Enhanced Corneal Penetration of a Poorly Permeable Drug Using Bioadhesive Multiple Microemulsion Technology
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Animals
2.3. Methods
2.3.1. Ribavirin HPLC Assay
2.3.2. Preparation of Bioadhesive Multiple W/O/W Microemulsion
2.3.3. Microemulsion Physical Stability Tests
- (a)
- Freeze–Thaw Cycles Test
- (b)
- Centrifugation Test
2.3.4. Microemulsion In Vitro Evaluations
- (a)
- Droplet Size, Polydispersity Index (PDI) and Zeta Potential Measurements
- (b)
- Transmission Electron Microscopy Examination
- (c)
- Microemulsion Viscosity Determination
- (d)
- Measuring Microemulsion Bioadhesive Force
- (e)
- In Vitro Drug Release
- (f)
- In Vitro Transcorneal Permeability Study
- (g)
- In Vitro Evaluation of ME Effect on Cell Viability
2.3.5. In Vivo Safety and Ocular Tolerance Evaluation
- (a)
- Acute Ocular Toxicity Evaluation (Modified Draize Test)
- (b)
- Chronic Ocular Toxicity Evaluation
2.3.6. Statistical Analysis
3. Results and Discussion
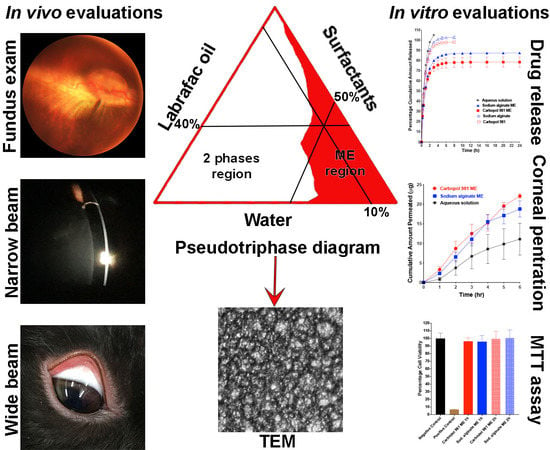
3.1. Preparation of the Bioadhesive Multiple W/O/W Microemulsion
3.2. Microemulsion Physical Stability
3.3. Microemulsion In Vitro Evaluations
3.3.1. Droplet Size, Polydispersity Index (PDI) and Zeta Potential Measurements
3.3.2. Transmission Electron Microscopy Examination
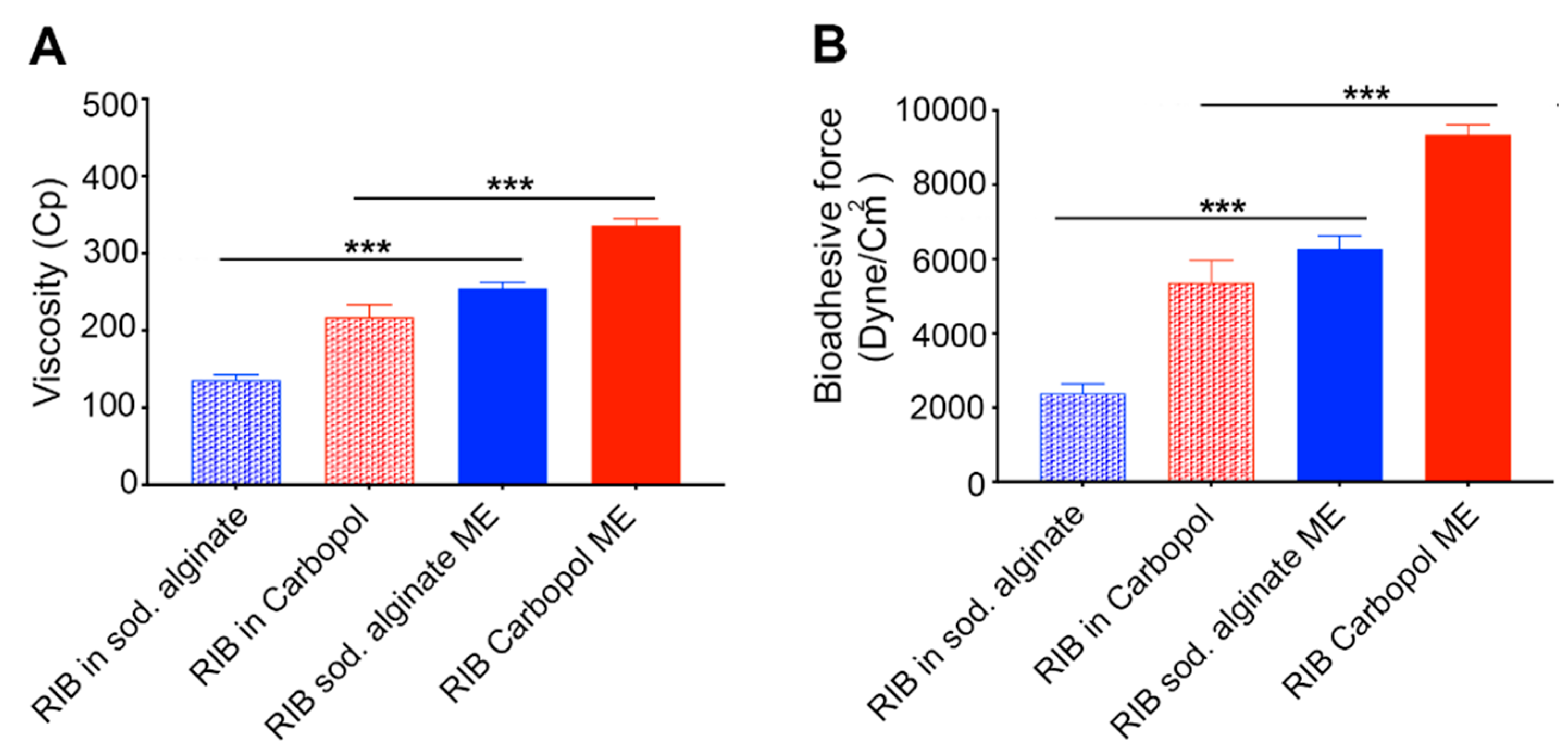
3.3.3. Microemulsion Viscosity Determination
3.3.4. Measuring Microemulsion Bioadhesive Force
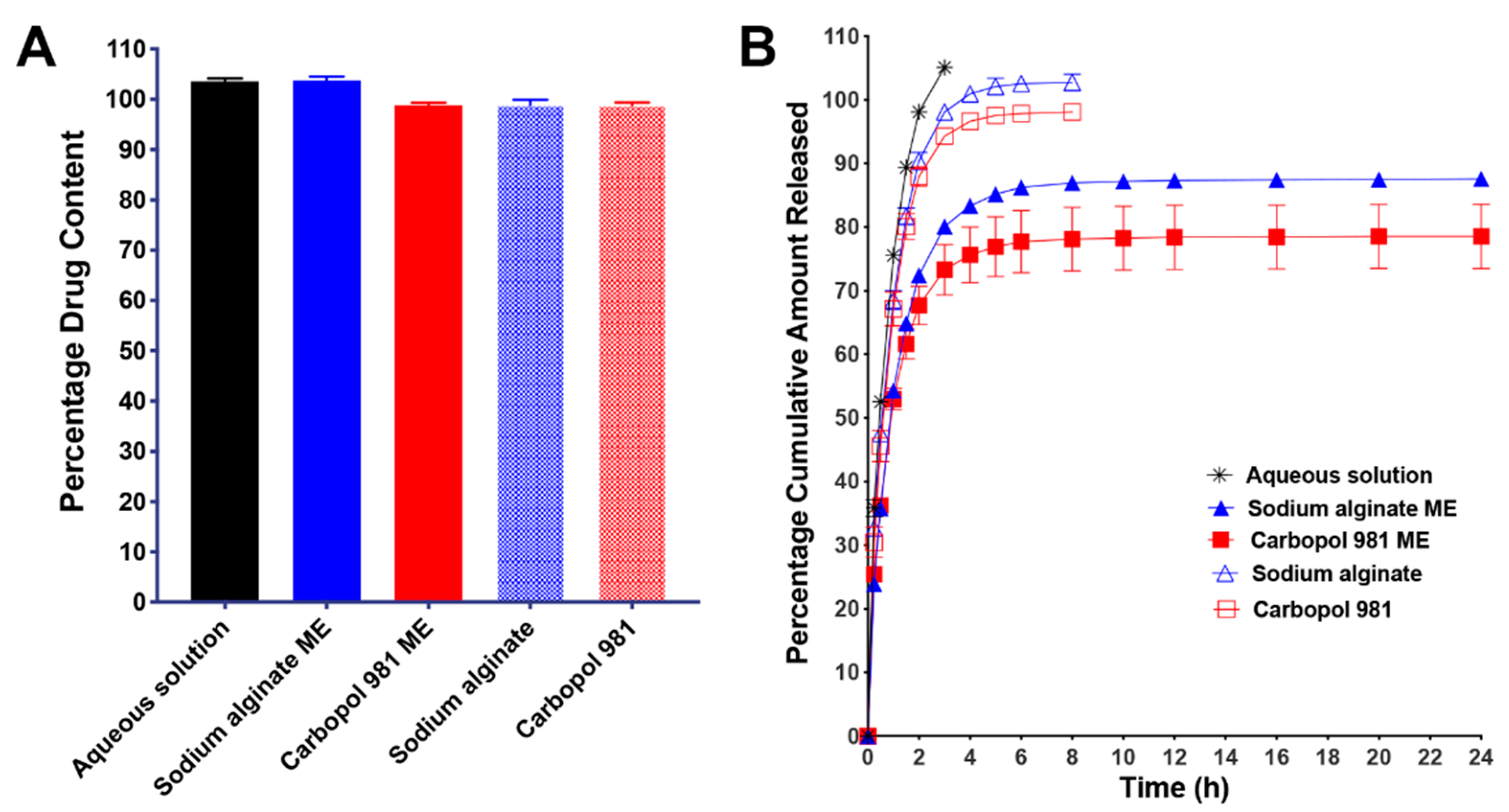
3.3.5. In Vitro Drug Release
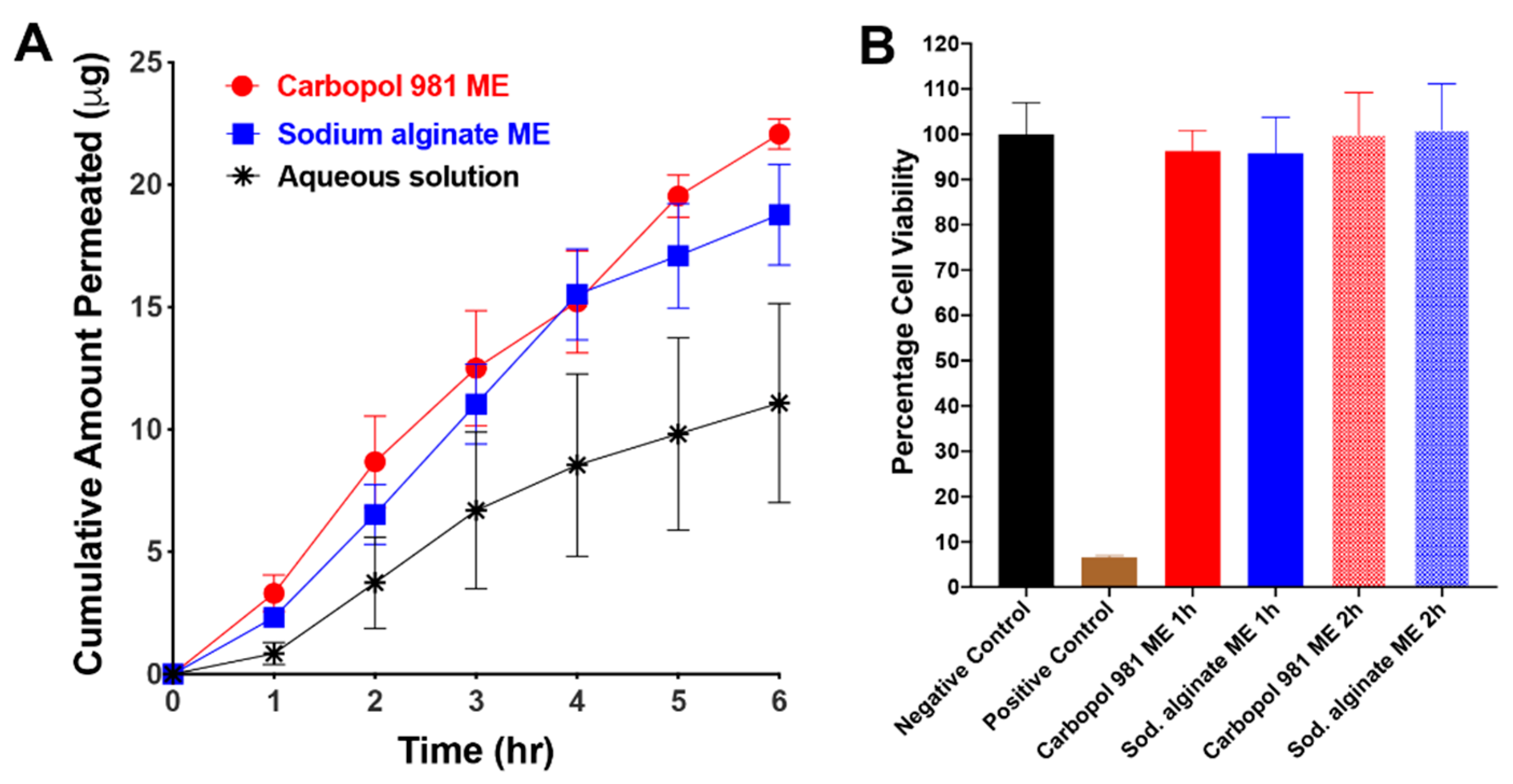
3.3.6. In Vitro Transcorneal Permeability
3.3.7. In Vitro Evaluation of ME Effect on Cell Viability
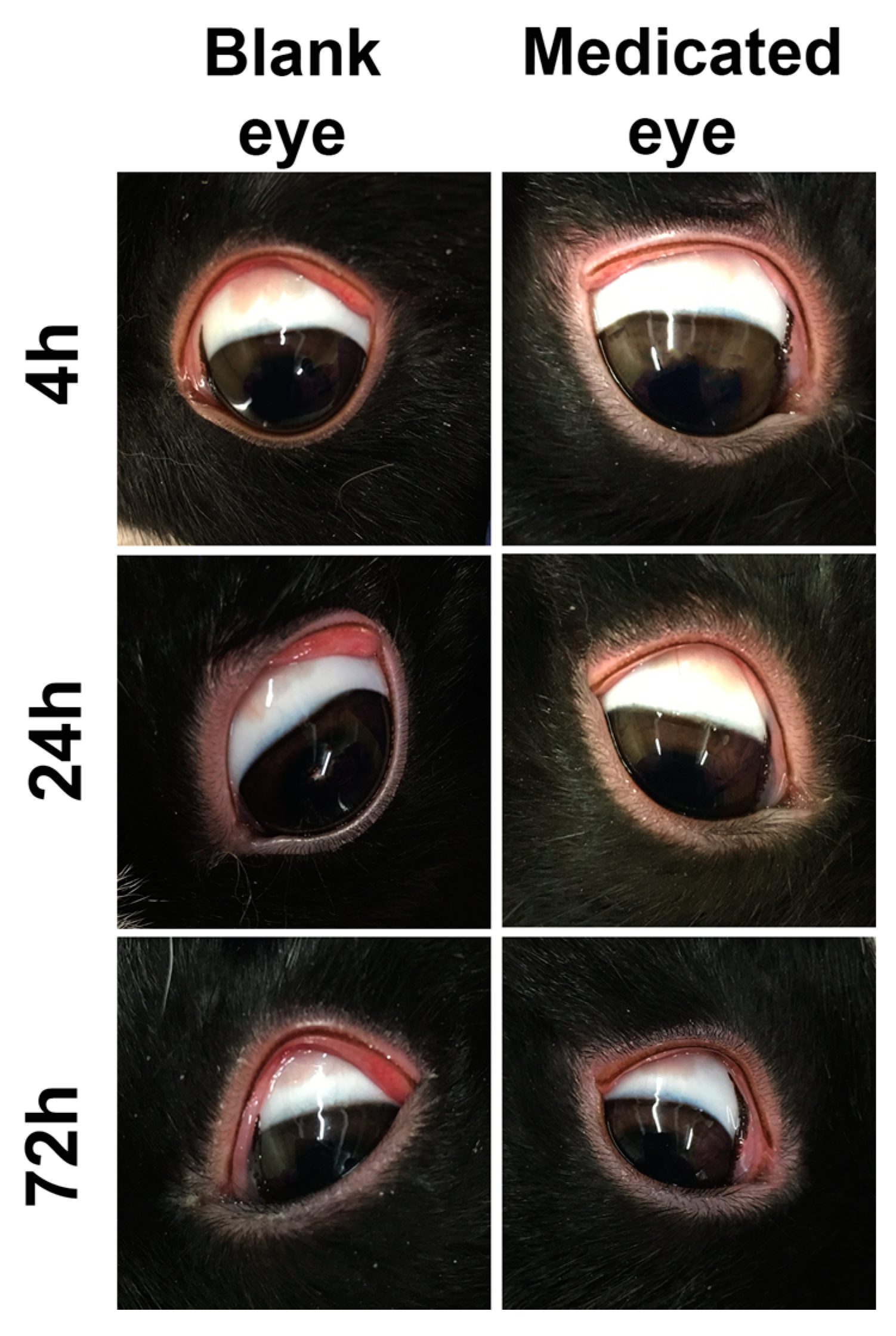
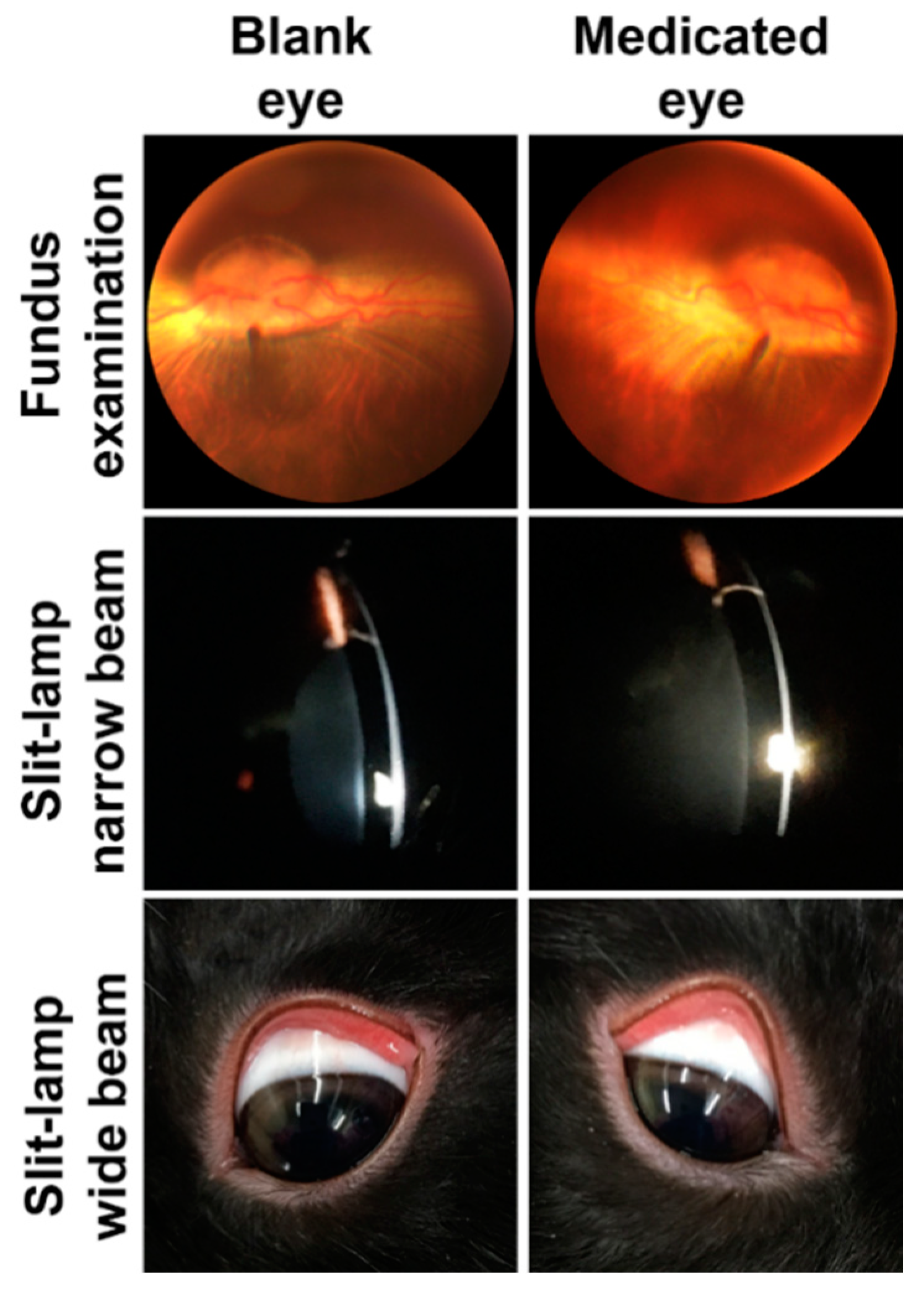
3.4. In Vivo Safety and Ocular Tolerance Evaluation
3.4.1. Acute Ocular Toxicity Evaluation (Modified Draize Test)
3.4.2. Chronic Ocular Toxicity Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Javadzadeh, A.R.; Omidi, Y. Ocular novel drug delivery: Impacts of membranes and barriers. Expert Opin. Drug Deliv. 2008, 5, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Ananthula, H.K.; Vaishya, R.D.; Barot, M.; Mitra, A.K. Duane’s Ophthalmology. In Bioavailability; Tasman, W., Jaeger, E.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Ahmed, I. The noncorneal route in ocular drug delivery. In Ophthalmic Drug Delivery Systems; Mitra, A.K., Ed.; Marcel Dekker: New York, NY, USA, 2003; pp. 335–363. [Google Scholar]
- Andres-Guerrero, V.; Vicario-de-la-Torre, M.; Molina-Martinez, I.T.; Benitez-del-Castillo, J.M.; Garcia-Feijoo, J.; Herrero-Vanrell, R. Comparison of the in vitro tolerance and in vivo efficacy of traditional timolol maleate eye drops versus new formulations with bioadhesive polymers. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3548–3556. [Google Scholar] [CrossRef]
- De Figueirêdo, E.S.; de Macedo, A.C.; de Figueirêdo, P.F.R.; de Figueirêdo, R.S. Use of hyaluronic acid in Ophthalmology. Arq. Bras. Oftalmol. 2010, 73, 92–95. [Google Scholar]
- Yamaguchi, M.; Ueda, K.; Isowaki, A.; Ohtori, A.; Takeuchi, H.; Ohguro, N.; Tojo, K. Mucoadhesive properties of chitosan-coated ophthalmic lipid emulsion containing indomethacin in tear fluid. Biol. Pharm. Bull. 2009, 32, 1266–1271. [Google Scholar] [CrossRef]
- Piras, A.M.; Zambito, Y.; Burgalassi, S.; Monti, D.; Tampucci, S.; Terreni, E.; Fabiano, A.; Balzano, F.; Uccello-Barretta, G.; Chetoni, P. A water-soluble, mucoadhesive quaternary ammonium chitosan-methyl-beta-cyclodextrin conjugate forming inclusion complexes with dexamethasone. J. Mater. Sci. Mater. Med. 2018, 29, 42. [Google Scholar] [CrossRef]
- Ding, D.; Kundukad, B.; Somasundar, A.; Vijayan, S.; Khan, S.A.; Doyle, P.S. Design of Mucoadhesive PLGA Microparticles for Ocular Drug Delivery. ACS Appl. Bio Mater. 2018, 1, 561–571. [Google Scholar] [CrossRef]
- Rupenthal, I.D.; Green, C.R.; Alany, R.G. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: Physicochemical characterisation and in vitro release. Int. J. Pharm. 2011, 411, 69–77. [Google Scholar] [CrossRef]
- Kaur, I.P.; Smitha, R. Penetration enhancers and ocular bioadhesives: Two new avenues for ophthalmic drug delivery. Drug Dev. Ind. Pharm. 2002, 28, 353–369. [Google Scholar] [CrossRef]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Saettone, M.F. Ocular toxicity of some corneal penetration enhancers evaluated by electrophysiology measurements on isolated rabbit corneas. Toxicol. In Vitro 2003, 17, 497–504. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo. Int. J. Pharm. 2016, 501, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Al-Kinani, A.A.; Arshad, M.S.; Chang, M.W.; Alany, R.G.; Ahmad, Z. Development and characterisation of electrospun timolol maleate-loaded polymeric contact lens coatings containing various permeation enhancers. Int. J. Pharm. 2017, 532, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cadena, M.D.L.A.; Spaeth, G.L. Ocular surface disease and glaucoma. Expert Rev. Ophthalmol. 2016, 11, 215–226. [Google Scholar] [CrossRef]
- Alvarado, H.L.; Abrego, G.; Garduno-Ramirez, M.L.; Clares, B.; Calpena, A.C.; Garcia, M.L. Design and optimization of oleanolic/ursolic acid-loaded nanoplatforms for ocular anti-inflammatory applications. Nanomedicine 2015, 11, 521–530. [Google Scholar] [CrossRef]
- Parra, A.; Mallandrich, M.; Clares, B.; Egea, M.A.; Espina, M.; Garcia, M.L.; Calpena, A.C. Design and elaboration of freeze-dried PLGA nanoparticles for the transcorneal permeation of carprofen: Ocular anti-inflammatory applications. Colloids Surf. B Biointerfaces 2015, 136, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, P.; Patil, A.; Wu, K.W.; Sweeney, C.; Tripathi, S.; Avula, B.; Taskar, P.; Khan, S.; Majumdar, S. Optimization, stabilization, and characterization of amphotericin B loaded nanostructured lipid carriers for ocular drug delivery. Int. J. Pharm. 2019, 572, 118771. [Google Scholar] [CrossRef]
- Terreni, E.; Chetoni, P.; Tampucci, S.; Burgalassi, S.; Al-Kinani, A.A.; Alany, R.G.; Monti, D. Assembling Surfactants-Mucoadhesive Polymer Nanomicelles (ASMP-Nano) for Ocular Delivery of Cyclosporine-A. Pharmaceutics 2020, 12, 253. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Topical application of polymeric nanomicelles in ophthalmology: A review on research efforts for the noninvasive delivery of ocular therapeutics. Expert Opin. Drug Deliv. 2019, 16, 397–413. [Google Scholar] [CrossRef]
- Smetanova, L.; Stetinova, V.; Svoboda, Z.; Kvetina, J. Caco-2 cells, biopharmaceutics classification system (BCS) and biowaiver. Acta Med. Hradec Kralove 2011, 54, 3–8. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Maria, D.N.; Mishra, S.R.; Guragain, D.; Wang, X.; Jablonski, M.M. Once Daily Pregabalin Eye Drops for Management of Glaucoma. ACS Nano 2019, 13, 13728–13744. [Google Scholar] [CrossRef]
- Goodarzi, N.; Barazesh Morgani, A.; Abrahamsson, B.; Cristofoletti, R.; Groot, D.W.; Langguth, P.; Mehta, M.U.; Polli, J.E.; Shah, V.P.; Dressman, J.B. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Ribavirin. J. Pharm. Sci. 2016, 105, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Te, H.S.; Randall, G.; Jensen, D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol. Hepatol. N. Y. 2007, 3, 218–225. [Google Scholar] [PubMed]
- Manns, M.P.; McHutchison, J.G.; Gordon, S.C.; Rustgi, V.K.; Shiffman, M.; Reindollar, R.; Goodman, Z.D.; Koury, K.; Ling, M.; Albrecht, J.K. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 2001, 358, 958–965. [Google Scholar] [CrossRef]
- Gipson, I.K.; Spurr-Michaud, S.; Argueso, P.; Tisdale, A.; Ng, T.F.; Russo, C.L. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2496–2506. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Joshi, S.; Jindal, K.C.; Khanna, S. Stability Indicating Hplc Method for Ribavirin and its Pharmaceutical Dosage Forms. Drug Dev. Ind. Pharm. 2008, 20, 85–91. [Google Scholar] [CrossRef]
- Radwan, S.A.A.; ElMeshad, A.N.; Shoukri, R.A. Microemulsion loaded hydrogel as a promising vehicle for dermal delivery of the antifungal sertaconazole: Design, optimization and ex vivo evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1351–1365. [Google Scholar] [CrossRef]
- Dehghani, F.; Farhadian, N.; Golmohammadzadeh, S.; Biriaee, A.; Ebrahimi, M.; Karimi, M. Preparation, characterization and in-vivo evaluation of microemulsions containing tamoxifen citrate anti-cancer drug. Eur. J. Pharm. Sci. 2017, 96, 479–489. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Abd-Elgawad, A.H.; Soliman, O.A.; Jablonski, M.M. Natural Bioadhesive Biodegradable Nanoparticle-Based Topical Ophthalmic Formulations for Management of Glaucoma. Transl. Vis. Sci. Technol. 2015, 4, 12. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Abd-Elgawad, A.H.; Soliman, O.A.; Jablonski, M.M. Stability and Ocular Pharmacokinetics of Celecoxib-Loaded Nanoparticles Topical Ophthalmic Formulations. J. Pharm. Sci. 2016, 105, 3691–3701. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: Design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int. J. Pharm. 2013, 443, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Abd-Elgawad, A.E.; Soliman, O.A.; Jablonski, M.M. Nanoparticle-based topical ophthalmic formulations for sustained celecoxib release. J. Pharm. Sci. 2013, 102, 1036–1053. [Google Scholar] [CrossRef] [PubMed]
- Balguri, S.P.; Adelli, G.R.; Janga, K.Y.; Bhagav, P.; Majumdar, S. Ocular disposition of ciprofloxacin from topical, PEGylated nanostructured lipid carriers: Effect of molecular weight and density of poly (ethylene) glycol. Int. J. Pharm. 2017, 529, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; McCanna, D.J.; Sivak, J.G. Use of the viability reagent PrestoBlue in comparison with alamarBlue and MTT to assess the viability of human corneal epithelial cells. J. Pharmacol. Toxicol. Methods 2015, 71, 1–7. [Google Scholar] [CrossRef]
- Janga, K.Y.; Tatke, A.; Dudhipala, N.; Balguri, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Gellan Gum Based Sol-to-Gel Transforming System of Natamycin Transfersomes Improves Topical Ocular Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 814–822. [Google Scholar] [CrossRef]
- Preston, S.L.; Drusano, G.L.; Glue, P.; Nash, J.; Gupta, S.K.; McNamara, P. Pharmacokinetics and absolute bioavailability of ribavirin in healthy volunteers as determined by stable-isotope methodology. Antimicrob. Agents Chemother. 1999, 43, 2451–2456. [Google Scholar] [CrossRef]
- Kimberlin, D.W. Antiviral Agents. In Principles and Practice of Pediatric Infectious Diseases; Elsevier: New York, NY, USA, 2018; pp. 1551–1567. [Google Scholar] [CrossRef]
- Ren, Q.; Deng, C.; Meng, L.; Chen, Y.; Chen, L.; Sha, X.; Fang, X. In vitro, ex vivo, and in vivo evaluation of the effect of saturated fat acid chain length on the transdermal behavior of ibuprofen-loaded microemulsions. J. Pharm. Sci. 2014, 103, 1680–1691. [Google Scholar] [CrossRef]
- Kaukonen, A.M.; Boyd, B.J.; Porter, C.J.; Charman, W.N. Drug solubilization behavior during in vitro digestion of simple triglyceride lipid solution formulations. Pharm. Res. 2004, 21, 245–253. [Google Scholar] [CrossRef]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTech 2009, 10, 808–819. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Li, J.; Liu, R.; Shu, L.; Jin, J. Effects of Labrasol on the corneal drug delivery of baicalin. Drug Deliv. 2009, 16, 399–404. [Google Scholar] [CrossRef]
- Fialho, S.L.; da Silva-Cunha, A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004, 32, 626–632. [Google Scholar] [CrossRef]
- Cremophor® EL. BASF Fine Chemicals; Technical Leaflet; Cremophor’ EL: Ludwigshafen, Germany, 1997. [Google Scholar]
- Grant, W.M.; Schuman, J.S. Toxicology of the Eye: Effects on the Eyes and Visual System from Chemicals, Drugs, Metals and Minerals, Plants, Toxins, and Venoms; Also, Systemic Side Effects from Eye Medications, 4th ed.; Charles C Thomas: Springfield, IL, USA, 1993. [Google Scholar]
- Benelli, U. Systane lubricant eye drops in the management of ocular dryness. Clin. Ophthalmol. 2011, 5, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a new drug carrier made from alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Sechoy, O.; Tissie, G.; Sebastian, C.; Maurin, F.; Driot, J.Y.; Trinquand, C. A new long acting ophthalmic formulation of carteolol containing alginic acid. Int. J. Pharm. 2000, 207, 109–116. [Google Scholar] [CrossRef]
- Lubrizol. Carbopol® Polymer Products. Available online: https://www.lubrizol.com/Health/Pharmaceuticals/Excipients/Carbopol-Polymer-Products (accessed on 6 April 2020).
- Best, B.P. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015, 18, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Mensch, C.D.; Davis, H.B.; Blue, J.T. Characterization of Propylene Glycol-Mitigated Freeze/Thaw Agglomeration of a Frozen Liquid nOMV Vaccine Formulation by Static Light Scattering and Micro-Flow Imaging. PDA J. Pharm. Sci. Technol. 2015, 69, 477–498. [Google Scholar] [CrossRef] [PubMed]
- Tenjaria, S. Microemulsions: an overview and pharmaceutical applications. Crit Rev. Ther. Drug Carrier Syst. 1999, 16, 461–521. [Google Scholar]
- Seijo, B.; Fattal, E.; Roblot-Treupel, L.; Couvreur, P. Design of nanoparticles of less than 50 nm diameter: Preparation, characterization and drug loading. Int. J. Pharm. 1990, 62, 1–7. [Google Scholar] [CrossRef]
- Scholes, P.D.; Coombes, A.G.A.; Illum, L.; Daviz, S.S.; Vert, M.; Davies, M.C. The preparation of sub-200 nm poly(lactide-co-glycolide) microspheres for site-specific drug delivery. J. Control. Release 1993, 25, 145–153. [Google Scholar] [CrossRef]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J. Microemulsion Based Bioadhesive Gel of Itraconazole Using Tamarind Gum: In-vitro and Ex-vivo Evaluation. Marmara Pharm. J. 2017, 21, 688. [Google Scholar] [CrossRef]
- Naga Sravan Kumar Varma, V.; Maheshwari, P.V.; Navya, M.; Reddy, S.C.; Shivakumar, H.G.; Gowda, D.V. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm. J. 2014, 22, 591–599. [Google Scholar] [CrossRef]
- Shaikh, R.; Raj Singh, T.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Dhawan, N.; Sharma, H.; Vaidya, S.; Vaidya, B. Bioadhesive polymers: Novel tool for drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 42, 274–283. [Google Scholar] [CrossRef]
- Ribavirin Compound, PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ribavirin-_-ddDAPR (accessed on 25 April 2020).
- Araie, M.; Shirasawa, E.; Hikita, M. Effect of oxidized glutathione on the barrier function of the corneal endothelium. Invest. Ophthalmol. Vis. Sci. 1988, 29, 1884–1887. [Google Scholar] [PubMed]
- Araie, M.; Shirasawa, E.; Ohashi, T. Intraocular irrigating solutions and permeability of the blood-aqueous barrier. Arch. Ophthalmol. 1990, 108, 882–885. [Google Scholar] [CrossRef]
- Van Hoogdalem, E.J.; de Boer, A.G.; Breimer, D.D. Intestinal drug absorption enhancement: An overview. Pharmacol. Ther. 1989, 44, 407–443. [Google Scholar] [CrossRef]
- Hosoya, K.; Lee, V.H. Cidofovir transport in the pigmented rabbit conjunctiva. Curr. Eye Res. 1997, 16, 693–697. [Google Scholar] [CrossRef]
- Hochman, J.H.; Fix, J.A.; LeCluyse, E.L. In vitro and in vivo analysis of the mechanism of absorption enhancement by palmitoylcarnitine. J. Pharmacol. Exp. Ther. 1994, 269, 813–822. [Google Scholar]

| ME Ingredient (% w/w) | |
|---|---|
| Ribavirin | 0.1 |
| Labrafac Lipophile WL1349 | 3.96 |
| Capryol 90 | 2.48 |
| Soybean lecithin | 2.48 |
| Labrasol | 4.5 |
| Cremophor EL | 4.5 |
| Propylene glycol | 18.0 |
| Polymer | X |
| Water (to) | 100 |
| Type of ME | Mean Droplet Size (nm) | PDI | Zeta Potential (mV) | |||
|---|---|---|---|---|---|---|
| Blank | Medicated | Blank | Medicated | Blank | Medicated | |
| Sodium alginate ME | 10.8 ± 0.2 | 10.7 ± 0.02 | 0.304 ± 0.0 | 0.303 ± 0.0 | −19.1 ± 0.56 | −20.4 ± 1.1 |
| Carbopol 981 ME | 9.2 ± 0.04 | 9.1 ± 0.02 | 0.155 ± 0.0 | 0.163 ± 0.0 | −23.7 ± 0.60 | −21.4 ± 1.0 |
| Formulation | Rate of Permeation (dM/dt) | Flux (µg/cm2/min) | Permeability Coefficient (P) ×104 (cm/min) | Relative Improvement |
|---|---|---|---|---|
| Aqueous solution | 0.021 ± 0.005 | 0.033 ± 0.008 | 3.322 ± 0.778 | 1 |
| Sodium alginate ME | 0.027 ± 0.005 | 0.043 ± 0.007 | 4.276 ± 0.725 | 1.29 |
| Carbopol 981 ME | 0.057 ± 0.019 | 0.090 ± 0.030 | 8.991 ± 2.975 | 2.71 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.M.; Maria, D.N.; Wang, X.; Simpson, R.N.; Hollingsworth, T.J.; Jablonski, M.M. Enhanced Corneal Penetration of a Poorly Permeable Drug Using Bioadhesive Multiple Microemulsion Technology. Pharmaceutics 2020, 12, 704. https://doi.org/10.3390/pharmaceutics12080704
Ibrahim MM, Maria DN, Wang X, Simpson RN, Hollingsworth TJ, Jablonski MM. Enhanced Corneal Penetration of a Poorly Permeable Drug Using Bioadhesive Multiple Microemulsion Technology. Pharmaceutics. 2020; 12(8):704. https://doi.org/10.3390/pharmaceutics12080704
Chicago/Turabian StyleIbrahim, Mohamed Moustafa, Doaa Nabih Maria, XiangDi Wang, Raven N. Simpson, T.J. Hollingsworth, and Monica M. Jablonski. 2020. "Enhanced Corneal Penetration of a Poorly Permeable Drug Using Bioadhesive Multiple Microemulsion Technology" Pharmaceutics 12, no. 8: 704. https://doi.org/10.3390/pharmaceutics12080704
APA StyleIbrahim, M. M., Maria, D. N., Wang, X., Simpson, R. N., Hollingsworth, T. J., & Jablonski, M. M. (2020). Enhanced Corneal Penetration of a Poorly Permeable Drug Using Bioadhesive Multiple Microemulsion Technology. Pharmaceutics, 12(8), 704. https://doi.org/10.3390/pharmaceutics12080704