Advancement on Sustained Antiviral Ocular Drug Delivery for Herpes Simplex Virus Keratitis: Recent Update on Potential Investigation
Abstract
:1. Introduction
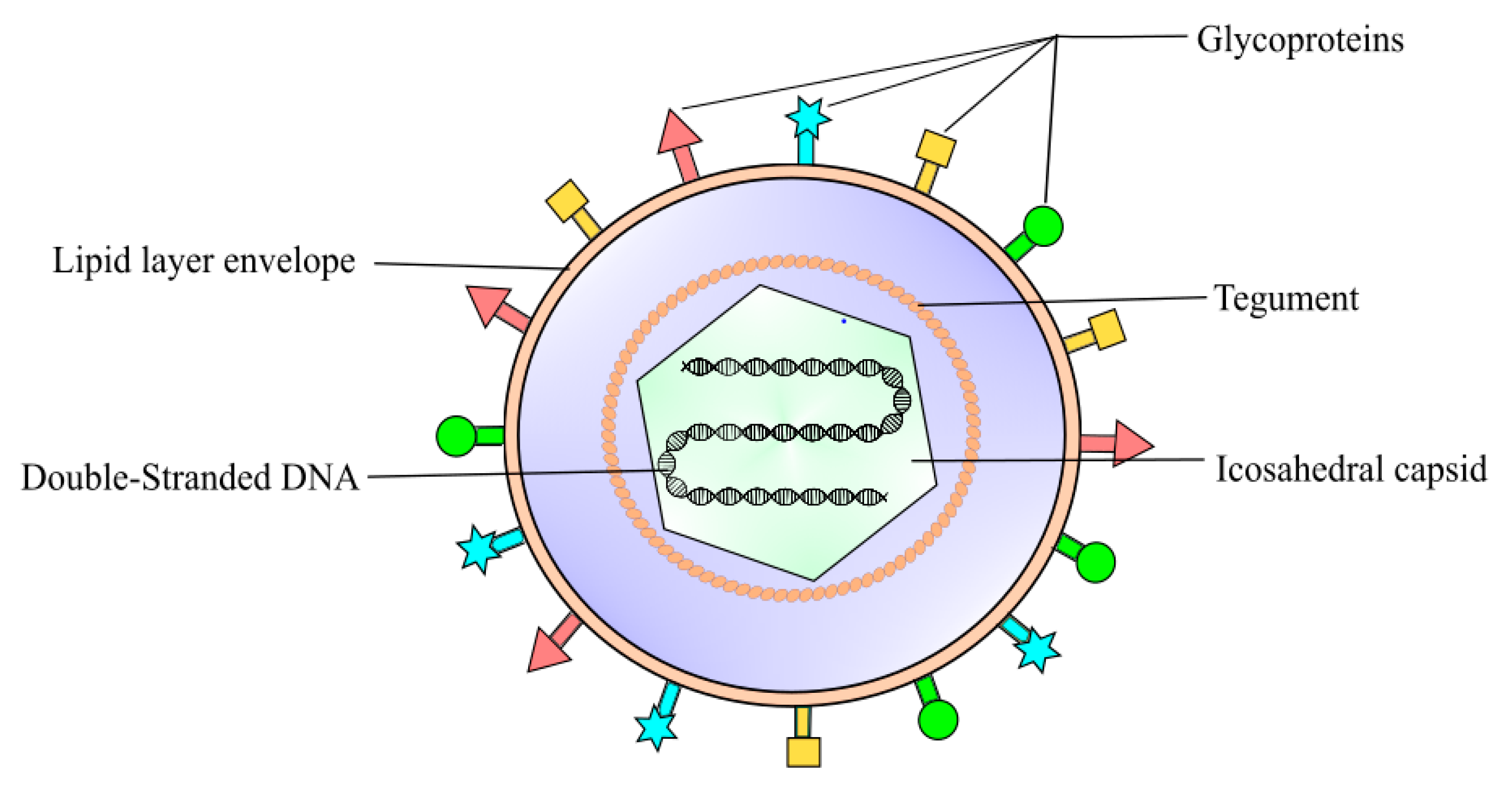
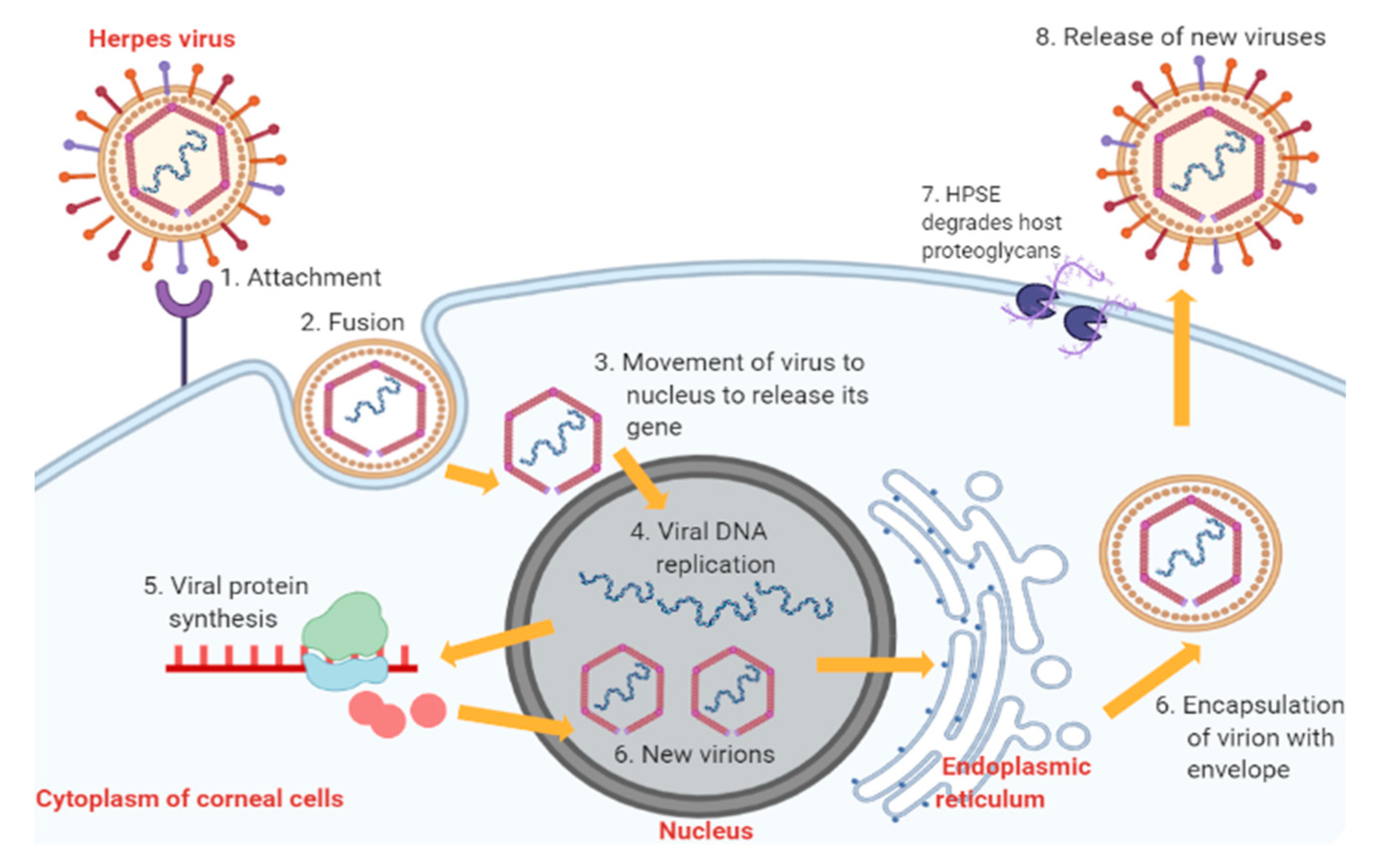
2. Pathophysiology of Herpes Simplex Virus Keratitis Condition
3. Available Treatments for Herpes Simplex Virus Keratitis and Associated Limitations
4. Novel Approach for Ocular Drug Delivery against Herpes Simplex Keratitis
4.1. Lipid-Based Nanocarriers for Ocular Drug Delivery
4.1.1. Liposomes and Niosomes for Ocular Drug Delivery
4.1.2. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Ocular Drug Delivery
4.1.3. Advancements of Nanoemulsions for Ocular Drug Delivery
4.2. Polymeric-Based Nanocarriers for Ocular Drug Delivery
4.2.1. Nanomicelles for Ocular Drug Delivery
4.2.2. Polymeric Nanoparticles and Nanosuspensions for Ocular Drug Delivery
4.3. Prodrug Approach for Ocular Drug Delivery
4.4. Peptide Delivery Approaches for Ocular Drug Delivery
4.5. In Situ and other Approaches for Ocular Drug Delivery
4.5.1. In Situ Ocular Gel for Ocular Drug Delivery
4.5.2. In Situ Minitablets for Ocular Drug Delivery
4.5.3. Ocular Inserts for Ocular Drug Delivery
5. Clinical and Safety Aspect of Novel Ocular Delivery Approaches
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, M.L.; Chodosh, J. Herpes Simplex Virus Keratitis: A Treatment Guideline - 2014 - American Academy of Ophthalmology. Available online: https://www.aao.org/clinical-statement/herpes-simplex-virus-keratitis-treatment-guideline (accessed on 4 September 2020).
- Sugar, A.; Jacobs, D.; Hirsch, M.; Givens, J. Herpes Simplex Keratitis. Available online: https://www.uptodate.com/contents/herpes-simplex-keratitis (accessed on 4 September 2020).
- Farooq, A.V.; Shukla, D. Herpes Simplex Epithelial and Stromal Keratitis: An Epidemiologic Update. Surv. Ophthalmol. 2012, 57, 448–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, B.; Patel, B.C. Herpes Simplex Keratitis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Valerio, G.S.; Lin, C.C. Ocular manifestations of herpes simplex virus. Curr. Opin. Ophthalmol. 2019, 30, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Wald, A.; Hirsch, M.; Mitty, J. Epidemiology, Clinical Manifestations, and Diagnosis of Herpes Simplex Virus Type 1 Infection. Available online: https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection (accessed on 4 September 2020).
- Kalezic, T.; Mazen, M.; Kuklinski, E.; Asbell, P. Herpetic eye disease study: Lessons learned. Curr. Opin. Ophthalmol. 2018, 29, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Khadr, L.; Harfouche, M.; Omori, R.; Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J. The epidemiology of herpes simplex virus type 1 in Asia: Systematic review, meta-analyses, and meta-regressions. Clin. Infect. Dis. 2019, 68, 757–772. [Google Scholar] [CrossRef]
- Chou, T.Y.; Hong, B.Y. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: Background, effectiveness, tolerability, safety, and future applications. Ther. Clin. Risk Manag. 2014, 10, 665–681. [Google Scholar] [CrossRef] [Green Version]
- Lobo, A.M.; Agelidis, A.M.; Shukla, D. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul. Surf. 2019, 17, 40–49. [Google Scholar] [CrossRef]
- Cabrera-Aguas, M.; Robaei, D.; McCluskey, P.; Watson, S. Clinical translation of recommendations from randomized trials for management of herpes simplex virus keratitis. Clin. Exp. Ophthalmol. 2018, 46, 1008–1016. [Google Scholar] [CrossRef] [Green Version]
- Vadoothker, S.; Andrews, L.; Jeng, B.H.; Levin, M.R. Management of Herpes Simplex Virus Keratitis in the Pediatric Population. Pediatr. Infect. Dis. J. 2018, 37, 949–951. [Google Scholar] [CrossRef]
- Duxfield, L.; Sultana, R.; Wang, R.; Englebretsen, V.; Deo, S.; Rupenthal, I.D.; Al-Kassas, R. Ocular delivery systems for topical application of anti-infective agents. Drug Dev. Ind. Pharm. 2016, 42, 1–11. [Google Scholar] [CrossRef]
- Tsatsos, M.; MacGregor, C.; Athanasiadis, I.; Moschos, M.M.; Hossain, P.; Anderson, D. Herpes simplex virus keratitis: An update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin. Exp. Ophthalmol. 2016, 44, 824–837. [Google Scholar] [CrossRef]
- Koganti, R.; Yadavalli, T.; Shukla, D. Current and emerging therapies for ocular herpes simplex virus type-1 infections. Microorganisms 2019, 7, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynaud, C.; Rousseau, A.; Kaswin, G.; M’garrech, M.; Barreau, E.; Labetoulle, M. Persistent Impairment of Quality of Life in Patients with Herpes Simplex Keratitis. Ophthalmology 2017, 124, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.M.; Castillo, E.; Duarte, L.F.; Arriagada, J.; Corrales, N.; Farías, M.A.; Henríquez, A.; Agurto-Muñoz, C.; González, P.A. Current Antivirals and Novel Botanical Molecules Interfering With Herpes Simplex Virus Infection. Front. Microbiol. 2020, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Roozbahani, M.; Hammersmith, K.M. Management of herpes simplex virus epithelial keratitis. Curr. Opin. Ophthalmol. 2018, 29, 360–364. [Google Scholar] [CrossRef]
- Cabrera-Aguas, M.; Kerdraon, Y.; Symes, R.J.; McCluskey, P.; Samarawickrama, C.; Rawlinson, W.; Watson, S.L. Development, Implementation, and Evaluation of Treatment Guidelines for Herpes Simplex Keratitis in Sydney, Australia. Cornea 2020, 39, 834–840. [Google Scholar] [CrossRef]
- Wilhelmus, K.R. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef]
- Sozen, E.; Avunduk, A.M.; Akyol, N. Comparison of Efficacy of Oral Valacyclovir and Topical Acyclovir in the Treatment of Herpes Simplex Keratitis: A Randomized Clinical Trial. Chemotherapy 2006, 52, 29–31. [Google Scholar] [CrossRef]
- Vikas, R.; Prabhu, S.G.; Mudgal, P.P.; Shetty, U.; Karunakaran, K.; Jagadesh, A.; Auti, A.; Stansilaus, R.P.; Nair, S.; Arunkumar, G. HSV susceptibility to acyclovir—Genotypic and phenotypic characterization. Antivir. Ther. 2019, 24, 141–145. [Google Scholar] [CrossRef]
- Piret, J.; Goyette, N.; Boivin, G. Novel method based on real-time cell analysis for drug susceptibility testing of herpes simplex virus and human cytomegalovirus. J. Clin. Microbiol. 2016, 54, 2120–2127. [Google Scholar] [CrossRef] [Green Version]
- Caliaro, O.; Barbani, M.T.; Klenja, S.; Morfin, F.; Frobert, E.; Gorgievski, M.; Steinlin-Schopfer, J.; Suter-Riniker, F. Phenotypic testing of patient herpes simplex virus type 1 and 2 isolates for acyclovir resistance by a novel method based on real-time cell analysis. J. Clin. Virol. 2020, 125, 104303. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Moghadam, R.S.; Elmi, R.; Nosrati, A.; Taghiabadi, E.; Aghdami, N. Topical tacrolimus as an adjunct to conventional therapy for stromal herpetic keratitis: A randomized clinical trial. J. Ophthalmic Vis. Res. 2019, 14, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Brocks, D.; Mead, O.G.; Tighe, S.; Tseng, S.C.G. Self-retained cryopreserved amniotic membrane for the management of corneal ulcers. Clin. Ophthalmol. 2020, 14, 1437–1443. [Google Scholar] [CrossRef]
- Field, A.J.; Gottsch, J.D. Persisting epithelial herpes simplex keratitis while on cyclosporin-A ointment. Aust. N. Z. J. Ophthalmol. 1995, 23, 333–334. [Google Scholar] [CrossRef]
- Sharif, Z.; Sharif, W. Corneal neovascularization: Updates on pathophysiology, investigations & management. Rom. J. Ophthalmol. 2019, 63, 15–22. [Google Scholar] [CrossRef]
- Castro-Balado, A.; Mondelo-García, C.; Zarra-Ferro, I.; Fernández-Ferreiro, A. New ophthalmic drug delivery systems. Farm. Hosp. 2020, 44, 149–157. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular drug delivery: Present innovations and future challenges. J. Pharmacol. Exp. Ther. 2020, 374, 602–624. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Tandel, H.T.; Parmar, H.K.; Pandya, K.K.; Pardasani, L.J.; Panchal, V.S. A Systematic Review on Mucoadhesive Drug Delivery System. World J. Pharm. Res. 2017, 6, 337–366. [Google Scholar] [CrossRef] [Green Version]
- Anil, A.; Sudheer, P. Mucoadhesive Polymers: A Review. J. Pharm. Res. 2018, 17, 47–55. [Google Scholar] [CrossRef]
- Ibrahim, Y.H.E.Y.; Regdon, G.; Hamedelniel, E.I.; Sovány, T. Review of recently used techniques and materials to improve the efficiency of orally administered proteins/peptides. DARU J. Pharm. Sci. 2020, 28, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, H.; Maheshwari, R.; Pandey, M.; Tekade, M.; Gorain, B.; Tekade, R.K. Advanced nanoscale carrier-based approaches to overcome biopharmaceutical issues associated with anticancer drug ‘Etoposide’. Mater. Sci. Eng. C 2020, 106, 110275. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Nair, A.B.; Dubey, S.K.; Kesharwani, P. Theranostic application of nanoemulsions in chemotherapy. Drug Discov. Today 2020, 25, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Qamar, Z.; Qizilbash, F.F.; Iqubal, M.K.; Ali, A.; Narang, J.K.; Ali, J.; Baboota, S. Nano-Based Drug Delivery System: Recent Strategies for the Treatment of Ocular Disease and Future Perspective. Recent Pat. Drug Deliv. Formul. 2019, 13, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.M.; Petrilli, R.; Lopez, R.F.V. The prominence of the dosage form design to treat ocular diseases. Int. J. Pharm. 2020, 586, 119577. [Google Scholar] [CrossRef]
- Chetoni, P.; Rossi, S.; Burgalassi, S.; Monti, D.; Mariotti, S.; Saettone, M.F. Comparison of Liposome-Encapsulated Acyclovir with Acyclovir Ointment: Ocular Pharmacokinetics in Rabbits. J. Ocul. Pharmacol. Ther. 2004, 20, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Law, S.L.; Huang, K.J.; Chiang, C.H. Acyclovir-containing liposomes for potential ocular delivery Corneal penetration and absorption. J. Control. Release 2000, 63, 135–140. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Managit, C.; Phanaksri, T.; Treesuppharat, W.; Fuongfuchat, A. Formulation development and in vitro evaluation of transferrin-conjugated liposomes as a carrier of ganciclovir targeting the retina. Int. J. Pharm. 2020, 577, 119084. [Google Scholar] [CrossRef]
- Akhter, S.; Ramazani, F.; Ahmad, M.Z.; Ahmad, F.J.; Rahman, Z.; Bhatnagar, A.; Storm, G. Ocular pharmacoscintigraphic and aqueous humoral drug availability of ganciclovir-loaded mucoadhesive nanoparticles in rabbits. Eur. J. Nanomedicine 2013, 5, 159–167. [Google Scholar] [CrossRef]
- Hassan, H.; Adam, S.K.; Othman, F.; Shamsuddin, A.F.; Basir, R. Antiviral Nanodelivery Systems: Current Trends in Acyclovir Administration. J. Nanomater. 2016. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Sinha, V.R. Lipid Nanocarrier: An Efficient Approach Towards Ocular Delivery of Hydrophilic Drug (Valacyclovir). AAPS PharmSciTech 2017, 18, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Seyfoddin, A.; Al-Kassas, R. Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir. Drug Dev. Ind. Pharm. 2013, 39, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Seyfoddin, A.; Sherwin, T.; Patel, D.V.; McGhee, C.N.; Rupenthal, I.D.; Taylor, J.A.; Al-Kassas, R. Ex vivo and In vivo Evaluation of Chitosan Coated Nanostructured Lipid Carriers for Ocular Delivery of Acyclovir. Curr. Drug Deliv. 2016, 13, 923–934. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent Update on Nanoemulgel as Topical Drug Delivery System. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Yeun, O.C.; Yin, H.M.; Lynn, T.W.; Tine, C.L.Y.; Wi, N.S.; Yen, K.C.C.; Phing, C.S.; Kesharwani, P.; et al. Perspectives of Nanoemulsion Strategies in The Improvement of Oral, Parenteral and Transdermal Chemotherapy. Curr. Pharm. Biotechnol. 2018, 19, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Jain, P.; Parkhe, G. Formulation and evaluation of acyclovir loaded novel nano-emulsion gel for topical treatment of herpes simplex viral infections. J. Drug Deliv. Ther. 2018, 8, 265–270. [Google Scholar] [CrossRef]
- Mitra, A.K.; Kwatra, D.; Vadlapudi, A.D. (Eds.) Drug Delivery, 1st ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2015; ISBN 9781284025682. [Google Scholar]
- Vadlapudi, A.D.; Mitra, A.K. Nanomicelles: An emerging platform for drug delivery to the eye. Ther. Deliv. 2013, 4, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular drug delivery barriers—role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef]
- Al-Ghabeish, M.; Xu, X.; Krishnaiah, Y.S.R.; Rahman, Z.; Yang, Y.; Khan, M.A. Influence of drug loading and type of ointment base on the in vitro performance of acyclovir ophthalmic ointment. Int. J. Pharm. 2015, 495, 783–791. [Google Scholar] [CrossRef]
- Sun, F.; Zheng, Z.; Lan, J.; Li, X.; Li, M.; Song, K.; Wu, X. New micelle myricetin formulation for ocular delivery: Improved stability, solubility, and ocular anti-inflammatory treatment. Drug Deliv. 2019, 26, 575–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Zhang, Y.; Yang, Z.; Li, M.; Li, F.; Cui, F.; Liu, T.; Shi, W.; Wu, X. Nanomicelle formulation for topical delivery of cyclosporine A into the cornea: In vitro mechanism and in vivo permeation evaluation. Sci. Rep. 2015, 5, 12968. [Google Scholar] [CrossRef] [Green Version]
- Vadlapudi, A.D.; Cholkar, K.; Vadlapatla, R.K.; Mitra, A.K. Aqueous nanomicellar formulation for topical delivery of biotinylated lipid prodrug of acyclovir: Formulation development and ocular biocompatibility. J. Ocul. Pharmacol. Ther. 2014, 30, 49–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wempe, M.F.; Wright, C.; Little, J.L.; Lightner, J.W.; Large, S.E.; Caflisch, G.B.; Buchanan, C.M.; Rice, P.J.; Wacher, V.J.; Ruble, K.M.; et al. Inhibiting efflux with novel non-ionic surfactants: Rational design based on vitamin E TPGS. Int. J. Pharm. 2008, 370, 93–102. [Google Scholar] [CrossRef]
- Meng, X.; Liu, J.; Yu, X.; Li, J.; Lu, X.; Shen, T. Pluronic F127 and D-α-Tocopheryl Polyethylene Glycol Succinate (TPGS) Mixed Micelles for Targeting Drug Delivery across The Blood Brain Barrier. Sci. Rep. 2017, 7, 2964. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Kumbhar, S.A.S.A.; Tekade, R.K.; Iyer, A.K.A.K.; Kesharwani, P. Recent advances in TPGS-based nanoparticles of docetaxel for improved chemotherapy. Int. J. Pharm. 2017, 529, 506–522. [Google Scholar] [CrossRef]
- Bu, H.-Z.; Gukasyan, H.; Goulet, L.; Lou, X.-J.; Xiang, C.; Koudriakova, T. Ocular Disposition, Pharmacokinetics, Efficacy and Safety of Nanoparticle-Formulated Ophthalmic Drugs. Curr. Drug Metab. 2007, 8, 91–107. [Google Scholar] [CrossRef]
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141. [Google Scholar] [CrossRef]
- Rajendran, N.; Natrajan, R.; Kumar, R.; Selvaraj, S. Acyclovir-loaded chitosan nanoparticles for ocular delivery. Asian J. Pharm. 2010, 4. [Google Scholar] [CrossRef]
- Selvaraj, S.; Niraimathi, V.; Nappinnai, M. Formulation and evaluation of acyclovir loaded chitosan nanoparticles. Int. J. Pharm. Anal. Res. 2016, 5, 619–629. [Google Scholar]
- Alkholief, M.; Albasit, H.; Alhowyan, A.; Alshehri, S.; Raish, M.; Abul Kalam, M.; Alshamsan, A. Employing a PLGA-TPGS based nanoparticle to improve the ocular delivery of Acyclovir. Saudi Pharm. J. 2019, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lee, S.H.; Gan, C.W.; Feng, S.S. In vitro and in vivo investigation on PLA-TPGS nanoparticles for controlled and sustained small molecule chemotherapy. Pharm. Res. 2008, 25, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Acyclovir—Drug Bank. Available online: https://www.drugbank.ca/drugs/DB00787 (accessed on 4 September 2020).
- Taskar, P.; Tatke, A.; Majumdar, S. Advances in the use of prodrugs for drug delivery to the eye. Expert Opin. Drug Deliv. 2017, 14, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Yellepeddi, V.K.; Palakurthi, S. Recent Advances in Topical Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2016, 32, 67–82. [Google Scholar] [CrossRef]
- Singh, A.; Negi, D.; Mishra, N.; Baldi, A. Recent trends in ocular drug delivery. Pharmaspire 2018, 10, 55–63. [Google Scholar]
- Gote, V.; Ansong, M.; Pal, D. Prodrugs and nanomicelles to overcome ocular barriers for drug penetration. Expert Opin. Drug Metab. Toxicol. 2020, 1–22. [Google Scholar] [CrossRef]
- Katragadda, S.; Gunda, S.; Hariharan, S.; Mitra, A.K. Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: Evaluation of their utility in treating ocular HSV infections. Int. J. Pharm. 2008, 359, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Suresh, K.; Xiadong, Z.; Ravi, T.S.; Mitra, A.K. Small Neutral Amino Acid Ester Prodrugs of Acyclovir Targeting Amino Acid Transporters on the Cornea: Possible Antiviral Agents against Ocular HSV-1 Infections. Ophthalmol. Eye Dis. 2010, 2, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, S.; Nashed, Y.E.; Patel, K.; Jain, R.; Itahashi, M.; Neumann, D.M.; Hill, J.M.; Mitra, A.K. Dipeptide monoester ganciclovir prodrugs for treating HSV-1-induced corneal epithelial and stromal keratitis: In vitro and in vivo evaluations. J. Ocul. Pharmacol. Ther. 2005, 21, 463–474. [Google Scholar] [CrossRef]
- Gunda, S.; Hariharan, S.; Mitra, A.K. Corneal absorption and anterior chamber pharmacokinetics of dipeptide monoester prodrugs of ganciclovir (GCV): In vivo comparative evaluation of these prodrugs with val-GCV and GCV in rabbits. J. Ocul. Pharmacol. Ther. 2006, 22, 465–476. [Google Scholar] [CrossRef]
- Vadlapudi, A.D.; Vadlapatla, R.K.; Earla, R.; Sirimulla, S.; Bailey, J.B.; Pal, D.; Mitra, A.K. Novel biotinylated lipid prodrugs of acyclovir for the treatment of herpetic keratitis (HK): Transporter recognition, tissue stability and antiviral activity. Pharm. Res. 2013, 30, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Pal, D.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Mitra, A.K. Ocular delivery of proteins and peptides: Challenges and novel formulation approaches. Adv. Drug Deliv. Rev. 2018, 126, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.K.; Al-Attraqchi, O.; Chandrasekaran, B.; Paradkar, A.; Tekade, R.K. Protein/peptide drug delivery systems: Practical considerations in pharmaceutical product development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 651–684. ISBN 9780128179093. [Google Scholar]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Huang, L.C.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 2005, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Buznyk, O.; Kuffova, L.; Rajendran, V.; Forrester, J.V.; Phopase, J.; Islam, M.M.; Skog, M.; Ahlqvist, J.; Griffith, M. Cathelicidin LL-37 and HSV-1 Corneal Infection: Peptide Versus Gene Therapy. Transl. Vis. Sci. Technol. 2014, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, C.R. Peptide therapeutics for treating ocular surface infections. J. Ocul. Pharmacol. Ther. 2014, 30, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; Liu, J.; Valyi-Nagy, T.; Shukla, D. Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J. Biol. Chem. 2011, 286, 25406–25415. [Google Scholar] [CrossRef] [Green Version]
- Elnagdy, S.; AlKhazindar, M. The Potential of Antimicrobial Peptides as an Antiviral Therapy against COVID-19. ACS Pharmacol. Transl. Sci. 2020, 3, 780–782. [Google Scholar] [CrossRef]
- Jaishankar, D.; Buhrman, J.S.; Valyi-Nagy, T.; Gemeinhart, R.A.; Shukla, D. Extended release of an anti–heparan sulfate peptide from a contact lens suppresses corneal herpes simplex virus-1 infection. Investig. Ophthalmol. Vis. Sci. 2016, 57, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, P.S.; Neumann, D.M.; Foster, T.P.; Clement, C.; Singh, G.; Thompson, H.W.; Kaufman, H.E.; Hill, J.M. Effective treatment of ocular HSK with a human apolipoprotein E mimetic peptide in a mouse eye model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4263–4268. [Google Scholar] [CrossRef]
- Bhattacharjee, P.S.; Neumann, D.M.; Hill, J.M. A human apolipoprotein E mimetic peptide effectively inhibits HSV-1 TK-positive and TK-negative acute epithelial keratitis in rabbits. Curr. Eye Res. 2009, 34, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Terreni, E.; Burgalassi, S.; Chetoni, P.; Tampucci, S.; Zucchetti, E.; Fais, R.; Ghelardi, E.; Lupetti, A.; Monti, D. Development and Characterization of a Novel Peptide-Loaded Antimicrobial Ocular Insert. Biomolecules 2020, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Madan, M.; Bajaj, A.; Lewis, S.; Udupa, N.; Baig, J.A. In situ forming polymeric drug delivery systems. Indian J. Pharm. Sci. 2009, 71, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shah, S.J.; Wang, Z.; Agrahari, V.; Pal, D.; Mitra, A.K. Nanoparticle-based topical ophthalmic formulation for sustained release of stereoisomeric dipeptide prodrugs of ganciclovir. Drug Deliv. 2016, 23, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Mahboobian, M.M.; Mohammadi, M.; Mansouri, Z. Development of thermosensitive in situ gel nanoemulsions for ocular delivery of acyclovir. J. Drug Deliv. Sci. Technol. 2020, 55, 101400. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.; Chen, H.; Zhang, S.; Yu, S.; Li, Y.; Cui, M.; Pan, W.; Yang, X. A novel ion-activated in situ gelling ophthalmic delivery system based on κ-carrageenan for acyclovir. Drug Dev. Ind. Pharm. 2018, 44, 829–836. [Google Scholar] [CrossRef]
- Ramyadevi, D.; Sandhya, P. Dual sustained release delivery system for multiple route therapy of an antiviral drug. Drug Deliv. 2014, 21, 276–292. [Google Scholar] [CrossRef]
- Lin, T.; Gong, L.; Sun, X.H.; Zhao, N.Q.; Chen, W.; Yuan, H.P.; Shao, Y.; Gao, M.H.; Tang, H. Effectiveness and safety of 0.15% ganciclovir in situ ophthalmic gel for herpes simplex keratitis—A multicenter, randomized, investigator-masked, parallel group study in Chinese patients. Drug Des. Devel. Ther. 2013, 7, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.V.; Chetty, C.M.; Reddy, Y.D.; Ugandar, R.E.; Gladiola, B.D. Formulation and in vitro Characterization of Ocular in situ Gels of Valcyclovir. J. Pharm. Sci. Res. 2019, 11, 2974–2979. [Google Scholar]
- Kapadia, R.; Khambete, H.; Katara, R.; Ramteke, S. A Novel Approach for Ocular Delivery of Acyclovir Via Niosomes Entrapped In Situ Hydrogel System. J. Pharm. Res. 2009, 2, 745–751. [Google Scholar]
- Popa, L.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Irimia, T. Chitosan: A Good Candidate for Sustained Release Ocular Drug Delivery Systems. In Chitin-Chitosan—Myriad Functionalities in Science and Technology; InTech: London, UK, 2018; pp. 283–310. [Google Scholar]
- Refai, H.; Tag, R. Development and characterization of sponge-like acyclovir ocular minitablets. Drug Deliv. 2011, 18, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Jervis, L. A Summary of Recent Advances in Ocular Inserts and Implants. J. Bioequiv. Availab. 2016, 09, 320–323. [Google Scholar] [CrossRef]
- Pavan-Langston, D.; Langston, R.H.S.; Geary, P.A. Idoxuridine Ocular Insert Therapy: Use in Treatment of Experimental Herpes Simplex Keratitis. Arch. Ophthalmol. 1975, 93, 1349–1351. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration Zirgan. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022211_zirgan_toc.cfm (accessed on 4 September 2020).
- Kaufman, H.E.; Haw, W.H. Ganciclovir ophthalmic gel 0.15%: Safety and efficacy of a new treatment for herpes simplex keratitis. Curr. Eye Res. 2012, 37, 654–660. [Google Scholar] [CrossRef]
- Safety and Efficacy of CRISPR/Cas9 mRNA Instantaneous Gene Editing Therapy to Treat Refractory Viral Keratitis Full Text View ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04560790?term=ocular&cond=Herpes+Simplex+Virus+Keratitis&draw=2&rank=2 (accessed on 17 December 2020).
| Objectives | Drug | Type of Formulation | Polymer Used | Membrane/Cell Line/Animal Model | Outcome | Source |
|---|---|---|---|---|---|---|
| To investigate the pharmacokinetics of acyclovir liposomes delivered to aqueous humour. | Acyclovir | Liposomes | Cholesterol, L-phosphatidylcholine, stearylamine | New Zealand (NZ) albino rabbits | Particle size: 370.9 ± 5.6 nm. Entrapment efficiency: 22.8%. Loading ACV concentration in liposome dispersion: 0.20 mg/mL. In vivo efficacy: 11-fold greater drug availability in the aqueous humour vs. reference ointment. In vitro release: higher drug release (50.25%). | [40] |
| To develop and optimise formulations of transferrin-conjugated liposomes containing ganciclovir. | Ganciclovir | Liposomes | Cholesterol, 1,2- distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2- distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (DSPE-PEG), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (polyethylene glycol) − 2000] (DSPE-PEG-MAL) | Human retinal pigment epithelial cells (ARPE-19) | Particle size: 88–113 nm. Zeta potential: ~−32 mV. Entrapment efficiency: 32–36%. In vitro release: prolonged drug release of over 12 h. In vivo efficacy: higher drug uptake by ARPE-19. In vitro cytotoxic (APRE-19): cell viability of 80–100% based on MTT assay. | [42] |
| To evaluate the ocular retention and intraocular delivery of mucoadhesive niosomal ganciclovir. | Ganciclovir | Niosomes coated with chitosan | Cholesterol, Span 60, chitosan | NZ albino rabbits | Particle size: 190 nm. Zeta potential: +41.8 mV (successfully coated with cationic chitosan). Entrapment efficiency: 47.2%. In vitro release: sustained drug release over 12 h. In vivo efficacy: drug concentrations obtained in the aqueous humour of niosome-treated albino rabbits were significantly greater. In vivo irritation: no visual irritation or damaging effect to ocular tissues of tested rabbits. | [43] |
| To fabricate and achieve efficient delivery of valacyclovir into the eye via solid lipid nanoparticles (SLNs). | Valacyclovir | SLNs | Stearic acid, tristearin, poloxamer 188, sodium taurocholate | Chorioallantoic membrane (CAM) | Particle size: 202.5 ± 2.56 nm. Zeta potential: −34.4 ± 3.04 mV. Entrapment efficiency: 58.82 ± 2.45%. In vitro release: sustained drug release over 12 h. In vivo efficacy: improved ocular bioavailability. Ex vivo irritation: no irritation in CAM and histopathology result. | [45] |
| To improve the ocular bioavailability of acyclovir using SLN and nanostructured lipid carriers (NLC) delivery systems. | Acyclovir | SLNs and NLCs | Stearic acid, Capryol® 90 Lauroglycol® 90, Compritol® 888 ATO, and Cithrol GMS, Tween® 40, Tween® 80, Poloxamer® 188, Brij® 78 | Bovine cornea | Particle size: 185–766 nm. Zeta potential: −30 to 34 mV. Entrapment efficiency: 4–34%. In vitro release: Both NLCs and SLNs showed extended drug release (8 h) compared to the reference solution (4 h). Faster diffusion and release of drug from the NLCs. Hydration level: no signs of toxicity to the cornea based on hydration level test. | [46] |
| To conduct an ex vivo and in vivo evaluation of chitosan-coated NLCs for acyclovir ocular delivery. | Acyclovir | NLCs | Lauroglycol® 90, Compritol® 888 ATO, Cithrol GMS, Tween® 40, chitosan | Vero cells | In vivo efficacy: 3.5-fold reduction in effective concentration to achieve 50% inhibition of viral replication (IC50) was observed with acyclovir NLC-treated monkey kidney cells (CV-1). Acyclovir uptake by primary human corneal epithelial cells (HCEC) was higher in NLCs. In vitro cytotoxicity: MTT assay found no toxic effects on Vero cells. | [47] |
| To develop and characterise a nanoemulsion of acyclovir as a topical gel. | Acyclovir | Nanoemulsion | Castor oil, Span 40, PEG 400 | - | Mean vesicle size: 41.6 nm. Zeta potential: −32.4 mV. Loading capacity: ~62–89%. In vitro release: 88% drug release within 8 h. | [50] |
| Objectives | Drug | Type of Formulation | Polymer Used | Membrane/Cell Line/Animal Model | Outcome | Source |
|---|---|---|---|---|---|---|
| To evaluate the solubility of acyclovir, corneal permeability, and sclera penetration of Soluplus and Solutol polymeric micelles. | Acyclovir | Polymeric micelle | Soluplus® (polyvinyl co- prolactam-polyvinyl acetate-polyethylene glycol copolymer) | Chorioallantoic membrane (CAM) | Particle size: 219 nm. Zeta potential: +0.35 mV. Encapsulated acyclovir solubility: 2-fold in both water medium and phosphate-buffered saline (PBS) compared to unencapsulated acyclovir. In vivo permeability: 2.8-fold and 3.4-fold higher permeability flux than aqueous acyclovir in both cornea and sclera. In vitro irritation: no toxicity in fertilised eggs. | [54] |
| To develop a clear aqueous nanomicelle formulation and evaluate its biocompatibility. | Biotinylated lipid prodrug of acyclovir | Surfactant nanomicelle | D-α-tocopheryl polyethylene glycol 1000 succinate (Vitamin E TPGS) and octoxynol-40 | Human corneal epithelial cells (HCECs) | Particle size: 10.78 nm. Zeta potential: −1.59 mV. Entrapment efficiency: ~90%. In vitro release: showed sustained release properties for up to 4 days. In vitro ocular irritation: no cytotoxic effect in HCECs. | [58] |
| To validate the effect of acyclovir concentration on the physicochemical characteristic and release profile of chitosan nanoparticles. | Acyclovir | Polymeric nanosuspension | Chitosan and Tween-80 | - | Particle size: 200 ± 30 nm. Zeta potential: +36.7 ± 1.5 mV. Encapsulation efficiency: 56%. Loading capacity: 25%. In vitro release: release for up to 24 h. | [64] |
| To validate the effect of chitosan concentration on the physicochemical characteristic and release profile of chitosan nanoparticles. | Acyclovir | Polymeric nanosuspension | Chitosan and Tween-80 | - | Particle size: 250 nm. Zeta potential: +42.8 mV. Encapsulation efficiency: 90%. Loading capacity: 50%. In vitro release: release for up to 24 h. | [65] |
| To increase ocular bioavailability of acyclovir through poly (lactic-co-glycolic acid) (PLGA)-based nanoparticles stabilised with vitamin E TPGS. | Acyclovir | Polymeric nanosuspension | PLGA and vitamin E TPGS | Albino rabbits | Particle size: 262.38 ± 11.85 nm. Zeta potential: 15.14 ± 2.81 mV. Encapsulation efficiency: 74.12 ± 6.19%. Loading capacity: 8.65 ± 1.09%. In vitro release: showed sustained release for up to 72 h. In vivo permeability: 1.4-fold higher permeability flux compared to drug solution. In vivo distribution: bioavailability was 2.76-fold higher than drug solution. In vivo ocular irritation: demonstrated mild irritation but subsided after 6 h. | [66] |
| Objectives | Drug | Type of Formulation | Polymer Used | Membrane/Cell Line/Animal Model | Outcome | Source |
|---|---|---|---|---|---|---|
| To evaluate the corneal absorption of amino acid prodrugs. | Acyclovir (ACV) | Ophthalmic prodrug | - | Primary corneal epithelial cell cultures | Stability: L-Serine-ACV (SACV) was the most stable among the other prodrugs. In vivo ocular absorption: SACV and L-Valine- ACV (VACV) showed a 2-fold increase in area under concentration time curve (AUC) and maximum aqueous humor concentration (Cmax) of prodrug and regenerated ACV compared to ACV. Cytotoxicity studies: cellular toxicity of ACV prodrugs was significant lower compared to trifluridine. | [73] |
| To characterise the amino acid prodrugs based on affinity and permeability. | Acyclovir | Ophthalmic prodrug | - | Rabbit primary corneal epithelial cell culture (rPCEC) | In vitro antiviral studies: SACV displayed anti-HSV-1 activity and the concentration required to inhibit viral cytopathogenicity by 50% (EC50) was 6.3 μM. Corneal permeability: SACV exhibited higher corneal permeability and superior anti-HSV-1 activity relative to ACV. | [74] |
| To evaluate dipeptide monoester ganciclovir (GCV) prodrugs. | Ganciclovir (GCV) | Ophthalmic prodrug | - | New Zealand White (NZW) rabbits | Solubility: the prodrugs showed better aqueous solubility compared to parent drug. Transcorneal permeability: valine-GCV (VGCV) and divaline-GCV (VVGCV) were 7- to 8-fold higher than GCV. In vivo efficacy studies: 1% VVGCV has better therapeutic activity against HSV-1 epithelial keratitis compared to 1% trifluridine. | [75] |
| To evaluate the corneal absorption of dipeptide monoester prodrugs. | Ganciclovir | Ophthalmic prodrug | - | NZW rabbits | In vivo studies: The area under the concentration–time profile (AUCinfinity)of the regenerated GCV from tyrosine-valine-GCV (YVGCV) was 8.6-fold higher than GCV, whereas VVGCV was 1.8-fold higher than GCV. Both YVGCV and VVGCV demonstrated enhanced permeability and superior corneal absorption. | [76] |
| To develop sodium-dependent multivitamin transporter (SMVT)-targeted biotinylated lipid prodrugs to improve cellular absorption. | Acyclovir | Ophthalmic prodrug | - | Human corneal epithelial cells (HCECs) | Uptake study: the uptake of biotin-ricinoleicacid-acyclovir (B-R-ACV) and biotin-12hydroxystearicacid-acyclovir (B-12HS-ACV) was nearly 13.6-fold and 13.1-fold higher than parent drug, respectively. Stability: B-R-ACV and B-12HS-ACV possessed better stability. In vitro antiviral activity: B-R-ACV: ~4.5-fold and 8.7-fold more potent against HSV-1 and HSV-2, respectively, compared to parent drug. B-12HS-ACV: ~200-fold and 21-fold more potent against HSV-1 and HSV-2, respectively, compared to parent drug. | [77] |
| Objectives | Drug/Peptide Used | Type of Formulation | Polymer Used | Membrane/Cell Line/Animal Model | Outcome | Source |
|---|---|---|---|---|---|---|
| To evaluate antimicrobial activity of LL-37. | LL-37 | Ophthalmic peptide delivery | - | Human corneal and conjunctival epithelial cells (LL-37 expression study) | Antiviral assay: 500 µg/mL of LL-37 reduced HSV-1 viral load by more than 100-fold compared to the phosphate-buffered saline (PBS) and scrambled peptide. | [82] |
| To compare the release of LL-37 from nanoparticle–hydrogel corneal implants and human corneal epithelial cell (HCEC)-produced LL-37. | LL-37 | Peptide delivery, nanoparticle–hydrogel corneal implants, human corneal epithelial cell (HCEC)-produced LL-37. | - | HCECs | In vitro studies: the viral binding was reduced by LL-37, but the virus was not completely cleared from the already infected cells. | [83] |
| To identify peptides that bind specifically to heparan sulfate (HS). To investigate their effectiveness in inhibiting HSV-1. | G1 and G2 peptide | Ophthalmic peptide delivery | - | Mouse cornea | In vivo studies: the G1 and G2 peptides significantly reduced the severity of keratitis when administered prophylactically. | [85] |
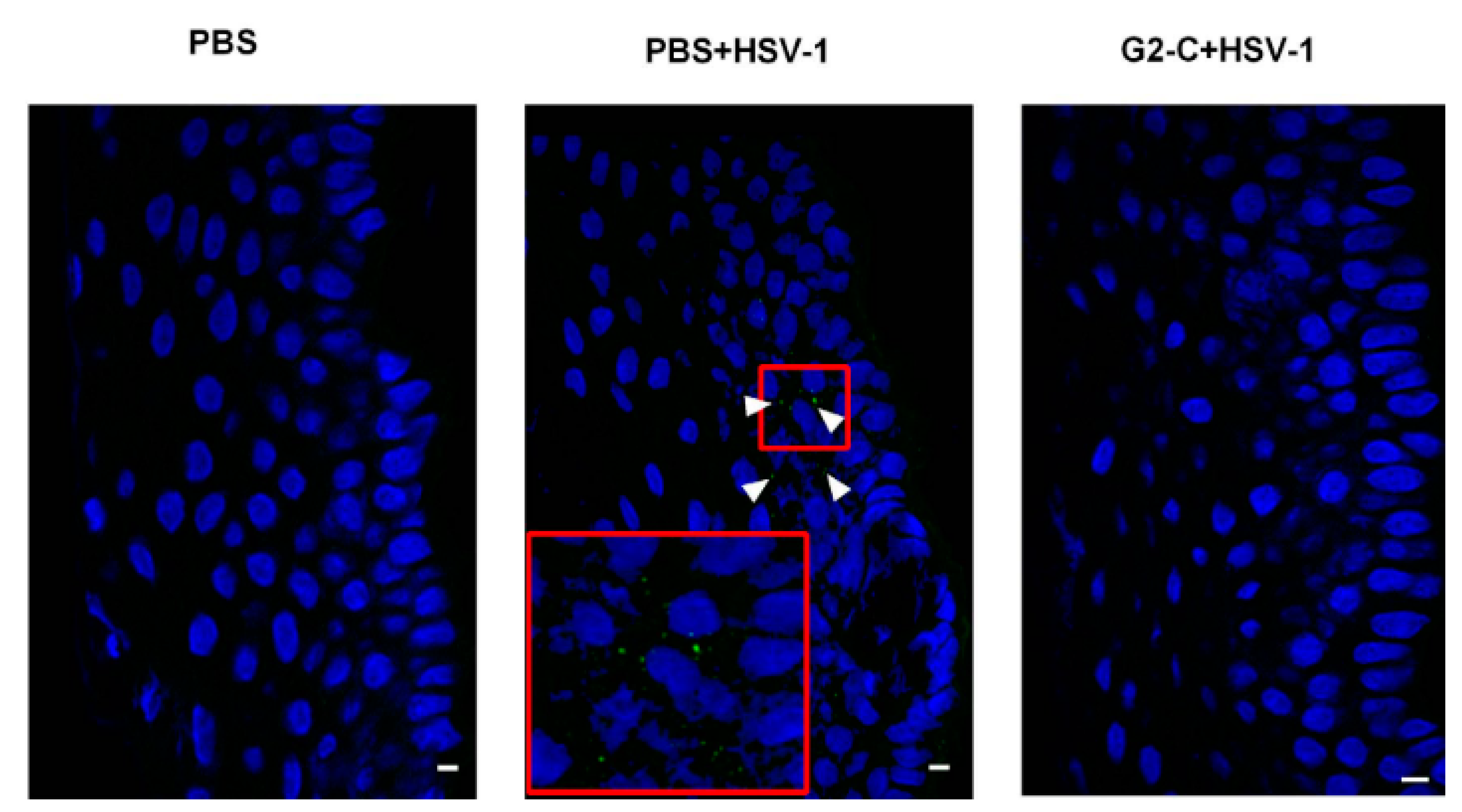
| To develop and evaluate G2-C contact lens to lengthen the release of G2-C peptide. | G2-C peptide | Ophthalmic peptide delivery using contact lens. | - | Human cornea epithelial cells (ex vivo virus spread assay), pig corneas (ex vivo study of inhibition of HSV-1), mouse model (in vivo efficacy study) | In vitro release: the release of G2-C was prolonged with the use of the contact lens. In vivo and ex vivo studies: the G2-C lens were effective in inhibiting HSV-1 entry in both ex vivo and in vivo studies. | [87] |
| To evaluate of the therapeutic efficacy of 1% apoEdp. | apoEdp | Ophthalmic peptide delivery | - | Mouse | In vivo studies: 1% apoEdp was as effective as 1% trifluridine in reducing the incidence and severity of herpes simplex keratitis (HSK). The expression of several proinflammatory cytokines was downregulated compared to the control. | [88] |
| To evaluate the efficacy of 1% apoEdp against HSV-1 thymidine kinase (TK)-positive and HSV-1 TK negative virus. | apoEdp | Ophthalmic peptide delivery | - | NZW rabbits | In vivo studies: apoEdp was as effective as trifluridine and foscarnet in reducing the severity of keratitis in both TK-positive and TK-negative HSV groups. | [89] |
| To develop ocular insert for antimicrobial peptide delivery. | hLF 1-11 | Ophthalmic peptide delivery | - | hLF 1-11 was found to be stable in a freeze-dried solid matrix of hydroxypropyl methylcellulose (HPMCs) and it released the peptide in sustained manner. | [90] |
| Objective | Drug | Types of Stimuli/Polymer Used | Membrane/Cell Line/Animal Model | Outcome | Source |
|---|---|---|---|---|---|
| Preparation of ocular in situ micelles to enhance ocular permeation. | Acyclovir | Thermo-responsive micelles/Soluplus | Rabbits | Higher corneal and sclera permeability compared to conventional formulation. | [54] |
| Preparation and evaluation of ion-activated in situ gel ophthalmic delivery system of acyclovir based on kappa-carrageenan. | Acyclovir- hydroxypropyl-β-cyclodextrin complex | Ion-activated/kappa-carrageenan | New Zealand White (NZW) rabbits | Rheology: pseudoplastic fluid Gelling capacity: gel formed rapidly after contact with tear fluid, maintained for a long time. In vitro release: 80% of drug released after 6 h In vitro permeability: 2.16-fold higher apparent permeability. In vivo irritation: no irritation to rabbits’ eyes. | [95] |
| To develop sustained release nanoparticles loaded with ganciclovir prodrug. | Ganciclovir prodrug | Thermo-responsive/poly (lactic-co-glycolic acid) (PLGA), PLGA-polyethylene glycol-PLGA (PLGA-PEG-PLGA) | Human corneal epithelial cells (HCECs) | NPs were small in size with higher drug loading and entrapment. Biphasic release pattern: burst release followed by sustained release. Efficient permeation of prodrug with accumulation in cul-de-sac. | [93] |
| To develop and evaluate thermo-responsive in situ gel nanoemulsions in delivering acyclovir. | Acyclovir (ACV) | Thermo-responsive nanoemulsion/Triacetin and Transcutol® P (nanoemulsion) poloxamer 407 and poloxamer 188 (in situ) | NZW rabbits (in vivo ocular irritation test) and Hen’s Egg-Chorioallantoic Membrane (HET-CAM) (in vitro ocular irritation test) | Gelation temperature 30.9 °C. pH: 4.58 ± 0.068 Viscosity: 103.03 ± 4.68 mPa.s In vitro drug release efficiency: 80.78 ± 1.82% The optimised formulations displayed sustained release. Ex vivo permeation: permeation of ACV was 2.83-fold higher in optimised formulation compared to ACV solution. In vivo ocular irritation test: minimal conjunctival redness but disappeared after 2 h of administration. In vitro ocular irritation (HET-CAM) test: cumulative score of 0.33 ± 0.58, indicating non-irritant | [94] |
| To design polymeric nanoparticles of acyclovir incorporated in in situ gelling system to provide dual sustained release effect, whereby the duration of action and bioavailability through different routes of administration could be improved. | Acyclovir | Thermo-activated/Pluronic F-127 and pH-activated/Carbopol | - | Gelation temperature: 25 ± 0.20 to 35 ± 0.46 °C Gelation time: 2 to 4 min In vitro drug release study: better sustained release characteristics, with non-Fickian diffusion mechanism of drug release. | [96] |
| Formulation of valcyclovir in situ gels. | Valcyclovir | pH-activated/Carbopol 940, HPMC K 100M | - | In situ gels show sustained release with profile. | [98] |
| Development of acyclovir-loaded niosomes entrapped in hydrogel | Acyclovir | pH-activated/Span 20 or Span 60, cholesterol, Carbopol 934, methylcellulose | Rabbits | Sustained release with no sign of irritation | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, M.; Choudhury, H.; Abdul-Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Su, J.S.T.; Tan, C.L.; Chin, W.Y.; Yip, K.Y. Advancement on Sustained Antiviral Ocular Drug Delivery for Herpes Simplex Virus Keratitis: Recent Update on Potential Investigation. Pharmaceutics 2021, 13, 1. https://doi.org/10.3390/pharmaceutics13010001
Pandey M, Choudhury H, Abdul-Aziz A, Bhattamisra SK, Gorain B, Su JST, Tan CL, Chin WY, Yip KY. Advancement on Sustained Antiviral Ocular Drug Delivery for Herpes Simplex Virus Keratitis: Recent Update on Potential Investigation. Pharmaceutics. 2021; 13(1):1. https://doi.org/10.3390/pharmaceutics13010001
Chicago/Turabian StylePandey, Manisha, Hira Choudhury, Azila Abdul-Aziz, Subrat Kumar Bhattamisra, Bapi Gorain, Jocelyn Sziou Ting Su, Choo Leey Tan, Woon Yee Chin, and Khar Yee Yip. 2021. "Advancement on Sustained Antiviral Ocular Drug Delivery for Herpes Simplex Virus Keratitis: Recent Update on Potential Investigation" Pharmaceutics 13, no. 1: 1. https://doi.org/10.3390/pharmaceutics13010001
APA StylePandey, M., Choudhury, H., Abdul-Aziz, A., Bhattamisra, S. K., Gorain, B., Su, J. S. T., Tan, C. L., Chin, W. Y., & Yip, K. Y. (2021). Advancement on Sustained Antiviral Ocular Drug Delivery for Herpes Simplex Virus Keratitis: Recent Update on Potential Investigation. Pharmaceutics, 13(1), 1. https://doi.org/10.3390/pharmaceutics13010001









