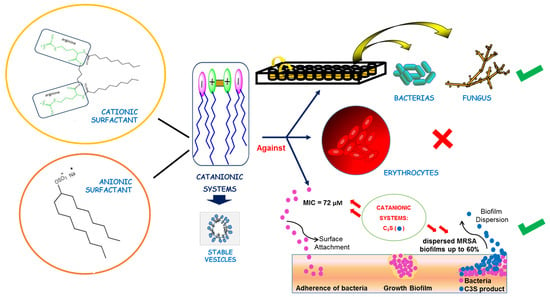
Biocompatible Catanionic Vesicles from Arginine-Based Surfactants: A New Strategy to Tune the Antimicrobial Activity and Cytotoxicity of Vesicular Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparations of Catanionic Mixtures
2.3. Fluorescence Measurements
2.4. Conductivity
2.5. NMR Measurements
2.6. ς-Potential and Size Distribution Analysis
2.7. Antimicrobial Activity
2.8. Antibiofilm Activity
2.9. Hemolysis Assay
2.10. Ethidium Bromide Fluorescence
3. Results and Discussion
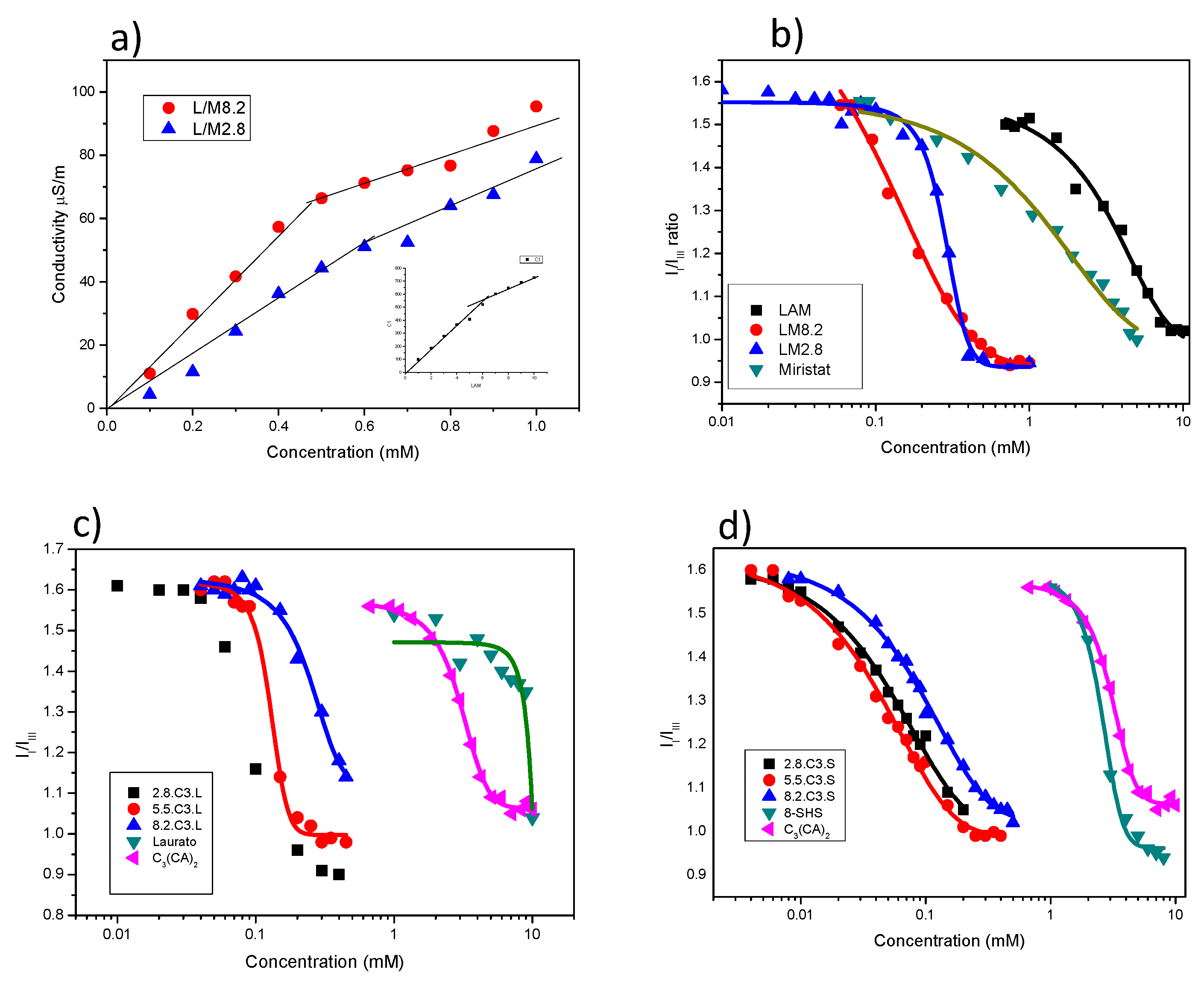
3.1. Critical Aggregation Concentration
3.2. Size Distribution and ς-Potential
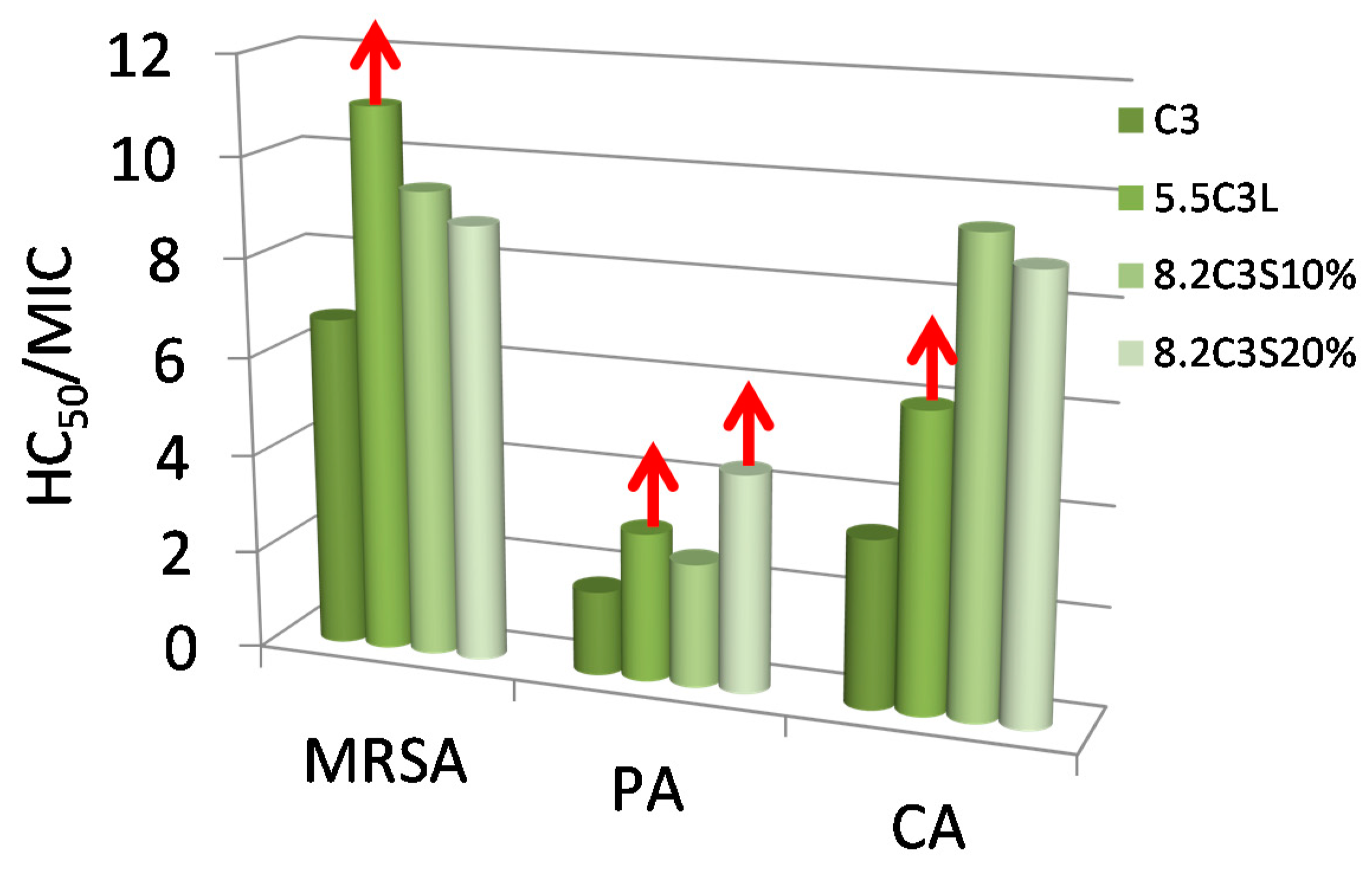
3.3. Antimicrobial Activity
3.4. Antibiofilm Activity
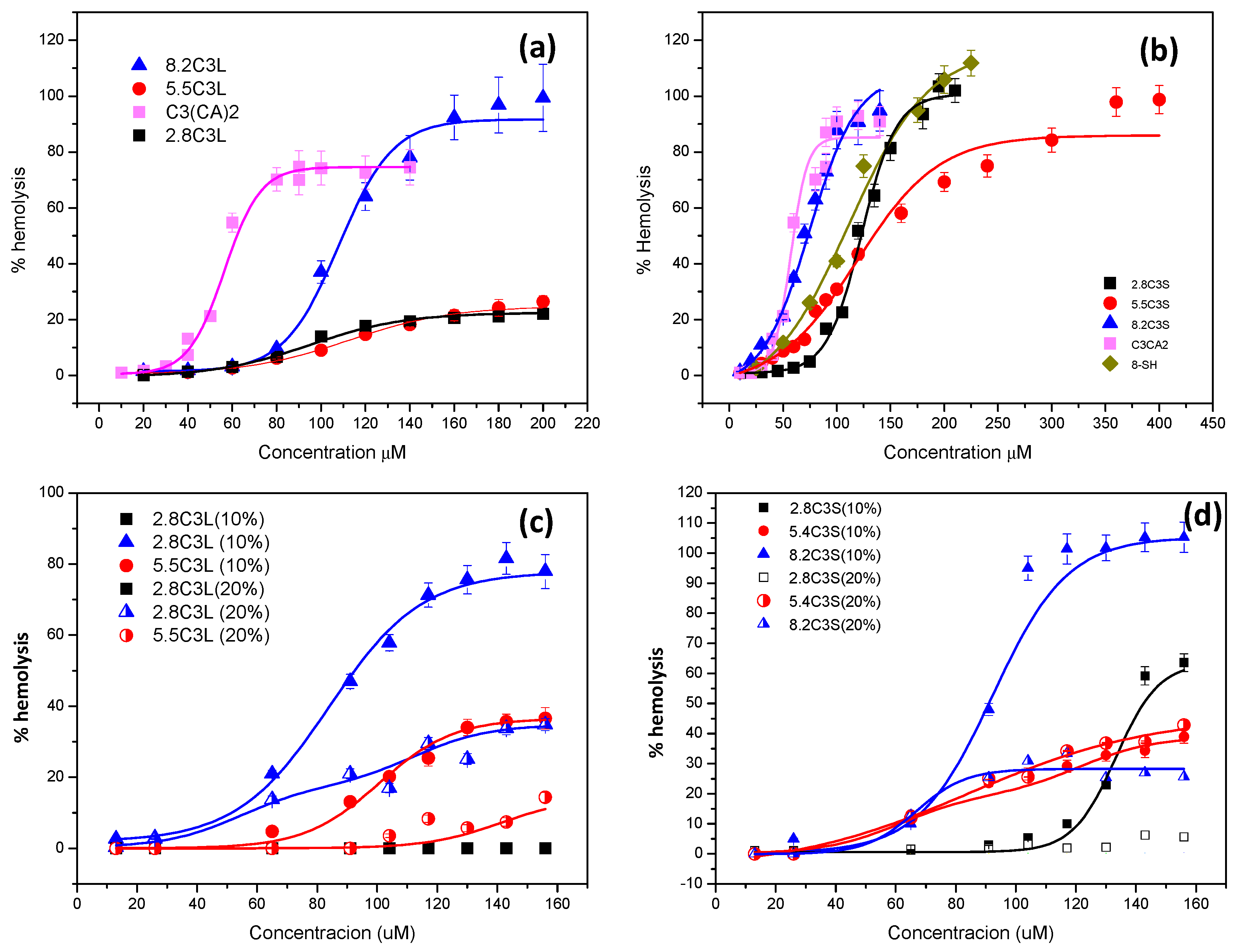
3.5. Hemolytic Activity
3.6. DNA Binding Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barratt, G. Colloidal drug carriers: Achievements and perspectives. Cell. Mol. Life Sci. 2003, 60, 21–37. [Google Scholar] [CrossRef]
- Bangham, A.; Horne, R. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660-IN10. [Google Scholar] [CrossRef]
- Zhu, J.; Xue, J.; Guo, Z.; Zhang, L.; Marchant, R. Biomimetic glycoliposomes as nanocarriers for targeting p-selectin on activated platelets. Bioconjug. Chem. 2007, 18, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Muzykantov, V.R.; Sourkas, T. Multifunctional nanoparticles: Cost versus benefit of adding targeting. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V. Liposomes Methods and Protocols; Weissig, V., Ed.; Humana Press: New York, NY, USA, 2010; Volume 1. [Google Scholar] [CrossRef]
- Sultana, Y.; Aqil, M.; Samad, A. Liposomal drug delivery systems: An update review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef]
- Winterhalter, M.; Lasic, D.D. Liposome stability and formation: Experimental parameters and theories on the size distribution. Chem. Phys. Lipids 1993, 64, 35–43. [Google Scholar] [CrossRef]
- Dhawan, V.V.; Nagarsenker, M.S. Catanionic systems in nanotherapeutics—Biophysical aspects and novel trends in drug delivery applications. J. Control. Release 2017, 266, 331–345. [Google Scholar] [CrossRef]
- Marques, E.F. Size and stability of catanionic vesicles: Effects of formation path, sonication, and aging. Langmuir 2000, 16, 4798–4807. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, F.; Luan, Y.; Cao, W.; Ji, X.; Zhao, L.; Zhang, L.; Li, Z. Formation of drug/surfactant catanionic vesicles and their application in sustained drug release. Int. J. Pharm. 2012, 436, 806–814. [Google Scholar] [CrossRef]
- Dias, R.S.; Lindman, B.; Miguel, M.G. DNA interaction with catanionic vesicles. J. Phys. Chem. B 2002, 106, 12600–12607. [Google Scholar] [CrossRef]
- Yuan, J.; Zheng, L.; Zhao, M. The formation of vesicles by N-Dodecyl-N-Methylpyrrolidinium bromide ionic liquid/copper dodecyl sulfate and application in the synthesis of leaflike CuO nanosheets. Colloid Polym. Sci. 2012, 290, 1361–1369. [Google Scholar] [CrossRef]
- Dowling, M.B.; Javvaji, V.; Payne, G.F.; Raghavan, S.R. Vesicle capture on patterned surfaces coated with amphiphilic biopolymers. Soft Matter 2011, 7, 1219–1226. [Google Scholar] [CrossRef]
- Kahe, H.; Chamsaz, M.; Zavar, M.H.A. A novel supramolecular aggregated liquid-solid microextraction method for the preconcentration and determination of trace amounts of lead in saline solutions and food samples using electrothermal atomic absorption spectrometry. RSC Adv. 2016, 6, 49076–49082. [Google Scholar] [CrossRef]
- Jurašin, D.D.; Šegota, S.; Čadež, V.; Atiđa Selmani, M.D.S. Recent Advances in Catanionic Mixtures. In Application and Characterization of Surfactants; Najjar, R., Ed.; IntechOpen Limited: London, UK, 2017; ISBN 978-953-51-4768-8. [Google Scholar] [CrossRef]
- Soussan, E.; Mille, C.; Blanzat, M.; Bordat, P.; Rico-Lattes, I. Sugar-derived tricatenar catanionic surfactant: Synthesis, self-assembly properties, and hydrophilic probe encapsulation by vesicles. Langmuir 2008, 24, 2326–2330. [Google Scholar] [CrossRef] [PubMed]
- Morán, M.C.; Pinazo, A.; Pérez, L.; Clapés, P.; Angelet, M.; García, M.T.; Vinardell, M.P.; Infante, M.R. “Green” amino acid-based surfactants. Green Chem. 2004, 6. [Google Scholar] [CrossRef]
- Blanzat, M.; Perez, E.; Rico-Lattes, I.; Lattes, A. Synthesis and anti-HIV activity of catanionic analogs of galactosylceramide. New J. Chem. 1999, 23, 1063–1065. [Google Scholar] [CrossRef]
- Blanzat, M.; Perez, E.; Rico-Lattes, I.; Prome, D.; Prome, J.C.; Lattes, A. New catanionic glycolipids. 1. synthesis, characterization, and biological activity of double-chain and gemini catanionic analogues of galactosylceramide (Galβ1cer). Langmuir 1999, 15, 6163–6169. [Google Scholar] [CrossRef]
- Lozano, N.; Pérez, L.; Pons, R.; Pinazo, A. Diacyl glycerol arginine-based surfactants: Biological and physicochemical properties of catanionic formulations. Amino Acids 2011, 40. [Google Scholar] [CrossRef]
- Richard, C.; Souloumiac, E.; Jestin, J.; Blanzat, M.; Cassel, S. Influence of dermal formulation additives on the physicochemical characteristics of catanionic vesicles. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 373–383. [Google Scholar] [CrossRef]
- Rosa, M.; Infante, M.R.; Miguel, M.D.G.; Lindman, B. Spontaneous formation of vesicles and dispersed cubic and hexagonal particles in amino acid-based catanionic surfactant systems. Langmuir 2006, 22, 5588–5596. [Google Scholar] [CrossRef]
- Pinazo, A.; Manresa, M.A.; Marques, A.M.; Bustelo, M.; Espuny, M.J.; Pérez, L. Amino acid–based surfactants: New antimicrobial agents. Adv. Colloid Interface Sci. 2016, 228, 17–39. [Google Scholar] [CrossRef]
- Pérez, L.; Torres, J.L.; Manresa, A.; Solans, C.; Infante, M.A.R. Synthesis, aggregation, and biological properties of a new class of gemini cationic amphiphilic compounds from arginine, Bis(Args). Langmuir 1996, 12, 5296–5301. [Google Scholar] [CrossRef]
- Pucci, C.; Pérez, L.; La Mesa, C.; Pons, R. Characterization and stability of catanionic vesicles formed by pseudo-tetraalkyl surfactant mixtures. Soft Matter 2014, 10, 9657–9667. [Google Scholar] [CrossRef] [PubMed]
- Method, J.B.; Patel, F.C.; Tenover, I.D.; Turnidge, J.H.J. Manual of Clinical Microbiology, 10th ed.; Versalovic, K.C., Carrol, G., Funke, J.H., Jorgensen, M.L., landry, D.W.W., Eds.; ASM Press: Washinton, DC, USA, 2011. [Google Scholar]
- Pape, W.J.; Pfannenbecker, U.; Hoppe, U. Validation of the red blood cell test system as in vitro assay for the rapid screening of irritation potential of surfactants. Mol. Toxicol. 1987, 1, 525–536. [Google Scholar] [PubMed]
- Soussan, E.; Cassel, S.; Blanzat, M.; Rico-Lattes, I. Drug delivery by soft matter: Matrix and vesicular carriers. Angew. Chem. Int. Ed. 2009, 48, 274–288. [Google Scholar] [CrossRef]
- Pérez, L.; Pinazo, A.; Rosen, M.J.; Infante, M.R. Surface activity properties at equilibrium of novel gemini cationic amphiphilic compounds from arginine, Bis(Args). Langmuir 1998, 14, 2307–2315. [Google Scholar] [CrossRef]
- Herrington, K.L.; Kaler, E.W.; Miller, D.D.; Zasadzinski, J.A.; Chiruvolu, S. Phase Behavior of Aqueous Mixtures of Dodecyltrimethylammonium Bromide (DTAB) and Sodium Dodecyl Sulfate (SDS). J. Phys. Chem. 1993, 97, 13792–13802. [Google Scholar] [CrossRef]
- Li, S.J.; Lai, L.; Mei, P.; Li, Y.; Cheng, L.; Ren, Z.H.; Liu, Y. Equilibrium and dynamic surface properties of cationic/anionic surfactant mixtures based on bisquaternary ammonium salt. J. Mol. Liq. 2018, 254, 248–254. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Bustelo, M.; Pinazo, A.; Manresa, M.A.; Mitjans, M.; Vinardell, M.P.; Pérez, L. Monocatenary histidine-based surfactants: Role of the alkyl chain length in antimicrobial activity and their selectivity over red blood cells. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 501–509. [Google Scholar] [CrossRef]
- Pinazo, A.; Angelet, M.; Pons, R.; Lozano, M.; Infante, M.R.; Pérez, L. Lysine-bisglycidol conjugates as novel lysine cationic surfactants. Langmuir 2009, 25, 7803–7814. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dong, S.; Jia, X.; Song, A.; Hao, J. Vesicles of a new salt-free cat-anionic fluoro/hydrocarbon surfactant system. Chem. A Eur. J. 2007, 13, 9495–9502. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.F.; Regev, O.; Khan, A.; Da Graça Miguel, M.; Lindman, B. Vesicle formation and general phase behavior in the catanionic mixture SDS-DDAB-water. The cationic-rich side. J. Phys. Chem. B 1999, 103, 8353–8363. [Google Scholar] [CrossRef]
- Kaler, E.W.; Herrington, K.L.; Murthy, A.K.; Zasadzinski, J.A.N. Phase behavior and structures of mixtures of anionic and cationic surfactants. J. Phys. Chem. 1992, 96, 6698–6707. [Google Scholar] [CrossRef]
- Wang, Y.; Marques, E.F. Non-ideal behavior of mixed micelles of cationic gemini surfactants with varying spacer length and anionic surfactants: A conductimetric study. J. Mol. Liq. 2008, 142, 136–142. [Google Scholar] [CrossRef]
- Wen, C.F.; Hsieh, Y.L.; Wang, C.W.; Yang, T.Y.; Chang, C.H.; Yang, Y.M. Effects of ethanol and cholesterol on thermotropic phase behavior of ion-pair amphiphile bilayers. J. Oleo Sci. 2018, 67, 295–302. [Google Scholar] [CrossRef]
- Kuo, J.H.S.; Jan, M.S.; Chang, C.H.; Chiu, H.W.; Li, C.T. Cytotoxicity characterization of catanionic vesicles in RAW 264.7 murine macrophage-like cells. Colloids Surf. B Biointerfaces 2005, 41, 189–196. [Google Scholar] [CrossRef]
- Mannock, D.A.; Lewis, R.N.A.H.; McMullen, T.P.W.; McElhaney, R.N. The effect of variations in phospholipid and sterol structure on the nature of lipid-sterol interactions in lipid bilayer model membranes. Chem. Phys. Lipids 2010, 163, 403–448. [Google Scholar] [CrossRef]
- Kuo, A.T.; Tu, C.L.; Yang, Y.M.; Chang, C.H. Enhanced physical stability of positively charged catanionic vesicles: Role of cholesterol-adjusted molecular packing. J. Taiwan Inst. Chem. Eng. 2018, 92, 29–35. [Google Scholar] [CrossRef]
- González-Bello, C. Antibiotic adjuvants—A strategy to unlock bacterial resistance to antibiotics. Bioorganic Med. Chem. Lett. 2017, 27, 4221–4228. [Google Scholar] [CrossRef]
- Durão, P.; Balbontín, R.; Gordo, I. Evolutionary mechanisms shaping the maintenance of antibiotic resistance. Trends Microbiol. 2018, 26, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.A.; Clapés, P.; Infante, M.; Comas, J.; Manresa, A. Comparative study of the antimicrobial activity of bis(Nα-caproyl-l-arginine)-1,3-propanediamine dihydrochloride and chlorhexidine dihydrochloride against Staphylococcus aureus and Escherichia coli. J. Antimicrob. Chemoth. 2006, 57, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, M.; Furutani, T.; Azuma, M.; Ooshima, H.; Kato, J. Acyl amino acid derivatives as novel inhibitors of influenza neuraminidase. Biosci. Biotechnol. Biochem. 1997, 61, 870–874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinazo, A.; Manresa, M.A.; Vinardell, M.P.; Mitjans, M.; Colomer, A.; Pinazo, A.; Manresa, M.A.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; et al. Cationic surfactants derived from lysine: Effects of their structure and charge type on antimicrobial and hemolytic activities. J. Med. Chem. 2011, 54, 989–1002. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial activity, in vitro cytotoxicity, and cell cycle arrest of gemini quaternary ammonium surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef]
- Morente, E.O.; Fernández-fuentes, M.A.; José, M.; Burgos, G.; Abriouel, H.; Pulido, R.P.; Gálvez, A. Biocide tolerance in bacteria. Int. J. Food Microbiol. Biocide Toler. Bact. 2013, 162, 13–25. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Structure—Activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Qi, R.; Zhang, P.; Liu, J.; Zhou, L.; Zhou, C.; Zhang, N.; Han, Y.; Wang, S.; Wang, Y. Peptide amphiphiles with distinct supramolecular nanostructures for controlled antibacterial activities. ACS Appl. Bio Mater. 2018, 1, 21–26. [Google Scholar] [CrossRef]
- Tavano, L.; Infante, M.R.; Riya, M.A.; Pinazo, A.; Vinardell, M.P.; Mitjans, M.; Manresa, M.A.; Perez, L. Role of aggregate size in the hemolytic and antimicrobial activity of colloidal solutions based on single and gemini surfactants from arginine. Soft Matter 2013, 9, 306–319. [Google Scholar] [CrossRef]
- Buzhor, M.; Fuentes, E.; Pujals, S.; Amir, R.J.; Feiner-Gracia, N.; Pujals, S.; Amir, R.J.; Albertazzi, L. Micellar stability in biological media dictates internalization in living cells. J. Am. Chem. Soc. 2017, 139, 16677–16687. [Google Scholar] [CrossRef]
- Han, Y.; Perez, J.; Kirkpatrick, S.; Wang, Y.; Rodrigues de Almeida, N.; Sheridan, M.C. Design, synthesis, and nanostructure-dependent antibacterial activity of cationic peptide amphiphiles. ACS Appl. Mater. Interfaces 2018, 11, 2790–2801. [Google Scholar] [CrossRef]
- Makovitzki, A.; Baram, J.; Shai, Y. Antimicrobial lipopolypeptides composed of palmitoyl Di- and tricationic peptides: In vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry 2008, 47, 10630–10636. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Costantini, P.E.; Fedi, S.; Cappelletti, M.; Abruzzo, A.; Parolin, C.; Foschi, C.; Frisco, G.; Calonghi, N.; Cerchiara, T.; et al. Liposomes containing biosurfactants isolated from lactobacillus gasseri exert antibiofilm activity against methicillin resistant Staphylococcus Aureus strains. Eur. J. Pharm. Biopharm. 2019, 139, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.C.; Ator, L.E.; Paniak, T.J.; Minbiole, K.P.C.; Wuest, W.M. Biofilm-eradicating properties of quaternary ammonium amphiphiles: Simple mimics of antimicrobial peptides. ChemBioChem 2014, 15, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Park, H.D. Lauroyl arginate ethyl: An effective antibiofouling agent applicable for reverse osmosis processes producing potable water. J. Memb. Sci. 2016, 507, 24–33. [Google Scholar] [CrossRef]
- Manaargadoo-Catin, M.; Ali-Cherif, A.; Pougnas, J.-L.; Perrin, C. Hemolysis by surfactants—A review. Adv. Colloid Interface Sci. 2016, 228, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Pinazo, A.; Pérez, L.; Manresa, A.; Marqués, A.M. Green catanionic gemini surfactant-lichenysin mixture: Improved surface, antimicrobial, and physiological properties. ACS Appl. Mater. Interfaces 2017, 9, 22121–22131. [Google Scholar] [CrossRef]
- Rosa, M.; da Miguel, M.G.; Lindman, B. DNA encapsulation by biocompatible catanionic vesicles. J. Colloid Interface Sci. 2007, 312, 87–97. [Google Scholar] [CrossRef]
- Rosa, M.; del Carmen Morán, M.; da Miguel, M.G.; Lindman, B. The association of DNA and stable catanionic amino acid-based vesicles. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 361–375. [Google Scholar] [CrossRef]
- Dasgupta, A.; Das, P.K.; Dias, R.S.; Miguel, M.G.; Lindman, B. Effect of headgroup on DNA—Cationic surfactant interactions. J. Phys. Chem. B 2007, 111, 8502–8508. [Google Scholar] [CrossRef] [PubMed]
- da Ramos Silva, A.; Manresa, M.Á.; Pinazo, A.; García, M.T.; Pérez, L. Rhamnolipids functionalized with basic amino acids: Synthesis, aggregation behavior, antibacterial activity and biodegradation studies. Colloids Surf. B Biointerfaces 2019, 181. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Baek, J.; Bai, C.Z.; Park, J. Arginine-conjugated polypropylenimine dendrimer as a non-toxic and efficient gene delivery carrier. Biomaterials 2007, 28, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.M.; Valaske, R.; Maiti, S. Interaction between 14mer DNA oligonucleotide and cationic surfactants of various chain lengths. J. Phys. Chem. B 2008, 112, 8824–8831. [Google Scholar] [CrossRef] [PubMed]
- Bhadani, A.; Singh, S. Novel gemini pyridinium surfactants: Synthesis and study of their surface activity, DNA binding, and cytotoxicity. Langmuir 2009, 25, 11703–11712. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, A.; Pons, R.; Bustelo, M.; Manresa, M.Á.; Morán, C.; Raluy, M.; Pérez, L. Gemini histidine based surfactants: Characterization; Surface properties and biological activity. J. Mol. Liq. 2019, 289, 111156. [Google Scholar] [CrossRef]
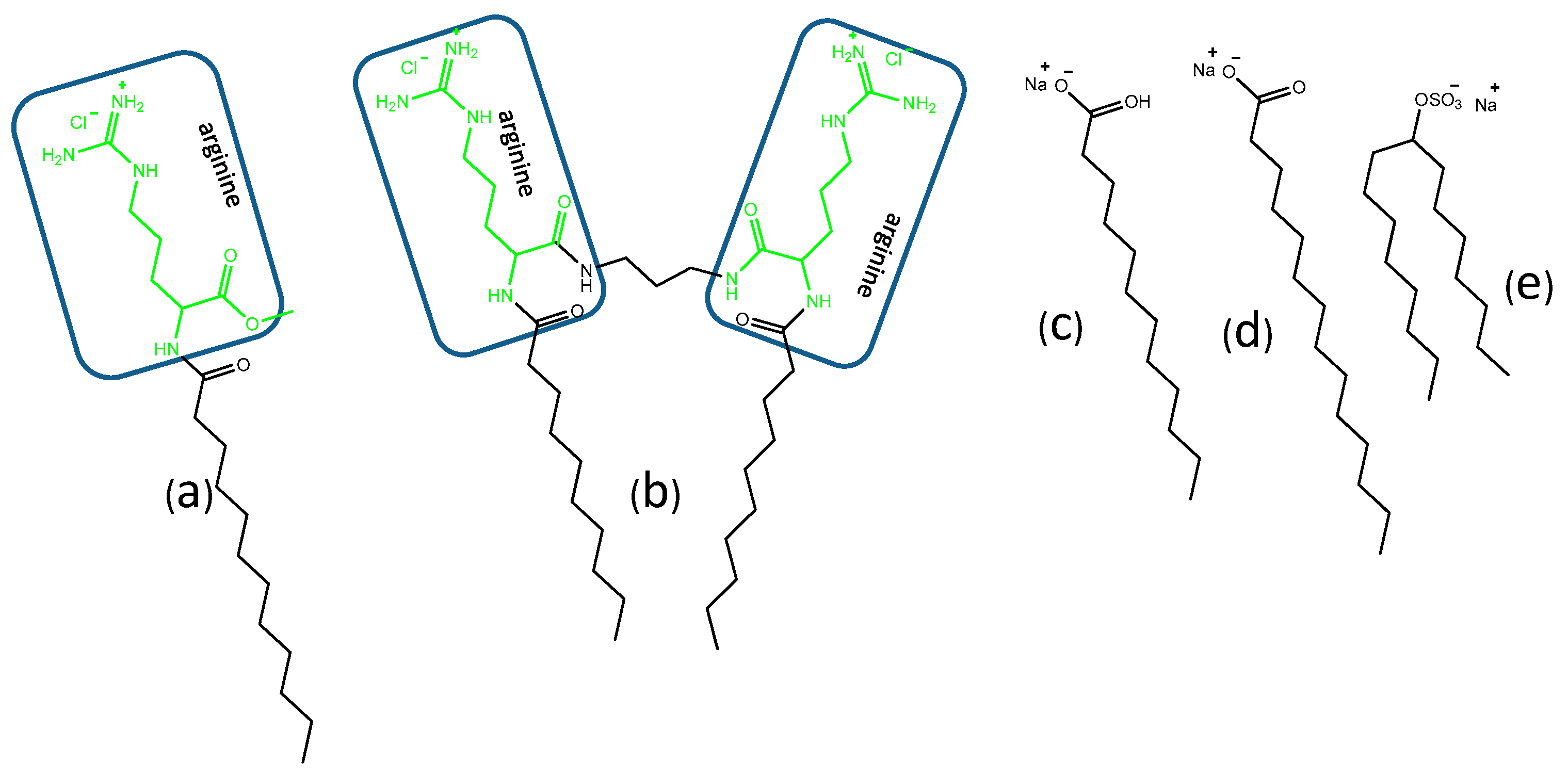
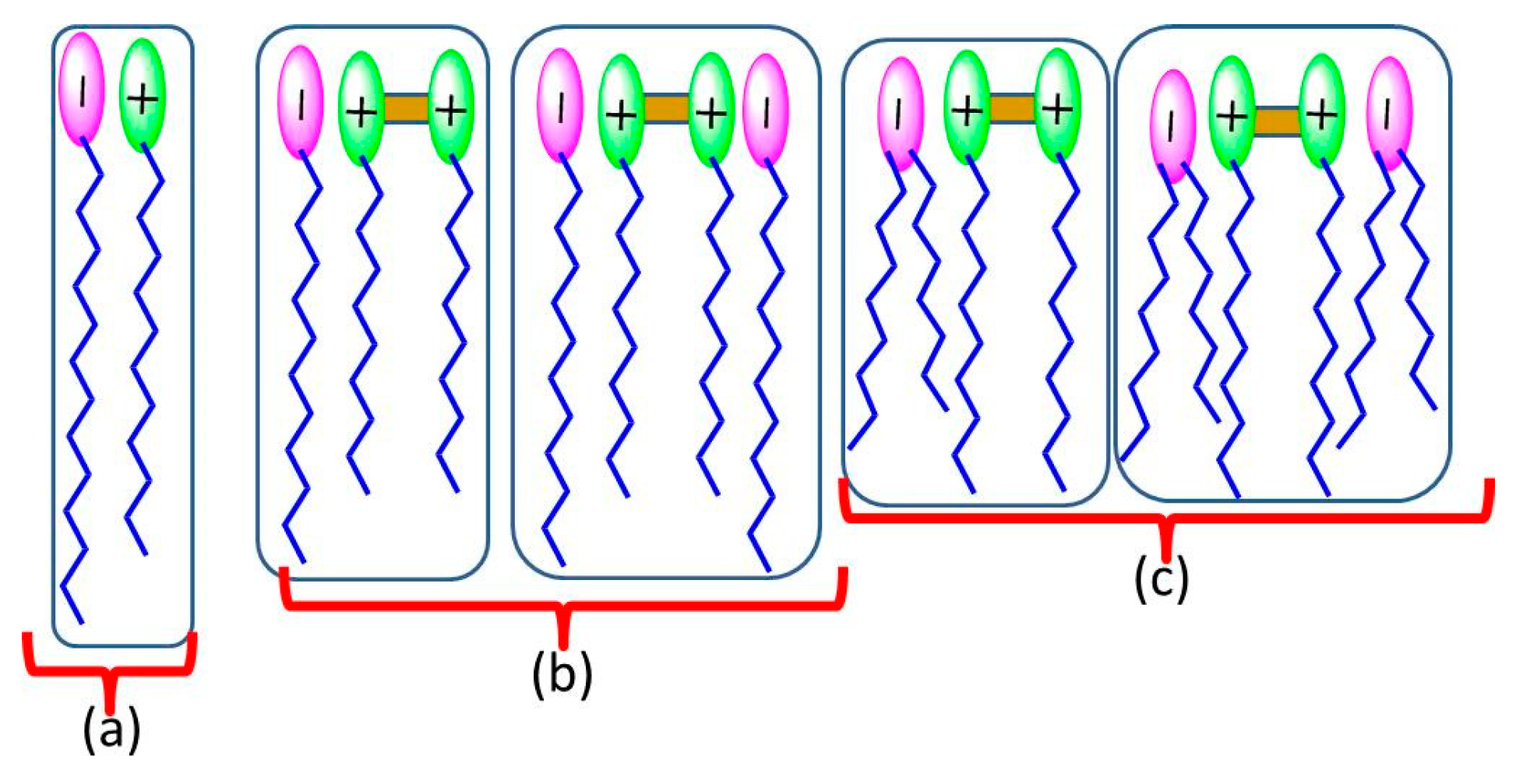
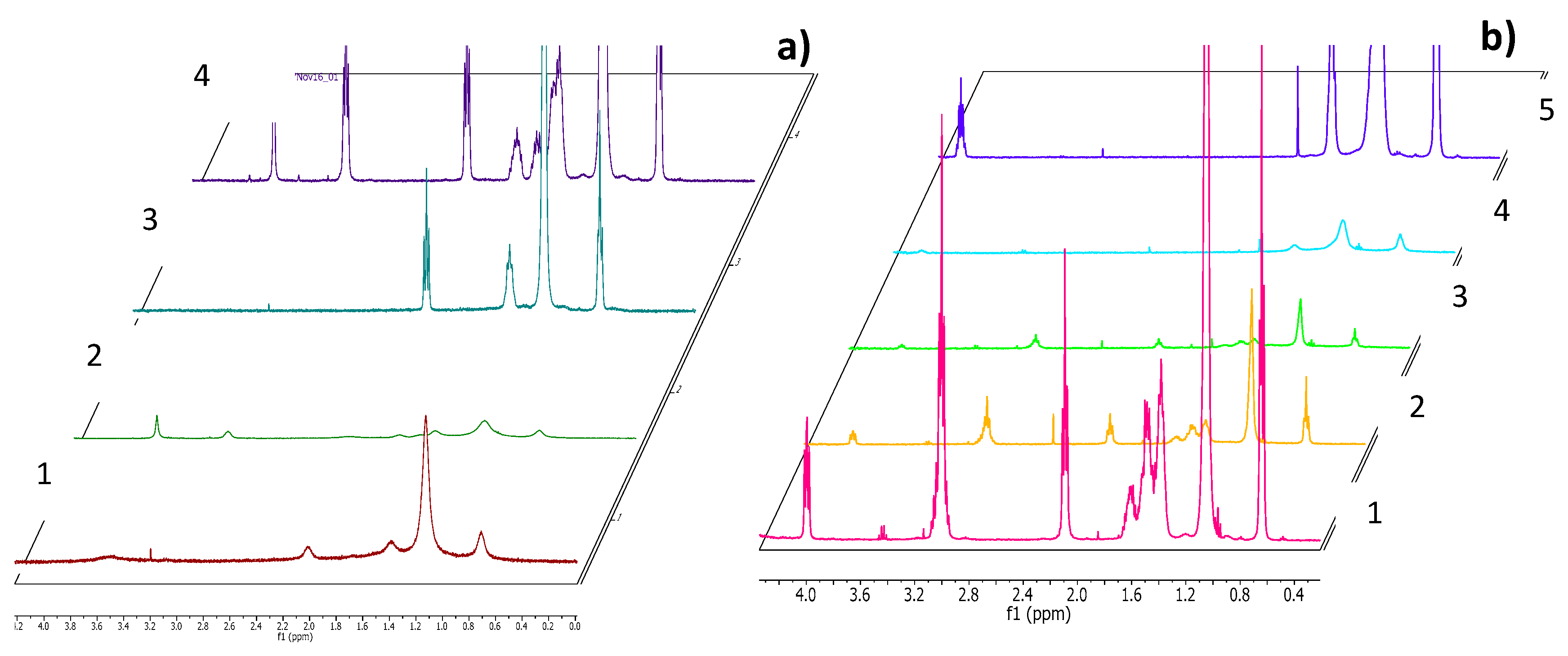
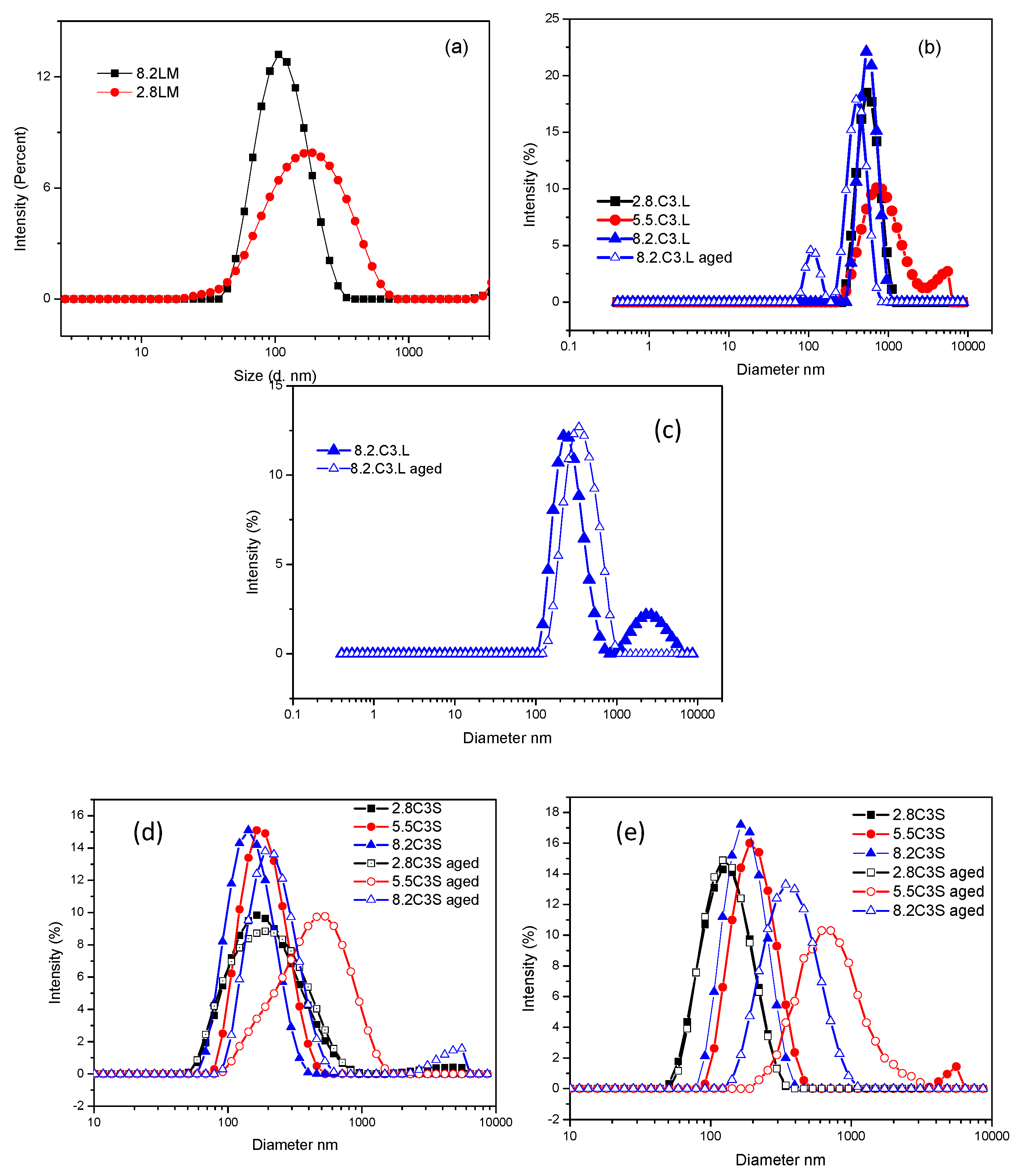
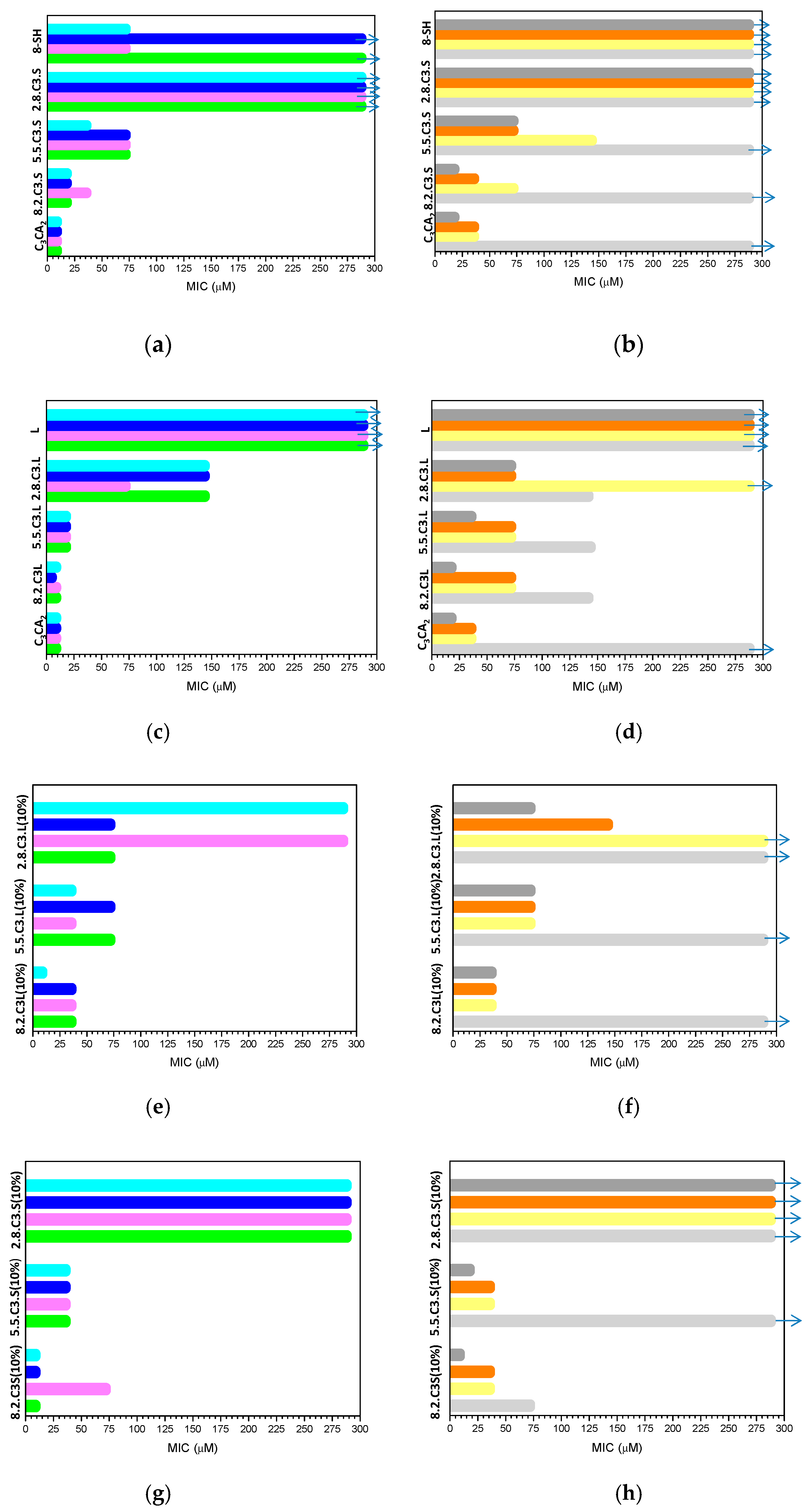
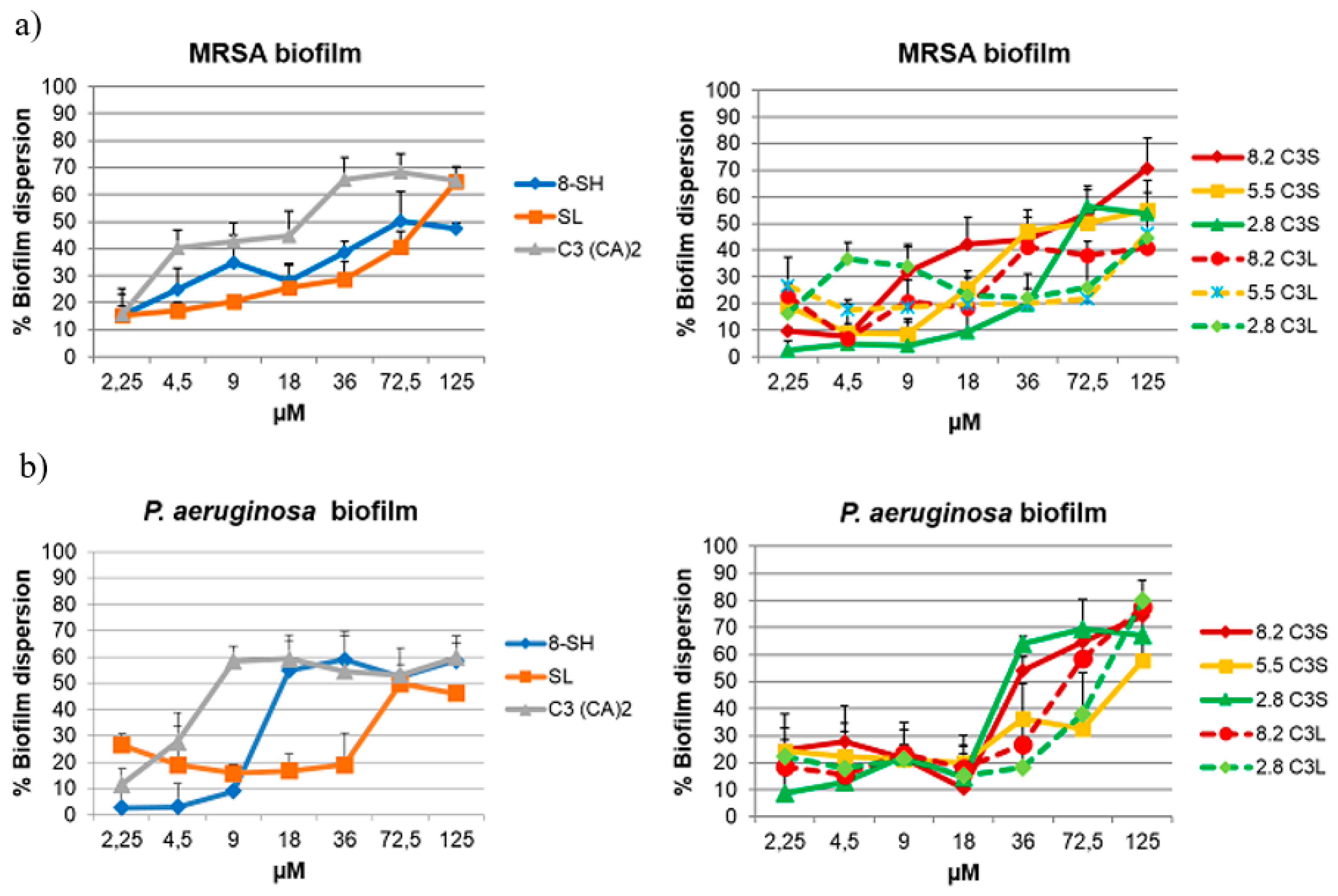

| Catanionic Mixtures | |||||||||
| Acronym | LAM (%) | Sodium Myristate (%) | Acronym | C3(CA)2 (%) | 8SH (%) | Acronym | C3(CA)2 (%) | Sodium Laurate (%) | |
| 8.2.LM | 80 | 20 | 2.8.C3S | 20 | 80 | 2.8.C3L | 20 | 80 | |
| 2.8.LM | 20 | 80 | 5.5.C3S | 50 | 50 | 5.5.C3L | 50 | 50 | |
| 8.2.C3S | 80 | 20 | 8.2.C3L | 80 | 20 | ||||
| Cholesterol Containing Catanionic Mixtures | |||||||||
| Acronym | Cholesterol (%) | C3(CA)2 (%) | 8SH (%) | Acronym | Cholesterol (%) | C3(CA)2 (%) | Sodium Laurate (%) | ||
| 2.8.C3S(10%) | 10 | 18 | 72 | 2.8.C3L (10%) | 10 | 18 | 72 | ||
| 2.8.C3S(20%) | 20 | 16 | 64 | 2.8.C3L (20%) | 20 | 16 | 64 | ||
| 5.5.C3S(10%) | 10 | 45 | 45 | 5.5.C3L (10%) | 10 | 45 | 45 | ||
| 5.5.C3S(20%) | 20 | 40 | 40 | 5.5.C3L (20%) | 20 | 40 | 40 | ||
| 8.2.C3S(10%) | 10 | 72 | 18 | 8.2.C3L (10%) | 10 | 72 | 18 | ||
| 8.2.C3S(20%) | 20 | 64 | 16 | 8.2.C3L (20%) | 20 | 64 | 16 | ||
| Single Chain Surfactants | Double Chain Surfactants | |||
|---|---|---|---|---|
| System | cac (mM) Fluorescente | cac (mM) Conductivity | System | cac (mM) Fluorescence |
| LAM | 4.7 | 6.2 | C3(CA)2 | 3.2 |
| Sodium myristate | 1.6 | - | 8-SH | 2.5 |
| 8.2 LM | 0.3 | 0.45 | Sodium laurate | 9.1 |
| 2.8 LM | 0.4 | 0.6 | 2.8C3S | 0.064 |
| 5.5C3S | 0.048 | |||
| 8.2C3S | 0.105 | |||
| 2.8C3L | 0.08 | |||
| 5.5C3L | 0.13 | |||
| 8.2C3L | 0.28 | |||
| Concentration | Formulation | Size Dh(nm) | PdI | ς-Potential (mV) | Visual Aspect |
|---|---|---|---|---|---|
| 1 mM | 2.8 LM | 190 | 0.400 | −65.7 |  |
| 5.5 LM | Precipitation | ||||
| 8.2 LM | 121 | 0.198 | +64.7 | ||
| 2.8 C3S | 138 | 0.109 | −33.4 |  | |
| 5.5 C3S | 212 | 0.180 | +15.3 | ||
| 8.2 C3S | 183 | 0.090 | +40.4 | ||
| 2.8C3L | 581 | 0.133 | −5.4 |  | |
| 5.5C3L | 924 | 0.360 | +27.3 | ||
| 8.2C3L | 572 | 0.320 | +37.4 | ||
| 5 mM | 2.8 C3S | 217 | 0.223 | −52.5 |  |
| 5.5 C3S | 194 | 0.151 | +36.4 | ||
| 8.2 C3S | 156 | 0.174 | +61.5 | ||
| 2.8C3L | 272 | 0.262 | −20 |  | |
| 5.5C3L | GEL | ||||
| 8.2C3L | GEL | ||||
| Cholesterol | Formulation | Size Dh(nm) | PdI | ς-Potential (mV) | Visual Aspect |
|---|---|---|---|---|---|
| 10% | 2.8C3L | 566 | 0.175 | −15 |  |
| 5.5C3L | 218 | 0.202 | 40 | ||
| 8.2C3L | 58 | 0.400 | 44 | ||
| 2.8 C3S | 242 | 0.390 | −17 |  | |
| 5.5 C3S | 463 | 0.343 | 17 | ||
| 8.2 C3S | 118 | 0.225 | 40 | ||
| 20% | 2.8C3L | 251 | 0.216 | −12 |  |
| 5.5C3L | 191 | 0.179 | 24 | ||
| 8.2C3L | 207 | 0.335 | 29 | ||
| 2.8 C3S | 255 | 0.410 | −10 |  | |
| 5.5 C3S | 272 | 0.332 | 16 | ||
| 8.2 C3S | 218 | 0.447 | 39 |
| Pure Compounds | HC50 μM | Catanionic Mixtures | HC50 μM | 10%Chol Mixtures | HC50 μM | 20% Chol Mixtures | HC50 μM |
|---|---|---|---|---|---|---|---|
| C3(CA)2 | 60 ± 3.1 | 2.8C3S | 120 ± 6 | 2.8C3S (10%) | >156 | 2.8C3S (20%) | >156 |
| 8-SH | 100 ± 4.2 | 5.5.C3S | 160 ± 12 | 5.5.C3S (10%) | >156 | 5.5.C3S (20%) | >156 |
| SL | >200 | 8.2.C3S | 79 ± 5 | 8.2.C3S (10%) | 85 ± 5 | 8.2.C3S (20%) | >156 |
| 2.8C3L | >200 | 2.8C3L (10%) | >156 | 2.8C3L (20%) | >156 | ||
| 5.5.C3L | >200 | 5.5.C3L (10%) | >156 | 5.5.C3L (20%) | >156 | ||
| 8.2.C3L | 110 ± 11 | 8.2.C3L (10%) | 100 ± 8 | 8.2.C3L (20%) | >156 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinazo, A.; Pons, R.; Marqués, A.; Farfan, M.; da Silva, A.; Perez, L. Biocompatible Catanionic Vesicles from Arginine-Based Surfactants: A New Strategy to Tune the Antimicrobial Activity and Cytotoxicity of Vesicular Systems. Pharmaceutics 2020, 12, 857. https://doi.org/10.3390/pharmaceutics12090857
Pinazo A, Pons R, Marqués A, Farfan M, da Silva A, Perez L. Biocompatible Catanionic Vesicles from Arginine-Based Surfactants: A New Strategy to Tune the Antimicrobial Activity and Cytotoxicity of Vesicular Systems. Pharmaceutics. 2020; 12(9):857. https://doi.org/10.3390/pharmaceutics12090857
Chicago/Turabian StylePinazo, Aurora, Ramon Pons, Ana Marqués, Maribel Farfan, Anderson da Silva, and Lourdes Perez. 2020. "Biocompatible Catanionic Vesicles from Arginine-Based Surfactants: A New Strategy to Tune the Antimicrobial Activity and Cytotoxicity of Vesicular Systems" Pharmaceutics 12, no. 9: 857. https://doi.org/10.3390/pharmaceutics12090857
APA StylePinazo, A., Pons, R., Marqués, A., Farfan, M., da Silva, A., & Perez, L. (2020). Biocompatible Catanionic Vesicles from Arginine-Based Surfactants: A New Strategy to Tune the Antimicrobial Activity and Cytotoxicity of Vesicular Systems. Pharmaceutics, 12(9), 857. https://doi.org/10.3390/pharmaceutics12090857