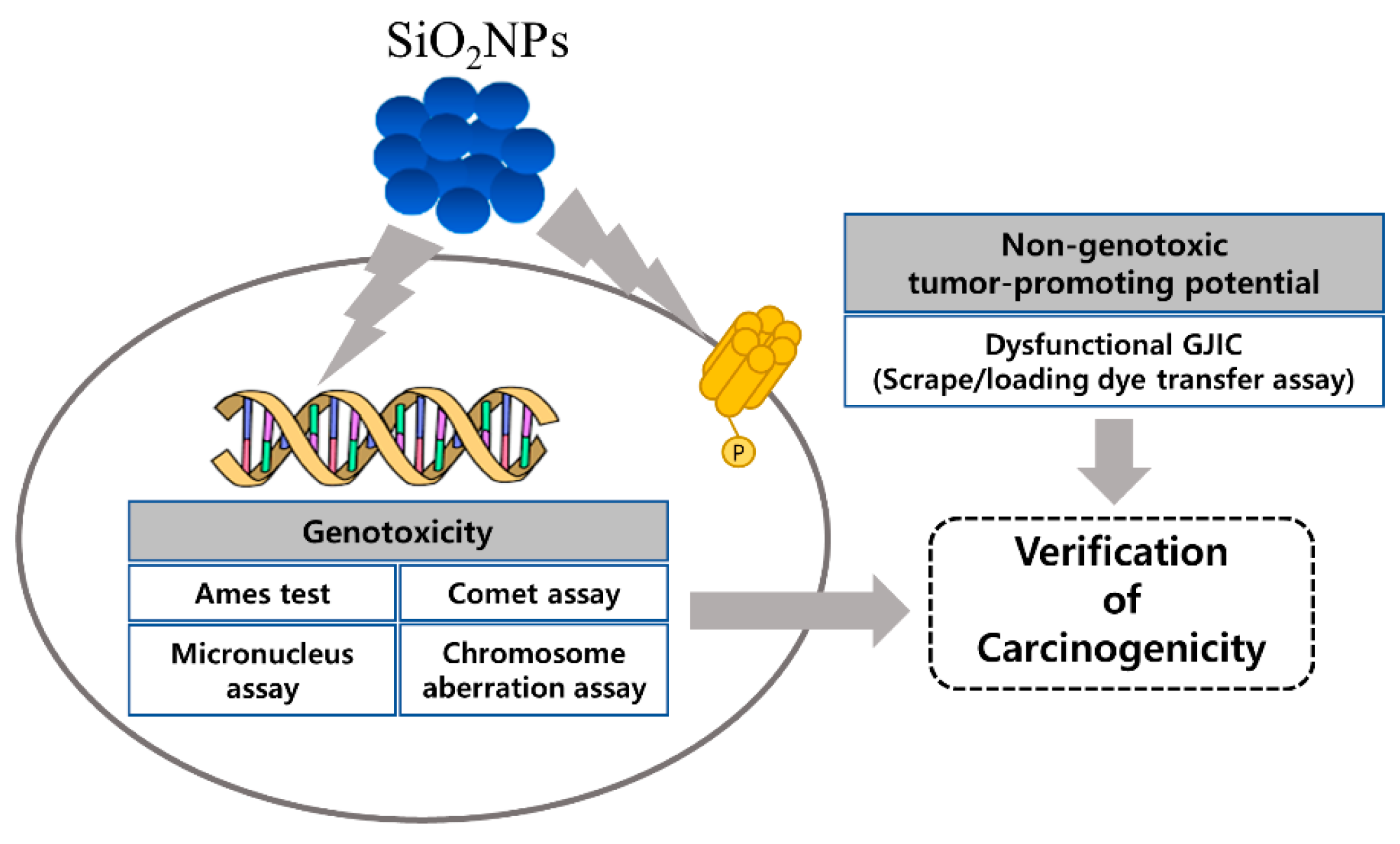
Toxicologic Evaluation for Amorphous Silica Nanoparticles: Genotoxic and Non-Genotoxic Tumor-Promoting Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Physicochemical Characterization
2.2. Ames Test
2.3. In Vitro Chromosomal Aberration Study
2.4. In Vivo Bone Marrow Micronucleus Test
2.5. In Vitro Comet Assay
2.6. Cell Culture and Treatments for GJIC Analysis
2.7. SL/DT Assay for GJIC Analysis
2.8. Immunofluorescence Staining of Cx43
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Characterization of SiO2NPs
3.2. SiO2NPs Exposure Results in Ames Test
3.3. SiO2NPs Exposure Results In Vitro Chromosomal Aberration Assay
3.4. SiO2NPs Exposure Results In Vivo Bone Marrow Micronucleus Assay
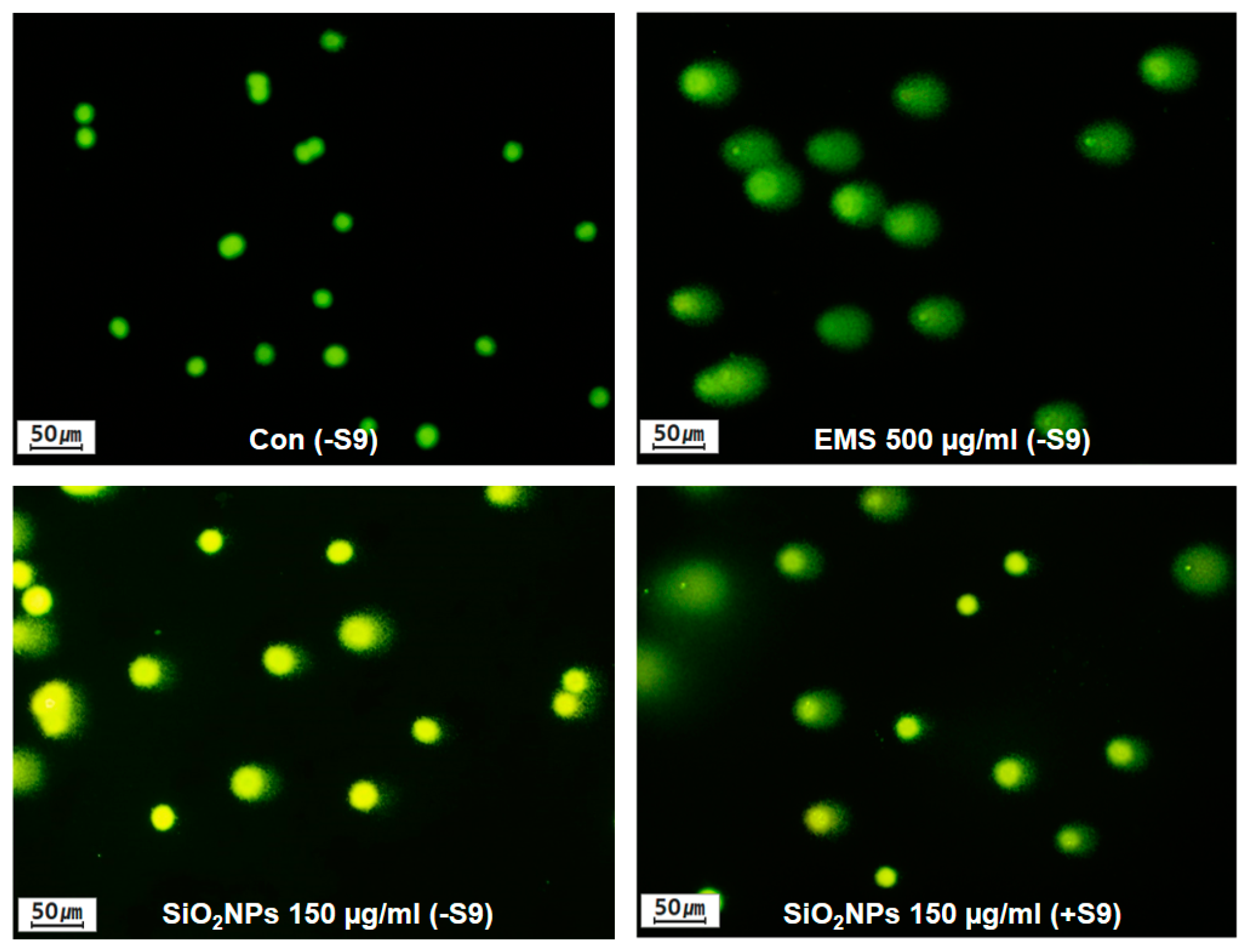
3.5. SiO2NPs Exposure Results In Vitro Comet Assay
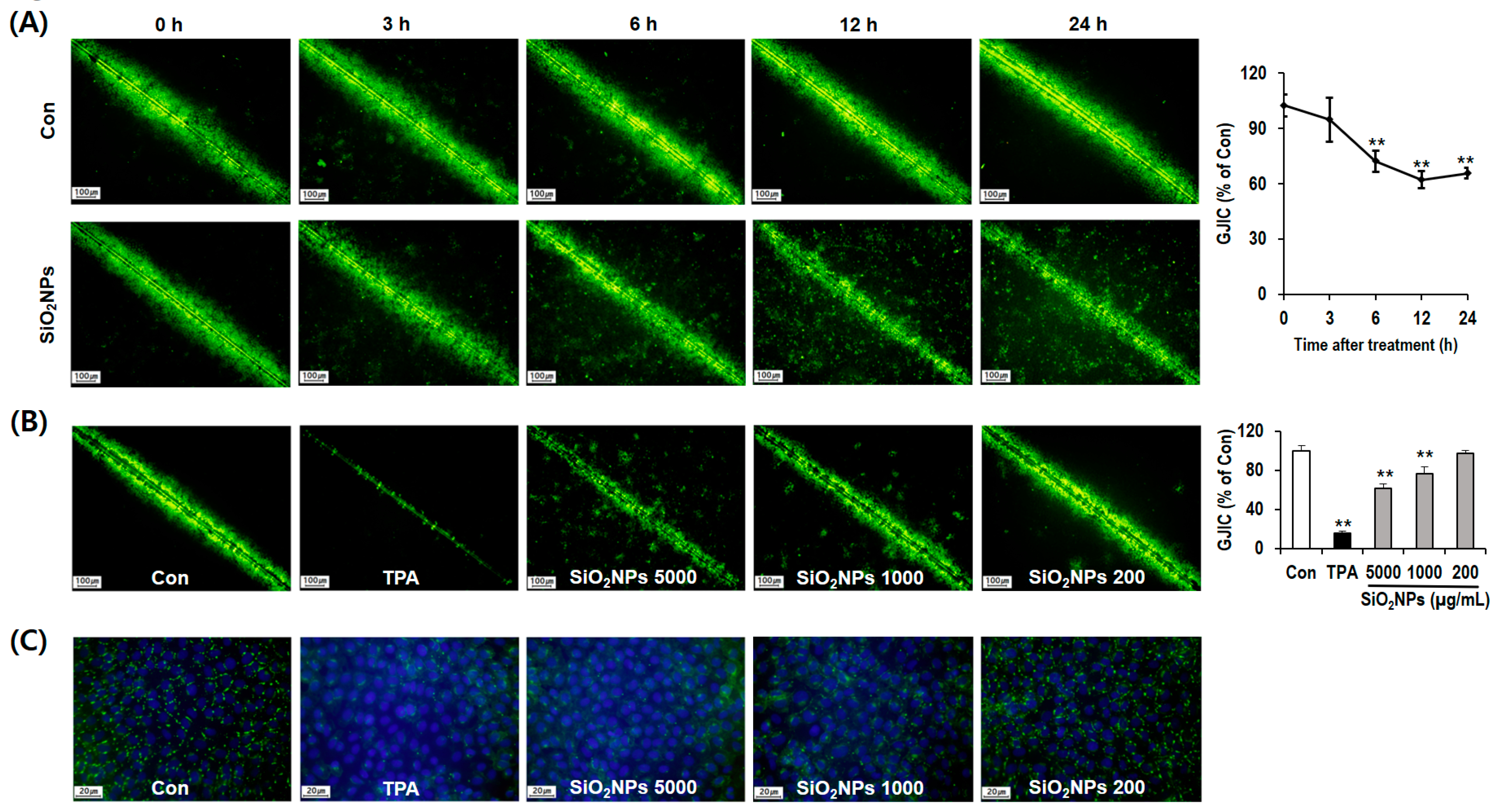
3.6. SiO2NPs Exposure Results in GJIC Analysis
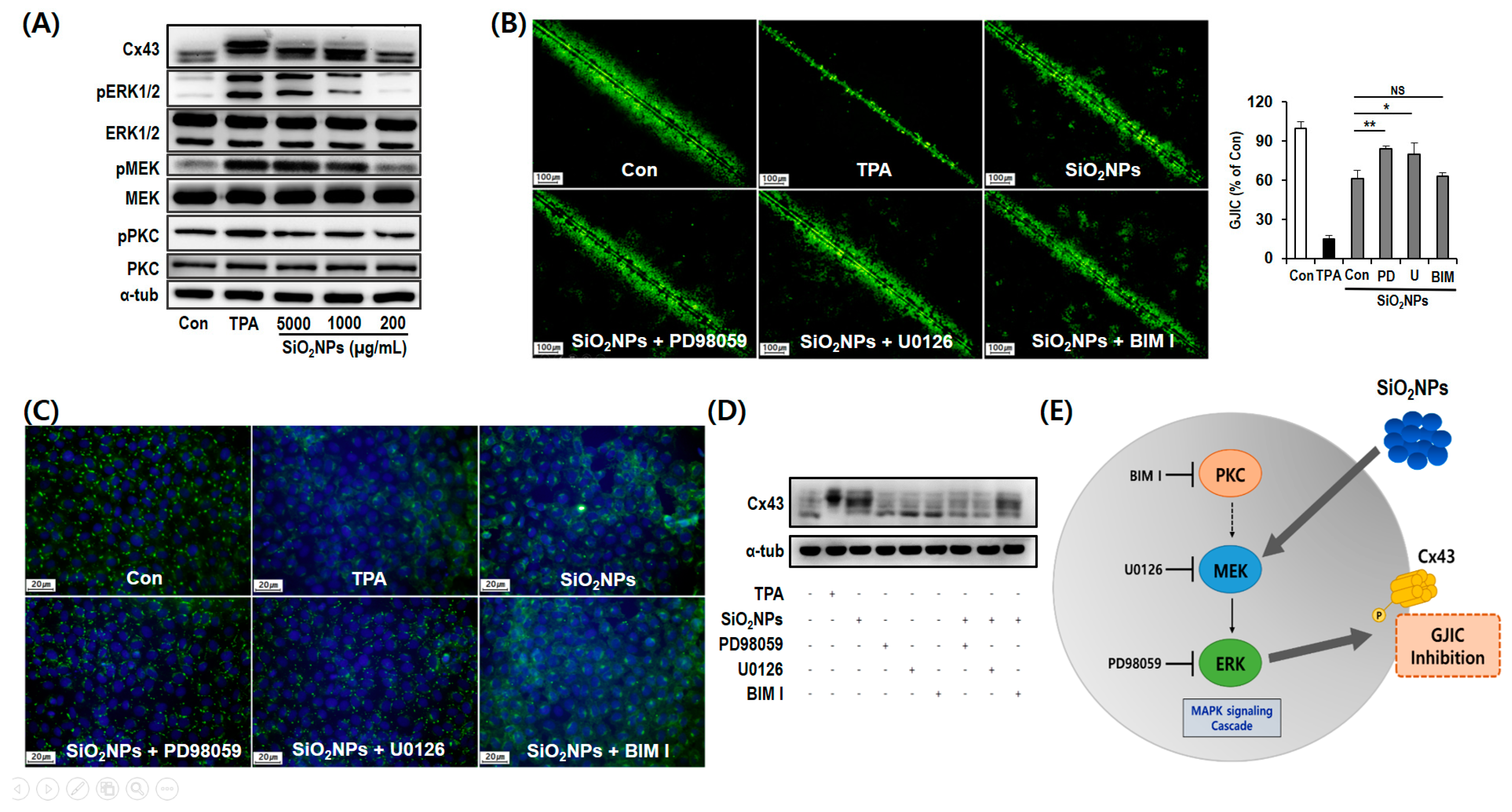
3.7. Mechanism of GJIC Inhibition by SiO2NPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lux-Research. Nanomaterials State of the Market Q3 2008: Stealth Success, Broad Impact. Available online: https://portal.luxresearchinc.com/research/document_excerpt/3735 (accessed on 1 July 2008).
- Chun, A.L. Will the public swallow nanofood? Nat. Nanotechnol. 2009, 4, 790–791. [Google Scholar] [CrossRef]
- Hristozov, D.R.; Gottardo, S.; Critto, A.; Marcomini, A. Risk assessment of engineered nanomaterials: A review of available data and approaches from a regulatory perspective. Nanotoxicology 2012, 6, 880–898. [Google Scholar] [CrossRef]
- Könczöl, M.; Ebeling, S.; Goldenberg, E.; Treude, F.; Gminski, R.; Gieré, R.; Grobéty, B.; Rothen-Rutishauser, B.; Merfort, I.; Mersch-Sundermann, V. Cytotoxicity and genotoxicity of size-fractionated iron oxide (magnetite) in A549 human lung epithelial cells: Role of ROS, JNK, and NF-κB. Chem. Res. Toxicol. 2011, 24, 1460–1475. [Google Scholar] [CrossRef]
- Fubini, B.; Ghiazza, M.; Fenoglio, I. Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology 2010, 4, 347–363. [Google Scholar] [CrossRef]
- Cho, W.S.; Kang, B.C.; Lee, J.K.; Jeong, J.; Che, J.H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Webb, T.R.; Sayes, C.M.; Colvin, V.L.; Reed, K.L. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area. Toxicol. Sci. 2006, 91, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.H.; Park, K.; Yi, J.; Ryu, D.Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. In Vitro 2009, 23, 1076–1084. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Devey, M.; Hawkins, S.; Hails, L.; Davis, S.A.; Mann, S.; Chang, I.T.; Ingham, E.; Malhas, A.; Vaux, D.J.; et al. Influence of particle size and reactive oxygen species on cobalt chrome nanoparticle-mediated genotoxicity. Biomaterials 2013, 34, 3559–3570. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Cytotoxic, genotoxic and pro-inflammatory effects of zinc oxide nanoparticles in human nasal mucosa cells in vitro. Toxicol. In Vitro 2011, 25, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Magaye, R.; Zhao, J.; Bowman, L.; Ding, M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp. Ther. Med. 2012, 4, 551–561. [Google Scholar] [CrossRef]
- Merget, R.; Bauer, T.; Küpper, H.U.; Philippou, S.; Bauer, H.D.; Breitstadt, R.; Bruening, T. Health hazards due to the inhalation of amorphous silica. Arch. Toxicol. 2002, 75, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, W.J.; Låg, M.; Holme, J.A.; Friede, B.; Gualtieri, M.; Kruszewski, M.; Schwarze, P.E.; Skuland, T.; Refsnes, M. Comparison of non-crystalline silica nanoparticles in IL-1β release from macrophages. Part. Fibre Toxicol. 2012, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Uboldi, C.; Giudetti, G.; Broggi, F.; Gilliland, D.; Ponti, J.; Rossi, F. Amorphous silica nanoparticles do not induce cytotoxicity, cell transformation or genotoxicity in Balb/3T3 mouse fibroblasts. Mutat. Res. 2012, 745, 11–20. [Google Scholar] [CrossRef]
- Ahamed, M. Silica nanoparticles-induced cytotoxicity, oxidative stress and apoptosis in cultured A431 and A549 cells. Hum. Exp. Toxicol. 2013, 32, 186–195. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Wang, J.; Beer, C.; Thorsen, K.; Sutherland, D.S.; Autrup, H. Biological effects induced by BSA-stabilized silica nanoparticles in mammalian cell lines. Chem. Biol. Interact. 2013, 204, 28–38. [Google Scholar] [CrossRef]
- Tan, X.; Liu, X.; Zhang, Y.; Zhang, H.; Lin, X.; Pu, C.; Gou, J.; He, H.; Yin, T.; Zhang, Y.; et al. Silica nanoparticles on the oral delivery of insulin. Expert Opin. Drug Deliv. 2018, 15, 805–820. [Google Scholar] [CrossRef]
- Ryu, H.J.; Seong, N.W.; So, B.J.; Seo, H.S.; Kim, J.H.; Hong, J.S.; Park, M.K.; Kim, M.S.; Kim, Y.R.; Cho, K.B.; et al. Evaluation of silica nanoparticle toxicity after topical exposure for 90 days. Int. J. Nanomed. 2014, 9, 127–136. [Google Scholar] [CrossRef][Green Version]
- Guichard, Y.; Fontana, C.; Chavinier, E.; Terzetti, F.; Gaté, L.; Binet, S.; Darne, C. Cytotoxic and genotoxic evaluation of different synthetic amorphous silica nanomaterials in the V79 cell line. Toxicol. Ind. Health 2016, 32, 1639–1650. [Google Scholar] [CrossRef]
- Good Laboratory Practice Regulation for Non-Clinical Laboratory Studies; Notification no. 2005-79; Ministry of Food and Drug Safety (MFDS): Cheongju, Korea, 2005.
- Matesic, D.F.; Rupp, H.L.; Bonney, W.J.; Ruch, R.J.; Trosko, J.E. Changes in gap-junction permeability, phosphorylation, and number mediated by phorbol ester and non-phorbol-ester tumor promoters in rat liver epithelial cells. Mol. Carcinog. 1994, 10, 226–236. [Google Scholar] [CrossRef]
- Hofer, A.; Sáez, J.C.; Chang, C.C.; Trosko, J.E.; Spray, D.C.; Dermietzel, R. C-erbB2/neu transfection induces gap junctional communication incompetence in glial cells. J. Neurosci. 1996, 16, 4311–4321. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E.; Ruch, R.J. Gap junctions as targets for cancer chemoprevention and chemotherapy. Curr. Drug Targets 2002, 3, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Mesnil, M.; Crespin, S.; Avanzo, J.L.; Zaidan-Dagli, M.L. Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta 2005, 1719, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Nimlamool, W.; Andrews, R.M.; Falk, M.M. Connexin43 phosphorylation by PKC and MAPK signals VEGF-mediated gap junction internalization. Mol. Biol. Cell 2015, 26, 2755–2768. [Google Scholar] [CrossRef]
- OECD. OECD Guideline for Testing of Chemicals, Test. No. 471: Bacterial Reverse Mutation Test; OECD: Paris, France, 1997. [Google Scholar]
- OECD. OECD Guideline for Testing of Chemicals, Test. No. 473: In Vitro Mammalian Chromosome Aberration Test; OECD: Paris, France, 1997. [Google Scholar]
- OECD. OECD Guideline for Testing of Chemicals, Test. No. 474: Mammalian Erythrocyte Micronucleus Test; OECD: Paris, France, 1997. [Google Scholar]
- Heddle, J.A.; Stuart, E.; Salamone, M.F. The Bone Marrow Micronucleus Test in Handbook of Mutagenicity Test Procedures; Kilbey, B.J., Legator, M., Nichols, W., Ramel, C., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 441–457. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P.; Durand, R.E. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat. Res. 1990, 122, 86–94. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001, 161, 351–354. [Google Scholar] [CrossRef]
- Babica, P.; Sovadinová, I.; Upham, B.L. Scrape Loading/Dye Transfer Assay. Methods Mol. Biol. 2016, 1437, 133–144. [Google Scholar] [CrossRef]
- Kaszuba, M.; Connah, M.T. Protein and Nanoparticle Characterisation Using Light Scattering Techniques. Part. Part. Syst. Charact. 2006, 23, 193–196. [Google Scholar] [CrossRef]
- Antonio Alves Júnior, J.; Baptista Baldo, J. The Behavior of Zeta Potential of Silica Suspensions. New J. Glass Ceram. 2014, 4, 29–37. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Mahmoud, N.N.; Hashemi, F.; Hajipour, M.J.; Farvadi, F.; Mahmoudi, M. Misinterpretation in Nanotoxicology: A Personal Perspective. Rev. Chem. Res. Toxicol. 2016, 29, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, T.; Santos, H.; Vihola, H.; Salonen, J.; Riikonen, J.; Heikkila, T.; Peltonen, L.; Kumar, N.; Murzin, D.Y.; Lehto, V.; et al. Failure of MTT as a toxicity testing agent for mesoporous silicon microparticles. Chem. Res. Toxicol. 2007, 20, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramírez, I.E.; Díaz de León Olmos, M.A.; Muñoz Ortega, M.H.; Zapien, J.A.; Betancourt, I.; Santoyo-Elvira, N. Development and Assessment of Nano-Technologies for Cancer Treatment: Cytotoxicity and Hyperthermia Laboratory Studies. Cancer Investig. 2020, 38, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.B.; McCullough, K.D.; Esch, G.L.; Faris, R.A.; Hixson, D.C.; Smith, G.J.; Grisham, J.W. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am. J. Pathol. 1997, 151, 353–359. [Google Scholar]
- Guo, Y.; Martinez-Williams, C.; Gilbert, K.A.; Rannels, D.E. Inhibition of gap junction communication in alveolar epithelial cells by 18alpha-glycyrrhetinic acid. Am. J. Physiol. 1999, 276, L1018–L1026. [Google Scholar] [CrossRef]
- Leithe, E.; Rivedal, E. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J. Biol. Chem. 2004, 279, 50089–50096. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yun, J.W.; Kim, Y.S.; Kwon, E.; Choi, H.J.; Yeom, S.C.; Kang, B.C. Mutagenicity and tumor-promoting effects of Tiglium seed extract via PKC and MAPK signaling pathways. Biosci. Biotechnol. Biochem. 2015, 79, 374–383. [Google Scholar] [CrossRef]
- Jaganathan, H.; Godin, B. Biocompatibility assessment of Si-based nano- and micro-particles. Adv. Drug Deliv. Rev. 2012, 64, 1800–1819. [Google Scholar] [CrossRef]
- Yu, K.O.; Grabinski, C.M.; Schrand, A.M.; Murdock, R.C.; Wang, W.; Gu, B.H.; Schlager, J.J.; Hussain, S.M. Toxicity of amorphous silica nanoparticles in mouse keratinocytes. J. Nanopart. Res. 2009, 11, 15–24. [Google Scholar] [CrossRef]
- Park, E.J.; Park, K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol. Lett. 2009, 184, 18–25. [Google Scholar] [CrossRef]
- Kim, H.W.; Ahn, E.K.; Jee, B.K.; Yoon, H.K.; Lee, K.H.; Lim, Y. Nanoparticulate-induced toxicity and related mechanism in vitro and in vivo. J. Nanopart. Res. 2009, 11, 55–65. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Jin, M.; Du, Z.; Liu, X.; Guo, C.; Li, Y.; Huang, P.; Sun, Z. Size-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma HepG2 cells. Toxicol. In Vitro 2011, 25, 1343–1352. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Yang, M.; Li, Y.; Cui, S.; Zhou, X.; Li, Y.; Sun, Z. Amorphous silica nanoparticles induce malignant transformation and tumorigenesis of human lung epithelial cells via P53 signaling. Nanotoxicology 2017, 11, 1176–1194. [Google Scholar] [CrossRef]
- Xie, D.; Zhou, Y.; Luo, X. Amorphous silica nanoparticles induce tumorigenesis via regulating ATP5H/SOD1-related oxidative stress, oxidative phosphorylation and EIF4G2/PABPC1-associated translational initiation. PeerJ 2019, 7, e6455. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.M.; Ames, B.N. Revised methods for the mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Hakura, A.; Suzuki, S.; Satoh, T. Advantage of the use of human liver S9 in the Ames test. Mutat. Res. 1999, 438, 29–36. [Google Scholar] [CrossRef]
- Kaleeswaran, S.; Sriram, P.; Prabhu, D.; Vijayakumar, C.; Mathuram, L.N. Anti- and pro-mutagenic effects of silymarin in the Ames bacterial reverse mutation assay. Phytother. Res. 2009, 23, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Seo, C.S.; Kim, J.Y.; Shin, H.K. Evaluation of a water extract of So-Cheong-Ryong-Tang for acute toxicity and genotoxicity using in vitro and in vivo tests. BMC Complement. Altern. Med. 2015, 15, 235. [Google Scholar] [CrossRef]
- Ishidate, M., Jr.; Sofuni, T. The in vitro chromosomal aberration test using Chinese hamster lung (CHL) fibroblast cells in culture. Prog. Mutat. Res. 1985, 5, 427–432. [Google Scholar]
- Akyıl, D.; Konuk, M. Detection of genotoxicity and mutagenicity of chlorthiophos using micronucleus, chromosome aberration, sister chromatid exchange, and Ames tests. Environ. Toxicol. 2015, 30, 937–945. [Google Scholar] [CrossRef]
- Landsiedel, R.; Kapp, M.D.; Schulz, M.; Wiench, K.; Oesch, F. Genotoxicity investigations on nanomaterials: Methods, preparation and characterization of test material, potential artifacts and limitations--many questions, some answers. Mutat. Res. 2009, 681, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Doak, S.H.; Manshian, B.; Jenkins, G.J.; Singh, N. In vitro genotoxicity testing strategy for nanomaterials and the adaptation of current OECD guidelines. Mutat. Res. 2012, 745, 104–111. [Google Scholar] [CrossRef]
- Oesch, F.; Landsiedel, R. Genotoxicity investigations on nanomaterials. Arch. Toxicol. 2012, 86, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.S.; Li, Y.; Yan, J.; Bishop, M.; Jones, M.Y.; Watanabe, F.; Biris, A.S.; Rice, P.; Zhou, T.; Chen, T. Genotoxicity evaluation of titanium dioxide nanoparticles using the Ames test and Comet assay. J. Appl. Toxicol. 2012, 32, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Grek, C.L.; Prasad, G.M.; Viswanathan, V.; Armstrong, D.G.; Gourdie, R.G.; Ghatnekar, G.S. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: A multicenter, randomized trial. Wound Repair Regen. 2015, 23, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, I.; Fujimoto, A.; Hatakeyama, K.; Aoki, T.; Nishikawa, A.; Noda, T.; Minowa, O.; Kurebayashi, N.; Ikeda, K.; Kamiya, K. In Vitro Models of GJB2-Related Hearing Loss Recapitulate Ca2+ Transients via a Gap Junction Characteristic of Developing Cochlea. Stem Cell Rep. 2016, 7, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhao, J.; Wu, D.; Zhang, J. Ultraviolet A exposure induces reversible disruption of gap junction intercellular communication in lens epithelial cells. Int. J. Mol. Med. 2011, 28, 239–245. [Google Scholar] [CrossRef][Green Version]
- Berger, A.C.; Kelly, J.J.; Lajoie, P.; Shao, Q.; Laird, D.W. Mutations in Cx30 that are linked to skin disease and non-syndromic hearing loss exhibit several distinct cellular pathologies. J. Cell Sci. 2014, 127, 1751–1764. [Google Scholar] [CrossRef]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Güldenagel, M.; Deutsch, U.; Söhl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef]
- Evans, W.H.; Martin, P.E. Gap junctions: Structure and function. Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef]
- Hayashi, Y. Overview of genotoxic carcinogens and non-genotoxic carcinogens. Exp. Toxicol. Pathol. 1992, 44, 465–471. [Google Scholar] [CrossRef]
- Tacheau, C.; Laboureau, J.; Mauviel, A.; Verrecchia, F. TNF-alpha represses connexin43 expression in HaCat keratinocytes via activation of JNK signaling. J. Cell. Physiol. 2008, 216, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, Z.; Cohen, D.M. MAPK signaling and the kidney. Am. J. Physiol. Renal Physiol. 2000, 279, F593–F604. [Google Scholar] [CrossRef]
- Ueda, Y.; Hirai, S.; Osada, S.; Suzuki, A.; Mizuno, K.; Ohno, S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J. Biol. Chem. 1996, 271, 23512–23519. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Novak, R.F. DDT stimulates c-erbB2, c-met, and STATS tyrosine phosphorylation, Grb2-Sos association, MAPK phosphorylation, and proliferation of human breast epithelial cells. Biochem. Biophys. Res. Commun. 1997, 231, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Suzuki, D.; Honma, M.; Uehara, G.; Sakai, T.; Umezono, T.; Sakai, H. High expression of PKC-MAPK pathway mRNAs correlates with glomerular lesions in human diabetic nephropathy. Kidney Int. 2004, 66, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Ariano, P.; Zamburlin, P.; Gilardino, A.; Mortera, R.; Onida, B.; Tomatis, M.; Ghiazza, M.; Fubini, B.; Lovisolo, D. Interaction of spherical silica nanoparticles with neuronal cells: Size-dependent toxicity and perturbation of calcium homeostasis. Small 2011, 7, 766–774. [Google Scholar] [CrossRef]
- Yu, T.; Malugin, A.; Ghandehari, H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano 2011, 5, 5717–5728. [Google Scholar] [CrossRef]
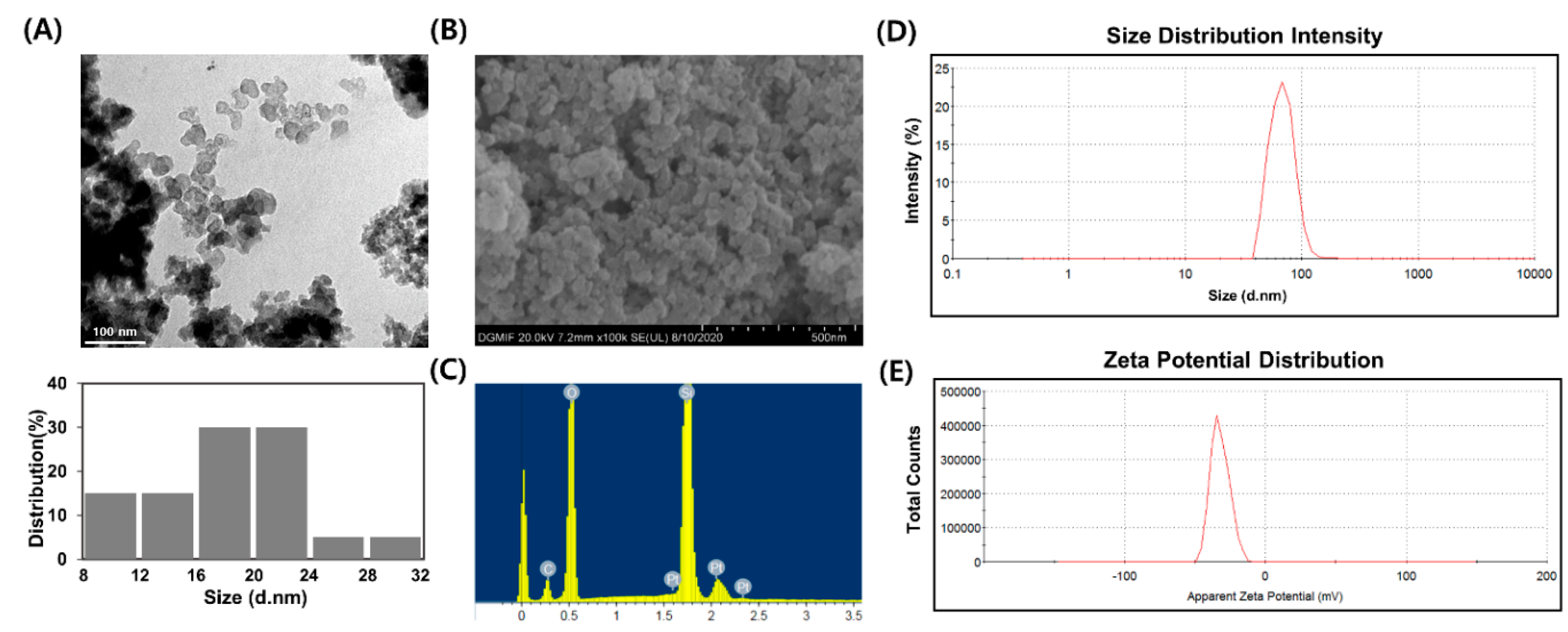

| Particle Diameter (nm) a | Hydrodynamic Size (nm) b | Zeta-Potential (mV) c |
|---|---|---|
| 18.54 ± 5.05 | 68.78 ± 2.43 | −32.07 ± 0.32 |
| S9 | Substance | Dose (µg/100 µL/plate) | His+ Revertant Colony/Plate | ||||
|---|---|---|---|---|---|---|---|
| TA98 | TA100 | WP2uvrA | TA1535 | TA1537 | |||
| - | Distilled water a | - | 28 ± 5.1 | 125 ± 8.7 | 22 ± 2.1 | 12 ± 1.7 | 16 ± 3.0 |
| 2-nitrofluorene b | 10 | 640 ± 54.1 * | - | - | - | - | |
| Sodium azide b | 5 | - | 884 ± 29.8 * | - | - | - | |
| Mitomycin C b | 0.5 | - | - | 413 ± 12.5 * | - | - | |
| Sodium azide b | 0.5 | - | - | - | 419 ± 22.1 * | - | |
| 9-aminoacridine b | 80 | - | - | - | - | 894 ± 44.5 * | |
| SiO2NPs | 1250 | 29 ± 1.7 | 130 ± 2.6 | 26±2.6 | 8 ± 2.5 | 17 ± 1.2 | |
| 2500 | 30 ± 4.5 | 129 ± 5.0 | 25 ± 0.6 | 11 ± 4.2 | 18 ± 2.6 | ||
| 5000 | 24 ± 4.0 | 130 ± 8.5 | 22 ± 2.5 | 9 ± 1.2 | 18 ± 2.5 | ||
| + | Distilled water a | - | 31 ± 7.4 | 117 ± 6.6 | 38 ± 1.5 | 14 ± 2.5 | 20 ± 2.1 |
| 2-aminoanthracene b | 2.0 | 405 ± 16.5 * | 431 ± 14.0 * | - | - | - | |
| 5.0 | - | - | 397 ± 19.9 * | 411 ± 8.0 * | 408 ± 16.1 * | ||
| SiO2NPs | 1250 | 34 ± 0.6 | 113 ± 3.1 | 30 ± 7.5 | 14 ± 0.6 | 18 ± 1.5 | |
| 2500 | 34 ± 5.9 | 114 ± 4.0 | 35 ± 1.0 | 12 ± 2.5 | 18 ± 0.6 | ||
| 5000 | 30 ± 7.2 | 121 ± 11.9 | 31 ± 2.3 | 14 ± 3.1 | 19 ± 1.0 | ||
| Substance | Dose (µg/mL) | Number of Cells Scored | No. of Cells with Aberrations | ||
|---|---|---|---|---|---|
| −S9 | +S9 | ||||
| 6 h | 24 h | 6 h | |||
| MEM a | - | 200 | 0.5 ± 0.71 | 1.0 ± 0.00 | 0 |
| Mitomycin C b | 0.1 | 200 | 26.0 ± 2.83 * | 36.5 ± 2.12 * | - |
| Cyclophosphamide b | 5 | 200 | - | - | 48.5 ± 0.71 * |
| Distilled water | - | 200 | 0 | 0.5 ± 0.71 | 0.5 ± 0.71 |
| SiO2NPs | 133.38 | 200 | 0 | 0.5 ± 0.71 | 0.5 ± 0.71 |
| 153.39 | 200 | 1.0 ± 1.41 | 0.5 ± 0.71 | 0 | |
| 176.40 | 200 | 0 | 0 | 0 | |
| Substance | Dose (mg/kg BW) | Number of Mice | MNPCE c | PCE/(PCE + NCE) d |
|---|---|---|---|---|
| Distilled water a | - | 5 | 2.0 ± 1.00 | 49.4 ± 4.40 |
| SiO2NPs | 500 | 5 | 2.0 ± 1.22 | 42.9 ± 10.99 |
| 1000 | 5 | 5.4 ± 2.19 | 46.2 ± 4.25 | |
| 2000 | 5 | 3.8 ± 0.84 | 45.7 ± 4.53 | |
| Mitomycin C b | 2 | 5 | 137.0 ± 11.96 ** | 39.8 ± 2.97 * |
| Substance | Dose (µg/mL) | S-9 Mix | Time (h) | % Tail DNA | Olive Tail Moment | Relative Cell Count (%) |
|---|---|---|---|---|---|---|
| Distilled water a | - | - | 4 | 5.66 ± 3.66 | 0.46 ± 0.31 | 100 |
| SiO2NPs | 37.5 | 4 | 12.16 ± 6.02 ** | 1.29 ± 1.05 ** | 98 | |
| 75.0 | 4 | 13.94 ± 6.66 ** | 1.40 ± 1.13 ** | 83 | ||
| 150.0 | 4 | 13.11 ± 6.12 ** | 1.19 ± 1.09 ** | 78 | ||
| EMS b | 500 | 4 | 25.18 ± 12.13 ** | 2.86 ± 2.34 ** | 73 | |
| Distilled water a | - | - | 24 | 5.74 ± 3.32 | 0.48 ± 0.45 | 100 |
| SiO2NPs | 37.5 | 24 | 11.65 ± 6.39 ** | 1.11 ± 1.02 | 95 | |
| 75.0 | 24 | 12.17 ± 6.64 ** | 1.07 ± 1.01 | 86 | ||
| 150.0 | 24 | 20.51 ± 10.47 ** | 2.50 ± 1.98 ** | 77 | ||
| EMS b | 500 | 24 | 39.44 ± 14.26 ** | 8.29 ± 6.25 ** | 67 | |
| Distilled water a | - | + | 4 | 5.20 ± 3.20 | 0.43 ± 0.32 | 100 |
| SiO2NPs | 37.5 | 4 | 6.13 ± 3.34 | 0.47 ± 0.38 | 94 | |
| 75.0 | 4 | 7.47 ± 3.8 | 0.62 ± 0.53 | 85 | ||
| 150.0 | 4 | 10.10 ± 5.32 ** | 1.00 ± 0.84 * | 79 | ||
| EMS b | 500 | 4 | 24.59 ± 11.21 ** | 3.12 ± 2.34 ** | 76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-H.; Kim, Y.-S.; Kwon, E.; Yun, J.-W.; Kang, B.-C. Toxicologic Evaluation for Amorphous Silica Nanoparticles: Genotoxic and Non-Genotoxic Tumor-Promoting Potential. Pharmaceutics 2020, 12, 826. https://doi.org/10.3390/pharmaceutics12090826
Lee G-H, Kim Y-S, Kwon E, Yun J-W, Kang B-C. Toxicologic Evaluation for Amorphous Silica Nanoparticles: Genotoxic and Non-Genotoxic Tumor-Promoting Potential. Pharmaceutics. 2020; 12(9):826. https://doi.org/10.3390/pharmaceutics12090826
Chicago/Turabian StyleLee, Gwang-Hoon, Yun-Soon Kim, Euna Kwon, Jun-Won Yun, and Byeong-Cheol Kang. 2020. "Toxicologic Evaluation for Amorphous Silica Nanoparticles: Genotoxic and Non-Genotoxic Tumor-Promoting Potential" Pharmaceutics 12, no. 9: 826. https://doi.org/10.3390/pharmaceutics12090826
APA StyleLee, G.-H., Kim, Y.-S., Kwon, E., Yun, J.-W., & Kang, B.-C. (2020). Toxicologic Evaluation for Amorphous Silica Nanoparticles: Genotoxic and Non-Genotoxic Tumor-Promoting Potential. Pharmaceutics, 12(9), 826. https://doi.org/10.3390/pharmaceutics12090826






