Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives
Abstract
1. Introduction
2. Intraocular Pharmacokinetics of Current Intravitreal Drugs
| Drug | Species | Molecular Weight (kDa) | Intravitreal t1/2 (day) | Reference |
|---|---|---|---|---|
| Pegaptanib | Rhesus monkey | 40 | 3.92 | Drolet et al. [44] |
| Ranibizumab | New Zealand rabbit | 48 | 2.51 (vitrectomized) | Ahn et al. [30] |
| 2.75 (nonvitrectomized) | ||||
| 2.18 | Park et al. [34] | |||
| 3.0 | Gaudreault et al. [10] | |||
| 3.2 | Shatz et al. [45] | |||
| Dutch belted rabbit | 2.88 2.81 | Bakri et al. [8] Christoforidis et al. [46] | ||
| Cynomolgus monkey | 1.4 (vitrectomized) | Niwa et al. [31] | ||
| 2.3 (nonvitrectomized) | ||||
| 2.54 | Gaudreault et al. [47] | |||
| 2.63 | ||||
| Owl monkey | 2.73 | Christoforidis et al. [48] | ||
| Human | 7.19 (nonvitrectomized) | Krohne et al. [27] | ||
| Bevacizumab | New Zealand rabbit | 149 | 7.56 | Ahn et al. [33] |
| 6.51 | Sinapis et al. [49] | |||
| Dutch belted rabbit | 4.32 | Bakri et al. [9] | ||
| 6.0 | Nomoto et al. [50] | |||
| 4.22 | Christoforidis et al. [46] | |||
| Owl monkey | 3.60 | Christoforidis et al. [48] | ||
| Human | 9.82 (nonvitrectomized) | Krohne et al. [26] | ||
| 11.67 | Meyer et al. [51] | |||
| Aflibercept | New Zealand rabbit | 145 | 3.92 | Park et al. [34] |
| Cynomolgus monkey | 1.5 (vitrectomized) 2.2 (nonvitrectomized) | Niwa et al. [31] | ||
| Owl monkey | 2.44 | Christoforidis et al. [48] | ||
| Abicipar pegol | Human | 34 | ≥13 days | Campochiaro et al. [52] |
| Brolucizumab | New Zealand rabbit | 26 | 2.94 | FDA review [36] |
| Cynomolgus monkey | 2.08 | |||
| Human | 5 ± 2 | |||
| Faricimab | Cynomolgus monkey | 150 | 2.83 (aqueous) | Regula et al. [37] |
| Conbercept | Chinchilla rabbit | 143 | 4.24 | Li et al. [38] |
3. Efforts to Enhance the Intraocular Pharmacokinetics and Pharmacodynamics of Intravitreal Drugs
3.1. Dose Escalation of Intravitreal Drugs
3.2. Increasing the Molecular Weight of Intravitreal Drug Molecules
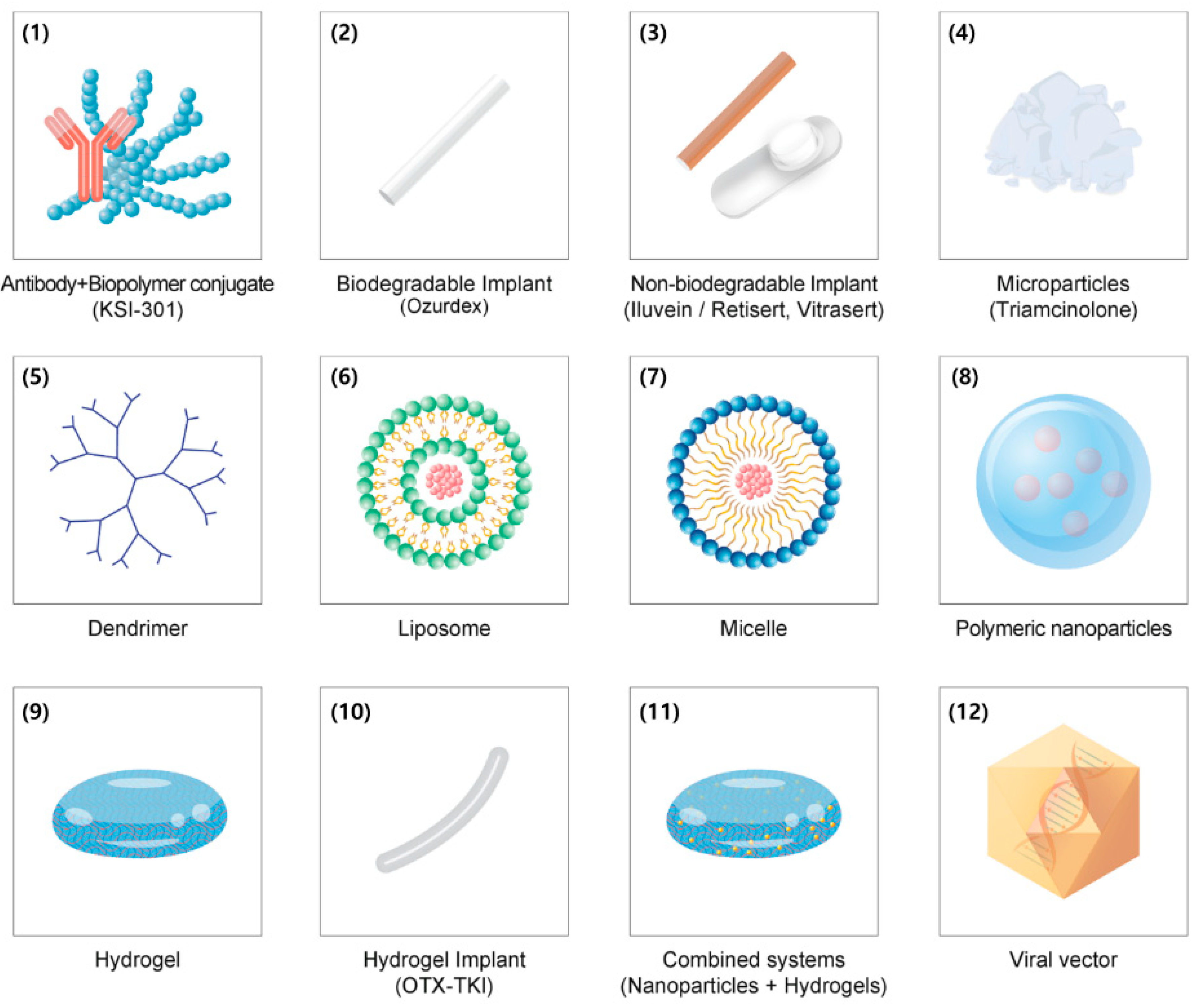
3.3. Sustained-Release Intravitreal Implants
3.4. Micro- and Nanoparticles
3.4.1. Microparticles
3.4.2. Dendrimer
3.4.3. Liposome
3.4.4. Polymeric Micelles
3.4.5. Polymeric Nanoparticles
3.4.6. Solid Lipid Nanoparticles
3.4.7. Coated Nanoparticles
3.4.8. Inorganic Nanoparticles
3.5. Hydrogels
3.6. Combined Drug Delivery Systems
3.7. Port Delivery Systems
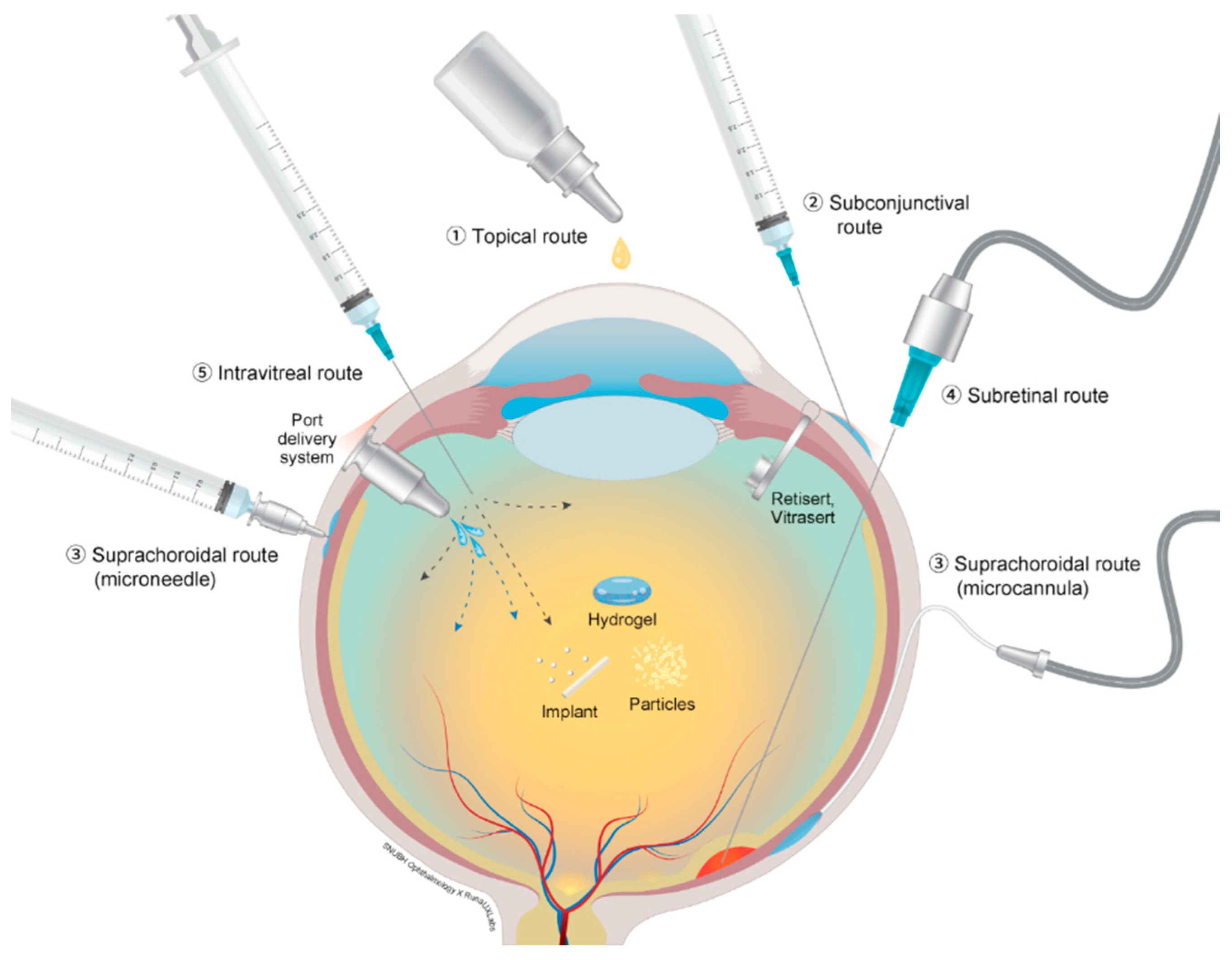
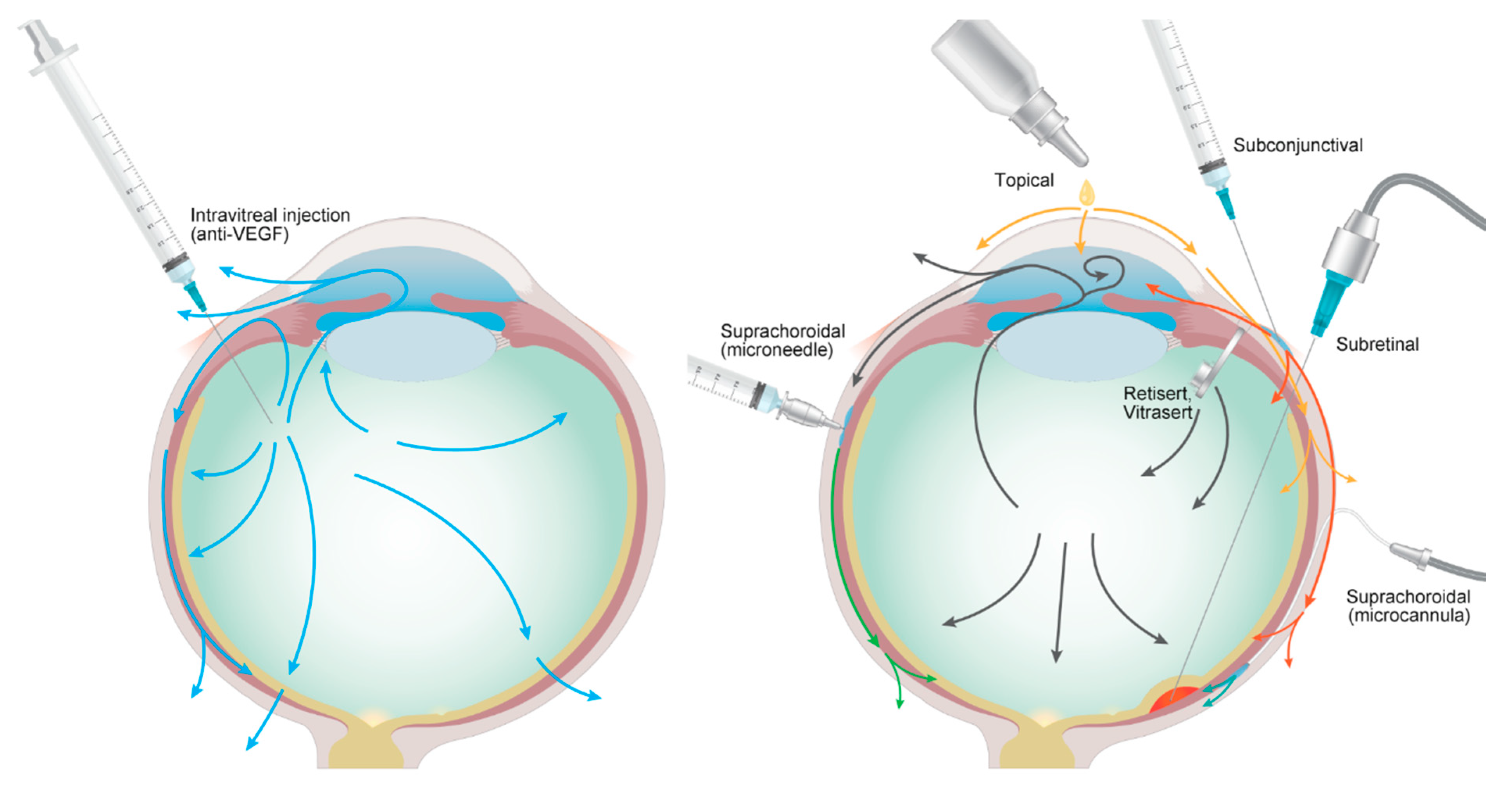
4. Drug Administration Routes besides the Intravitreal Route
4.1. Topical Routes
4.2. Subconjunctival and Subtenon Routes
4.3. Suprachoroidal Routes
4.4. Subretinal Routes
4.5. Trans-Scleral Routes
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; Group, M.S. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Antoszyk, A.N.; Pavan, P.R.; Leff, S.R.; Rosenfeld, P.J.; Ciulla, T.A.; Dreyer, R.F.; Gentile, R.C.; Sy, J.P.; Hantsbarger, G.; et al. Ranibizumab for treatment of neovascular age-related macular degeneration: A phase I/II multicenter, controlled, multidose study. Ophthalmology 2006, 113, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C.M.; Miller, J.W. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr. Opin. Ophthalmol. 2007, 18, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Campochiaro, P.A.; Singh, R.P.; Li, Z.; Gray, S.; Saroj, N.; Rundle, A.C.; Rubio, R.G.; Murahashi, W.Y.; Investigators, C. Ranibizumab for macular edema following central retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology 2010, 117, 1124–1133.e1121. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Campochiaro, P.A.; Bhisitkul, R.B.; Ho, A.C.; Gray, S.; Saroj, N.; Adamis, A.P.; Rubio, R.G.; Murahashi, W.Y. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011, 118, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Group, C.R.; Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef]
- Ferrara, N.; Damico, L.; Shams, N.; Lowman, H.; Kim, R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006, 26, 859–870. [Google Scholar] [CrossRef]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Ezzat, M.K.; Singh, R.J. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 2007, 114, 2179–2182. [Google Scholar] [CrossRef]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Singh, R.J. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007, 114, 855–859. [Google Scholar] [CrossRef]
- Gaudreault, J.; Fei, D.; Beyer, J.C.; Ryan, A.; Rangell, L.; Shiu, V.; Damico, L.A. Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina 2007, 27, 1260–1266. [Google Scholar] [CrossRef]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K.; Group, S.-U.S. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.C.; Campain, A.; Barthelmes, D.; Simpson, J.M.; Arnold, J.J.; Guymer, R.H.; McAllister, I.L.; Essex, R.W.; Morlet, N.; Hunyor, A.P.; et al. Long-Term Outcomes of Treatment of Neovascular Age-Related Macular Degeneration: Data from an Observational Study. Ophthalmology 2015, 122, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Tadayoni, R.; Beatty, S.; Berger, A.; Cereda, M.G.; Cortez, R.; Hoyng, C.B.; Hykin, P.; Staurenghi, G.; Heldner, S.; et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br. J. Ophthalmol. 2015, 99, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.; Tufail, A.; Daien, V.; Lee, A.Y.; Nguyen, V.; Ozturk, M.; Barthelmes, D.; Gillies, M.C. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog. Retin. Eye Res. 2018, 65, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Chen, E.; Mariani, A.; Major, J.C., Jr.; Group, S.S. Super-dose anti-VEGF (SAVE) trial: 2.0 mg intravitreal ranibizumab for recalcitrant neovascular macular degeneration-primary end point. Ophthalmology 2013, 120, 349–354. [Google Scholar] [CrossRef]
- Busbee, B.G.; Ho, A.C.; Brown, D.M.; Heier, J.S.; Suner, I.J.; Li, Z.; Rubio, R.G.; Lai, P.; Group, H.S. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013, 120, 1046–1056. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Brown, D.M.; Croft, D.E.; Wong, T.P. Two Year SAVE Outcomes: 2.0 mg ranibizumab for recalcitrant neovascular AMD. Ophthalmology 2013, 120, 1945–1946.e1941. [Google Scholar] [CrossRef]
- Ho, A.C.; Busbee, B.G.; Regillo, C.D.; Wieland, M.R.; Van Everen, S.A.; Li, Z.; Rubio, R.G.; Lai, P.; Group, H.S. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014, 121, 2181–2192. [Google Scholar] [CrossRef]
- Kompella, U.B.; Amrite, A.C.; Pacha Ravi, R.; Durazo, S.A. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog. Retin. Eye Res. 2013, 36, 172–198. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Osswald, C.R.; Mieler, W.F. Advances in ocular drug delivery: Emphasis on the posterior segment. Expert Opin. Drug. Deliv. 2014, 11, 1647–1660. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Dosmar, E.; Liu, W.; Mieler, W.F. Extended ocular drug delivery systems for the anterior and posterior segments: Biomaterial options and applications. Expert Opin. Drug. Deliv. 2017, 14, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat-Binstock, E.; Peer, J.; Domb, A.J. New techniques for drug delivery to the posterior eye segment. Pharm. Res. 2010, 27, 530–543. [Google Scholar] [CrossRef]
- Rafiei, F.; Tabesh, H.; Farzad, F. Sustained subconjunctival drug delivery systems: Current trends and future perspectives. Int. Ophthalmol. 2020, 40, 2385–2401. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernandez, R.; Diaz-Tome, V.; Luaces-Rodriguez, A.; Conde-Penedo, A.; Garcia-Otero, X.; Luzardo-Alvarez, A.; Fernandez-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Krohne, T.U.; Eter, N.; Holz, F.G.; Meyer, C.H. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am. J. Ophthalmol. 2008, 146, 508–512. [Google Scholar] [CrossRef]
- Krohne, T.U.; Liu, Z.; Holz, F.G.; Meyer, C.H. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am. J. Ophthalmol. 2012, 154, 682–686.e682. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, H.; Woo, S.J.; Park, J.H.; Park, S.; Hwang, D.J.; Park, K.H. Pharmacokinetics of intravitreally injected bevacizumab in vitrectomized eyes. J. Ocul. Pharmacol. Ther. 2013, 29, 612–618. [Google Scholar] [CrossRef]
- Xu, L.; Lu, T.; Tuomi, L.; Jumbe, N.; Lu, J.; Eppler, S.; Kuebler, P.; Damico-Beyer, L.A.; Joshi, A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: A population approach. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1616–1624. [Google Scholar] [CrossRef]
- Ahn, S.J.; Ahn, J.; Park, S.; Kim, H.; Hwang, D.J.; Park, J.H.; Park, J.Y.; Chung, J.Y.; Park, K.H.; Woo, S.J. Intraocular pharmacokinetics of ranibizumab in vitrectomized versus nonvitrectomized eyes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 567–573. [Google Scholar] [CrossRef]
- Niwa, Y.; Kakinoki, M.; Sawada, T.; Wang, X.; Ohji, M. Ranibizumab and Aflibercept: Intraocular Pharmacokinetics and Their Effects on Aqueous VEGF Level in Vitrectomized and Nonvitrectomized Macaque Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6501–6505. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Oh, J.; Kim, Y.K.; Park, J.H.; Park, J.Y.; Hong, H.K.; Park, K.H.; Lee, J.E.; Kim, H.M.; Chung, J.Y.; et al. Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-Trap in a rabbit model. Eye 2015, 29, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Hong, H.K.; Na, Y.M.; Park, S.J.; Ahn, J.; Oh, J.; Chung, J.Y.; Park, K.H.; Woo, S.J. Use of Rabbit Eyes in Pharmacokinetic Studies of Intraocular Drugs. J. Vis. Exp. 2016, 113, e53878. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Choi, Y.; Na, Y.M.; Hong, H.K.; Park, J.Y.; Park, K.H.; Chung, J.Y.; Woo, S.J. Intraocular Pharmacokinetics of Intravitreal Aflibercept (Eylea) in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Park, K.H.; Chung, J.Y.; Woo, S.J. A Prediction Model for the Intraocular Pharmacokinetics of Intravitreally Injected Drugs Based on Molecular Physicochemical Properties. Ophthalmic. Res. 2020, 63, 41–49. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research Application Number 761125Orig1s000, Clinical Pharmacology Reviews. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761125Orig1s000ClinPharmR.pdf (accessed on 13 October 2020).
- Regula, J.T.; von Leithner, P.L.; Foxton, R.; Barathi, V.A.; Cheung, C.M.; Bo Tun, S.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288. [Google Scholar] [CrossRef]
- Li, H.; Lei, N.; Zhang, M.; Li, Y.; Xiao, H.; Hao, X. Pharmacokinetics of a long-lasting anti-VEGF fusion protein in rabbit. Exp. Eye Res. 2012, 97, 154–159. [Google Scholar] [CrossRef]
- Van Deemter, M.; Kuijer, R.; Harm Pas, H.; van der Worp, R.J.; Hooymans, J.M.; Los, L.I. Trypsin-mediated enzymatic degradation of type II collagen in the human vitreous. Mol. Vis. 2013, 19, 1591–1599. [Google Scholar]
- Hutton-Smith, L.A.; Gaffney, E.A.; Byrne, H.M.; Maini, P.K.; Schwab, D.; Mazer, N.A. A Mechanistic Model of the Intravitreal Pharmacokinetics of Large Molecules and the Pharmacodynamic Suppression of Ocular Vascular Endothelial Growth Factor Levels by Ranibizumab in Patients with Neovascular Age-Related Macular Degeneration. Mol. Pharm. 2016, 13, 2941–2950. [Google Scholar] [CrossRef]
- Hutton-Smith, L.A.; Gaffney, E.A.; Byrne, H.M.; Maini, P.K.; Gadkar, K.; Mazer, N.A. Ocular Pharmacokinetics of Therapeutic Antibodies Given by Intravitreal Injection: Estimation of Retinal Permeabilities Using a 3-Compartment Semi-Mechanistic Model. Mol. Pharm. 2017, 14, 2690–2696. [Google Scholar] [CrossRef]
- Hutton-Smith, L.A.; Gaffney, E.A.; Byrne, H.M.; Caruso, A.; Maini, P.K.; Mazer, N.A. Theoretical Insights into the Retinal Dynamics of Vascular Endothelial Growth Factor in Patients Treated with Ranibizumab, Based on an Ocular Pharmacokinetic/Pharmacodynamic Model. Mol. Pharm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.J.; Muether, P.S.; Fauser, S. A model of the ocular pharmacokinetics involved in the therapy of neovascular age-related macular degeneration with ranibizumab. Br. J. Ophthalmol. 2015, 99, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Drolet, D.W.; Nelson, J.; Tucker, C.E.; Zack, P.M.; Nixon, K.; Bolin, R.; Judkins, M.B.; Farmer, J.A.; Wolf, J.L.; Gill, S.C.; et al. Pharmacokinetics and safety of an anti-vascular endothelial growth factor aptamer (NX1838) following injection into the vitreous humor of rhesus monkeys. Pharm. Res. 2000, 17, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Shatz, W.; Hass, P.E.; Mathieu, M.; Kim, H.S.; Leach, K.; Zhou, M.; Crawford, Y.; Shen, A.; Wang, K.; Chang, D.P.; et al. Contribution of Antibody Hydrodynamic Size to Vitreal Clearance Revealed through Rabbit Studies Using a Species-Matched Fab. Mol. Pharm. 2016, 13, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, J.B.; Carlton, M.M.; Knopp, M.V.; Hinkle, G.H. PET/CT imaging of I-124-radiolabeled bevacizumab and ranibizumab after intravitreal injection in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5899–5903. [Google Scholar] [CrossRef]
- Gaudreault, J.; Fei, D.; Rusit, J.; Suboc, P.; Shiu, V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Investig. Ophthalmol. Vis. Sci. 2005, 46, 726–733. [Google Scholar] [CrossRef]
- Christoforidis, J.B.; Briley, K.; Binzel, K.; Bhatia, P.; Wei, L.; Kumar, K.; Knopp, M.V. Systemic Biodistribution and Intravitreal Pharmacokinetic Properties of Bevacizumab, Ranibizumab, and Aflibercept in a Nonhuman Primate Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5636–5645. [Google Scholar] [CrossRef]
- Sinapis, C.I.; Routsias, J.G.; Sinapis, A.I.; Sinapis, D.I.; Agrogiannis, G.D.; Pantopoulou, A.; Theocharis, S.E.; Baltatzis, S.; Patsouris, E.; Perrea, D. Pharmacokinetics of intravitreal bevacizumab (Avastin(R)) in rabbits. Clin. Ophthalmol. 2011, 5, 697–704. [Google Scholar] [CrossRef]
- Nomoto, H.; Shiraga, F.; Kuno, N.; Kimura, E.; Fujii, S.; Shinomiya, K.; Nugent, A.K.; Hirooka, K.; Baba, T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4807–4813. [Google Scholar] [CrossRef]
- Meyer, C.H.; Krohne, T.U.; Holz, F.G. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina 2011, 31, 1877–1884. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Channa, R.; Berger, B.B.; Heier, J.S.; Brown, D.M.; Fiedler, U.; Hepp, J.; Stumpp, M.T. Treatment of diabetic macular edema with a designed ankyrin repeat protein that binds vascular endothelial growth factor: A phase I/II study. Am. J. Ophthalmol. 2013, 155, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Abraham, P.; Sarraf, D.; Nuthi, A.S.; Lin, S.G.; McCannel, C.A. Earlier therapeutic effects associated with high dose (2.0 mg) Ranibizumab for treatment of vascularized pigment epithelial detachments in age-related macular degeneration. Eye 2015, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Sepah, Y.J.; Sadiq, M.A.; Boyer, D.; Callanan, D.; Gallemore, R.; Bennett, M.; Marcus, D.; Halperin, L.; Hassan, M.; Campochiaro, P.A.; et al. Twenty-four-Month Outcomes of the Ranibizumab for Edema of the Macula in Diabetes–Protocol 3 with High Dose (READ-3) Study. Ophthalmology 2016, 123, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Park, Y.J.; Lee, S.; Son, J.Y.; Hong, H.K.; Ham, M.H.; Jin, X.; Chung, J.Y.; Park, K.H.; Park, K.D.; et al. Intraocular Pharmacokinetics of 10-fold Intravitreal Ranibizumab Injection Dose in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Dugel, P.U.; Weissgerber, G.; Hamilton, R.; Silva, R.; Bandello, F.; Larsen, M.; Weichselberger, A.; Wenzel, A.; Schmidt, A.; et al. Single-Chain Antibody Fragment VEGF Inhibitor RTH258 for Neovascular Age-Related Macular Degeneration: A Randomized Controlled Study. Ophthalmology 2016, 123, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Jaffe, G.J.; Sallstig, P.; Warburton, J.; Weichselberger, A.; Wieland, M.; Singerman, L. Brolucizumab Versus Aflibercept in Participants with Neovascular Age-Related Macular Degeneration: A Randomized Trial. Ophthalmology 2017, 124, 1296–1304. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Rimpela, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- KODIAK. A Study to Evaluate the Efficacy and Safety of KSI-301, an Anti-VEGF Antibody Biopolymer Conjugate, Versus Aflibercept in Patients with Neovascular (Wet) Age-Related Macular Degeneration. Available online: https://kodiak.com/our-pipeline/ (accessed on 29 September 2020).
- ARVO Annual Meeting Abstract, J. Updated Results of Phase 1b Study of KSI-301, an Anti-VEGF Antibody Biopolymer Conjugate with Extended Durability, in wAMD, DME, and RVO. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2769586 (accessed on 29 September 2020).
- Jaffe, G.J.; McCallum, R.M.; Branchaud, B.; Skalak, C.; Butuner, Z.; Ashton, P. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology 2005, 112, 1192–1198. [Google Scholar] [CrossRef]
- Kuppermann, B.D.; Blumenkranz, M.S.; Haller, J.A.; Williams, G.A.; Weinberg, D.V.; Chou, C.; Whitcup, S.M.; Dexamethasone, D.D.S.P.I.I.S.G. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch. Ophthalmol. 2007, 125, 309–317. [Google Scholar] [CrossRef]
- Kane, F.E.; Burdan, J.; Cutino, A.; Green, K.E. Iluvien: A new sustained delivery technology for posterior eye disease. Expert Opin. Drug. Deliv. 2008, 5, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann, B.D.; Patel, S.S.; Boyer, D.S.; Augustin, A.J.; Freeman, W.R.; Kerr, K.J.; Guo, Q.; Schneider, S.; Lopez, F.J. Phase 2 Study of the Safety and Efficacy of Brimonidine Drug Delivery System (Brimo Dds) Generation 1 in Patients with Geographic Atrophy Secondary to Age-Related Macular Degeneration. Retina 2020. [Google Scholar] [CrossRef] [PubMed]
- Peeters, L.; Sanders, N.N.; Braeckmans, K.; Boussery, K.; Van de Voorde, J.; De Smedt, S.C.; Demeester, J. Vitreous: A barrier to nonviral ocular gene therapy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3553–3561. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wang, J.; Zhao, Y.; Chen, D.; Zhu, C.; Liu, J.; Gan, Y. Hyaluronan-modified core-shell liponanoparticles targeting CD44-positive retinal pigment epithelium cells via intravitreal injection. Biomaterials 2013, 34, 5978–5987. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.S.; Rupenthal, I.D. Hyaluronic Acid Coated Albumin Nanoparticles for Targeted Peptide Delivery to the Retina. Mol. Pharm. 2017, 14, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Ha, S.; Hong, H.K.; Hwang, Y.; Kim, P.; Yang, E.; Chung, J.Y.; Park, S.; Park, Y.J.; Park, K.H.; et al. Intraocular Distribution and Kinetics of Intravitreally Injected Antibodies and Nanoparticles in Rabbit Eyes. Transl. Vis. Sci. Technol. 2020, 9, 20. [Google Scholar] [CrossRef]
- Chen, H.; Sun, S.M.; Li, J.; Du, W.N.; Zhao, C.H.; Hou, J.P.; Xu, Y.; Cheng, L.Y. Different Intravitreal Properties of Three Triamcinolone Formulations and Their Possible Impact on Retina Practice. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2178–2185. [Google Scholar] [CrossRef]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef]
- Missel, P.J.; Horner, M.; Muralikrishnan, R. Simulating dissolution of intravitreal triamcinolone acetonide suspensions in an anatomically accurate rabbit eye model. Pharm. Res. 2010, 27, 1530–1546. [Google Scholar] [CrossRef]
- Beer, P.M.; Bakri, S.J.; Singh, R.J.; Liu, W.; Peters, G.B., 3rd; Miller, M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology 2003, 110, 681–686. [Google Scholar] [CrossRef]
- Kim, H.; Csaky, K.G.; Gravlin, L.; Yuan, P.; Lutz, R.J.; Bungay, P.M.; Tansey, G.; de Monasterio, F.; Potti, G.K.; Grimes, G.; et al. Safety and pharmacokinetics of a preservative-free triamcinolone acetonide formulation for intravitreal administration. Retina 2006, 26, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Dib, E.; Maia, M.; Lima Ade, S.; de Paula Fiod Costa, E.; de Moraes-Filho, M.N.; Rodrigues, E.B.; Penha, F.M.; Coppini, L.P.; de Barros, N.M.; Coimbra Rde, C.; et al. In vivo, in vitro toxicity and in vitro angiogenic inhibition of sunitinib malate. Curr. Eye Res. 2012, 37, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, F.; Hiemstra, C.; Steendam, R.; Kazazi-Hyseni, F.; Van Nostrum, C.F.; Storm, G.; Kiessling, F.; Lammers, T.; Hennink, W.E.; Kok, R.J. Sunitinib microspheres based on [PDLLA-PEG-PDLLA]-b-PLLA multi-block copolymers for ocular drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Narvekar, P.; Lalani, R.; Chougule, M.B.; Pathak, Y.; Sutariya, V. An in vitro Assessment of Thermo-Reversible Gel Formulation Containing Sunitinib Nanoparticles for Neovascular Age-Related Macular Degeneration. AAPS PharmSciTech 2019, 20, 281. [Google Scholar] [CrossRef] [PubMed]
- Tsujinaka, H.; Fu, J.; Shen, J.; Yu, Y.; Hafiz, Z.; Kays, J.; McKenzie, D.; Cardona, D.; Culp, D.; Peterson, W.; et al. Sustained treatment of retinal vascular diseases with self-aggregating sunitinib microparticles. Nat. Commun. 2020, 11, 694. [Google Scholar] [CrossRef]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef]
- Marano, R.J.; Toth, I.; Wimmer, N.; Brankov, M.; Rakoczy, P.E. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: A long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene Ther. 2005, 12, 1544–1550. [Google Scholar] [CrossRef]
- Iezzi, R.; Guru, B.R.; Glybina, I.V.; Mishra, M.K.; Kennedy, A.; Kannan, R.M. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials 2012, 33, 979–988. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Mishra, M.K.; Mastorakos, P.; Oh, Y.; Lutty, G.A.; Kannan, R.M. Intracellular delivery of dendrimer triamcinolone acetonide conjugates into microglial and human retinal pigment epithelial cells. Eur. J. Pharm. Biopharm. 2015, 95, 239–249. [Google Scholar] [CrossRef]
- Yavuz, B.; Bozdag Pehlivan, S.; Sumer Bolu, B.; Nomak Sanyal, R.; Vural, I.; Unlu, N. Dexamethasone—PAMAM dendrimer conjugates for retinal delivery: Preparation, characterization and in vivo evaluation. J. Pharm. Pharmacol. 2016, 68, 1010–1020. [Google Scholar] [CrossRef]
- Dabkowska, M.; Roginska, D.; Klos, P.; Sobus, A.; Adamczak, M.; Litwinska, Z.; Machalinska, A.; Machalinski, B. Electrostatic complex of neurotrophin 4 with dendrimer nanoparticles: Controlled release of protein in vitro and in vivo. Int. J. Nanomed. 2019, 14, 6117–6131. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Li, L.; Han, M.; Tang, S.; Wang, T.; Han, J.; He, X.; He, X.; Wang, A.; et al. A novel dendrimer-based complex co-modified with cyclic RGD hexapeptide and penetratin for noninvasive targeting and penetration of the ocular posterior segment. Drug Deliv. 2019, 26, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Hu, C.; Wei, H.; Lu, Y.; Zhang, Y.; Yang, J.; Yun, G.; Zou, W.; Song, B. Intravitreal pharmacokinetics of liposome-encapsulated amikacin in a rabbit model. Ophthalmology 1993, 100, 1640–1644. [Google Scholar] [CrossRef]
- Budai, L.; Hajdu, M.; Budai, M.; Grof, P.; Beni, S.; Noszal, B.; Klebovich, I.; Antal, I. Gels and liposomes in optimized ocular drug delivery: Studies on ciprofloxacin formulations. Int. J. Pharm. 2007, 343, 34–40. [Google Scholar] [CrossRef]
- Habib, F.S.; Fouad, E.A.; Abdel-Rhaman, M.S.; Fathalla, D. Liposomes as an ocular delivery system of fluconazole: In-Vitro studies. Acta Ophthalmol. 2010, 88, 901–904. [Google Scholar] [CrossRef]
- Abrishami, M.; Zarei-Ghanavati, S.; Soroush, D.; Rouhbakhsh, M.; Jaafari, M.R.; Malaekeh-Nikouei, B. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (avastin) for intravitreal administration. Retina 2009, 29, 699–703. [Google Scholar] [CrossRef]
- Liu, H.A.; Liu, Y.L.; Ma, Z.Z.; Wang, J.C.; Zhang, Q. A lipid nanoparticle system improves siRNA efficacy in RPE cells and a laser-induced murine CNV model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4789–4794. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- York, A.W.; Kirkland, S.E.; McCormick, C.L. Advances in the synthesis of amphiphilic block copolymers via RAFT polymerization: Stimuli-responsive drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1018–1036. [Google Scholar] [CrossRef]
- Elsaid, N.; Somavarapu, S.; Jackson, T.L. Cholesterol-poly(ethylene) glycol nanocarriers for the transscleral delivery of sirolimus. Exp. Eye Res. 2014, 121, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Di Prima, G.; Saladino, S.; Bongiovi, F.; Adamo, G.; Ghersi, G.; Pitarresi, G.; Giammona, G. Novel inulin-based mucoadhesive micelles loaded with corticosteroids as potential transcorneal permeation enhancers. Eur. J. Pharm. Biopharm. 2017, 117, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, R.; Xu, M.; Qiao, J.; Yan, L.; Guo, X.D. Hyaluronic acid modified MPEG-b-PAE block copolymer aqueous micelles for efficient ophthalmic drug delivery of hydrophobic genistein. Drug Deliv. 2018, 25, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Ye, Z.; Ji, Y.L.; Ma, X.; Wen, J.G.; Wei, W.; Huang, S.M. Pharmacokinetics and distributions of bevacizumab by intravitreal injection of bevacizumab-PLGA microspheres in rabbits. Int. J. Ophthalmol. 2015, 8, 653–658. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Li, G.; Xu, F.; Li, S.; Teng, L.; Li, Y.; Sun, F. Anti-Angiogenic Activity Of Bevacizumab-Bearing Dexamethasone-Loaded PLGA Nanoparticles For Potential Intravitreal Applications. Int. J. Nanomed. 2019, 14, 8819–8834. [Google Scholar] [CrossRef]
- Qiu, F.; Meng, T.; Chen, Q.; Zhou, K.; Shao, Y.; Matlock, G.; Ma, X.; Wu, W.; Du, Y.; Wang, X.; et al. Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-Related Macular Degeneration. Mol. Pharm. 2019, 16, 1958–1970. [Google Scholar] [CrossRef]
- Abrishami, M.; Abrishami, M.; Mahmoudi, A.; Mosallaei, N.; Roodi, M.V.A.; Malaekeh-Nikouei, B. Solid Lipid Nanoparticles Improve the Diclofenac Availability in Vitreous after Intraocular Injection. J. Drug Deliv. 2016, 2016, 1368481. [Google Scholar] [CrossRef]
- Freitas, L.G.A.; Isaac, D.L.C.; Lima, E.M.; Souza, L.G.; Abud, M.A.; Reis, R.G.D.; Tannure, W.T.; Avila, M.P. Retinal changes in rabbit after intravitreal injection of sunitinib encapsulated into solid lipid nanoparticles and polymeric nanocapsules. Arq. Bras. Oftalmol. 2018, 81, 408–413. [Google Scholar] [CrossRef]
- Huang, D.; Chen, Y.S.; Green, C.R.; Rupenthal, I.D. Hyaluronic acid coated albumin nanoparticles for targeted peptide delivery in the treatment of retinal ischaemia. Biomaterials 2018, 168, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Tzameret, A.; Ketter-Katz, H.; Edelshtain, V.; Sher, I.; Corem-Salkmon, E.; Levy, I.; Last, D.; Guez, D.; Mardor, Y.; Margel, S.; et al. In vivo MRI assessment of bioactive magnetic iron oxide/human serum albumin nanoparticle delivery into the posterior segment of the eye in a rat model of retinal degeneration. J. Nanobiotechnol. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Jang, I.; Moon, H.; Kim, Y.J.; Jeoung, J.W.; Park, K.H.; Kim, H. Neuroprotective Effects of Human Serum Albumin Nanoparticles Loaded With Brimonidine on Retinal Ganglion Cells in Optic Nerve Crush Model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5641–5649. [Google Scholar] [CrossRef] [PubMed]
- Luis de Redin, I.; Boiero, C.; Martinez-Oharriz, M.C.; Agueros, M.; Ramos, R.; Penuelas, I.; Allemandi, D.; Llabot, J.M.; Irache, J.M. Human serum albumin nanoparticles for ocular delivery of bevacizumab. Int. J. Pharm. 2018, 541, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Varshochian, R.; Riazi-Esfahani, M.; Jeddi-Tehrani, M.; Mahmoudi, A.R.; Aghazadeh, S.; Mahbod, M.; Movassat, M.; Atyabi, F.; Sabzevari, A.; Dinarvand, R. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J. Biomed. Mater. Res. A 2015, 103, 3148–3156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Jacobs, K.M.; Ohr, M.P.; Swindle-Reilly, K.E. Chitosan-Polycaprolactone Core-Shell Microparticles for Sustained Delivery of Bevacizumab. Mol. Pharm. 2020, 17, 2570–2584. [Google Scholar] [CrossRef]
- Barathmanikanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.; Youn, H.S.; Eom, S.; Gurunathan, S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010, 8, 16. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Kalishwaralal, K.; Sheikpranbabu, S.; Deepak, V.; Haribalaganesh, R.; Gurunathan, S. Gold nanoparticles downregulate VEGF-and IL-1beta-induced cell proliferation through Src kinase in retinal pigment epithelial cells. Exp. Eye Res. 2010, 91, 769–778. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.H.; Jo, D.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. The inhibition of retinal neovascularization by gold nanoparticles via suppression of VEGFR-2 activation. Biomaterials 2011, 32, 1865–1871. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; Busch, M.; Asin-Prieto, E.; Peynshaert, K.; Rathod, R.; Remaut, K.; Dunker, N.; Gopferich, A. Hyaluronic acid coating of gold nanoparticles for intraocular drug delivery: Evaluation of the surface properties and effect on their distribution. Exp. Eye Res. 2020, 198, 108151. [Google Scholar] [CrossRef]
- Kyosseva, S.V.; Chen, L.; Seal, S.; McGinnis, J.F. Nanoceria inhibit expression of genes associated with inflammation and angiogenesis in the retina of Vldlr null mice. Exp. Eye Res. 2013, 116, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Giannaccini, M.; Giannini, M.; Calatayud, M.P.; Goya, G.F.; Cuschieri, A.; Dente, L.; Raffa, V. Magnetic nanoparticles as intraocular drug delivery system to target retinal pigmented epithelium (RPE). Int. J. Mol. Sci. 2014, 15, 1590–1605. [Google Scholar] [CrossRef] [PubMed]
- Giannaccini, M.; Pedicini, L.; De Matienzo, G.; Chiellini, F.; Dente, L.; Raffa, V. Magnetic nanoparticles: A strategy to target the choroidal layer in the posterior segment of the eye. Sci. Rep. 2017, 7, 43092. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.L.; Velez-Montoya, R.; Mandava, N.; Stoldt, C.R. Intravitreal silicon-based quantum dots as neuroprotective factors in a model of retinal photoreceptor degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5713–5721. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.D.; Li, Q.N.; Li, C.M.; Bi, H.S. Zinc oxide nanoparticles inhibit murine photoreceptor-derived cell proliferation and migration via reducing TGF-beta and MMP-9 expression in vitro. Cell Prolif. 2015, 48, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Zhou, Y.; Wang, X.; Zhang, Y.; Fan, Y.; Huang, Y.; Liu, Y. Chitosan-based thermosensitive hydrogel as a promising ocular drug delivery system: Preparation, characterization, and in vivo evaluation. J. Biomater. Appl. 2012, 27, 391–402. [Google Scholar] [CrossRef]
- Kushwaha, S.K.; Saxena, P.; Rai, A. Stimuli sensitive hydrogels for ophthalmic drug delivery: A review. Int. J. Pharm. Investig. 2012, 2, 54–60. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Fathi, M.; Barar, J.; Aghanejad, A.; Omidi, Y. Hydrogels for ocular drug delivery and tissue engineering. Bioimpacts 2015, 5, 159–164. [Google Scholar] [CrossRef]
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M. OTX-IVT (anti-VEGF Antibody Implant). Available online: https://www.ocutx.com/research/pipeline/ (accessed on 25 September 2020).
- Elhayek, R.F.; Jarrett, T.; Lattrell, Z.; Takach, S.; Jarrett, P.K.; McGrath, M.; Talamo, J.H.; Sawhney, A. Efficacy of a 6 month Sustained Hydrogel Delivery System for Tyrosine Kinase Inhibitors in a VEGF Induced Retinal Leakage Model. Investig. Ophthalmol. Vis. Sci. 2017, 58. [Google Scholar]
- Tyagi, P.; Barros, M.; Stansbury, J.W.; Kompella, U.B. Light-activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Mol. Pharm. 2013, 10, 2858–2867. [Google Scholar] [CrossRef] [PubMed]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bisht, R.; Jaiswal, J.K.; Chen, Y.S.; Jin, J.; Rupenthal, I.D. Light-responsive in situ forming injectable implants for effective drug delivery to the posterior segment of the eye. Expert Opin. Drug Deliv. 2016, 13, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Rauck, B.M.; Friberg, T.R.; Medina Mendez, C.A.; Park, D.; Shah, V.; Bilonick, R.A.; Wang, Y. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Investig. Ophthalmol. Vis. Sci. 2014, 55, 469–476. [Google Scholar] [CrossRef]
- Yu, Y.; Lau, L.C.; Lo, A.C.; Chau, Y. Injectable Chemically Crosslinked Hydrogel for the Controlled Release of Bevacizumab in Vitreous: A 6-Month In Vivo Study. Transl. Vis. Sci. Technol. 2015, 4, 5. [Google Scholar] [CrossRef]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and Extended In Vitro Release of Bioactive Anti-Vascular Endothelial Growth Factors from a Microsphere-Hydrogel Drug Delivery System. Curr. Eye Res. 2016, 41, 1216–1222. [Google Scholar] [CrossRef]
- Osswald, C.R.; Guthrie, M.J.; Avila, A.; Valio, J.A., Jr.; Mieler, W.F.; Kang-Mieler, J.J. In Vivo Efficacy of an Injectable Microsphere-Hydrogel Ocular Drug Delivery System. Curr. Eye Res. 2017, 42, 1293–1301. [Google Scholar] [CrossRef]
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and Extended Release of a Model Protein from a Microsphere-Hydrogel Drug Delivery System. Ann. Biomed. Eng. 2015, 43, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Borrell, M.A.; Venerus, D.C.; Mieler, W.F.; Kang-Mieler, J.J. Characterization of Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Ranibizumab. Transl. Vis. Sci. Technol. 2019, 8, 12. [Google Scholar] [CrossRef]
- Liu, W.; Lee, B.S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. Curr. Eye Res. 2019, 44, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang-Mieler, J.J.; Liu, W.; Wang, Z.; Yiu, G.; Teixeira, L.B.C.; Mieler, W.F.; Thomasy, S.M. Safety and Biocompatibility of Aflibercept-Loaded Microsphere Thermo-Responsive Hydrogel Drug Delivery System in a Nonhuman Primate Model. Transl. Vis. Sci. Technol. 2020, 9, 30. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Marcus, D.M.; Awh, C.C.; Regillo, C.; Adamis, A.P.; Bantseev, V.; Chiang, Y.; Ehrlich, J.S.; Erickson, S.; Hanley, W.D.; et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology 2019, 126, 1141–1154. [Google Scholar] [CrossRef]
- Loftsson, T.; Stefansson, E. Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int. J. Pharm. 2017, 531, 413–423. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Konraethsdottir, F.; Loftsson, T.; Stefansson, E. Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol. Scand. 2007, 85, 598–602. [Google Scholar] [CrossRef]
- Joussen, A.M.; Wolf, S.; Kaiser, P.K.; Boyer, D.; Schmelter, T.; Sandbrink, R.; Zeitz, O.; Deeg, G.; Richter, A.; Zimmermann, T.; et al. The Developing Regorafenib Eye drops for neovascular Age-related Macular degeneration (DREAM) study: An open-label phase II trial. Br. J. Clin. Pharmacol. 2019, 85, 347–355. [Google Scholar] [CrossRef]
- PanOptica. PAN-90806: Once-Daily Topical Anti-VEGF Eye Drop for Wet AMD and Other Neovascular Eye Disease. Available online: https://www.panopticapharma.com/wp-content/uploads/2019/10/PAN-90806-Data-at-OIS@AAO.pdf (accessed on 2 October 2020).
- Barar, J.; Javadzadeh, A.R.; Omidi, Y. Ocular novel drug delivery: Impacts of membranes and barriers. Expert Opin. Drug Deliv. 2008, 5, 567–581. [Google Scholar] [CrossRef]
- Chu, H.S.; Hu, F.R.; Yang, C.M.; Yeh, P.T.; Chen, Y.M.; Hou, Y.C.; Chen, W.L. Subconjunctival injection of bevacizumab in the treatment of corneal neovascularization associated with lipid deposition. Cornea 2011, 30, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Bandi, N.; Ayalasomayajula, S.P. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Van Quill, K.R.; Dioguardi, P.K.; Tong, C.T.; Gilbert, J.A.; Aaberg, T.M., Jr.; Grossniklaus, H.E.; Edelhauser, H.F.; O’Brien, J.M. Subconjunctival carboplatin in fibrin sealant in the treatment of transgenic murine retinoblastoma. Ophthalmology 2005, 112, 1151–1158. [Google Scholar] [CrossRef]
- Ayalasomayajula, S.P.; Kompella, U.B. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur. J. Pharmacol. 2005, 511, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Misra, G.P.; Singh, R.S.; Aleman, T.S.; Jacobson, S.G.; Gardner, T.W.; Lowe, T.L. Subconjunctivally implantable hydrogels with degradable and thermoresponsive properties for sustained release of insulin to the retina. Biomaterials 2009, 30, 6541–6547. [Google Scholar] [CrossRef]
- Kang, S.J.; Durairaj, C.; Kompella, U.B.; O’Brien, J.M.; Grossniklaus, H.E. Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Arch. Ophthalmol. 2009, 127, 1043–1047. [Google Scholar] [CrossRef]
- Rieke, E.R.; Amaral, J.; Becerra, S.P.; Lutz, R.J. Sustained subconjunctival protein delivery using a thermosetting gel delivery system. J. Ocul. Pharmacol. Ther. 2010, 26, 55–64. [Google Scholar] [CrossRef]
- Peng, Y.; Ang, M.; Foo, S.; Lee, W.S.; Ma, Z.; Venkatraman, S.S.; Wong, T.T. Biocompatibility and biodegradation studies of subconjunctival implants in rabbit eyes. PLoS ONE 2011, 6, e22507. [Google Scholar] [CrossRef]
- Nagai, N.; Kaji, H.; Onami, H.; Ishikawa, Y.; Nishizawa, M.; Osumi, N.; Nakazawa, T.; Abe, T. A polymeric device for controlled transscleral multi-drug delivery to the posterior segment of the eye. Acta Biomater. 2014, 10, 680–687. [Google Scholar] [CrossRef]
- Imai, H.; Misra, G.P.; Wu, L.; Janagam, D.R.; Gardner, T.W.; Lowe, T.L. Subconjunctivally Implanted Hydrogels for Sustained Insulin Release to Reduce Retinal Cell Apoptosis in Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7839–7846. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharm. 2018, 107, 1564–1582. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Chiang, B.; Wu, X.; Prausnitz, M.R. Ocular delivery of macromolecules. J. Control. Release 2014, 190, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Ranta, V.P.; Mannermaa, E.; Lummepuro, K.; Subrizi, A.; Laukkanen, A.; Antopolsky, M.; Murtomaki, L.; Hornof, M.; Urtti, A. Barrier analysis of periocular drug delivery to the posterior segment. J. Control. Release 2010, 148, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.R.; Lee, S.S.; Kim, H.; Kim, S.; Lutz, R.J.; Galban, C.; Bungay, P.M.; Yuan, P.; Wang, N.S.; Kim, J.; et al. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp. Eye Res. 2006, 82, 479–487. [Google Scholar] [CrossRef]
- Raghava, S.; Hammond, M.; Kompella, U.B. Periocular routes for retinal drug delivery. Expert Opin. Drug Deliv. 2004, 1, 99–114. [Google Scholar] [CrossRef]
- Vellonen, K.S.; Soini, E.M.; Del Amo, E.M.; Urtti, A. Prediction of Ocular Drug Distribution from Systemic Blood Circulation. Mol. Pharm. 2016, 13, 2906–2911. [Google Scholar] [CrossRef]
- Olsen, T.W.; Feng, X.; Wabner, K.; Conston, S.R.; Sierra, D.H.; Folden, D.V.; Smith, M.E.; Cameron, J.D. Cannulation of the suprachoroidal space: A novel drug delivery methodology to the posterior segment. Am. J. Ophthalmol. 2006, 142, 777–787. [Google Scholar] [CrossRef]
- Olsen, T.W.; Feng, X.; Wabner, K.; Csaky, K.; Pambuccian, S.; Cameron, J.D. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4749–4756. [Google Scholar] [CrossRef]
- Patel, S.R.; Lin, A.S.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm. Res. 2011, 28, 166–176. [Google Scholar] [CrossRef]
- Patel, S.R.; Berezovsky, D.E.; McCarey, B.E.; Zarnitsyn, V.; Edelhauser, H.F.; Prausnitz, M.R. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Ebert, F.G.; Bartolo, E.D.; Barca, F.; Cresti, F.; Augustin, C.; Augustin, A. Suprachoroidal drug infusion for the treatment of severe subfoveal hard exudates. Retina 2012, 32, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Edelhauser, H.F.; Verhoeven, R.S.; Burke, B.; Struble, C.B.; Patel, S.R. Intraocular Distribution and Targeting of Triamcinolone Acetonide Suspension Administered Into the Suprachoroidal Space. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5259. [Google Scholar]
- Chen, M.; Li, X.; Liu, J.; Han, Y.; Cheng, L. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J. Control. Release 2015, 203, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Wykoff, C.C.; Brown, D.M.; Boyer, D.S.; Barakat, M.; Taraborelli, D.; Noronha, G.; Tanzanite Study, G. Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study. Ophthalmol. Retin. 2018, 2, 320–328. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Khurana, R.N.; Lampen, S.I.R.; Noronha, G.; Brown, D.M.; Ou, W.C.; Sadda, S.R.; Group, H.S. Suprachoroidal Triamcinolone Acetonide for Diabetic Macular Edema: The HULK Trial. Ophthalmol. Retin. 2018, 2, 874–877. [Google Scholar] [CrossRef]
- Yeh, S.; Khurana, R.N.; Shah, M.; Henry, C.R.; Wang, R.C.; Kissner, J.M.; Ciulla, T.A.; Noronha, G.; Investigators, P.S. Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: Phase 3 Randomized Trial. Ophthalmology 2020, 127, 948–955. [Google Scholar] [CrossRef]
- Janoria, K.G.; Gunda, S.; Boddu, S.H.; Mitra, A.K. Novel approaches to retinal drug delivery. Expert Opin. Drug Deliv. 2007, 4, 371–388. [Google Scholar] [CrossRef]
- Qi, Y.; Dai, X.; Zhang, H.; He, Y.; Zhang, Y.; Han, J.; Zhu, P.; Zhang, Y.; Zheng, Q.; Li, X.; et al. Trans-Corneal Subretinal Injection in Mice and Its Effect on the Function and Morphology of the Retina. PLoS ONE 2015, 10, e0136523. [Google Scholar] [CrossRef]
- Johnson, C.J.; Berglin, L.; Chrenek, M.A.; Redmond, T.M.; Boatright, J.H.; Nickerson, J.M. Technical brief: Subretinal injection and electroporation into adult mouse eyes. Mol. Vis. 2008, 14, 2211–2226. [Google Scholar]
- Rajala, A.; Wang, Y.; Zhu, Y.; Ranjo-Bishop, M.; Ma, J.X.; Mao, C.; Rajala, R.V. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 2014, 14, 5257–5263. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Maeno, H.; Gujrati, M.; Schur, R.; Maeda, A.; Maeda, T.; Palczewski, K.; Lu, Z.R. Self-Assembly of a Multifunctional Lipid With Core-Shell Dendrimer DNA Nanoparticles Enhanced Efficient Gene Delivery at Low Charge Ratios into RPE Cells. Macromol. Biosci. 2015, 15, 1663–1672. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; Del Pozo-Rodriguez, A.; Torrecilla, J.; Rodriguez-Gascon, A.; Rodriguez, J.M.; Friedrich, U.; Weber, B.H.; Solinis, M.A. Solid lipid nanoparticle-based vectors intended for the treatment of X-linked juvenile retinoschisis by gene therapy: In vivo approaches in Rs1h-deficient mouse model. J. Control. Release 2015, 217, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Regenxbio. RGX-314 Gene Therapy for Neovascular AMD Trial. Available online: https://www.regenxbio.com/therapeutic-programs (accessed on 29 September 2020).
- Papangkorn, K.; Prendergast, E.; Higuchi, J.W.; Brar, B.; Higuchi, W.I. Noninvasive Ocular Drug Delivery System of Dexamethasone Sodium Phosphate in the Treatment of Experimental Uveitis Rabbit. J. Ocul. Pharmacol. Ther. 2017, 33, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat-Binstock, E.; Orucov, F.; Frucht-Pery, J.; Peer, J.; Domb, A.J. Methylprednisolone delivery to the back of the eye using hydrogel iontophoresis. J. Ocul. Pharmacol. Ther. 2008, 24, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, S.; Ferrari, G.; Quarta, M.; Macaluso, C.; Santi, P. In vitro transscleral iontophoresis of high molecular weight neutral compounds. Eur. J. Pharm. Sci. 2009, 36, 486–492. [Google Scholar] [CrossRef]
- Molokhia, S.; Papangkorn, K.; Butler, C.; Higuchi, J.W.; Brar, B.; Ambati, B.; Li, S.K.; Higuchi, W.I. Transscleral Iontophoresis for Noninvasive Ocular Drug Delivery of Macromolecules. J. Ocul. Pharmacol. Ther. 2020, 36, 247–256. [Google Scholar] [CrossRef]
- Parkinson, T.M.; Ferguson, E.; Febbraro, S.; Bakhtyari, A.; King, M.; Mundasad, M. Tolerance of ocular iontophoresis in healthy volunteers. J. Ocul. Pharmacol. Ther. 2003, 19, 145–151. [Google Scholar] [CrossRef]
- Chopra, P.; Hao, J.; Li, S.K. Iontophoretic transport of charged macromolecules across human sclera. Int. J. Pharm. 2010, 388, 107–113. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Domb, A.J.; Orucov, F.; Frucht-Pery, J.; Peer, J. Methotrexate delivery to the eye using transscleral hydrogel iontophoresis. Curr. Eye Res. 2007, 32, 639–646. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Domb, A.J.; Orucov, F.; Dagan, A.; Frucht-Pery, J.; Peer, J. In vitro and in vivo evaluation of carboplatin delivery to the eye using hydrogel-iontophoresis. Curr. Eye Res. 2008, 33, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Bourlais, C.L.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems--recent advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Rodrigues, G.A.; Lutz, D.; Shen, J.; Yuan, X.; Shen, H.; Cunningham, J.; Rivers, H.M. Topical Drug Delivery to the Posterior Segment of the Eye: Addressing the Challenge of Preclinical to Clinical Translation. Pharm. Res. 2018, 35, 245. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Jwala, J.; Boddu, S.H.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Perez-Aranda, M.; Martinez, G.; Merinero, M.; Arguelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), S3–S9. [Google Scholar] [CrossRef]
- Chong, D.Y.; Johnson, M.W.; Huynh, T.H.; Hall, E.F.; Comer, G.M.; Fish, D.N. Vitreous penetration of orally administered famciclovir. Am. J. Ophthalmol. 2009, 148, 38–42.e31. [Google Scholar] [CrossRef]
- Srinivas, A.; Azad, R.V.; Sharma, Y.R.; Kumar, A.; Satpathy, G.; Velpandian, T. Evaluation of vitreous levels of gatifloxacin after systemic administration in inflamed and non-inflamed eyes. Acta Ophthalmol. 2009, 87, 648–652. [Google Scholar] [CrossRef]
- Kim, H.; Robinson, M.R.; Lizak, M.J.; Tansey, G.; Lutz, R.J.; Yuan, P.; Wang, N.S.; Csaky, K.G. Controlled drug release from an ocular implant: An evaluation using dynamic three-dimensional magnetic resonance imaging. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2722–2731. [Google Scholar] [CrossRef]
- Samtani, S.; Amaral, J.; Campos, M.M.; Fariss, R.N.; Becerra, S.P. Doxycycline-mediated inhibition of choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5098–5106. [Google Scholar] [CrossRef]
- Takahashi, K.; Saishin, Y.; Saishin, Y.; King, A.G.; Levin, R.; Campochiaro, P.A. Suppression and regression of choroidal neovascularization by the multitargeted kinase inhibitor pazopanib. Arch. Ophthalmol. 2009, 127, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Ladas, I.D.; Karagiannis, D.A.; Rouvas, A.A.; Kotsolis, A.I.; Liotsou, A.; Vergados, I. Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: Our experience after 2,000 injections. Retina 2009, 29, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.J.; Veith, M.; Hamouz, J.; Ernest, J.; Zalewski, D.; Studnicka, J.; Vajas, A.; Papp, A.; Gabor, V.; Luu, J.; et al. Efficacy and Safety of a Proposed Ranibizumab Biosimilar Product vs a Reference Ranibizumab Product for Patients With Neovascular Age-Related Macular Degeneration: A Randomized Clinical Trial. JAMA Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Baumal, C.R.; Spaide, R.F.; Vajzovic, L.; Freund, K.B.; Walter, S.D.; John, V.; Rich, R.; Chaudhry, N.; Lakhanpal, R.R.; Oellers, P.R.; et al. Retinal Vasculitis and Intraocular Inflammation after Intravitreal Injection of Brolucizumab. Ophthalmology 2020, 127, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Haug, S.J.; Hien, D.L.; Uludag, G.; Ngoc, T.T.T.; Lajevardi, S.; Halim, M.S.; Sepah, Y.J.; Do, D.V.; Khanani, A.M. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am. J. Ophthalmol. Case Rep. 2020, 18, 100680. [Google Scholar] [CrossRef]
- Witkin, A.J.; Hahn, P.; Murray, T.G.; Arevalo, J.F.; Blinder, K.J.; Choudhry, N.; Emerson, G.G.; Goldberg, R.A.; Kim, S.J.; Pearlman, J.; et al. Occlusive Retinal Vasculitis Following Intravitreal Brolucizumab. J. Vitreoretin. Dis. 2020, 4, 269–279. [Google Scholar] [CrossRef]

| Route | Delivery Platform | Characteristics | Drug/Cargo | Development | Reference | |
|---|---|---|---|---|---|---|
| Intravitreal | Implants |
| Dexamethasone(Ozurdex) Fluocinolone acetonide (Retiset, Illuvien) Ganciclovir (Vitrasert) Brimonidine (Brimo DDS) | Launched Phase 2 | [62,63,64,65] | |
| Micro-, Nano-particles | Microparticles |
| Triamcinolone (Kenalog, MaQaid, Trivaris, Triesence) Sunitinib Malate (GB-102) | Launched Phase 2 | [70,71,72,73,74,75,76,77,78] | |
| Dendrimer |
| Anti-VEGF (Bevacizumab) Fluocinolone Acetonide Dexamethasone Triamcinolone Acetonide Neurotrophin 4 | Pre-clinical | [79,80,81,82,83,84,85] | ||
| Liposome |
| siRNA DNA Anti-VEGF (Bevacizumab) | Pre-clinical | [86,87,88,89,90] | ||
| Micelles |
| Dexamethasone Triamcinolone Acetonide Sirolimus Genistein | Pre-clinical | [91,92,93,94,95,96,97] | ||
| Polymeric |
| Anti-VEGF (Bevacizumab) Fenofibrate | Pre-clinical | [98,99,100,101] | ||
| Others |
| Anti-VEGF (Bevacizumab) Brimonidine | Pre-clinical | [102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118] | ||
| Hydrogel |
| Anti-VEGF (Ranibizumab, Bevacizumab) Tyrosine kinase inhibitor | Phase 1 (Ocular therapeutix) | [119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135] | ||
| Combined systems |
| Anti-VEGF (Ranibizumab, Bevacizumab) | Pre-clinical | [136,137,138,139] | ||
| Port delivery system |
| Anti-VEGF (Ranibizumab) | Phase 3 | [140] | ||
| Topical |
| Dexamethasone Regorafenib PAN-90806 | Phase 2 | [141,142,143,144] | ||
| Subconjunctival | Hydrogel |
| Insulin Ovalbumin | Pre-clinical | [145,146,147,148,149,150,151,152,153,154,155,156,157] | |
| Polymeric Nanoparticles | Budesonide Carboplatin Celecoxib | Pre-clinical | ||||
| Suprachoroidal | Microneedle Microcannulation |
| Triamcinolone Acetonide | Phase 3 | [158,159,160,161,162,163,164,165,166,167,168,169,170,171,172] | |
| Subretinal | Liposome Dendrimer Viral vector |
| siRNA Plasmid DNA Anti-VEGF genome | Pre-clinical Phase 1/2 | [173,174,175,176,177,178,179] | |
| Trans-scleral | Iontophoresis |
| Dexamethasone Anti-VEGF (Bevacizumab) | Pre-clinical | [180,181,182,183,184,185,186,187] | |
| Drug Delivery Systems | Trial Name | Drug (Sponsor) | Description | Results |
|---|---|---|---|---|
| Dose escalation | HARBOR (NCT00891735) | Ranibizumab (Novartis) | Phase 3, interventional, multicenter, randomized, dose-comparison | 2.0 mg (4-fold dose escalation) showed similar treatment effect compared to 0.5 mg dose, whereas fewer required injections. |
| SAVE (NCT01025232) | Ranibizumab (Novartis) | Phase 1/2a, interventional, open-label, multicenter | 2.0 mg (4-fold dose escalation) showed visual and anatomic gains in recalcitrant neovascular AMD. | |
| READ 3 (NCT01077401) | Ranibizumab (Novartis) | Phase 3, interventional, multicenter, randomized, controlled, double-masked | 2.0 mg (4-fold dose escalation) showed no superior 24 months visual improvements compared to 0.5 mg dose in diabetic macular edema. | |
| PHOTON (NCT04429503) | Aflibercept (Bayer) | Phase 3, interventional, multicenter, randomized, controlled, double-masked | Ongoing 8.0 mg (4-fold dose escalation) every 12 or 16 weeks after a loading phase in participants with diabetic retinopathy. | |
| PULSAR (NCT04423718) | Aflibercept (Bayer) | Ongoing 8.0 mg (4-fold dose escalation) every 12 or 16 weeks after a loading phase in participants with neovascular AMD. | ||
| HAWK (NCT02307682) | Brolucizumab (Novartis) | Phase 3, interventional, multicenter, randomized, active-controlled, double-masked | Brolucizumab 3 mg showed similar visual outcomes compared to aflibercept and resulted in fewer injections. | |
| HARRIER (NCT02434328) | Brolucizumab (Novartis) | Brolucizumab 3 mg and 6 mg showed similar visual outcomes compared to aflibercept and resulted in fewer injections. | ||
| Large molecule biopolymer | DAZZLE (NCT04049266) | KSI-301 (KODIAK) | Phase 2b/3, interventional, multicenter, randomized, controlled, double-masked | Ongoing//KSI-301 5 mg vs. Aflibercept 2 mg Prior phase 1 study (NCT03790852) resulted in visual improvements with 6-month interval intravitreal injections in neovascular AMD, diabetic macular edema and retinal vein occlusion subjects. |
| Intravitreal implant | (NCT00658619) | Brimonidine (Allergan) | Phase 2, interventional, randomized, multicenter | Mean area of geographic atrophy secondary to neovascular AMD was reduced at month 12 in brimonidine implant compared to sham. |
| Microparticles | ADAGIO (NCT03249740) | Sunitinib Malate (GB-102, GrayBug vision) | Phase 1/2a, interventional, randomized, multicenter | 88% patients at 3 months and 68% patients at 6 months were maintained on a single intravitreal injection. |
| ALTISSIMO (NCT03953079) | Phase 2b, interventional, randomized, multicenter | Ongoing Compare the visual outcome after intravitreal administration of 1 mg and 2 mg GB-102, and aflibercept 2 mg dose in neovascular AMD. | ||
| (NCT04085341) | Phase 2a, interventional, randomized, multicenter | Ongoing Diabetic macular edema and retinal vein occlusion. | ||
| Hydrogel | CLN-0046 (NCT03630315) | OTX-TKI (Ocular Therapeutix) | Phase 1, interventional, open-label, randomized, controlled, multicenter | Ongoing Low dose vs. middle dose vs. high dose vs. OTX-TKI + Anti-VEGF. |
| Port delivery system | LADDER (NCT02510794) | Ranibizumab (Novartis) | Phase 2, interventional, randomized, controlled, multicenter | Visual outcomes were similar in the ranibizumab-loaded port 100 mg/mL and monthly intravitreal injections at 9 months with the median time to initial refill was 15 months in the 100 mg/mL group. |
| ARCHWAY (NCT03677934) | Ranibizumab (Novartis) | Phase 3, interventional, randomized, visual assessor-masked, active-comparator study | Ongoing Port delivery system 100 mg/mL vs. monthly intravitreal injection 0.5 mg (10 mg/mL) in neovascular AMD. | |
| PAVILION (NCT04503551) | Ranibizumab (Novartis) | Ongoing Diabetic retinopathy. | ||
| PAGODA (NCT04108156) | Ranibizumab (Novartis) | Ongoing Diabetic macular edema. | ||
| Topical | DREAM (NCT02222207) | Regorafenib (Bayer) | Phase 2a/2b, interventional, randomized, multicenter | Among 51 subjects, 21 patients (41%) required intravitreal ranibizumab rescue due to the ocular treatment-emergent adverse events by week 12. |
| (NCT03479372) | PAN-90806 (PanOptica) | Phase 1/2a, interventional, randomized, uncontrolled, double-masked, multicenter | Two, 6, or 10 mg/mL eye drops daily for 12 weeks No rescue therapy for 51% of patients. Eighty-eight percent of nonrescued patients showed clinical improvement or stability. | |
| Suprachoroidal | TANZANITE (NCT02303184) | Triamcinolone Acetonide (Clearside Biomedical) | Phase 2, interventional, randomized, controlled, masked | Improved visual outcomes than aflibercept monotherapy. Lesser number of intravitreal injections. |
| TYBEE (NCT03126786) | Phase 2, interventional, randomized, controlled, double-masked | No definite advantages of visual outcomes in diabetic macular edema with suprachoroidal CLS-TA + intravitreal aflibercept combined. | ||
| PEACHTREE (NCT02595398) | Phase 3, interventional, randomized, controlled, double-masked | Better visual outcome and less ocular complication in noninfectious uveitis complicated by macular edema. | ||
| Subretinal | (NCT03066258) | RGX-314 (Regenxbio) | Phase 1/2a, interventional, open-label, non-randomized, multiple-cohort, dose-escalation | Ongoing After initial ranibizumab injection, genomes were injected and anti-VEGF was treated after 4 weeks and as needed. At 6 months, visual outcomes were improved with less required intravitreal injections. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. https://doi.org/10.3390/pharmaceutics13010108
Kim HM, Woo SJ. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics. 2021; 13(1):108. https://doi.org/10.3390/pharmaceutics13010108
Chicago/Turabian StyleKim, Hyeong Min, and Se Joon Woo. 2021. "Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives" Pharmaceutics 13, no. 1: 108. https://doi.org/10.3390/pharmaceutics13010108
APA StyleKim, H. M., & Woo, S. J. (2021). Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics, 13(1), 108. https://doi.org/10.3390/pharmaceutics13010108






