Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters
Abstract
1. Introduction
2. Materials
3. Methods
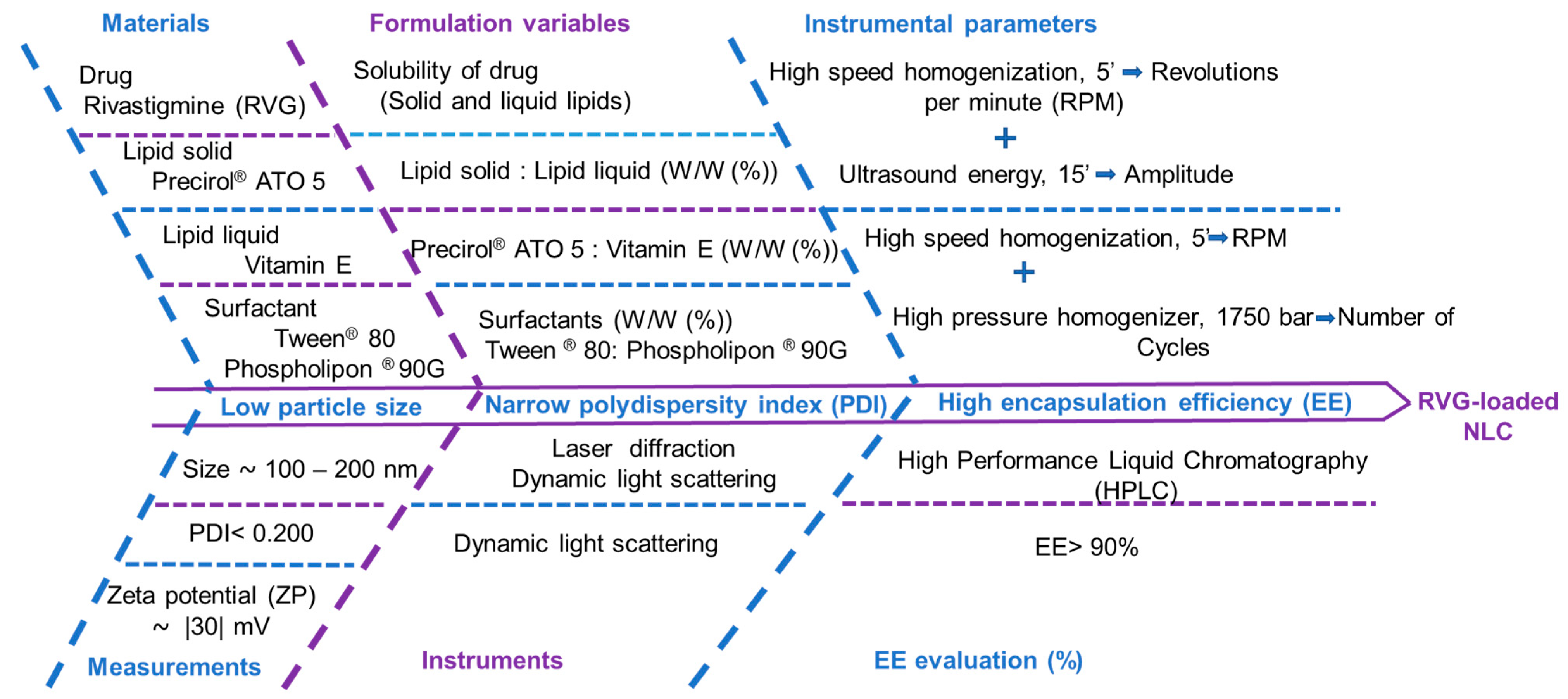
3.1. Screening of Drug and Excipients
3.2. Preparation of Rivastigmine-Loaded NLC Formulations
3.3. Determination of Particle Size, Polydispersity Index (PDI), and Zeta Potential (ZP)
3.4. Rivastigmine Quantification
3.4.1. Development and Validation of a High-Performance Liquid Chromatography (HPLC) Method
3.4.2. Chromatographic Conditions
3.4.3. Preparation of Standard Solutions
3.4.4. Assessment of Encapsulation Parameters
3.5. Design of Experiment (DoE) for the Optimization of Rivastigmine-Loaded NLC Formulation
3.5.1. Part 1: Optimization of Formulation Variables by Central Composite Design (CCD)
3.5.2. Part 2: Optimization of Instrumental Parameters by Box–Behnken Design (BBD)
3.6. pH and Osmolarity
3.7. In Vitro Drug Release Studies
Kinetic Mechanism of Drug Release
- (1)
- Zero order model: M0 − M = kt
- (2)
- First order model: ln m = kt
- (3)
- Higuchi equation: M0 − M = kt1/2
- (4)
- Korsmeyer–Peppas model: log (M0 − M) = log k + n log t
3.8. Statistical Analysis
3.9. Stability Studies
4. Results
4.1. Screening of Drug and Excipients
4.2. Suitability of the HPLC Method for Rivastigmine Quantification
4.3. Part 1: Optimization of Formulation Cariables by CCD
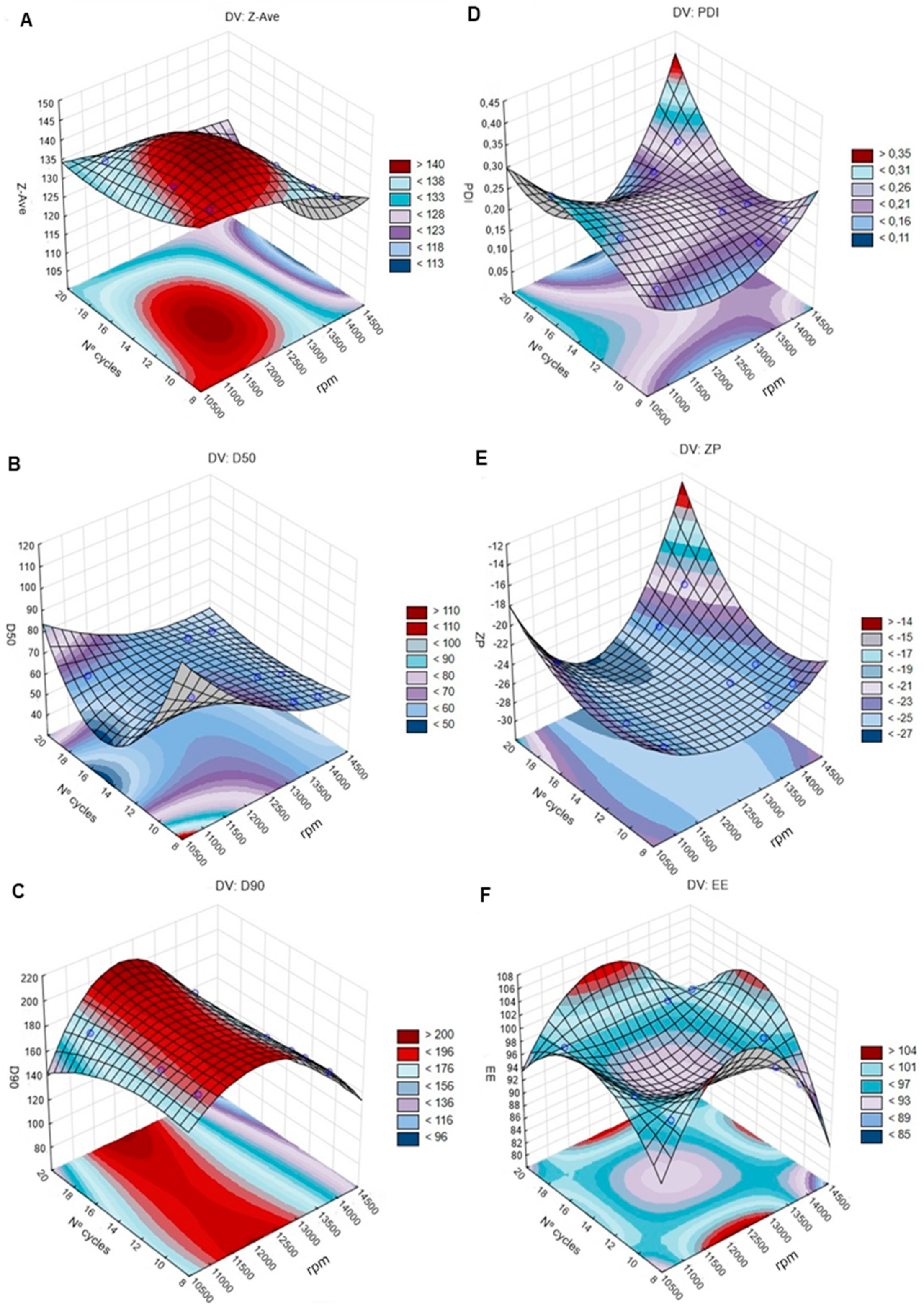
4.3.1. Effect of Lipids and Surfactants Ratio on Particle Size (Z-Ave, D50, and D90)
4.3.2. Effect of Lipid and Surfactant Ratios on PDI, ZP, and EE
4.4. Part 2: Optimization of Instrumental Parameters by BBD
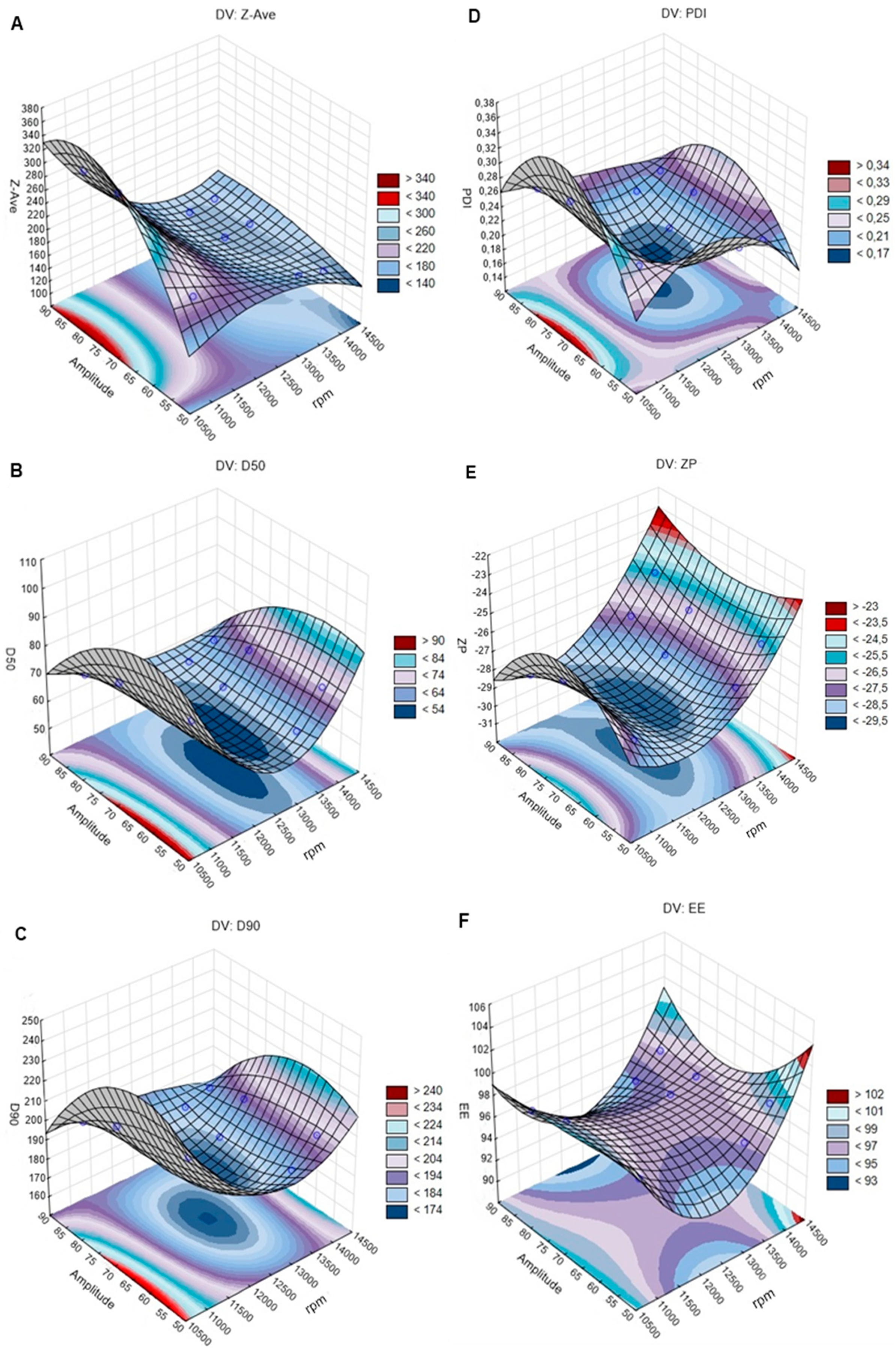
4.4.1. Effects of Emulsification Speed and HPH Cycles on Particles Size (Z-Ave, D50, and D90), PDI, ZP, and EE
Particle Size
PDI, ZP, and EE
4.4.2. Effects of Ultrasound Technique on Particles Size (Z-Ave, D50, and D90), PDI, ZP, and EE
Particle Size
PDI, ZP, and EE
4.5. Model Validation
4.6. pH and Osmolarity
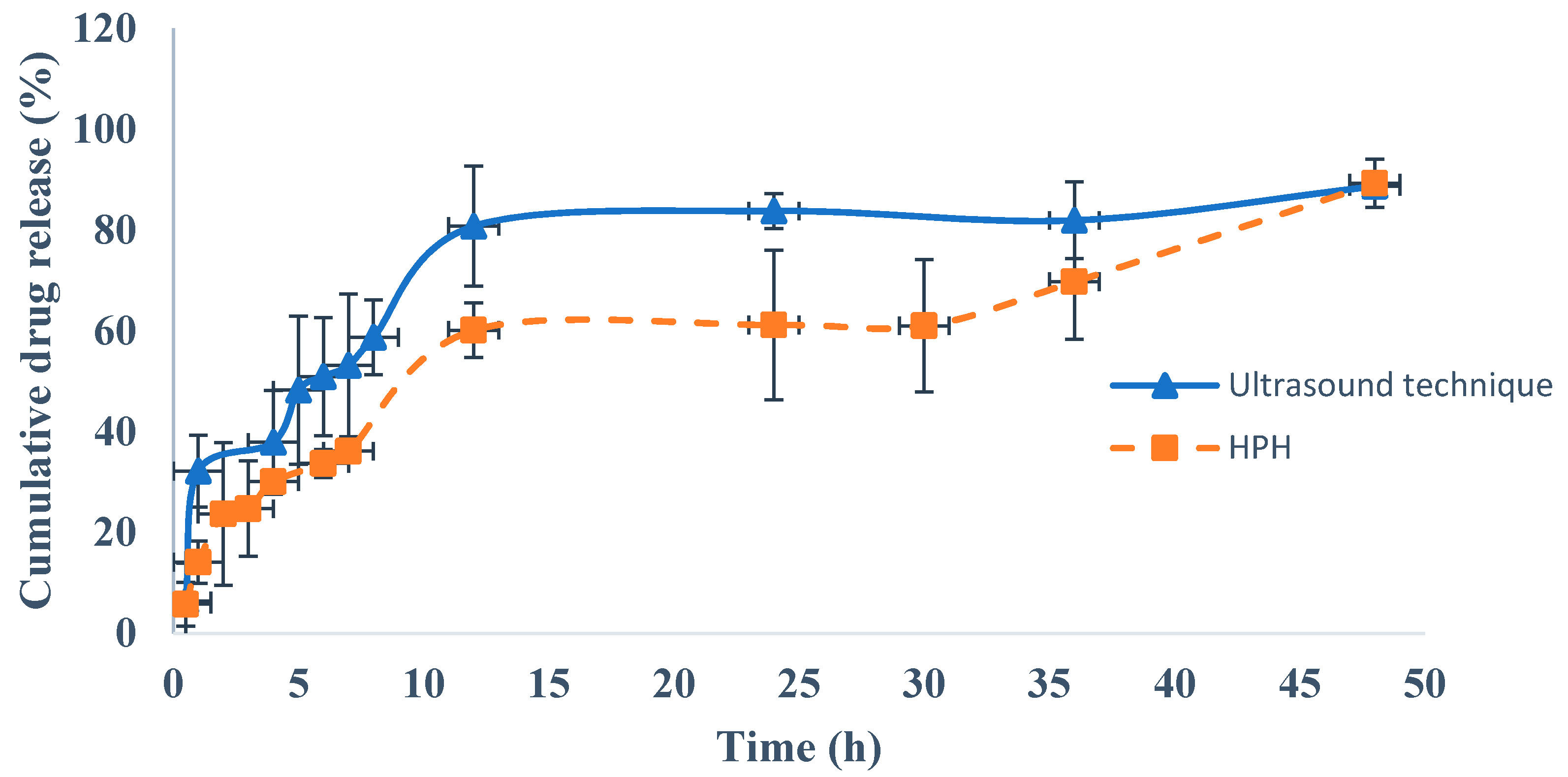
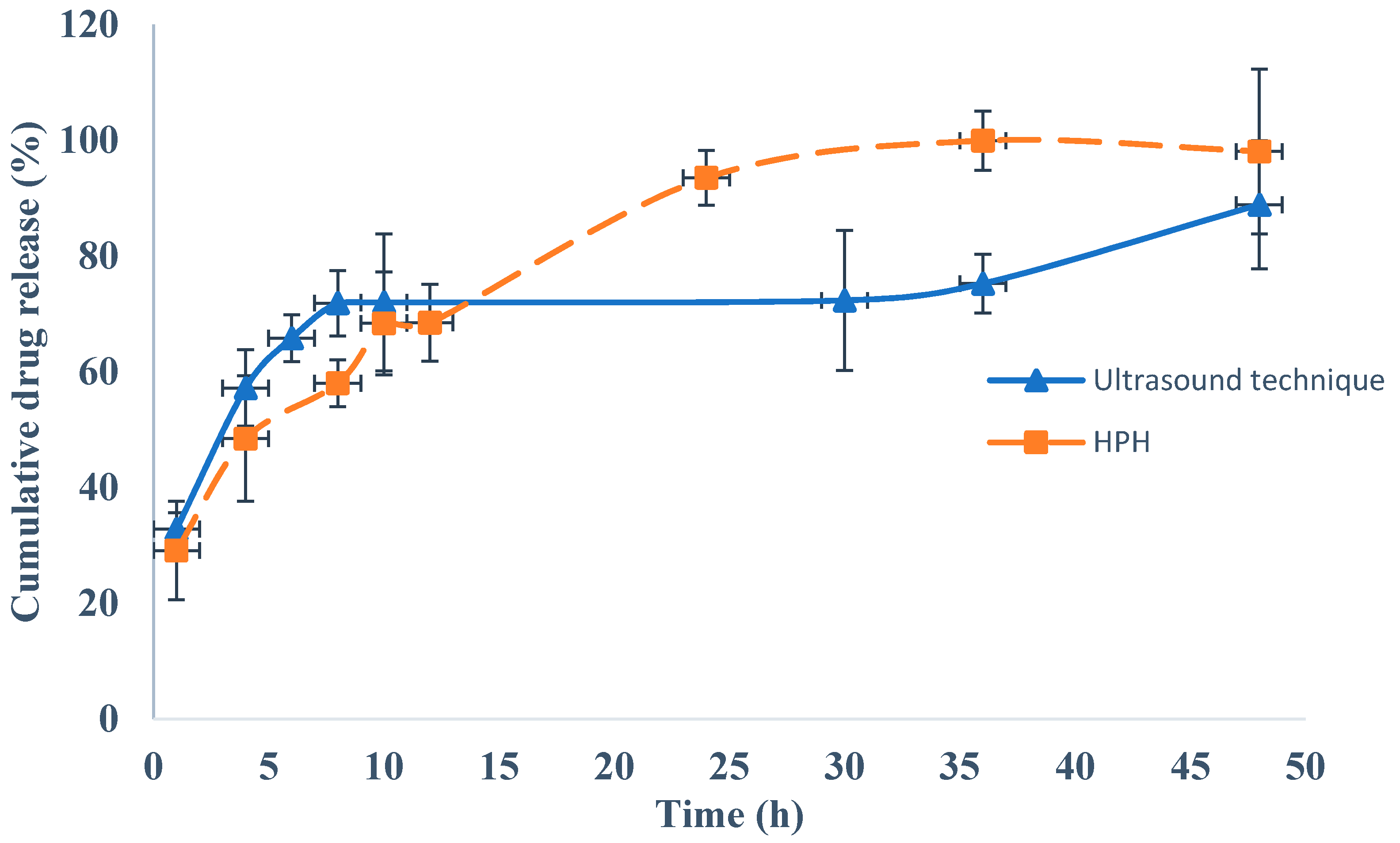
4.7. In Vitro Drug Release Studies
4.8. Stability Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khoury, R.; Rajamanickam, J.; Grossberg, G.T. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Ther. Adv. Drug Saf. 2018, 9, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Karch, C.M.; Goate, A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry 2015, 77, 43–51. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- Cunha, S.; Almeida, H.; Amaral, M.H.; Lobo, J.M.S.; Silva, A. Intranasal lipid nanoparticles for the treatment of neurodegenerative diseases. Curr. Pharm. Des. 2017, 23, 6553–6562. [Google Scholar] [CrossRef] [PubMed]
- Fazil, M.; Md, S.; Haque, S.; Kumar, M.; Baboota, S.; Kaur Sahni, J.; Ali, J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef]
- Moreira, F.T.; Sale, M.G.F.; Di Lorenzo, M. Towards timely Alzheimer diagnosis: A self-powered amperometric biosensor for the neurotransmitter acetylcholine. Biosens. Bioelectron. 2017, 87, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Kalambate, P.K.; Biradar, M.R.; Karna, S.P.; Srivastava, A.K. Adsorptive stripping differential pulse voltammetry determination of rivastigmine at graphene nanosheet-gold nanoparticle/carbon paste electrode. J. Electroanal. Chem. 2015, 757, 150–158. [Google Scholar] [CrossRef]
- Birks, J.S.; Evans, J.G. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Abouhussein, D.M.; Khattab, A.; Bayoumi, N.A.; Mahmoud, A.F.; Sakr, T.M. Brain targeted rivastigmine mucoadhesive thermosensitive In Situ gel: Optimization, In Vitro evaluation, radiolabeling, In Vivo pharmacokinetics and biodistribution. J. Drug Deliv. Sci. Technol. 2018, 43, 129–140. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.; Mishra, D. Optimization of brain targeted chitosan nanoparticles of Rivastigmine for improved efficacy and safety. Int. J. Biol. Macromol. 2013, 59, 72–83. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: Effect on formulation and characterization parameters. Eur. J. Pharm. Sci. 2015, 78, 54–66. [Google Scholar] [CrossRef]
- Nageeb El-Helaly, S.; Abd Elbary, A.; Kassem, M.A.; El-Nabarawi, M.A. Electrosteric stealth Rivastigmine loaded liposomes for brain targeting: Preparation, characterization, ex vivo, bio-distribution and In Vivo pharmacokinetic studies. Drug Deliv. 2017, 24, 692–700. [Google Scholar] [CrossRef]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Lobo, J.S.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019. [Google Scholar] [CrossRef]
- Schwarz, B.; Merkel, O.M. Nose-to-brain delivery of biologics. Ther. Deliv. 2019. [Google Scholar] [CrossRef]
- Joshi, S.A.; Chavhan, S.S.; Sawant, K.K. Rivastigmine-loaded PLGA and PBCA nanoparticles: Preparation, optimization, characterization, In Vitro and pharmacodynamic studies. Eur. J. Pharm. Biopharm. 2010, 76, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.; Rosch, J.; Putnam, D. Concepts, technologies, and practices for drug delivery past the blood–brain barrier to the central nervous system. J. Control. Release 2016, 240, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K. Intranasal administration of nanostructured lipid carriers containing CNS acting drug: Pharmacodynamic studies and estimation in blood and brain. J. Psychiatr. Res. 2012, 46, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; González-Mira, E.; García, M.L.; Egea, M.A.; Fonseca, J.; Silva, R.; Santos, D.; Souto, E.B.; Ferreira, D. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): High pressure homogenization versus ultrasound. Colloids Surf. B Biointerfaces 2011, 86, 158–165. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Buenestado, A.; Naline, E.; Faisy, C.; Blouquit-Laye, S.; Couderc, L.J.; Le Guen, M.; Fischler, M.; Devillier, P. Intranasal drug delivery: An efficient and non-invasive route for systemic administration: Focus on opioids. Pharmacol. Ther. 2012, 134, 366–379. [Google Scholar] [CrossRef]
- Miyake, M.M.; Bleier, B.S. The blood-brain barrier and nasal drug delivery to the central nervous system. Am. J. Rhinol. Allergy 2015, 29, 124–127. [Google Scholar] [CrossRef]
- Illum, L. Intranasal delivery to the central nervous system. In Blood-Brain Barrier in Drug Discovery: Optimizing Brain Exposure of CNS Drugs and Minimizing Brain Side Effects for Peripheral Drugs; Di, L., Kerns, E.H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 535–565. [Google Scholar]
- Cunha, S.; Amaral, M.H.; Lobo, J.S.; Silva, A.C. Lipid nanoparticles for nasal/intranasal drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Forbes, B.; Bommer, R.; Goole, J.; Hellfritzsch, M.; De Kruijf, W.; Lambert, P.; Caivano, G.; Regard, A.; Schiaretti, F.; Trenkel, M.; et al. A consensus research agenda for optimising nasal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Salade, L.; Wauthoz, N.; Goole, J.; Amighi, K. How to characterize a nasal product. The state of the art of in-vitro and ex-vivo specific methods. Int. J. Pharm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. 3), S5. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiao, Y.; Wu, Z. Nanosystem trends in drug delivery using quality-by-design concept. J. Control. Release 2017, 256, 9–18. [Google Scholar] [CrossRef]
- Silva, A.; González-Mira, E.; Lobo, J.S.; Amaral, M.H. Current progresses on nanodelivery systems for the treatment of neuropsychiatric diseases: Alzheimer’s and Schizophrenia. Curr. Pharm. Des. 2013, 19, 7185–7195. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Badran, M.M.; Ali, H.M.; Abdel-Bakky, M.S.; Ibrahim, H.M. Multifunctional carbamazepine loaded nanostructured lipid carrier (NLC) formulation. Int. J. Pharm. 2018, 550, 359–371. [Google Scholar] [CrossRef]
- Silva, A.C.; Amaral, M.H.; Lobo, J.M.S.; Lopes, C.M. Lipid nanoparticles for the delivery of biopharmaceuticals. Curr. Pharm. Biotechnol. 2015, 16, 291–302. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Kokare, C.R. Nanostructured lipid carriers engineered for intranasal delivery of teriflunomide in multiple sclerosis: Optimization and In Vivo studies. Drug Dev. Ind. Pharm. 2019, 45, 839–851. [Google Scholar] [CrossRef]
- Iqbal, B.; Ali, J.; Baboota, S. Silymarin loaded nanostructured lipid carrier: From design and dermatokinetic study to mechanistic analysis of epidermal drug deposition enhancement. J. Mol. Liq. 2018, 255, 513–529. [Google Scholar] [CrossRef]
- Madane, R.G.; Mahajan, H.S. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: Design, characterization, and In Vivo study. Drug Deliv. 2016, 23, 1326–1334. [Google Scholar] [PubMed]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Intranasal delivery of venlafaxine loaded nanostructured lipid carrier: Risk assessment and QbD based optimization. J. Drug Deliv. Sci. Technol. 2016, 33, 37–50. [Google Scholar] [CrossRef]
- Tambe, V.; Maheshwari, R.; Chourasiya, Y.; Choudhury, H.; Gorain, B.; Tekade, R.K. Clinical aspects and regulatory requirements for nanomedicines. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 733–752. [Google Scholar]
- Pallagi, E.; Ambrus, R.; Szabó-Révész, P.; Csóka, I. Adaptation of the quality by design concept in early pharmaceutical development of an intranasal nanosized formulation. Int. J. Pharm. 2015, 491, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Beck-Broichsitter, M.; Bøtker, J.P.; Malmsten, M.; Rantanen, J.; Bohr, A. Transforming nanomedicine manufacturing toward Quality by Design and microfluidics. Adv. Drug Deliv. Rev. 2018, 128, 115–131. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonised Tripartite Guideline. Pharmaceutical development. Q8 (2R). As revised in August 2009. Available online: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (accessed on 15 July 2019).
- Food and Drug Administration. Guidance for Industry: Q9 Quality Risk Management; Food and Drug Administration: Rockville, MD, USA, 2006. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatory-Information/Guidances/ucm073511.pdf (accessed on 15 July 2019).
- Bakonyi, M.; Berkó, S.; Kovács, A.; Budai-Szűcs, M.; Kis, N.; Erős, G.; Csóka, I.; Csányi, E. Application of quality by design principles in the development and evaluation of semisolid drug carrier systems for the transdermal delivery of lidocaine. J. Drug Deliv. Sci. Technol. 2018, 44, 136–145. [Google Scholar] [CrossRef]
- Awotwe-Otoo, D.; Agarabi, C.; Wu, G.K.; Casey, E.; Read, E.; Lute, S.; Brorson, K.A.; Khan, M.A.; Shah, R.B. Quality by design: Impact of formulation variables and their interactions on quality attributes of a lyophilized monoclonal antibody. Int. J. Pharm. 2012, 438, 167–175. [Google Scholar] [CrossRef]
- Tzeyung, A.S.; Md, S.; Bhattamisra, S.K.; Madheswaran, T.; Alhakamy, N.A.; Aldawsari, H.M.; Radhakrishnan, A.K. Fabrication, Optimization, and Evaluation of Rotigotine-Loaded Chitosan Nanoparticles for Nose-To-Brain Delivery. Pharmaceutics 2019, 11, 26. [Google Scholar] [CrossRef]
- Gadgil, P.; Shah, J.; Chow, D.-L. Enhanced brain delivery with lower hepatic exposure of lazaroid loaded nanostructured lipid carriers developed using a design of experiment approach. Int. J. Pharm. 2018, 544, 265–277. [Google Scholar] [CrossRef]
- Sarma, A.; Das, M.K. Formulation by Design (FbD) approach to develop Tenofovir Disoproxil Fumarate loaded Nanostructured Lipid Carriers (NLCs) for the aptness of nose to brain delivery. J. Drug Deliv. Ther. 2019, 9, 148–159. [Google Scholar] [CrossRef]
- Aqil, M.; Kamran, M.; Ahad, A.; Imam, S.S. Development of clove oil based nanoemulsion of olmesartan for transdermal delivery: Box–Behnken design optimization and pharmacokinetic evaluation. J. Mol. Liq. 2016, 214, 238–248. [Google Scholar] [CrossRef]
- Alam, T.; Khan, S.; Gaba, B.; Haider, M.F.; Baboota, S.; Ali, J. Adaptation of Quality by Design-Based Development of Isradipine Nanostructured–Lipid Carrier and Its Evaluation for In Vitro Gut Permeation and In Vivo Solubilization Fate. J. Pharm. Sci. 2018, 107, 2914–2926. [Google Scholar] [CrossRef] [PubMed]
- Barkat, M.A.; Rizwanullah, M.; Beg, S.; Pottoo, F.H.; Siddiqui, S.; Ahmad, F.J. Paclitaxel-loaded nanolipidic carriers with improved oral bioavailability and anticancer activity against human liver carcinoma. AAPS Pharmscitech 2019, 20, 87. [Google Scholar]
- EDQM. European Pharmacopeia, 5th ed.; EDQM: Strasbourg, France, 2005. [Google Scholar]
- Guideline, I.H.T. Validation of analytical procedures: Text and methodology Q2 (R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, November 2005. [Google Scholar]
- Chauhan, M.K.; Sharma, P.K. Optimization and characterization of rivastigmine nanolipid carrier loaded transdermal patches for the treatment of dementia. Chem. Phys. Lipids 2019, 224, 104794. [Google Scholar] [CrossRef]
- De Jesus, H.E.P.P. Aplicação de Polímeros Sensíveis a Estímulos em Sistemas de Libertação Modificada de Fármacos Para Uso Oftálmico. Doctoral Dissertation, Faculty of Pharmacy, University of Porto, Porto, Portugal, 2016. [Google Scholar]
- Eiras, F. Desenvolvimento, Caracterização E avaliação da Biocompatibilidade e do Potencial Irritativo de Formulações Cosméticas à Base de Nanopartículas Lipídicas. Master’s Thesis, Faculty of Pharmacy, University of Porto, Porto, Portugal, 2016. [Google Scholar]
- Eiras, F.; Amaral, M.H.; Silva, R.; Martins, E.; Lobo, J.S.; Silva, A.C. Characterization and biocompatibility evaluation of cutaneous formulations containing lipid nanoparticles. Int. J. Pharm. 2017, 519, 373–380. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Wu, P.C.; Huang, Y.B.; Chang, J.S.; Lin, C.L.; Tsai, Y.H.; Fang, J.Y. Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. Int. J. Pharm. 2012, 423, 461–470. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. EDTA and α-tocopherol improve the chemical stability of astaxanthin loaded into nanostructured lipid carriers. Eur. J. Lipid Sci. Technol. 2014, 116, 968–977. [Google Scholar] [CrossRef]
- Grande, G.; Vetrano, D.L.; Mangialasche, F. Risk factors and prevention in Alzheimer’s disease and dementia. In Neurodegenerative Diseases; Springer: Berlin, Germany, 2018; pp. 93–112. [Google Scholar]
- Tichota, D.M.; Silva, A.C.; Lobo, J.M.S.; Amaral, M.H. Design, characterization, and clinical evaluation of argan oil nanostructured lipid carriers to improve skin hydration. Int. J. Nanomed. 2014, 9, 3855. [Google Scholar]
- Mendes, A.; Silva, A.C.; Catita, J.A.M.; Cerqueira, F.; Gabriel, C.; Lopes, C.M. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: Improving antifungal activity. Colloids Surf. B Biointerfaces 2013, 111, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, D.D.; Pokharkar, V.B. Engineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: Physicochemical investigations. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 32–42. [Google Scholar] [CrossRef]
- Mendes, I.; Ruela, A.L.M.; Carvalho, F.C.; Freitas, J.T.J.; Bonfilio, R.; Pereira, G.R. Development and characterization of nanostructured lipid carrier-based gels for the transdermal delivery of donepezil. Colloids Surf. B Biointerfaces 2019, 177, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, K.; Mishra, M.K.; Srivastava, R. Fabrication and Characterization of Pluronic F68 and Phospholipon 90g Embedded Nanoformulation for Sertraline Delivery: An Optimized Factorial Design Approach and In Vivo Study. Asian J. Pharm. Res. Dev. 2019, 7, 59–66. [Google Scholar] [CrossRef]
- Anjum, R.; Lakshmi, P. A review on solid lipid nanoparticles; focus on excipients and formulation techniques. Int. J. Pharm. Sci. Res. 2019. [Google Scholar] [CrossRef]
- Bhatt, R.; Singh, D.; Prakash, A.; Mishra, N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv. 2015, 22, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Marple, B.; Roland, P.; Benninger, M. Safety review of benzalkonium chloride used as a preservative in intranasal solutions: An overview of conflicting data and opinions. Otolaryng Head Neck 2004, 130, 131–141. [Google Scholar] [CrossRef]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef]
- Wavikar, P.; Pai, R.; Vavia, P. Nose to brain delivery of rivastigmine by In Situ gelling cationic nanostructured lipid carriers: Enhanced brain distribution and pharmacodynamics. J. Pharm. Sci. 2017, 106, 3613–3622. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herrán, E.; Ruiz-Ortega, J.A.; Miguelez, C.; Igartua, M.; Lafuente, J.V.; Pedraz, J.L.; Ugedo, L.; Hernández, R.M. Intranasal administration of chitosan-coated nanostructured lipid carriers loaded with GDNF improves behavioral and histological recovery in a partial lesion model of Parkinson’s disease. J. Biomed. Nanotechnol. 2016, 12, 2220–2280. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Tagalpallewar, A.A.; Kokare, C.R. Agranulocytosis-protective olanzapine-loaded nanostructured lipid carriers engineered for CNS delivery: Optimization and hematological toxicity studies. AAPS Pharmscitech 2019, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hidau, M.K.; Gautam, S.; Gupta, K.; Singh, K.P.; Singh, S.K.; Singh, S. Glycol chitosan functionalized asenapine nanostructured lipid carriers for targeted brain delivery: Pharmacokinetic and teratogenic assessment. Int. J. Biol. Macromol. 2018, 108, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Lopes, C.M.; Fonseca, J.; Soares, M.E.; Santos, D.; Souto, E.B.; Ferreira, D. Risperidone release from solid lipid nanoparticles (SLN): Validated HPLC method and modelling kinetic profile. Curr. Pharm. Anal. 2012, 8, 307–316. [Google Scholar] [CrossRef]
- Alexander, A.; Agrawal, M.; Chougule, M.B.; Saraf, S.; Saraf, S. Nose-to-brain drug delivery: An alternative approach for effective brain drug targeting. In Nanopharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Wasan, E.K.; Syeda, J.; Strom, S.; Cawthray, J.; Hancock, R.E.; Wasan, K.M.; Gerdts, V. A lipidic delivery system of a triple vaccine adjuvant enhances mucosal immunity following nasal administration in mice. Vaccine 2019, 37, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.P.; Butani, S.B. Resveratrol anchored nanostructured lipid carrier loaded In Situ gel via nasal route: Formulation, optimization and In Vivo characterization. J. Drug Deliv. Sci. Technol. 2019, 51, 214–223. [Google Scholar] [CrossRef]
- Muller, R.H.; Keck, C.M. Challenges and solutions for the delivery of biotech drugs–a review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef]
- Mohtar, N.; Khan, N.A.; Darwis, Y. Solid lipid nanoparticles of atovaquone based on 24 full-factorial design. Iran J. Pharm. Res. 2015, 14, 989. [Google Scholar]
- Müller, R.H.; MaÈder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Campbell, C.; Morimoto, B.H.; Nenciu, D.; Fox, A.W. Drug development of intranasally delivered peptides. Ther. Deliv. 2012, 3, 557–568. [Google Scholar] [CrossRef]
- Silva, A.; Amaral, M.H.; González-Mira, E.; Santos, D.; Ferreira, D. Solid lipid nanoparticles (SLN)-based hydrogels as potential carriers for oral transmucosal delivery of Risperidone: Preparation and characterization studies. Colloids Surf. B Biointerfaces 2012, 93, 241–248. [Google Scholar] [CrossRef]
- Ofori-Kwakye, K.; Mfoafo, K.A.; Kipo, S.L.; Kuntworbe, N.; El Boakye-Gyasi, M. Development and evaluation of natural gum-based extended release matrix tablets of two model drugs of different water solubilities by direct compression. Saudi Pharm. J. 2016, 24, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, G.; Singh, M. In-vitro drug release characterization models. Int J. Pharm Stud. Res. 2011, 2, 77–84. [Google Scholar]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Baig, M.S.; Ahad, A.; Aslam, M.; Imam, S.S.; Aqil, M.; Ali, A. Application of Box–Behnken design for preparation of levofloxacin-loaded stearic acid solid lipid nanoparticles for ocular delivery: Optimization, In Vitro release, ocular tolerance, and antibacterial activity. Int. J. Biol. Macromol. 2016, 85, 258–270. [Google Scholar] [CrossRef]
- European Medicines Agency. Stability Testing of New Drug Substances and Products (CPMP/ICH/2736/99); European Medicines Agency: London, UK, 2003.
- Negi, L.M.; Jaggi, M.; Talegaonkar, S. Development of protocol for screening the formulation components and the assessment of common quality problems of nano-structured lipid carriers. Int. J. Pharm. 2014, 461, 403–410. [Google Scholar] [CrossRef]
- Bastogne, T. Quality-by-design of nanopharmaceuticals–A state of the art. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2151–2157. [Google Scholar] [CrossRef]
- Dhat, S.; Pund, S.; Kokare, C.; Sharma, P.; Shrivastava, B. Risk management and statistical multivariate analysis approach for design and optimization of satranidazole nanoparticles. Eur. J. Pharm. Sci. 2017, 96, 273–283. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Hommoss, A. Nanostructured Lipid Carriers (NLC) in Dermal and Personal Care Formulations. Doctoral Dissertation, Free University of Berlin, Berlin, Germany, 2009. [Google Scholar]
- Patil, S.S.; Kumbhar, D.D.; Manwar, J.V.; Jadhao, R.G.; Bakal, R.L.; Wakode, S. Ultrasound-Assisted Facile Synthesis of Nanostructured Hybrid Vesicle for the Nasal Delivery of Indomethacin: Response Surface Optimization, Microstructure, and Stability. AAPS Pharmscitech 2019, 20, 97. [Google Scholar] [CrossRef]
- Gabal, Y.M.; Kamel, A.O.; Sammour, O.A.; Elshafeey, A.H. Effect of surface charge on the brain delivery of nanostructured lipid carriers In Situ gels via the nasal route. Int. J. Pharm. 2014, 473, 442–457. [Google Scholar] [CrossRef]
- Rapp, B.E. Microfluidics: Modeling, Mechanics and Mathematics; William Andrew: Oxford, UK; Cambridge, MA, USA, 2016. [Google Scholar]
- Kaduk, J.A.; Zhong, K.; Gindhart, A.M.; Blanton, T.N. Crystal structure of rivastigmine hydrogen tartrate Form I (Exelon®), C14H23N2O2(C4H5O6). Powder Diffr. 2016, 31, 97–103. [Google Scholar] [CrossRef]
- Potter, P.E.; Kerecsen, L. Cholinesterase Inhibitors. Front. CNS Drug Discov. 2017, 3, 201–239. [Google Scholar]
- Ekambaram, P.; Sathali, A.A.H.; Priyanka, K. Solid lipid nanoparticles: A review. Sci Rev. Chem. Commun. 2012, 2, 80–102. [Google Scholar]
- Jain, K.; Sood, S.; Gowthamarajan, K. Optimization of artemether-loaded NLC for intranasal delivery using central composite design. Drug Deliv. 2015, 22, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Chavda, V.; Tandel, H.; Domadiya, K. Nasal Medication Conveyance Framework: An Approach for Brain Delivery from Essential to Cutting Edge. J. Med. 2019, 6, 14–27. [Google Scholar]
- Jazuli, I.; Nabi, B.; Alam, T.; Baboota, S.; Ali, J. Optimization of Nanostructured Lipid Carriers of Lurasidone Hydrochloride Using Box-Behnken Design for Brain Targeting: In Vitro and In Vivo Studies. J. Pharm. Sci. 2019. [Google Scholar] [CrossRef]
- Müller, R.H.; Rühl, D.; Runge, S.A. Biodegradation of solid lipid nanoparticles as a function of lipase incubation time. Int. J. Pharm. 1996, 144, 115–121. [Google Scholar] [CrossRef]
- Ribeiro, L.N.; Breitkreitz, M.C.; Guilherme, V.A.; da Silva, G.H.; Couto, V.M.; Castro, S.R.; de Paula, B.O.; Machado, D.; de Paula, E. Natural lipids-based NLC containing lidocaine: From pre-formulation to In Vivo studies. Eur. J. Pharm. Sci. 2017, 106, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Sharma, G.; Singh, B.; Nirbhavane, P.; Tyagi, R.K.; Shukla, R.; Katare, O.P. Quality by Design (QbD)-enabled development of aceclofenac loaded-nano structured lipid carriers (NLCs): An improved dermatokinetic profile for inflammatory disorder (s). Int. J. Pharm. 2017, 517, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, W.M.; Schwabe, K.; Müller, R.H.; Keck, C.M. Preservation of nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2010, 76, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: Preparation and In Vitro characterization studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]
- Cavalcanti, S.; Nunes, C.; Lima, S.C.; Soares-Sobrinho, J.L.; Reis, S. Optimization of nanostructured lipid carriers for Zidovudine delivery using a microwave-assisted production method. Eur. J. Pharm. Sci. 2018, 122, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Jojo, G.M.; Kuppusamy, G.; De, A.; Karri, V.N.R. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev. Ind. Pharm. 2019, 45, 1061–1072. [Google Scholar] [CrossRef]
| Formulation Variables | Levels | |||
|---|---|---|---|---|
| X1 Precirol® ATO 5: Vitamin E ratio (%, w/w) X2 Tween® 80: Phospholipon® 90G concentration (%, w/w) | X1 X2 | Low (−1) | Medium (0) | High (+1) |
| 5.94:3.94 | 6.94:2.94 | 7.94:1.94 | ||
| 2.00:1.00 | 2.50:0.50 | 2.50:1.50 | ||
| X1 X2 | 6.94:2.94 | 7.94:1.94 | 8.94:0.94 | |
| 2.00:1.00 | 2.50:0.50 | 2.50:1.50 | ||
| X1 X2 | 5.94:3.94 | 6.94:2.94 | 7.94:1.94 | |
| 3.00:1.00 | 3.50:0.50 | 3.50:1.50 | ||
| X1 X2 | 6.94:2.94 | 7.94:1.94 | 8.94:0.94 | |
| 3.00:1.00 | 3.50:0.50 | 3.50:1.50 | ||
| X1 X2 | 5.94:3.94 | 6.94:2.94 | 7.94:1.94 | |
| 4.00:0.40 | 4.00:1.00 | 4.50:0.50 | ||
| X1 X2 | 6.94:2.94 | 7.94:1.94 | 8.94:0.94 | |
| 4.00:0.40 | 4.00:1.00 | 4.50:0.50 | ||
| Instrumental Parameters | Levels | |||
|---|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | ||
| X1: Emulsification speed (rpm) + X2: HPH cycles | X1 | 11,000 | 13,400 | 14,000 |
| X2 | 9 | 12 | 18 | |
| X1: Emulsification speed (rpm) + X3: sonication amplitude | X1 | 11,000 | 13,400 | 14,000 |
| X3 | 55 | 75 | 85 | |
| Critical Quality Attributes (CQAs) | Z-Ave (nm) 1 | D50 (nm) 2 | D90 (nm) 2 | PDI 3 | ZP 4 (mV) | EE 5 (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Runs | O 6 | P 7 | O 6 | P 7 | O 6 | P 7 | O 6 | P 7 | O 6 | P 7 | O 6 | P 7 |
| 1 | 166.600 ± 1.911 | 175.000 | 58.400 ± 0.102 | 58.650 | 148.602 ± 0.570 | 163.740 | 0.221 ± 0.003 | 0.224 | −28.000 ± 0.253 | −28.900 | 94.001 ± 0.143 | 94.690 |
| 2 | 158.301 ± 0.852 | 150.450 | 61.401 ± 0.244 | 57.950 | 159.600 ± 0.992 | 155.040 | 0.224 ± 0.007 | 0.230 | −28.600 ± 0.251 | −29.020 | 94.890 ± 0.271 | 94.340 |
| 3 | 187.104 ± 0.980 | 190.190 | 51.100 ± 0.132 | 53.440 | 148.603 ± 0.793 | 161.200 | 0.263 ± 0.002 | 0.247 | −29.000 ± 0.192 | −29.670 | 92.900 ± 0.232 | 93.810 |
| 4 | 173.305 ± 1.231 | 160.140 | 75.900 ± 0.140 | 74.540 | 256.002 ± 0.651 | 248.900 | 0.234 ± 0.004 | 0.230 | −33.300 ± 0.231 | −33.490 | 93.594 ± 0.181 | 93.260 |
| 5 | 166.600 ± 0.893 | 165.220 | 59.901 ± 0.190 | 61.920 | 182.001 ± 0.733 | 176.180 | 0.225 ± 0.001 | 0.212 | −27.600 ± 0.280 | −26.890 | 93.761 ± 0.310 | 93.730 |
| 6 | 176.701 ± 0.972 | 182.820 | 70.900 ± 0.171 | 69.970 | 243.000 ± 0.651 | 240.760 | 0.224 ± 0.003 | 0.227 | −31.000 ± 0.300 | −30.600 | 92.702 ± 0.251 | 92.350 |
| 7 | 192.303 ± 0.114 | 183.180 | 51.200 ± 0.150 | 49.130 | 146.000 ± 0.910 | 128.040 | 0.242 ± 0.004 | 0.250 | −31.300 ± 0.371 | −30.400 | 96.390 ± 0.401 | 95.320 |
| 8 | 130.700 ± 0.791 | 144.560 | 60.401 ± 0.221 | 63.560 | 174.001 ± 0.882 | 183.900 | 0.251 ± 0.001 | 0.242 | −33.400 ± 0.193 | −33.190 | 94.001 ± 0.254 | 94.690 |
| 9 | 192.300 ± 0.150 | 192.300 | 61.301 ± 0.143 | 61.300 | 207.000 ± 0.980 | 207.000 | 0.243 ± 0.002 | 0.245 | −32.300 ± 0.204 | −32.300 | 95.500 ± 0.190 | 95.500 |
| 10 | 192.300 ± 0.150 | 192.300 | 61.301 ± 0.143 | 61.300 | 207.000 ± 0.980 | 207.000 | 0.234 ± 0.002 | 0.245 | −32.300 ± 0.204 | −32.300 | 95.500 ± 0.190 | 95.500 |
| Tested Ratios (w/w, %) | DoE 1 | LD 2 (Mean ± SD 3, n 4 = 5) | DLS 5 (Mean ± SD 3, n 4 = 5) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SL/LL 6 | Tw/Ph 7 | Levels | D50 8 (nm) | D90 9 (nm) | Z-Ave 10 (nm) | PDI 11 | ZP 12 (mV) | EE 13 (%) | |
| 5.94:3.94 | 4.0:0.4 | −1.00 | −1.00 | 58.402 ± 0.009 | 148.600 ± 0.009 | 166.602 ± 0.010 | 0.221 ± 0.011 | −28.000 ± 0.011 | 94.001 ± 0.012 |
| 5.94:3.94 | 4.5:0.5 | −1.00 | 1.00 | 61.400 ± 0.008 | 159.603 ± 0.010 | 158.300 ± 0.008 | 0.213 ± 0.010 | −28.601 ± 0.010 | 94.890 ± 0.008 |
| 7.94:1.94 | 4.0:0.4 | 1.00 | −1.00 | 51.101 ± 0.010 | 148.604 ± 0.011 | 187.101 ± 0.012 | 0.251 ± 0.010 | −29.000 ± 0.008 | 92.903 ± 0.011 |
| 7.94:1.94 | 4.5:0.5 | 1.00 | 1.00 | 75.903 ± 0.008 | 256.012 ± 0.008 | 173.302 ± 0.013 | 0.231 ± 0.009 | −33.300 ± 0.011 | 93.590 ± 0.013 |
| 5.94:3.94 | 4.0:1.0 | −1.41 | 0 | 59.902 ± 0.010 | 182.013 ± 0.007 | 166.603 ± 0.009 | 0.220 ± 0.010 | −27.600 ± 0.010 | 93.760 ± 0.007 |
| 6.94:2.94 | 4.5:0.5 | 0 | 1.41 | 60.401 ± 0.011 | 174.020 ± 0.011 | 130.703 ± 0.011 | 0.251 ± 0.011 | −33.400 ± 0.009 | 94.001 ± 0.010 |
| 8.94:0.94 | 4.5:0.5 | 1.00 | 1.00 | 63.200 ± 0.010 | 199.211 ± 0.010 | 174.200 ± 0.009 | 0.290 ± 0.010 | −32.900 ± 0.010 | 98.300 ± 0.011 |
| Observed Responses | Ultrasound Technique | High-Pressure Homogenization (HPH) Method |
| Z-Ave 1 (nm) | 114.000 ± 1.910 | 109.000 ± 0.850 |
| PDI 2 | 0.221 ± 0.003 | 0.196 ± 0.007 |
| ZP 3 (mV) | −30.633 ± 0.288 | −30.466 ± 0.252 |
| EE 4 (%) | 96.987 ± 0.446 | 97.174 ± 0.297 |
| Predicted Responses | Ultrasound Technique | High-Pressure Homogenization (HPH) Method |
| Z-Ave 1 (nm) | 155.000 | 124.000 |
| PDI 2 | 0.190 | 0.242 |
| ZP 3 (mV) | −28.400 | −29.100 |
| EE 4 (%) | 95.140 | 97.600 |
| Release Media | Formulation | R2 | n | |||
|---|---|---|---|---|---|---|
| Zero Order | First Order | Higuchi Model | Korsmeyer–Peppas | |||
| PBS, pH 6.4 | NLCs | 0.649 | 0.796 | 0.799 | 0.936 | 0.636 |
| NLCHPH | 0.773 | 0.796 | 0.919 | 0.978 | 0.670 | |
| SNE, pH 6.4 | NLCs | 0.630 | 0.785 | 0.757 | 0.978 | 0.599 |
| NLCHPH | 0.859 | 0.613 | 0.954 | 0.985 | 0.667 | |
| Formulation | Day | T 1 (°C) | D50 2 (nm) | D90 2 (nm) | Z-Ave 3 (nm) | PDI 4 | ZP 5 (mV) | EE 6 (%) |
|---|---|---|---|---|---|---|---|---|
| NLCs | 0 | - | 57.972 ± 0.971 | 184.300 ± 0.721 | 114.094 ± 0.990 | 0.221 ± 0.003 | −30.610 ± 0.321 | 96.983 ± 0.421 |
| 90 | 4.0 ± 0.5 | 60.590 ± 0.574 | 189.981 ± 0.995 | 116.230 ± 0.911 | 0.224 ± 0.020 | −30.901 ± 0.452 | 94.580 ± 0.111 | |
| 20.0 ± 0.5 | 67.653 ± 0.750 | 200.760 ± 0.651 | 125.630 ± 0.764 | 0.227 ± 0.005 | −31.073 ± 0.694 | 94.677 ± 0.140 | ||
| NLCHPH | 0 | - | 55.971 ± 0.831 | 144.322 ± 0.972 | 109.400 ± 0.895 | 0.196 ± 0.007 | −30.470 ± 0.394 | 97.152 ± 0.341 |
| 90 | 4.0 ± 0.5 | 65.293 ± 0.654 | 199.674 ± 0.913 | 111.780 ± 0.001 | 0.212 ± 0.004 | −29.971 ± 0.410 | 95.416 ± 0.980 | |
| 20.0 ± 0.5 | 68.890 ± 0.543 | 211.763 ± 0.742 | 114.980 ± 0.852 | 0.210 ± 0.003 | −30.050 ± 0.540 | 94.448 ± 0.991 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, S.; Costa, C.P.; Loureiro, J.A.; Alves, J.; Peixoto, A.F.; Forbes, B.; Sousa Lobo, J.M.; Silva, A.C. Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters. Pharmaceutics 2020, 12, 599. https://doi.org/10.3390/pharmaceutics12070599
Cunha S, Costa CP, Loureiro JA, Alves J, Peixoto AF, Forbes B, Sousa Lobo JM, Silva AC. Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters. Pharmaceutics. 2020; 12(7):599. https://doi.org/10.3390/pharmaceutics12070599
Chicago/Turabian StyleCunha, Sara, Cláudia Pina Costa, Joana A. Loureiro, Jorge Alves, Andreia F. Peixoto, Ben Forbes, José Manuel Sousa Lobo, and Ana Catarina Silva. 2020. "Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters" Pharmaceutics 12, no. 7: 599. https://doi.org/10.3390/pharmaceutics12070599
APA StyleCunha, S., Costa, C. P., Loureiro, J. A., Alves, J., Peixoto, A. F., Forbes, B., Sousa Lobo, J. M., & Silva, A. C. (2020). Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters. Pharmaceutics, 12(7), 599. https://doi.org/10.3390/pharmaceutics12070599









