Medicines Acceptability in Hospitalized Children: An Ongoing Need for Age-Appropriate Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Objective, Study Design and Setting
2.2. Participants
2.3. Data Collection
- results of intake (the required dose was fully, partly or not taken at all);
- patient reaction during the administration (positive, neutral or negative reaction);
- times needed to prepare (from opening any packaging to having a required dose of medication ready to use, including all handling and modifications), and to administer the required dose of medication (from a required dose of medication ready to use to the end of the intake). The sum of the times of preparation and administration time, both recorded using 10 s intervals, was classified as short (1 min and less), medium (from 1 min to 2 min and 30 s), or long (more than 2 min and 30 s).
- dividing the intake of a dose which could not be taken as a whole;
- altering the intended use (any modifications of dosage forms such as capsules opened or tablets crushed; use of another route/mode of administration);
- using food/drink to mask a bad taste or ease swallowing;
- using an administration device not provided with the medication;
- promising a reward;
- using restraint (administration was imposed upon the patient).
2.4. Data Analysis
3. Results
3.1. Patients and Medicines
3.2. Acceptability of Tablets
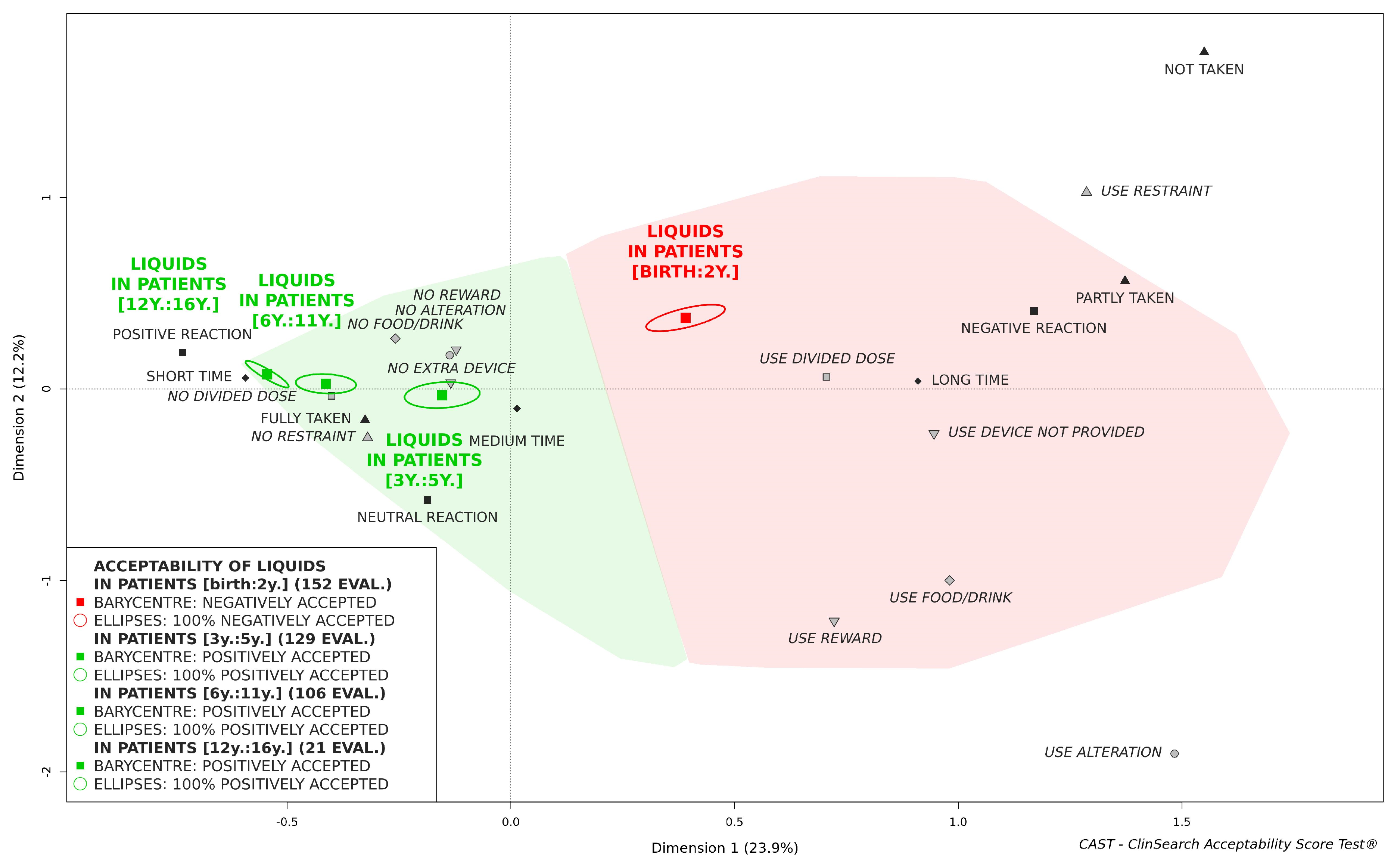
3.3. Acceptability of Oral Liquids Pharmaceutical Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guideline on Pharmaceutical Development of Medicines for Paediatric Use EMA/CHMP/QWP/805880/2012 Rev. 2; European Medicine Agency: London, UK, 2013.
- Davies, E.H.; Tuleu, C. Medicines for children: A matter of taste. J. Pediatr. 2008, 153, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Baguley, D.; Lim, E.; Bevan, A.; Pallet, A.; Faust, S.N. Prescribing for children–taste and palatability affect adherence to antibiotics: A review. Arch. Dis. Child. 2012, 97, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Toolkit for Research and Development of Paediatric Antiretroviral Drugs and Formulations; Module 5: Acceptability; World Health Organization: Geneva, Switzerland, 2018.
- Bjerknes, K.; Bøyum, S.; Kristensen, S.; Brustugun, J.; Wang, S. Manipulating tablets and capsules given to hospitalised children in Norway is common practice. Acta Paediatr. 2017, 106, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Richey, R.H.; Shah, U.U.; Peak, M.; Craig, J.V.; Ford, J.L.; Barker, C.E.; Nunn, A.J.; Turner, M.A. Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr. 2013, 13, 81. [Google Scholar] [CrossRef]
- Magalhães, J.; Rodrigues, A.T.; Roque, F.; Figueiras, A.; Falcão, A.; Herdeiro, M.T. Use of off-label and unlicenced drugs in hospitalised paediatric patients: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 1–13. [Google Scholar] [CrossRef]
- Bulletin Officiel n° 6396 du 3 Hija 1436 (17-9-2015). Dahir n° 1-15-110 du 18 Chaoual 1436 (4 août 2015) Portant Promulgation de la loi n° 28-13 Relative à la Protection des Personnes Participant aux Recherches Biomédicales; Gouvernement Marocain: Tétouan, Morocco, 2015.
- Ruiz, F.; Vallet, T.; Pense-Lheritier, A.M.; Aoussat, A. Standardized method to assess medicines’ acceptability: Focus on paediatric population. J. Pharm. Pharmacol. 2017, 69, 406–416. [Google Scholar] [CrossRef]
- Vallet, T.; Ruiz, F.; Lavarde, M.; Pense-Lheritier, A.M.; Aoussat, A. Standardized evaluation of medicine acceptability in paediatric population: Reliability of a model. J. Pharm. Pharmacol. 2018, 70, 42–50. [Google Scholar] [CrossRef]
- Reflection Paper: Formulations of Choice for the Paediatric Population; EMEA/CHMP/PEG/194810/2005; European Medicine Agency: London, UK, 2006.
- Babbitt, R.L.; Parrish, J.M.; Brierley, P.E.; Kohr, M.A. Teaching developmentally disabled children with chronic illness to swallow prescribed capsules. J. Dev. Behav. Pediatrics 1991, 12, 229–235. [Google Scholar] [CrossRef]
- Czyzewski, D.; Calles, N.; Runyan, R.; Lopez, M. Teaching and maintaining pill swallowing in HIV-infected children. Aids Read. N. Y. 2000, 10, 88–95. [Google Scholar]
- Ghuman, J.K.; Cataldo, M.D.; Beck, M.H.; Slifer, K.J. Behavioral training for pill-swallowing difficulties in young children with autistic disorder. J. Child Adolesc. Psychopharm. 2004, 14, 601–611. [Google Scholar] [CrossRef]
- Garvie, P.A.; Lensing, S.; Rai, S.N. Efficacy of a pill-swallowing training intervention to improve antiretroviral medication adherence in pediatric patients with HIV/AIDS. Pediatrics 2007, 119, 17353298. [Google Scholar] [CrossRef] [PubMed]
- Foy, T.; Czyzewski, D. Feeding Difficulties. In Nutrition in Pediatrics: Basic Science, Clinical Applications; Duggan, C.W., Watkins, J.B., Walker, W.A., Eds.; B.C. Decker Inc.: Hamilton, ON, Canada, 2008. [Google Scholar]
- Mennella, J.A.; Spector, A.C.; Reed, D.R.; Coldwell, S.E. The bad taste of medicines: Overview of basic research on bitter taste. Clin. Ther. 2013, 35, 1225–1246. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M. The mechanisms of pharmacokinetic food-drug interactions–A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Manrique, Y.; Lee, D.; Islam, F.; Nissen, L.; Cichero, J.A.; Stokes, J.; Steadman, K.J. Crushed tablets: Does the administration of food vehicles and thickened fluids to aid medication swallowing alter drug release? J. Pharm. Pharm. Sci. 2014, 17, 207–219. [Google Scholar] [CrossRef]
- Batchelor, H.K. Influence of food on paediatric gastrointestinal drug absorption following oral administration: A review. Children 2015, 2, 244–271. [Google Scholar] [CrossRef]
- Martir, J.; Flanagan, T.; Mann, J.; Fotaki, N. Recommended strategies for the oral administration of paediatric medicines with food and drinks in the context of their biopharmaceutical properties: A review. J. Pharm. Pharmacol. 2017, 69, 384–397. [Google Scholar] [CrossRef]
- Batchelor, H.; Kaukonen, A.M.; Klein, S.; Davit, B.; Ju, R.; Ternik, R.; Heimbach, T.; Lin, W.; Wang, J.; Storey, D. Food effects in paediatric medicines development for products Co-administered with food. Int. J. Pharm. 2018, 536, 530–535. [Google Scholar] [CrossRef]
- Thong, M.Y.; Manrique, Y.J.; Steadman, K.J. Drug loss while crushing tablets: Comparison of 24 tablet crushing devices. PLoS ONE 2018, 13, e0193683. [Google Scholar] [CrossRef]
- Skwierczynski, C.; Conroy, S. How long does it take to administer oral medicines to children? Paediatr. Perinat. Drug Ther. 2008, 8, 145–149. [Google Scholar] [CrossRef]
- Akram, G.; Mullen, A.B. Paediatric nurses’ knowledge and practice of mixing medication into foodstuff. Int. J. Pharm. Pract. 2012, 20, 191–198. [Google Scholar] [CrossRef]
- Venables, R.; Batchelor, H.; Hodson, J.; Stirling, H.; Marriott, J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int. J. Pharm. 2015, 480, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, V.; Mameli, C.; Zuccotti, G.V. Paediatric pharmacology: Remember the excipients. Pharmacol. Res. 2011, 63, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.S.; Mendelsohn, A.L.; Wolf, M.S.; Parker, R.M.; Fierman, A.; Van Schaick, L.; Bazan, I.S.; Kline, M.D.; Dreyer, B.P. Parents’ medication administration errors: Role of dosing instruments and health literacy. Arch. Pediatrics Adolesc. Med. 2010, 164, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Pohly, C.E.; Meissner, T.; Mayatepek, E.; Möltner, A.; Flunkert, K.; Breitkreutz, J.; Bosse, H.M. Acceptability of an Orodispersible Film Compared to Syrup in Neonates and Infants: A Randomized Controlled Trial. Eur. J. Pharm. Biopharm. 2020. [Google Scholar] [CrossRef]
- Thomson, S.A.; Tuleu, C.; Wong, I.C.; Keady, S.; Pitt, K.G.; Sutcliffe, A.G. Minitablets: New modality to deliver medicines to preschool-aged children. Pediatrics 2009, 123, e235–e238. [Google Scholar] [CrossRef]
- Spomer, N.; Klingmann, V.; Stoltenberg, I.; Lerch, C.; Meissner, T.; Breitkreutz, J. Acceptance of uncoated mini-tablets in young children: Results from a prospective exploratory cross-over study. Arch. Dis. Child. 2012, 97, 283–286. [Google Scholar] [CrossRef]
- Klingmann, V.; Seitz, A.; Meissner, T.; Breitkreutz, J.; Moeltner, A.; Bosse, H.M. Acceptability of Uncoated Mini-Tablets in Neonates-A Randomized Controlled Trial. J. Pediatr. 2015, 7, 732–735. [Google Scholar] [CrossRef]
- Expert Committee on Selection and Use of Essential Medicines, Report of the Informal Expert Meeting on Dosage Forms of Medicines for Children; World Health Organization: Geneva, Switzerland, 2008.
- Thabet, Y.; Klingmann, V.; Breitkreutz, J. Drug formulations: Standards and novel strategies for drug administration in pediatrics. J. Clin. Pharmacol. 2018, 58, S26–S35. [Google Scholar] [CrossRef]
- Zahálka, L.; Klovrzová, S.; Matysová, L.; Šklubalová, Z.; Solich, P. Furosemide ethanol-free oral solutions for paediatric use: Formulation, HPLC method and stability study. Eur. J. Hosp. Pharm. 2018, 25, 144–149. [Google Scholar] [CrossRef]

| Characteristics | Patient Age | Statistical Test | |||
|---|---|---|---|---|---|
| [Birth:2y.] (n = 191) | [3y.:5y.] (n = 169) | [6y.:11y.] (n = 165) | [12y.:16y.] (n = 45) | ||
| Sex of patients | χ2 b: p = 0.18 | ||||
| Boy | 87 (46) a | 95 (56) | 89 (54) | 26 (58) | |
| Girl | 102 (54) | 74 (44) | 75 (46) | 19 (42) | |
| missing data | 2 | 1 | |||
| Formulations of medicines | χ2: p < 0.001 | ||||
| Oral liquid preparations | 152 (80) | 129 (76) | 106 (64) | 21 (47) | |
| Solid oral dosage form | 39 (20) | 40 (24) | 59 (36) | 24 (53) | |
| Therapeutic areas of medicines | F c: p < 0.001 | ||||
| Antibacterials | 42 (22) | 49 (29) | 42 (25) | 8 (18) | |
| Corticosteroids | 46 (24) | 41 (24) | 27 (16) | 5 (11) | |
| Antiepileptics | 46 (24) | 29 (17) | 24 (15) | 7 (16) | |
| Antithrombotic agents | 2 (1) | 10 (6) | 18 (11) | 4 (9) | |
| Antianemic preparations | 17 (9) | 3 (2) | 3 (2) | 1 (2) | |
| Antivirals | 10 (5) | 4 (2) | 2 (1) | 7 (16) | |
| Psycholeptics | 5 (3) | 7 (4) | 8 (5) | 0 (0) | |
| Analgesics | 1 (1) | 6 (4) | 11 (7) | 0 (0) | |
| Ophthalmologicals | 0 (0) | 1 (1) | 12 (7) | 1 (2) | |
| Muscle relaxants | 1 (1) | 3 (2) | 4 (2) | 5 (11) | |
| Antimycobacterials | 2 (1) | 4 (2) | 2 (1) | 2 (4) | |
| Mineral supplements | 4 (2) | 1 (1) | 2 (1) | 2 (4) | |
| Urologicals | 0 (0) | 0 (0) | 1 (1) | 2 (4) | |
| Others (<5%) | 15 (8) | 11 (7) | 9 (5) | 1 (2) | |
| Outcomes | Patient Age | Statistical Test | |||
|---|---|---|---|---|---|
| [Birth:2y.] (n = 38) | [3y.:5y.] (n = 40) | [6y.:11y.] (n = 59) | [12y.:16y.] (n = 24) | ||
| Result intake | F b: p < 0.001 | ||||
| fully taken | 6 (16) a | 21 (52) | 53 (90) | 24 (100) | |
| partly taken | 32 (84) | 19 (48) | 6 (10) | 0 (0) | |
| not taken | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Patient’s reaction | χ2 c: p < 0.001 | ||||
| Positive | 0 (0) | 1 (2) | 15 (25) | 15 (62) | |
| Neutral | 2 (5) | 14 (35) | 29 (49) | 5 (21) | |
| Negative | 36 (95) | 25 (62) | 15 (25) | 4 (17) | |
| Manip.-admin. time | χ2: p < 0.001 | ||||
| short time | 1 (3) | 8 (20) | 29 (50) | 19 (79) | |
| medium time | 5 (13) | 21 (52) | 24 (41) | 5 (22) | |
| long time | 32 (84) | 11 (28) | 5 (9) | 0 (0) | |
| missing data | 1 | ||||
| Divided dose | χ2: p < 0.001 | ||||
| no divided dose | 4 (11) | 12 (30) | 43 (73) | 23 (96) | |
| use divided dose | 34 (89) | 28 (70) | 16 (27) | 1 (4) | |
| Alteration | χ2: p < 0.001 | ||||
| no alteration | 0 (0) | 4 (10) | 28 (47) | 23 (96) | |
| use alteration | 38 (100) | 36 (90) | 31 (53) | 1 (4) | |
| Device not provided | χ2: p < 0.001 | ||||
| no extra device | 12 (32) | 37 (92) | 55 (93) | 24 (100) | |
| use device not provided | 26 (68) | 3 (8) | 4 (7) | 0 (0) | |
| Food drink | χ2: p < 0.001 | ||||
| no food/drink | 2 (5) | 8 (20) | 35 (59) | 23 (96) | |
| use food/drink | 36 (95) | 32 (80) | 24 (41) | 1 (4) | |
| Reward | χ2: p < 0.001 | ||||
| no reward | 33 (87) | 8 (20) | 25 (42) | 20 (83) | |
| use reward | 5 (13) | 32 (80) | 34 (58) | 4 (17) | |
| Restraint | χ2: p < 0.001 | ||||
| no restraint | 5 (13) | 36 (90) | 56 (95) | 24 (100) | |
| use restraint | 33 (87) | 4 (10) | 3 (5) | 0 (0) | |
| Patient Age | Crushed into Powder | Dissolved into Liquid | Divided in Smallest Pieces | Unspecified |
|---|---|---|---|---|
| [Birth:2y.] (n = 38) | 28 (74) a | 7 (18) | 1 (3) | 2 (5) |
| [3y.:5y.] (n = 36) | 17 (47) | 11 (31) | 4 (11) | 4 (11) |
| [6y.:11y.] (n = 31) | 12 (39) | 5 (16) | 9 (29) | 5 (16) |
| [12y.:16y.] (n = 1) | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| Drugs | Alternatives to Conventional SODF | |
|---|---|---|
| Equivalence | Dosage Form | |
| Acenocoumarol | INN a | Mini-Tablet c |
| ATC b1: B01AA (Warfarine) | Oral suspension c | |
| Acetazolamide | INN | Powder and solvent for injection solution c,d |
| ATC: S01EC (Dorzolamide; Brinzolamide; Brimonidine) | Ophthalmic solutions | |
| Baclofen | INN | Oral solution c; Solution for perfusion c |
| ATC: M03BX (thiocolchicoside) | Solution for injection | |
| Clobazam | INN | Oral suspension |
| ATC: N05BA (Prazepam) | Drops Oral solution | |
| Hydrocortisone | INN | Lyophilisat and solution for parenteral use e |
| Pharmaco-Therapeutic b2: steroidal anti-inflammatory drugs (Ketotifen) | Syrup; Oral solution | |
| ATC: H02AB (Dexamethasone; Prednisolone; methylprednisolone) | Syrup; Oral solution; Powder for injection solution | |
| Levetiracetam | INN | Oral solution; Solution for perfusion; Coated Granules c |
| ATC: N03AX (Lamotrigine) | Dispersible tablet | |
| Paracetamol, Codeine | INN | Effervescent tablet |
| Pharmaco-Therapeutic: analgesic, antipyretic (Paracetamol) | Oral solution | |
| Phenobarbital | INN | Powder and solvent for injection solution e |
| Prednisone | Pharmaco-Therapeutic: steroidal anti-inflammatory drugs (Ketotifen) | Syrup; Oral solution |
| ATC: H02AB (Dexamethasone; Prednisolone) | Syrup; Oral solution | |
| Sildenafil | INN | Oral suspension c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallet, T.; Elhamdaoui, O.; Berraho, A.; Cherkaoui, L.O.; Kriouile, Y.; Mahraoui, C.; Mouane, N.; Pense-Lheritier, A.-M.; Ruiz, F.; Bensouda, Y. Medicines Acceptability in Hospitalized Children: An Ongoing Need for Age-Appropriate Formulations. Pharmaceutics 2020, 12, 766. https://doi.org/10.3390/pharmaceutics12080766
Vallet T, Elhamdaoui O, Berraho A, Cherkaoui LO, Kriouile Y, Mahraoui C, Mouane N, Pense-Lheritier A-M, Ruiz F, Bensouda Y. Medicines Acceptability in Hospitalized Children: An Ongoing Need for Age-Appropriate Formulations. Pharmaceutics. 2020; 12(8):766. https://doi.org/10.3390/pharmaceutics12080766
Chicago/Turabian StyleVallet, Thibault, Omar Elhamdaoui, Amina Berraho, Lalla Ouafae Cherkaoui, Yamna Kriouile, Chafiq Mahraoui, Nezha Mouane, Anne-Marie Pense-Lheritier, Fabrice Ruiz, and Yahya Bensouda. 2020. "Medicines Acceptability in Hospitalized Children: An Ongoing Need for Age-Appropriate Formulations" Pharmaceutics 12, no. 8: 766. https://doi.org/10.3390/pharmaceutics12080766
APA StyleVallet, T., Elhamdaoui, O., Berraho, A., Cherkaoui, L. O., Kriouile, Y., Mahraoui, C., Mouane, N., Pense-Lheritier, A.-M., Ruiz, F., & Bensouda, Y. (2020). Medicines Acceptability in Hospitalized Children: An Ongoing Need for Age-Appropriate Formulations. Pharmaceutics, 12(8), 766. https://doi.org/10.3390/pharmaceutics12080766






