 2015, 30, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.; De Vos, M.; Missotten, J.; Ovyn, A.; De Smet, S.; Van Ginneken, C. Maturation of digestive function is retarded and plasma antioxidant capacity lowered in fully weaned low birth weight piglets. Br. J. Nutr. 2012, 109, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Van Haver, E.R.; De Vooght, L.; Oste, M.; Vegge, A.; Thymann, T.; Weyns, A.L.M.; Van Ginneken, C.J. Postnatal and diet-dependent increases in enteric glial cells and VIP-containing neurones in preterm pigs. Neurogastroenterol. Motil. 2008, 20, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Walter, B.; Brust, P.; Füchtner, F.; Zwiener, U. Impact of asymmetric intrauterine growth restriction on organ function in newborn piglets. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S40–S49. [Google Scholar] [CrossRef]
- Vazquez-Gomez, M.; Garcia-Contreras, C.; Torres-Rovira, L.; Pesantez, J.L.; Gonzalez-Añover, P.; Gomez-Fidalgo, E.; Sanchez-Sanchez, R.; Ovilo, C.; Isabel, B.; Astiz, S.; et al. Polyphenols and IUGR pregnancies: Maternal hydroxytyrosol supplementation improves prenatal and early-postnatal growth and metabolism of the offspring. PLoS ONE 2017, 12, e0177593. [Google Scholar] [CrossRef] [PubMed]
- García-Contreras, C.; Vazquez-Gomez, M.; Pesantez, J.L.; Torres-Rovira, L.; Heras-Molina, A.; Encinas, T.; Astiz, S.; Gonzalez-Bulnes, A. Maternal Metformin Treatment Improves Developmental and Metabolic Traits of IUGR Fetuses. Biomolecules 2019, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- García-Contreras, C.; Vazquez-Gomez, M.; Barbero, A.; Pesantez, J.L.; Zinellu, A.; Berlinguer, F.; Gonzalez-Añover, P.; Gonzalez, J.; Encinas, T.; Torres-Rovira, L.; et al. Polyphenols and IUGR Pregnancies: Effects of Maternal Hydroxytyrosol Supplementation on Placental Gene Expression and Fetal Antioxidant Status, DNA-Methylation and Phenotype. Int. J. Mol. Sci. 2019, 20, 1187. [Google Scholar] [CrossRef]
- Che, L.; Hu, L.; Liu, Y.; Yan, C.; Peng, X.; Xu, Q.; Wang, R.; Cheng, Y.; Chen, H.; Fang, Z.; et al. Dietary Nucleotides Supplementation Improves the Intestinal Development and Immune Function of Neonates with Intra-Uterine Growth Restriction in a Pig Model. PLoS ONE 2016, 11, e0157314. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Chen, Y.; Zhang, L.; Wang, T. N-Acetylcysteine protects against intrauterine growth retardation-induced intestinal injury via restoring redox status and mitochondrial function in neonatal piglets. Eur. J. Nutr. 2018, 58, 3335–3347. [Google Scholar] [CrossRef]
- Cheng, K.; Ji, S.; Jia, P.; Zhang, H.; Wang, T.; Song, Z.; Zhang, L.; Wang, T. Resveratrol Improves Hepatic Redox Status and Lipid Balance of Neonates with Intrauterine Growth Retardation in a Piglet Model. BioMed Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Niu, Y.; He, J.; Ahmad, H.; Shen, M.; Zhao, Y.; Gan, Z.; Zhang, L.; Zhong, X.; Wang, C.; Wang, T.; et al. Dietary Curcumin Supplementation Increases Antioxidant Capacity, Upregulates Nrf2 and Hmox1 Levels in the Liver of Piglet Model with Intrauterine Growth Retardation. Nutrients 2019, 11, 2978. [Google Scholar] [CrossRef] [PubMed]
- Attig, L.; Djiane, J.; Gertler, A.; Rampin, O.; Larcher, T.; Boukthir, S.; Anton, P.M.; Madec, J.Y.; Gourdou, I.; Abdennebi-Najar, L. Study of hypothalamic leptin receptor expression in low-birth-weight piglets and effects of leptin supplementation on neonatal growth and development. Am. J. Physiol. Metab. 2008, 295, E1117–E1125. [Google Scholar] [CrossRef] [PubMed]
- Schubert, H.; Eiselt, M.; Walter, B.; Fritz, H.; Brodhun, M.; Bauer, R. Isoflurane/nitrous oxide anesthesia and stress-induced procedures enhance neuroapoptosis in intrauterine growth-restricted piglets. Intensiv. Care Med. 2012, 38, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Wixey, J.A.; Sukumar, K.R.; Pretorius, R.; Lee, K.M.; Colditz, P.B.; Bjorkman, S.T.; Chand, K.K. Ibuprofen Treatment Reduces the Neuroinflammatory Response and Associated Neuronal and White Matter Impairment in the Growth Restricted Newborn. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Engbers, A.G.; Flint, R.; Völler, S.; De Klerk, J.C.; Reiss, I.K.; Andriessen, P.; Liem, K.D.; Degraeuwe, P.L.; Croubels, S.; Millecam, J.; et al. Enantiomer specific pharmacokinetics of ibuprofen in preterm neonates with patent ductus arteriosus. Br. J. Clin. Pharmacol. 2020, 86, 2028–2039. [Google Scholar] [CrossRef]
- Stöger, R. The thrifty epigenotype: An acquired and heritable predisposition for obesity and diabetes? BioEssays 2008, 30, 156–166. [Google Scholar] [CrossRef]
- Feng, B.; Xuan, Y.; Hu, L.; Liu, Y.; Xu, Q.; Fang, Z.; Lin, Y.; Xu, S.; Wu, D.; Zhang, K.; et al. Effect of Postnatal Nutrition Restriction on the Oxidative Status of Neonates with Intrauterine Growth Restriction in a Pig Model. Neonatology 2014, 107, 93–99. [Google Scholar] [CrossRef]
- DuPriest, E.A.; Lin, B.; Kupfer, P.; Sekiguchi, K.; Bhusari, A.; Quackenbush, A.; Celebic, A.; Morgan, T.K.; Purnell, J.Q.; Bagby, S.P. Effects of postweaning calorie restriction on accelerated growth and adiponectin in nutritionally programmed microswine offspring. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R354–R368. [Google Scholar] [CrossRef]
- Ying, Z.; Ge, X.; Zhang, H.; Su, W.; Li, Y.; Zhou, L.; Zhang, L.; Wang, T. Effects of dietary methionine restriction on postnatal growth, insulin sensitivity, and glucose metabolism in intrauterine growth retardation pigs at 49 and 105 d of age1. J. Anim. Sci. 2018, 97, 610–619. [Google Scholar] [CrossRef]
- Panasevich, M.R.; Meers, G.M.; Linden, M.A.; Booth, F.W.; Perfield, J.W.; Fritsche, K.L.; Wankhade, U.D.; Chintapalli, S.V.; Shankar, K.; Ibdah, J.A.; et al. High-fat, high-fructose, high-cholesterol feeding causes severe NASH and cecal microbiota dysbiosis in juvenile Ossabaw swine. Am. J. Physiol. Metab. 2018, 314, E78–E92. [Google Scholar] [CrossRef]
- Dillard, K.; Coffin, M.; Hernandez, G.; Smith, V.; Johnson, C.; Immoos, C.; Blank, J.; Glanz, H.; Fanter, R.; Burrin, D.; et al. Differential Effects of Fatty Acid Saturation and Chain Length on NASH in Juvenile Iberian Pigs. Curr. Dev. Nutr. 2020, 4, 682. [Google Scholar] [CrossRef]
- La Frano, M.; Hernandez, G.; Smith, V.; Columbus, D.; Peterson, D.; Fanter, R.; Kitts, C.; Burrin, D.; Maj, M.; Manjarin, R. Metabolomic Characterization of a Novel Pig Model of Pediatric Non-alcoholic Fatty Liver Disease (P08-131-19). Curr. Dev. Nutr. 2019, 3, nzz044.P08-131-19. [Google Scholar] [CrossRef]
- McCommis, K.S.; Finck, B.N. Treating Hepatic Steatosis and Fibrosis by Modulating Mitochondrial Pyruvate Metabolism. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.V.; Smith, V.A.; Melnyk, M.; Burd, M.A.; Sprayberry, K.A.; Edwards, M.S.; Peterson, D.G.; Bennet, D.C.; Fanter, R.K.; Columbus, D.A.; et al. Dysregulated FXR-FGF19 signaling and choline metabolism are associated with gut dysbiosis and hyperplasia in a novel pig model of pediatric NASH. Am. J. Physiol. Liver Physiol. 2020, 318, G582–G609. [Google Scholar] [CrossRef] [PubMed]
- Manjarín, R.; Suryawan, A.; Koo, S.-J.; Wilson, F.A.; Nguyen, H.V.; Davis, T.A.; Orellana, R.A. Insulin modulates energy and substrate sensing and protein catabolism induced by chronic peritonitis in skeletal muscle of neonatal pigs. Pediatr. Res. 2016, 80, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Otten, W.; Tuchscherer, M.; Tuchscherer, A.; Domanska, G.; Brenmoehl, J.; Günther, J.; Ohde, D.; Weitschies, W.; Seidlitz, A.; et al. Effects of 1-Methyltryptophan on Immune Responses and the Kynurenine Pathway after Lipopolysaccharide Challenge in Pigs. Int. J. Mol. Sci. 2018, 19, 3009. [Google Scholar] [CrossRef]
- Hernandez-García, A.D.; Columbus, D.A.; Manjarín, R.; Nguyen, H.V.; Suryawan, A.; Orellana, R.A.; Davis, T.A. Leucine supplementation stimulates protein synthesis and reduces degradation signal activation in muscle of newborn pigs during acute endotoxemia. Am. J. Physiol. Metab. 2016, 311, E791–E801. [Google Scholar] [CrossRef]
- Holt, D.B.; Delaney, R.R.; Uyehara, C.F.T. Effects of Combination Dobutamine and Vasopressin Therapy on Microcirculatory Blood Flow in a Porcine Model of Severe Endotoxic Shock. J. Surg. Res. 2011, 171, 191–198. [Google Scholar] [CrossRef]
- Klymiuk, N.; Blutke, A.; Graf, A.; Krause, S.; Burkhardt, K.; Wuensch, A.; Krebs, S.; Kessler, B.; Zakhartchenko, V.; Kurome, M.; et al. Dystrophin-deficient pigs provide new insights into the hierarchy of physiological derangements of dystrophic muscle. Hum. Mol. Genet. 2013, 22, 4368–4382. [Google Scholar] [CrossRef]
- Rogers, C.S.; Hao, Y.; Rokhlina, T.; Samuel, M.; Stoltz, D.A.; Li, Y.; Petroff, E.; Vermeer, D.W.; Kabel, A.C.; Yan, Z.; et al. Production of CFTR-null and CFTR-ΔF508 heterozygous pigs by adeno-associated virus–mediated gene targeting and somatic cell nuclear transfer. J. Clin. Investig. 2008, 118, 1571–1577. [Google Scholar] [CrossRef]
- Steines, B.; Dickey, D.D.; Bergen, J.; Excoffon, K.J.; Weinstein, J.R.; Li, X.; Yan, Z.; Alaiwa, M.H.A.; Shah, V.S.; Bouzek, D.C.; et al. CFTR gene transfer with AAV improves early cystic fibrosis pig phenotypes. JCI Insight 2016, 1, e88728. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.D.; Nicolas, C.T.; Allen, K.; Mao, S.; Elgilani, F.; Glorioso, J.; Amiot, B.; VanLith, C.; Guthman, R.; Du, Z.; et al. Autologous Gene and Cell Therapy Provides Safe and Long-Term Curative Therapy in A Large Pig Model of Hereditary Tyrosinemia Type 1. Cell Transplant. 2019, 28, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ishikawa, K.; Fish, K.; Oh, J.G.; Motloch, L.J.; Kohlbrenner, E.; Lee, P.; Xie, C.; Lee, A.; Liang, L.; et al. Protein Phosphatase Inhibitor-1 Gene Therapy in a Swine Model of Nonischemic Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 1744–1756. [Google Scholar] [CrossRef] [PubMed]
- Bode, G.; Clausing, P.; Gervais, F.; Loegsted, J.; Luft, J.; Nogues, V.; Sims, J. The utility of the minipig as an animal model in regulatory toxicology. J. Pharmacol. Toxicol. Methods 2010, 62, 196–220. [Google Scholar] [CrossRef] [PubMed]
- Helke, K.; Swindle, M.M. Animal models of toxicology testing: The role of pigs. Expert Opin. Drug Metab. Toxicol. 2012, 9, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hoberman, A.M.; Lewis, E.M. Pediatric Nonclinical Drug Testing; Wiley Online Library: Hoboken, NA, USA, 2012. [Google Scholar]
- Barrow, P.C. Use of the Swine Pediatric Model. In Pediatric Nonclinical Drug Testing; Wiley: Hoboken, NJ, USA, 2012; Volume 2012, pp. 213–229. [Google Scholar]
- Negro Silva, L.F.; Li, C.; de Seadi Pereira, P.J.B.; Tan, W.; Dubuc-Mageau, M.; Sanfacon, A.; Forster, R.; Tavcar, R.; Makin, A.; Authier, S. Biochemical and Electroretinographic Characterization of the Minipig Eye in the Context of Drug Safety Investigations. Int. J. Toxicol. 2019, 38, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Shrader, S.M.; Greentree, W.F. Göttingen Minipigs in Ocular Research. Toxicol. Pathol. 2018, 46, 403–407. [Google Scholar] [CrossRef]
- Zhong, M.; Shoemake, C.; Fuller, A.; White, D.; Hanks, C.; Brocksmith, D.; Liu, J.; Gad, S.; Bouchard, G.; Stricker-Krongrad, A. Development of a Functional Observational Battery in the Minipig for Regulatory Neurotoxicity Assessments. Int. J. Toxicol. 2017, 36, 113–123. [Google Scholar] [CrossRef]
- O’Shea, J.P. Mechanistic Understanding of Bioenabling Formulation Approaches to Improve Oral Bioavailability Using Porcine In Vivo And In Silico Models; University College Cork: Cork, Ireland, 2018. [Google Scholar]
- Henze, L.J.; Koehl, N.J.; O’Shea, J.P.; Holm, R.; Reppas, C.; Griffin, B.T. Toward the establishment of a standardized pre-clinical porcine model to predict food effects-Case studies on fenofibrate and paracetamol. Int. J. Pharm. X 2019, 1, 100017. [Google Scholar] [CrossRef]
- Henze, L.J.; Koehl, N.J.; O’Shea, J.; Kostewicz, E.S.; Holm, R.; Griffin, B.T. The pig as a preclinical model for predicting oral bioavailability and in vivo performance of pharmaceutical oral dosage forms: A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 581–602. [Google Scholar] [CrossRef]
- Helke, K.L.; Nelson, K.N.; Sargeant, A.M.; Jacob, B.; McKeag, S.; Haruna, J.; Vemireddi, V.; Greeley, M.; Brocksmith, D.; Navratil, N. Pigs in toxicology: Breed differences in metabolism and background findings. Toxicol. Pathol. 2016, 44, 575–590. [Google Scholar] [CrossRef]
- Feyen, B.; Penard, L.; Van Heerden, M.; Fant, P.; Marsden, E.; De Jonghe, S.; DeSmidt, M.; Mousa, S.M.; Bailey, G.P. “All pigs are equal” Does the background data from juvenile Göttingen minipigs support this? Reprod. Toxicol. 2016, 64, 105–115. [Google Scholar] [CrossRef]
- Ettrup, K.S.; Sørensen, J.C.; Bjarkam, C.R. The anatomy of the Göttingen minipig hypothalamus. J. Chem. Neuroanat. 2010, 39, 151–165. [Google Scholar] [CrossRef]
- Siefert, J.; Hillebrandt, K.H.; Kluge, M.; Geisel, D.; Podrabsky, P.; Denecke, T.; Nösser, M.; Gassner, J.; Reutzel-Selke, A.; Strücker, B.; et al. Computed tomography-based survey of the vascular anatomy of the juvenile Göttingen minipig. Lab. Anim. 2017, 51, 388–396. [Google Scholar] [CrossRef]
- Egli, J.; Schmucki, R.; Loos, B.; Reichl, S.; Grabole, N.; Roller, A.; Ebeling, M.; Odermatt, A.; Iglesias, A. The genomic organization and expression pattern of the low-affinity Fc gamma receptors (FcγR) in the Göttingen minipig. Immunogenetics 2018, 71, 123–136. [Google Scholar] [CrossRef]
- Shrader, S.M.; Mowry, R.N. Histomorphometric evaluation of the Göttingen minipig eye. Veter. Ophthalmol. 2019, 22, 872–878. [Google Scholar] [CrossRef]
- Ellemann-Laursen, S.; Marsden, E.; Peter, B.; Downes, N.; Coulby, D.; Grossi, A. The incidence of congenital malformations and variations in Göttingen minipigs. Reprod. Toxicol. 2016, 64, 162–168. [Google Scholar] [CrossRef]
- Kuper, C.F.; Ernst, H.; Van Oostrum, L.C.M.; Rittinghausen, S.; Penninks, A.H.; Ganderup, N.-C.; Wolterbeek, A.P.M. Nasal Passages of Göttingen Minipigs from the Neonatal Period to Young Adult. Toxicol. Pathol. 2012, 40, 656–666. [Google Scholar] [CrossRef]
- Vrolyk, V.; Desmarais, M.-J.; Lambert, D.; Haruna, J.; Benoit-Biancamano, M.-O. Neonatal and Juvenile Ocular Development in Göttingen Minipigs and Domestic Pigs: A Histomorphological and Immunohistochemical Study. Veter. Pathol. 2020, 57, 889–914. [Google Scholar] [CrossRef]
- Descotes, J.; Allais, L.; Ancian, P.; Pedersen, H.D.; Friry-Santini, C.; Iglesias, A.; Rubic-Schneider, T.; Skaggs, H.; Vestbjerg, P. Nonclinical evaluation of immunological safety in Göttingen Minipigs: The CONFIRM initiative. Regul. Toxicol. Pharmacol. 2018, 94, 271–275. [Google Scholar] [CrossRef]
- Jelsing, J.; Gundersen, H.J.G.; Nielsen, R.; Hemmingsen, R.; Pakkenberg, B. The postnatal development of cerebellar Purkinje cells in the Gottingen minipig estimated with a new stereological sampling technique-the vertical bar fractionator. J. Anat. 2006, 209, 321–331. [Google Scholar] [CrossRef]
- Peter, B.; De Rijk, E.P.C.T.; Zeltner, A.; Emmen, H.H. Sexual Maturation in the Female Göttingen Minipig. Toxicol. Pathol. 2016, 44, 482–485. [Google Scholar] [CrossRef]
- Haagensen, A.M.; Grand, N.; Klastrup, S.; Skytte, C.; Sørensen, D.B. Spatial discrimination and visual discrimination. Behav. Pharmacol. 2013, 24, 172–179. [Google Scholar] [CrossRef]
- Gauthier, B.E.; Penard, L.; Bordier, N.F.; Briffaux, J.-P.J.; Ruty, B.M. Specificities of the Skin Morphology in Juvenile Minipigs. Toxicol. Pathol. 2018, 46, 821–834. [Google Scholar] [CrossRef]
- Dincer, Z.; Piccicuto, V.; Walker, U.J.; Mahl, A.; McKeag, S. Spontaneous and Drug-induced Arteritis/Polyarteritis in the Göttingen Minipig—Review. Toxicol. Pathol. 2018, 46, 121–130. [Google Scholar] [CrossRef]
- Bjarkam, C.R.; Glud, A.N.; Orlowski, D.; Sørensen, J.C.H.; Palomero-Gallagher, N. The telencephalon of the Göttingen minipig, cytoarchitecture and cortical surface anatomy. Brain Struct. Funct. 2016, 222, 2093–2114. [Google Scholar] [CrossRef]
- Ramot, Y.; Weber, K.; Lobato, B.M.; Sánchez-Margallo, F.M.; Caro, J.F.G.; Gómez, L.D.; Shabat, R.; Nyska, A. Trauma as a Cause for Hepatopathy in Newborn Göttingen Minipigs. Toxicol. Pathol. 2016, 44, 1123–1127. [Google Scholar] [CrossRef]
- Langston, J.L.; Myers, T.M. VX toxicity in the Göttingen minipig. Toxicol. Lett. 2016, 264, 12–19. [Google Scholar] [CrossRef]
- Tang, H.; Mayersohn, M. Porcine Prediction of Pharmacokinetic Parameters in People: A Pig in a Poke? Drug Metab. Dispos. 2018, 46, 1712–1724. [Google Scholar] [CrossRef]
- Li, D.-P.; Zhang, W.-H.; Yang, M.-L.; Liu, C.-B.; Zhang, X.; Cai, C.; Li, J.J. An improved urethral catheterization in female pigs: A pilot study. Chin. Med. J. 2017, 130, 1880. [Google Scholar] [CrossRef]
- Albl, B.; Haesner, S.; Braun-Reichhart, C.; Streckel, E.; Renner, S.; Seeliger, F.; Wolf, E.; Wanke, R.; Blutke, A. Tissue Sampling Guides for Porcine Biomedical Models. Toxicol. Pathol. 2016, 44, 414–420. [Google Scholar] [CrossRef]
- Søndergaard, L.V.; Dagnaes-Hansen, F.; Herskin, M. Welfare assessment in porcine biomedical research-Suggestion for an operational tool. Res. Veter. Sci. 2011, 91, e1–e9. [Google Scholar] [CrossRef]
- Jones, K.; Harding, J.; Makin, A.; Singh, P.; Jacobsen, B.; Mikkelsen, L.F. Perspectives From the 12th Annual Minipig Research Forum: Early Inclusion of the Minipig in Safety Assessment Species Selection Should be the Standard Approach. Toxicol. Pathol. 2019, 47, 891–895. [Google Scholar] [CrossRef]
- Prims, S.; Tambuyzer, B.; Vergauwen, H.; Huygelen, V.; Van Cruchten, S.; Van Ginneken, C.; Casteleyn, C. Intestinal immune cell quantification and gram type classification of the adherent microbiota in conventionally and artificially reared, normal and low birth weight piglets. Livest. Sci. 2016, 185, 1–7. [Google Scholar] [CrossRef]
- Everaert, N.; Van Cruchten, S.; Weström, B.; Bailey, M.; Van Ginneken, C.; Thymann, T.; Pieper, R. A review on early gut maturation and colonization in pigs, including biological and dietary factors affecting gut homeostasis. Anim. Feed. Sci. Technol. 2017, 233, 89–103. [Google Scholar] [CrossRef]
- De Zwart, L.; Haenen, H.; Versantvoort, C.; Wolterink, G.; Van Engelen, J.; Sips, A. Role of biokinetics in risk assessment of drugs and chemicals in children. Regul. Toxicol. Pharmacol. 2004, 39, 282–309. [Google Scholar] [CrossRef]
- Miller, B.G.; James, P.S.; Smith, M.W.; Bourne, F.J. Effect of weaning on the capacity of pig intestinal villi to digest and absorb nutrients. J. Agric. Sci. 1986, 107, 579–590. [Google Scholar] [CrossRef]
- Prims, S.; Jurgens, B.; Hole, C.V.; Van Cruchten, S.; Van Ginneken, C.; Casteleyn, C. The porcine tonsils and Peyer’s patches: A stereological morphometric analysis in conventionally and artificially reared piglets. Veter. Immunol. Immunopathol. 2018, 206, 9–15. [Google Scholar] [CrossRef]
- Myer, M.S. The presence of Paneth cells confirmed in the pig. Onderstepoort J. Veter. Res. 1982, 49, 131–132. [Google Scholar]
- Verdile, N.; Mirmahmoudi, R.; Brevini, T.; Gandolfi, F. Evolution of pig intestinal stem cells from birth to weaning. Animal 2019, 13, 2830–2839. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Williamson, I.A.; Piedrahita, J.A.; Blikslager, A.T.; Magness, S.T. Cell Lineage Identification and Stem Cell Culture in a Porcine Model for the Study of Intestinal Epithelial Regeneration. PLoS ONE 2013, 8, e66465. [Google Scholar] [CrossRef]
- Ayuso, M.; Michiels, J.; Wuyts, S.; Yan, H.; DeGroote, J.; Lebeer, S.; Le Bourgot, C.; Apper, E.; Majdeddin, M.; Van Noten, N.; et al. Short-chain fructo-oligosaccharides supplementation to suckling piglets: Assessment of pre- and post-weaning performance and gut health. PLoS ONE 2020, 15, e0233910. [Google Scholar] [CrossRef] [PubMed]
- Leser, T.D.; Amenuvor, J.Z.; Jensen, T.K.; Lindecrona, R.H.; Boye, M.; Møller, K. Culture-Independent Analysis of Gut Bacteria: The Pig Gastrointestinal Tract Microbiota Revisited. Appl. Environ. Microbiol. 2002, 68, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Dufrane, D.; Gianello, P. Pig Islet Xenotransplantation Into Non-human Primate Model. Transplantation 2008, 86, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Ouyang, H.; Yu, T.; Chen, X.; Pang, D.; Tang, X.; Chen, C. Preparation of a new type 2 diabetic miniature pig model via the CRISPR/Cas9 system. Cell Death Dis. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Bakhti, M.; Böttcher, A.; Lickert, H. Modelling the endocrine pancreas in health and disease. Nat. Rev. Endocrinol. 2018, 15, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Sherman, P.; Rowland, L.M.; Wijtenburg, S.A.; Acheson, A.; Fieremans, E.; Veraart, J.; Novikov, D.S.; Hong, L.E.; Sladky, J.; et al. Miniature pig model of human adolescent brain white matter development. J. Neurosci. Methods 2018, 296, 99–108. [Google Scholar] [CrossRef]
- Andersen, B.B.; Nielsen, R.; Olsen, A.K.; Grand, N.; Hemmingsen, R.; Pakkenberg, B. The postnatal development of neocortical neurons and glial cells in the Gottingen minipig and the domestic pig brain. J. Exp. Biol. 2006, 209, 1454–1462. [Google Scholar] [CrossRef]
- Helke, K.L.; Nelson, K.N.; Sargeant, A.M.; Jacob, B.; McKeag, S.; Haruna, J.; Vemireddi, V.; Greeley, M.; Brocksmith, D.; Navratil, N.; et al. Background pathological changes in minipigs: A comparison of the incidence and nature among different breeds and populations of minipigs. Toxicol. Pathol. 2016, 44, 325–337. [Google Scholar] [CrossRef]
- Brix, N.; Ernst, A.; Lauridsen, L.L.B.; Parner, E.; Støvring, H.; Olsen, J.; Henriksen, T.B.; Ramlau-Hansen, C.H. Timing of puberty in boys and girls: A population-based study. Paediatr. Périnat. Epidemiol. 2018, 33, 70–78. [Google Scholar] [CrossRef]
- Lorenzen, E.; Follmann, F.; Jungersen, G.; Agerholm, J.S. A review of the human vs. porcine female genital tract and associated immune system in the perspective of using minipigs as a model of human genital Chlamydia infection. Veter. Res. 2015, 46, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; Van Bilsen, J.H.M.; Cnossen, H.; Houben, G.; Garthoff, J.; Wolterbeek, A. Development of immune organs and functioning in humans and test animals: Implications for immune intervention studies. Reprod. Toxicol. 2016, 64, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.S. Pubertal growth and epiphyseal fusion. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Cone, S.G.; Warren, P.B.; Fisher, M.B. Rise of the Pigs: Utilization of the Porcine Model to Study Musculoskeletal Biomechanics and Tissue Engineering During Skeletal Growth. Tissue Eng. Part C Methods 2017, 23, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Wernersson, R.; Schierup, M.H.; Jørgensen, F.G.; Gorodkin, J.; Panitz, F.; Staerfeldt, H.-H.; Christensen, O.F.; Mailund, T.; Hornshøj, H.; Klein, A.; et al. Pigs in sequence space: A 0.66X coverage pig genome survey based on shotgun sequencing. BMC Genom. 2005, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.M.; Bendixen, E. Pig proteomics: A review of a species in the crossroad between biomedical and food sciences. J. Proteom. 2012, 75, 4296–4314. [Google Scholar] [CrossRef] [PubMed]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The pig as an animal model for human pathologies: A proteomics perspective. Proteom. Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Panitz, F.; Stengaard, H.; Hornshøj, H.; Gorodkin, J.; Hedegaard, J.; Cirera, S.; Thomsen, B.; Madsen, L.B.; Høj, A.; Vingborg, R.K.; et al. SNP mining porcine ESTs with MAVIANT, a novel tool for SNP evaluation and annotation. Bioinformatics 2007, 23, i387–i391. [Google Scholar] [CrossRef]
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef]
- Hesselager, M.O.; Codrea, M.C.; Sun, Z.; Deutsch, E.W.; Bennike, T.B.; Stensballe, A.; Bundgaard, L.; Moritz, R.L.; Bendixen, E. The Pig PeptideAtlas: A resource for systems biology in animal production and biomedicine. Proteomics 2016, 16, 634–644. [Google Scholar] [CrossRef]
- Carpentier, S.C.; Panis, B.; Vertommen, A.; Swennen, R.; Sergeant, K.; Renaut, J.; Laukens, K.; Witters, E.; Samyn, B.; Devreese, B. Proteome analysis of non-model plants: A challenging but powerful approach. Mass Spectrom. Rev. 2008, 27, 354–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xi, Q.-Y.; Sun, J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wang, S.-B.; Shu, G.; Wang, L.-N.; Zhu, X.-T.; et al. Revelation of mRNAs and proteins in porcine milk exosomes by transcriptomic and proteomic analysis. BMC Veter. Res. 2017, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Zhu, X.-Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; Van Wijnen, A.J.; Lerman, L.O. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0174303. [Google Scholar] [CrossRef] [PubMed]
- Ramayo-Caldas, Y.; Ballester, M.; Sánchez, J.P.; González-Rodríguez, O.; Revilla, M.; Reyer, H.; Wimmers†, K.; Torrallardona, D.; Quintanilla, R. Integrative approach using liver and duodenum RNA-Seq data identifies candidate genes and pathways associated with feed efficiency in pigs. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Ropka-Molik, K.; Żukowski, K.; Eckert, R.; Gurgul, A.; Piórkowska, K.; Oczkowicz, M. Comprehensive analysis of the whole transcriptomes from two different pig breeds using RNA-Seq method. Anim. Genet. 2014, 45, 674–684. [Google Scholar] [CrossRef]
- Shang, P.; Wang, Z.; Chamba, Y.; Zhang, B.; Zhang, H.; Wu, C. A comparison of prenatal muscle transcriptome and proteome profiles between pigs with divergent growth phenotypes. J. Cell. Biochem. 2018, 120, 5277–5286. [Google Scholar] [CrossRef]
- Yang, H.; Xu, X.-L.; Ma, H.; Jiang, J. Integrative analysis of transcriptomics and proteomics of skeletal muscles of the Chinese indigenous Shaziling pig compared with the Yorkshire breed. BMC Genet. 2016, 17, 1–13. [Google Scholar] [CrossRef]
- Ayuso, M.; Fernández, A.; Núñez, Y.; Benítez, R.; Isabel, B.; Barragán, C.; Fernández, A.I.; Rey, A.I.; Medrano, J.F.; Cánovas, Á.; et al. Comparative Analysis of Muscle Transcriptome between Pig Genotypes Identifies Genes and Regulatory Mechanisms Associated to Growth, Fatness and Metabolism. PLoS ONE 2015, 10, e0145162. [Google Scholar] [CrossRef]
- Ayuso, M.; Fernández, A.; Núñez, Y.; Benítez, R.; Isabel, B.; Fernández, A.I.; Rey, A.I.; González-Bulnes, A.; Medrano, J.F.; Cánovas, Á.; et al. Developmental Stage, Muscle and Genetic Type Modify Muscle Transcriptome in Pigs: Effects on Gene Expression and Regulatory Factors Involved in Growth and Metabolism. PLoS ONE 2016, 11, e0167858. [Google Scholar] [CrossRef]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R.K. Invited Review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef]
- Minipigs, G. Available online: https://minipigs.dk/knowledge-base/educational-package (accessed on 2 November 2020).
- Minipigs, G. Available online: https://minipigs.dk/news-events/courses (accessed on 2 November 2020).
- Schmitt, G.; Barrow, P.; Stephan-Gueldner, M. The Nonhuman Primate in Nonclinical Drug Development and Safety Assessment; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 337–355. [Google Scholar]
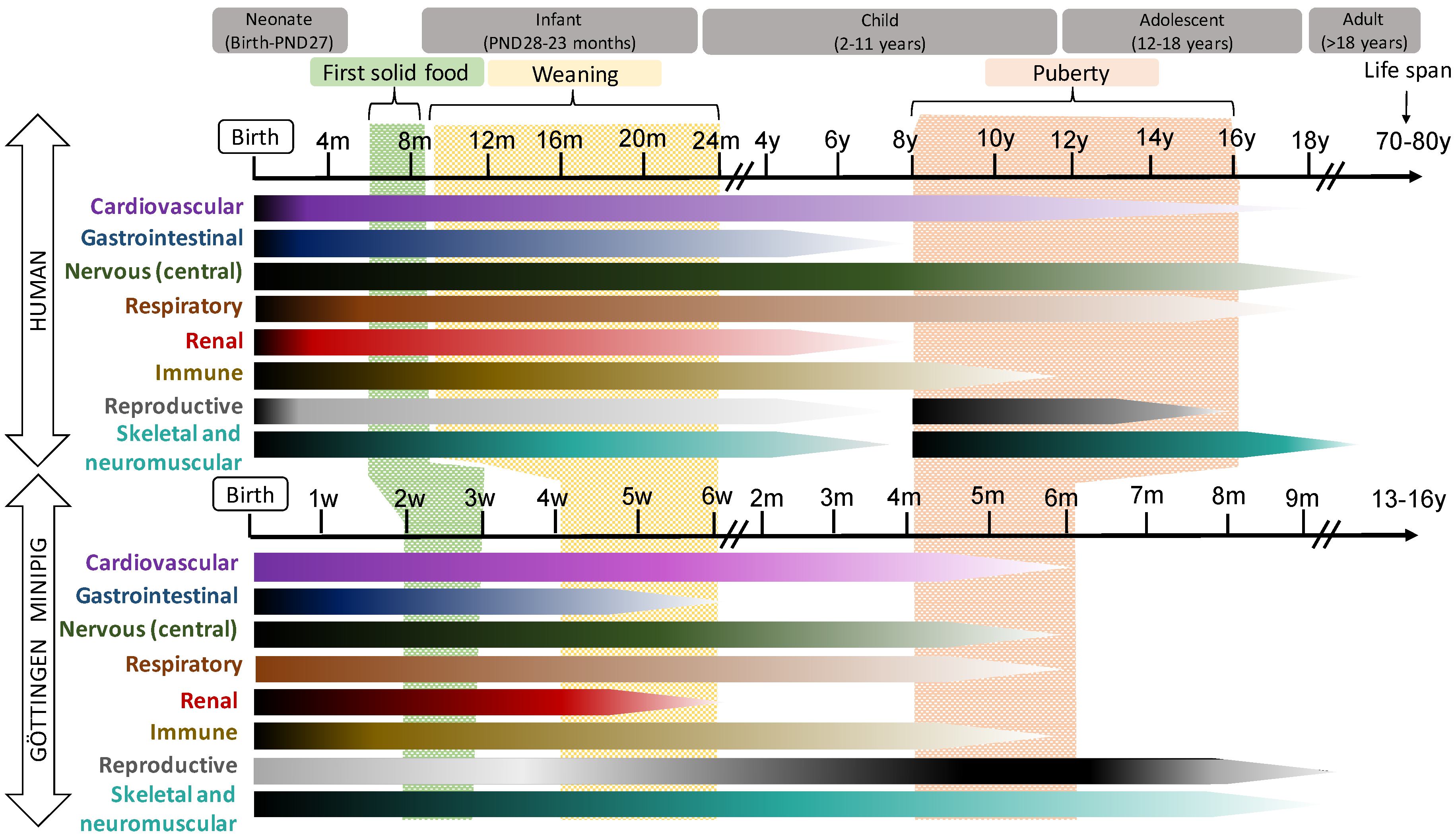
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayuso, M.; Buyssens, L.; Stroe, M.; Valenzuela, A.; Allegaert, K.; Smits, A.; Annaert, P.; Mulder, A.; Carpentier, S.; Van Ginneken, C.; et al. The Neonatal and Juvenile Pig in Pediatric Drug Discovery and Development. Pharmaceutics 2021, 13, 44. https://doi.org/10.3390/pharmaceutics13010044
Ayuso M, Buyssens L, Stroe M, Valenzuela A, Allegaert K, Smits A, Annaert P, Mulder A, Carpentier S, Van Ginneken C, et al. The Neonatal and Juvenile Pig in Pediatric Drug Discovery and Development. Pharmaceutics. 2021; 13(1):44. https://doi.org/10.3390/pharmaceutics13010044
Chicago/Turabian StyleAyuso, Miriam, Laura Buyssens, Marina Stroe, Allan Valenzuela, Karel Allegaert, Anne Smits, Pieter Annaert, Antonius Mulder, Sebastien Carpentier, Chris Van Ginneken, and et al. 2021. "The Neonatal and Juvenile Pig in Pediatric Drug Discovery and Development" Pharmaceutics 13, no. 1: 44. https://doi.org/10.3390/pharmaceutics13010044
APA StyleAyuso, M., Buyssens, L., Stroe, M., Valenzuela, A., Allegaert, K., Smits, A., Annaert, P., Mulder, A., Carpentier, S., Van Ginneken, C., & Van Cruchten, S. (2021). The Neonatal and Juvenile Pig in Pediatric Drug Discovery and Development. Pharmaceutics, 13(1), 44. https://doi.org/10.3390/pharmaceutics13010044










